Abstract
Background
Mesenchymal stromal cells, MSCs, show expression of specific antigens on their surface. The aim of the study is to assess the phenotype of stem cells like isolated from the umbilical cord with respect to the presence of surface antigens CD34, CD90, and CD105 and differences in the expression of surface antigens in cells isolated from freshly sampled material in comparison with the phenotype of cells from in vitro culture.
Material/Methods
Stem cells collected from the umbilical cord from healthy patients and then cultured in vitro. To assess the phenotype of stem cells, cytometric analysis was carried out. To assess the phenotype of cells we used fluorescently labelled monoclonal antibodies: APC Mouse anti-human CD34, PC5 Mouse anti-human CD90 and PE Mouse anti-human CD105.
Results
In the case of cells from the umbilical cord and then cultured in vitro for the period of 10–14 days CD34 expression is lower (69,5%) in comparison with the group of cells not cultured. Not cultured cells were demonstrated 37% of cells co-expression of antigens CD34 and CD105, over 21% of CD34/CD90 cells and over 24% of CD105/CD90. Cultured cells group was showed higher percentage of CD90, CD105, CD34/CD105, CD34/CD90, CD105/CD90 in comparison with not cultured cells.
Conclusions
Our reults suggested that adherent cells population from umbilical cord, demonstrate CD34 expression In vivo. Moreover, the phenotype of MSCs, mainly in the context of CD34 expression, may vary depending on the place of collection of cells and the length of growing the cell culture.
MeSH Keywords: Antigens, CD34; Antigens, Thy-1; Mesenchymal Stromal Cells; Umbilical Cord
Background
Mesenchymal stromal cells (MSCs) are also known as stromal stem cells, mesenchymal stem cells, and adherent non-hematopoietic stem cells. These cells derive from one of the germ layers, and have the potential to differentiate into tissues originating from the mesoderm, although some sources report that their plasticity is higher and they have the potential to differentiate into tissues of other germ layers as well. This topic is still controversial. Initially, these cells were identified in the bone marrow as a second population distinct from the hematopoietic stem cells [1,2], but currently it is believed that these cells are present in almost every tissue of the adult body [3,4], as well as in “perinatal” tissues. MSCs have been identified in may tissues, including skin, adipose tissue, brain, skeletal muscles, heart, liver, pancreas, blood vessels, peripheral blood, retina, breast tissue, prostate, testes and ovaries [5] dental pulp, synovial fluid, hair bulb [6], menstrual blood [7], breast milk [8], umbilical cord blood, placenta, and umbilical cord. Because of differences in number of cells present in various tissues and their different availability, several sources are currently used to obtain MSCs [4].
The number of MSCs depends on the tissue where they appear, and the number of stem cells in the body decreases with age. It has been suggested that bone marrow MSCs may be involved in aging, although their role in this process remains unclear [1,9,10].
There is no single characteristic parameter that can be used to identify MSCs. MSCs, although present in different tissues, do exhibit a variety of common features, including the ability of proliferation in vitro and adherence to the walls of plastic, and their appearance while growing is similar to fibroblast colonies. They show expression of specific antigens on their surface: CD44, CD71, CD73 (endoglin), CD90 (Thy-1), CD105 (ecto-5′-nucleotidase), CD166, and Stro-1, but they do not demonstrate expression of CD11, CD14, CD31, CD34, or CD45, although MSCs may also show CD34 [11–14]. They are capable of producing and secreting proangiogenic, antiapoptotic, immune-stimulating, and proliferation-stimulating factors (including VEGF, M-SCF, HGF, GM-CSF, G-CSF, SDF-1, TGF-β, PGE-2, and interleukins 6, 8, 11, 12, 14, and 15) [9,15]. It has been suggested that the common name of MSCs combines a heterogeneous group of mesenchymal cell potentials, including adipocytes, fibroblasts, osteoblasts, cells of adventitia, and pericytes [4,12,15,16].
In vitro studies show their relatively high proliferative potential, but it is not known whether they maintain the speed of division in vivo as suggested by some authors, or under physiological conditions in the body when they are in a “sleep mode” [17].
MSCs were also found in adipose tissue, which seems to be a much better source of MSCs due to its availability (less invasive procedure to obtain them) and abundance, as well as the number of MSCs, which is higher in comparison with bone marrow [2]. Because of the slightly different characteristics of MSCs derived from adipose tissue, it is proposed to call them adipose-derived stromal cells (ADSCs). These cells have greater proliferative potential in vitro when compared with cells isolated from bone marrow. They also have antigens not present on the surface of MSCs [2, 4,18,19].
Perinatal tissues such as umbilical cord (UC-MSCs), Wharton’s jelly (WJCs), placenta, and umbilical cord blood are also a rich source of MSCs. Because they contain fetal mesenchymal stromal cells (fMSCs), they have a lower degree of maturity, greater proliferative potential, and broader differentiation potential. They seem to be an excellent source of therapeutic cells with enormous regenerative potential [20,21].
Umbilical cord Wharton’s jelly has emerged as a good source of stem cells. According to several studies, cells obtained from this tissue have both the features of MSCs and characteristics of embryonic stem cells. MSCs have unique properties, including the high rate of proliferation, hyper-immunogenicity, broad multipotential, and anti-cancer properties, as well as the ability to modulate the immune response and secretion of cytokines that regulate apoptosis [22]. MSCs have been studied in the context of their use in therapy, but first it is necessary to better understand the phenotype of these cells and their physiological regulation in vivo and in vitro.
MSCs have the ability to differentiate mainly into cells of bone, adipose tissue, cartilage tissue, muscle tissue, and blood vessels [3]. According to numerous studies in cell cultures, using appropriate environmental conditions, growth factors, or culture vessels, it is possible to induce differentiation of these cells into a number of different cells, including cardiomyocytes, endothelial cells, chondrocytes, adipocytes, and muscle cells [15,23–25].
MSCs are used in therapy and the main therapeutic strategies associated with MSCs are related to their properties. The ability to differentiate into adipocytes, osteoblasts, and chondrocytes is used in regeneration of bones, cartilage, and muscles. The ability to secrete numerous cytokines is increasingly used in administration of MSCs to patients to improve functioning and regeneration of cardiac muscle after myocardial infarction. Transplantation of MSCs is also used in regenerating lungs, liver, or skin (e.g., after burns). The immunosuppressive potential of MSCs is utilized in organ and cell transplants, as well as in treating inflammatory diseases [15]. Numerous clinical studies using MSCs are currently being performed throughout the world.
It has not been clearly demonstrated if MSCs show increased plasticity in vitro, and are therefore capable of differentiating into cells from other germ layers in cell culture conditions [25,26]. There is no unified system of nomenclature and classification for isolated MSCs. A universal characteristic pattern of expression of antigens on the surface of each class of stem cells have not been created, creating difficulty in comparing studies undertaken by different groups of researchers to identify isolated cells in different matrices [27].
The aim of the study
The purpose of this study was to assess the phenotype of cells isolated from Wharton’s jelly with respect to the presence of surface antigens CD34, CD90, and CD105. We also attempt to assess differences in the expression of surface antigens CD34, CD90, and CD105 in cells isolated from freshly sampled material in comparison with the phenotype of cells from in vitro culture.
Material and Methods
Umbilical cords were collected from 10 healthy patients who shortly before had given birth in the Department of Obstetrics and Pathology of Pregnancy of the Independent Public Teaching Hospital No. 1 in Lublin. The material was preserved in DMEM medium. The procedure of isolating cells started within approximately 30 min after collecting samples. Women’s ages ranged from 24 to 33 years, the mean age was 29 years 8 months 12 days, and the median age was 30.5 years.
The study was performed according to the protocol and with the consent of the Bioethics Committee of the Medical University of Lublin (No. KE-0254/128/2014) and with the consent of the patients and the Head of the Department of Obstetrics and Pathology of Pregnancy of the Independent Public Teaching Hospital No. 1.
The studied material was divided into 2 groups:
WJC (Wharton’s Jelly-Derived Cells) – cells collected from Wharton’s jelly. The study group consisted of 5 samples.
WJC-CC (Wharton’s Jelly-Derived Cells – Cell Culture) – cells collected from Wharton’s jelly and then cultured in vitro in adherent conditions for 14 days. The study group consisted of 5 samples.
Isolation of cells from Wharton’s jelly from umbilical cords
Isolation of cells from Wharton jelly of umbilical cords was done through enzymatic digestion using collagenase type I (Sigma, USA). Isolation was conducted according to the following procedure: The umbilical cord immediately after birth was placed in a culture medium containing DMEM medium with L-glutamine and glucose, supplemented with 10% FBS and 1% antibiotic (as below). A fragment of the umbilical cord (about 10 cm long), was rinsed several times in sterile PBS supplemented with antibiotic (as below). Next, it was cut with a scalpel into 2–5-mm3 pieces. Fragments of Wharton’s jelly were placed in a sterile solution of collagenase type I (10 mg of collagenase/30 ml of solution) and digested for 2–3 h at 37°C. After incubation, samples were rinsed twice in PBS, followed by 10-min centrifugation at room temperature at 800 rpm (centrifuge 5810R f. Eppendorf). After rinsing, the cells were passed through a 100-μm sieve mesh to cleanse the mixture.
Cell culture
Cells isolated from the Wharton’s jelly were cultured in vitro for up to 14 days in an incubator and in a suitable DMEM culture medium supplemented with 10% fetal bovine serum and 1% antibiotics. Cell culture conditions are shown in Table 1. Culture conditions: Type of cell culture: Adherent (medium volume – 10 ml); Culture vessel – Culture bottle – surface area of the bottom: 25 cm2 TC Flask T25,Cell+, Sarstedt, Germany; Culture medium: DMEM (1x)+GlutaMAX™-I [+] 1 g/L D-Glucose [+] Pyruvate Gibco, UK; Serum - Heat Inactivated FBS (Fetal Bovine Serum) Gibco, USA; Antibiotics – Amphotericin B 250 μg/ml + Penicillin/Streptomycin (100×) PAA, Austria; Temperature: 37°C; O2 concentration: 4%; CO2 concentration: 5%; Moisture: 95%.
Table 1.
Cell culture conditions.
| Source of cells | Umbilical cord (after collagenase digestion) |
| Type of cell culture | Adherent (medium volume – 10 ml) |
| Culture vessel | Culture bottle – surface area of the bottom: 25 cm2 TC Flask T25, Cell+, Sarsted, Germany |
| Culture medium | DMEM (1×)+GlutaMAX™-I [+] 1g/L D-Glucos [+] Pyruvate Gibco, UK |
| Serum | Heat Inactivated FBS (Fetal Bovine Serum) Gibco, USA |
| Antibiotics | Amphotericin B 250 μg/ml + Penicillin/Streptomycin (100×) PAA, Austria |
| Temperature | 37°C |
| O2 concentration | 4% |
| CO2 concentration | 5% |
| Moisture | 95% |
Culture medium was changed every 3 days. In the end (P0), cells isolated from Wharton’s jelly were mechanically removed from the walls of the culture vessel with a sterile cell scraper, and rinsed in PBS. The cells were then prepared for flow cytometry analysis.
Cytometric analysis
To assess the phenotype of cells, cytometric analysis was carried out, using a digital flow cytometer with a MoFlow XDP Beckman Coulter (USA) cell sorter and flow cytometer with vision in real-time FlowSight Amnis (USA). Quantification data with MoFlow XPD were acquired.
The following fluorescently labelled monoclonal antibodies were used to assess the phenotype of cells:
APC Mouse anti-Human CD34 (Beckman Coulter, France);
PC5 Mouse anti-Human CD90 (Beckman Coulter, France);
PE Mouse anti-Human CD105 (Beckman Coulter, France).
The samples were prepared according to the following protocol:
Isolated cells/cultured cells were rinsed in PBS and centrifuged, then cell pellets were pipetted and resuspended in 200 μl of PBS without Ca and Mg ions (Biomed, Poland). The number of cells was determined using a TC20 Automated Cell Counter (BioRad) with trypan blue staining. Samples were adjusted to a concentration of 100 000 cells per ml, then we took 200 μl of the sample and added antibodies for surface antigens (CD34, CD90, CD105) in the amount of 10 μl of each antibody in a sample. Samples were mixed and incubated for 15 min at room temperature in the dark. We added 100 μl of lysis buffer (Versa Lyse, Beckman Coulter) to cell pellets to hemolyze the erythrocytes from the umbilical cord. Samples were mixed by shaking and incubated for 20 min at room temperature in the dark. After centrifugation, the supernatant was discarded and cell pellets were pipetted and resuspended in 200 μl of PBS without Ca and Mg ions.
Analysis of 10 000 events was recorded for each probe. Cells without any staining were used as a negative control and an isotype control was made. Single stained samples were used for compensation. FMO (fluorescence minus one) samples were used to determine the gating.
Statistical analysis
The means and standard deviations (SD) were calculated. Statistica software (StatSoft, Poland) was used to test whether data followed a normal distribution using the Shapiro-Wilke test. Statistical analysis was done using the unpaired t test and Pearson’s correlation. Results were considered statistically significant at p<0.05.
Results
Microscopic analysis
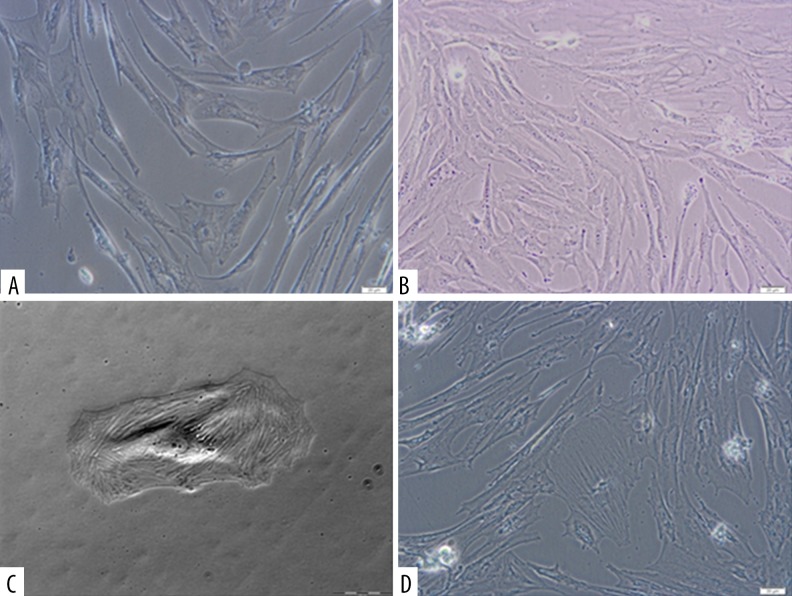
As a result of the growing Wharton’s jelly cell culture, proliferation capacity of cells was demonstrated in vitro, cells similar to fibroblast colonies were obtained, and the ability of cells to adhere to the plastic walls was shown. Cytometric analysis of cells tested showed the presence of CD90 and CD105 surface antigens, which are characteristic for MSCs, and CD34 antigen was observed as well. Microscopic assessment of cells was made in the course of culture. Figure 1A–1D presents photographs of WJC-CC from Wharton’s jelly on days 9, 10, and 14 of in vitro culture.
Figure 1.
(A) Microscopic image of sample 01WJC-CC from Wharton’s jelly. The photograph was taken on day 9 (magnification 200×, eyepiece 10×, lens 20×) using an Olympus CKX 41 inverted microscope and Olympus XC50 camera. (B) Microscopic image of sample 02WJC-CC from Wharton’s jelly. The photograph was taken on day 10 (magnification 200×, eyepiece 10×, lens 20×) using an Olympus CKX 41 inverted microscope and Olympus XC50 camera. (C) Microscopic image of sample 04WJC-CC from Wharton’s jelly. The photograph was taken on day 14 (magnification 200×, eyepiece 10×, lens 20×) using an Olympus CKX 41 inverted microscope and Olympus XC50 camera.
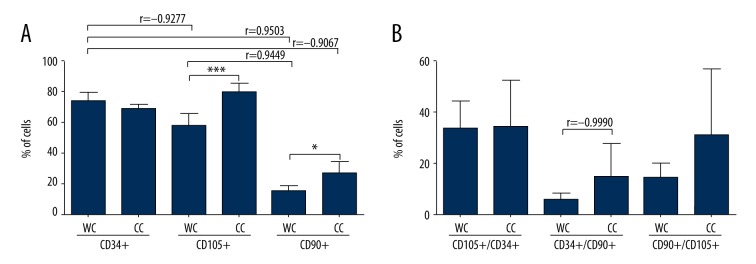
We found that over 60% of cells in both groups showed expression of CD34 antigen. A higher percentage of cells expressing CD34 (73.3±5%) was observed in the group of non-cultured cells from Wharton’s jelly (WJC), but no statistical significance was found. In cells from Wharton’s jelly cultured in vitro for 14 days (WJC-CC), CD34 expression was lower (69. 5±1.8%) than in the uncultured cells from Wharton’s jelly. Looking at CD90 expression, we observed that most (29±2.1%) CD90+ cells were in the WJC-CC group (cells from Wharton’s jelly cultured for 14 days). For CD105 antigen, the highest expression (77.6±2.7%) was observed in cells from Wharton’s jelly cultured in vitro (WJC-CC), while in cells from uncultured Wharton’s jelly, CD105 expression was lower and it was assessed at WJC: 57.9±6% (Figures 2, 3, 4A).
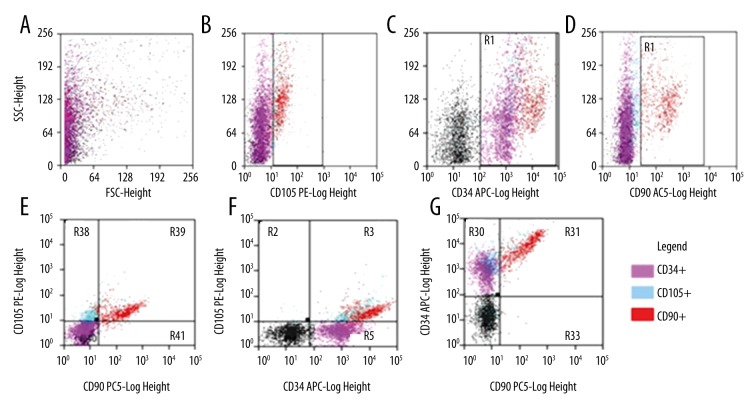
Figure 2.
Dot-plot charts of cell morphology and distribution of cells in terms of intensity of expression of surface antigens CD34, CD105, and CD90 in the analyzed 01WJC-CC sample on day 10 of cell culture – cells isolated from Wharton jelly and then cultured in vitro – test group WJC-CC. (A) Dependence of the analyzed cells on their shape and granularity: FSC-Forward Scatter/SSC-side scatter. (B) CD105 vs. SSC: R49 CD105+. (C) CD34 vs. SSC: R1 CD34+. (D) CD90 vs. SSC: R50 CD90+. (E) CD90 vs. CD105: R38 CD90−/CD105+; R39 CD90+/CD105+; R41 CD90+/CD105−, R41: CD90−/CD105−. (F) CD34 vs. CD105: R2 CD34−/CD105+; R3: CD34+/CD105+; R5: CD34+/CD105−; R4: CD34−/CD105− (G) CD90 vs. CD34: R30 CD90−/CD34+; R31 CD90+/CD34+; R33 CD90+/CD34−; R32 CD90−/CD34−. The analysis and chart were made using Summit™ Software on a digital flow.
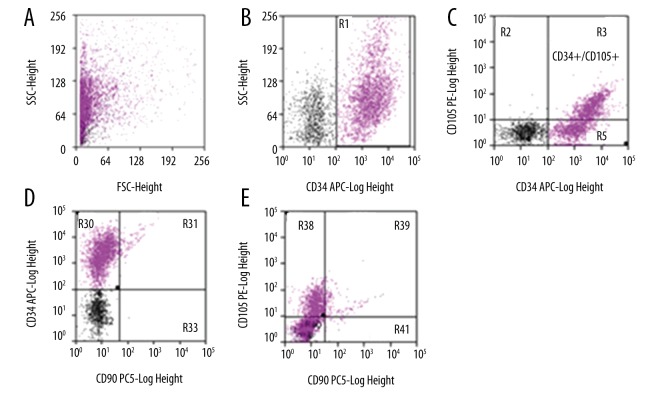
Figure 3.
Dot-plot charts of cell morphology and distribution of cells in terms of intensity of expression of surface antigens CD34, CD105, and CD90 in the analyzed 06WJC– cells isolated from Wharton’s jelly – test group WJC. (A) Dependence of the analyzed cells on their shape and granularity: FSC-Forward Scatter/SSC-side scatter. (B) CD34 vs. SSC: R1: CD34+. (C) CD34 vs. CD105: R2 CD34−/CD105+; R3: CD34+/CD105+; R5: CD34+/CD105−; R4: CD34−/CD105−. (D) CD90 vs. CD34: R30 CD90−/CD34+; R31 CD90+/CD34+; R33 CD90+/CD34−; R32 CD90−/CD34−. (E) CD90 vs. CD105: R38 CD90−/CD105+; R39 CD90+/CD105+; R41 CD90+/CD105−, R41: CD90−/CD105−. The analysis and chart were made using Summit™ Software on a digital flow cytometer with a MoFlow XDP Beckman Coulter cell sorter.
Figure 4.
(A) Frequency distribution of CD34, CD105, and CD90 on the cell surface, depending on the analyzed group (WJC, WJC-CC). The graph shows the average percentage of (±SD) cells demonstrating CD34, CD105, and CD90 expression in the test group. (B) Mean percentage of (±SD) cells demonstrating co-expression of CD34 and CD105, CD34 and CD90, CD90 and CD105 in the test group (n=5; * p<0,05; ** p<0.01; *** p<0.001 WC vs. CC, t test; Pearson’s r correlation coefficient).
The number of cells demonstrating co-expression of antigens CD34/CD105, CD34/CD90, and CD105/CD90 was analyzed, showing a statistically significant difference between those probes. The largest number of CD34+/CD105+ cells (37.7±2.4%) was found in group WJC-CC (cells from Wharton’s jelly cultured in vitro). The WJC group had lower (35.2±9.2%) CD34/CD105 co-expression, and the greatest dispersion of results was observed in this group. For co-expression of antigens CD34 and CD90, the highest expression (21.6±1.9%) was observed in cells cultured from Wharton’s jelly (WJC-CC). Non-cultured cells from the Wharton’s jelly (WJC: 5.3±2%) had lower expression compared to cultured cells. A similar pattern of results was observed for co-expression of antigens CD105 and CD90. The highest percentage of CD105+/CD90+ cells was in the WJC-CC group (24.4±10.1%), and the greatest dispersion of results was observed here. In non-cultured cells from Wharton’s jelly (WJC), the expression was lower (13.7±5.5%) than in cultured cells (WJC-CC) (Figures 2, 3, 4B).
Among non-cultured human adherent cells from Wharton’s jelly (WJC) there were cells with expression of surface antigens CD34, CD90, and CD105. The highest expression in this group was demonstrated by CD34+ cells (over 73%). This group was also characterized by high expression of CD105 (almost 60%). CD90+ cells accounted for over 15% of the analyzed group. There were also cells with co-expression of CD34/CD90 (over 5%), CD34/CD105 (over 35%), and CD105/CD90 (over 13%). This group had a higher percentage of CD34+ cells when compared with the WJC-CC group (Table 2, Figure 4, 5A).
Table 2.
The average percentage of cells demonstrating expression of surface antigens CD34, CD90, and CD105 in the studied groups (WJC, WJC-CC).
| The average percentage of cells (±SD) demonstrating expression of surface antigens in the studied groups | ||||||
|---|---|---|---|---|---|---|
| Studied group | CD34+ p=0.13542 |
CD90+* p=0.01152 |
CD105+*** p=0.00097 |
CD34+/CD105+ p=0.17355 |
CD34+/CD90+ p=0.13930 |
CD105+/CD90+ p=0.07096 |
| WJC | 73.3±5 | 15.3±4.2 | 57.9±6 | 35.2±9.2 | 5.3±2 | 13.7±5.5 |
| WJC-CC | 69.5±1.8 | 29±2.1 | 77.6±2.7 | 37.7±2.4 | 21.6±1.9 | 24.4±10.1 |
Figure 5.
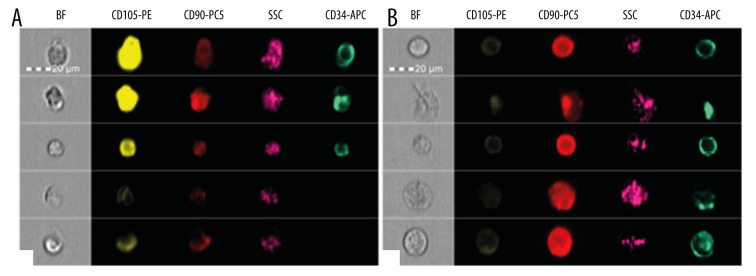
(A) Photographs of single samples of cells from the WJC group, presenting microscope image (BF) and fluorescence in channels, showing the expression of studied antigens. SSC-side scatter – assessment of cell morphology. The analysis and photographs were taken using the FlowSight f. Amnis flow cytometer. (B) Photographs of single samples of cells from the analyzed WJC-CC group, illustrating microscope image (BF) and fluorescence in channels showing the expression of the studied antigens. SSC-side scatter – assessment of cell morphology. The analysis and photographs were made using the FlowSight f. Amnis flow cytometer. (C) Photographs of single samples of cells from negative control from the analyzed WJC group, presenting microscope image (BF) and fluorescence in channels showing the expression of studied antigens. SSC-side scatter – assessment of cell morphology. The analysis and photographs were made using the FlowSight f. Amnis flow cytometer. (D) Photographs of single samples of cells from negative control from the analyzed WJC-CC group, illustrating microscope image (BF) and fluorescence in channels showing the expression of studied antigens. SSC-side scatter – assessment of cell morphology. The analysis and photographs were made using the FlowSight f. Amnis flow cytometer.
In a group of human adherent cells isolated from Wharton’s jelly and then cultured in vitro for 14 days (WJC-CC), the presence of analyzed surface antigens CD34, CD90, and CD105 was observed. CD105+ cells constituted the biggest part (over 77%); almost 70% of cells demonstrated expression of CD34 antigen and 29% of cells demonstrated expression of CD90+ antigen. During analysis of co-expression of antigens on the cells of the studied group, we found that 37% of cells demonstrated co-expression of antigens CD34 and CD105, over 21% of CD34+/CD90+ cells, and over 24% of CD105+/CD90+. The WJC-CC group had higher percentages of CD90+, CD105+, CD34+/CD105+, CD34+/CD90+, and CD105+/CD90+ in comparison with the WJC group. Detailed characteristics of WJC-CC cells, demonstrating expression of the analyzed antigens, are presented in Table 2 and Figure 4, 5B.
Discussion
While analyzing the phenotype of cells from Wharton’s jelly, we observed that isolated cells, apart from high expression of antigens characteristic of MSCs, also show expression of CD34 antigen characteristic of hematopoietic stem cells.
Minimum criteria to be met by mesenchymal stromal cells were set by the Mesenchymal and Tissue Stem Cell Committee of the International Society for Cellular Therapy. MSCs have to demonstrate adherence to the plastic surface of culture vessels and must demonstrate expression of CD105, CD90, and CD73 but not CD34, CD45, CD14, CD79, and CD11b. The ability to differentiate in vitro into adipocytes and chondroblasts has to be demonstrated [28]. According to current knowledge, MSCs do not demonstrate CD34 expression or the percentage of CD34+ cells in a culture of MSCs does not exceed 2%. The presence of CD34 antigen is considered to be a characteristic marker of undifferentiated hematopoietic stem cells, whose expression decreases along with the increase of differentiation of HSC cells into progenitor cells. Apart from HSCs, CD34 expression is also demonstrated by endothelial stem cells and cancer stem cells.
Evidence of the CD34-negative nature of MSCs is based mainly on experiments carried out on cells from cell cultures, often on a fixed cell line\s, after many passages. However, there have been few studies on CD34 expression on MSCs in vivo. In recent years, there have been a few reports on the presence of CD34 antigen on cells other than hematopoietic MSCs. These studies refer mainly to fresh populations isolated from adipose tissues (ADSCs) [13,29,32].
Studies suggest that MSCs present in tissues are CD34+, which is proved by the fact that freshly isolated cells with MSCs potential demonstrate CD34 expression, and its level decreases in proportion to the length of cell culture. The decrease in CD34 expression and its disappearance are results of cell culture rather than being the true nature of MSCs [13,30].
Suga et al. conducted research on human adipose-derived stem cells (hADSCs) by analyzing the phenotype of isolated cells using flow cytometry. Before starting cell culture in adherent conditions, they observed a significant proportion of CD34+ cells among the analyzed population. They sorted the isolated population into 2 groups of cells, CD34+ and CD34−, then they cultured them in vitro. After approximately 7 days, before a passage in CD34+ group of cells, they noticed that some of the cells lost CD34 antigen. After 21 days, they did not observe any difference in the morphology of CD34+ and CD34− cells. In culture of CD34+ cells, they noticed a higher number of colonies formed, which suggests that CD34 expression on cells from adipose tissue correlates with replication potential of these cells. It was also shown that both populations are able to migrate under the influence of the stimulation of growth factor and to form capillary networks. Higher expression of markers characteristic of endothelial progenitor cells was observed in the CD34+ population. Moreover, they suggested that a decrease in CD34 expression in the CD34+ cell population after 7 days of cell culture does not contribute to the change in their functionality. CD34 expression in cells with potential of MSCs from adipose tissue might be responsible for immaturity of the cell, its primordiality, or state of non-differentiation, and the decrease in CD34 expression in these cells is caused by a physiological process of maturation and differentiation in specific cell lines [31]. These studies suggest that high CD34 expression in cells other than HSCs may be typical of immature and undifferentiated cells.
The present study showed the presence of CD34 on cells freshly isolated from Wharton’s jelly, whereas CD34 expression was lower in cells cultured in vitro.
Kaiser et al. researched the in vivo phenotype of MSCs in bone marrow, with respect to expression of antigens CD34 and CD45. They sorted cell populations into CD34+ and CD34−, then cultured them in adherent conditions. The researchers observed that the CD34+ population of cells from bone marrow in adherent conditions show the ability to form colonies (CFU) and the growth of fibroblast-like cells. To confirm the phenotype of MSCs, their ability to differentiate into chondrocytes and adipocytes was verified as well. They proved that BMSCs CD34+ have the potential of MSCs. They also demonstrated that the population placed in in vitro culture lost CD34 expression [32].
In their studies, Busser et al. also observed differences in the phenotype of freshly isolated MSCs in comparison with cultured cells. They demonstrated that freshly isolated mesenchymal cells from bone marrow and MSCs from adipose tissue show CD34 expression [2].
Perinatal and fetal MSCs are extremely popular among scientists due to their proven greater ability of proliferation and broader differentiation potential, as well as an increase in immunosuppression and therefore greater therapeutic potential when compared to MSCs from adult tissues such as adipose tissue or bone marrow [33,34]. Patel et al. studied human MSCs from the placenta. The authors intended to isolate a population of fetal MSCs. They collected the placenta after birth by caesarean section from women who give birth to male infants. They observed that the population of cells freshly isolated from the placenta contains a fairly high percentage of CD34+. On the basis of reports by Anker et al., who demonstrated that CD34+ populations from the liver, spleen, lungs, and bone marrow of a fetus in the second trimester of pregnancy contain a much higher percentage of MSCs (positive MSC markers) when compared with the percentage of CD34− [35], Patel’s team used CD34 antigen as a positive marker for the fetal MSCs and sorted 2 cell populations, CD34+/CD45−/CD31− and CD34−/CD45−/CD31−, which were then cultured. After 7 days, formation of colonies was observed in both groups, and after 14 days both populations showed characteristics of MSCs, including the ability to differentiate into lines of osteoblasts and adipocytes. After FISH analysis aimed at assessment of sex chromosomes X and Y in both studied groups, it was found that 100% of sorted CD34+ MSCs are of fetal origin, whereas 100% of the population of mesenchymal CD34− stromal cells originate from the mother. The research shows that fresh MSCs isolated from the placenta demonstrate CD34 expression that declines in the course of culture. Furthermore, it was proved that the population of mesenchymal CD34+ stromal cells isolated from the placenta is of fetal origin and therefore is characterized by greater proliferation potential and broader differentiation potential [36]. Previously, Parant et al. suggested that CD34+ cells in the placenta are non-hematopoietic fetal cells of endothelial origin [37].
Trivanović et al. observed a high percentage of CD34+ cells (over 40%) in mesenchymal cells isolated form the umbilical cord and peripheral blood and suggested that cells from perinatal tissues may have a lower differentiation level and higher proliferation potential [3]. In another study, the same group demonstrated high CD34 expression in MSCs from adipose tissue surrounding the breast. They found that CD34 expression was not a result of the early passage of cells. It probably depends on the location from which adipose tissue was sampled [38].
In our study, adherent cells isolated from Wharton’s jelly, using enzymatic digestion with collagenase type I, demonstrated high expression of CD105 and CD90 antigens, and showed the presence of CD34 in more than 60% of these cells. In the course of the culture, we found the ability of cells to proliferate in vitro and to adhere to plastic walls. The appearance of cultured cells was similar to that of fibroblast colonies. Expression of SOX9 gene was demonstrated in MSCs; this gene is characteristic of differentiation of cells into chondrocytes (our own unpublished studies). We concluded that cells from Wharton’s jelly are mainly MSCs. After 14 days of cell culture (P0), cells from Wharton’s jelly are characterized by a decrease in the number of CD34+ cells, whereas the number of cells demonstrating expression of CD105, CD90, and CD105/CD90 co-expression increased significantly, suggesting variability of populations in the course of cell culture in adherent conditions. In cultured cells, there was also an increase in cells characterized by co-expression of CD34/CD90 and CD34/105, which confirms the possibility of occurrence of endothelial stem cells in the material isolated from Wharton’s jelly. This hypothesis, however, requires verification by analyzing antigens characteristic of endothelial stem cells (CD31+/CD34+) and hematopoietic stem cells (CD34+/CD45+) in the studied material.
Conclusions
Our study confirms results obtained by other groups suggesting that adherent cells from Wharton’s jelly demonstrate MSCs potential and CD34 expression in vivo. Moreover, the phenotype of MSCs, mainly in the context of CD34 expression, may vary depending on the place of collection and the duration of growing the cell culture.
The lack of established surface antigens that characterize MSCs in vivo significantly impedes their correct identification and isolation. There is a need for more research on freshly isolated cells and assessment of their phenotype before growing in culture.
Footnotes
Source of support: This study was supported in part by funds from the project “The equipment of innovative laboratories doing research on new medicines used in the therapy of civilization and neoplastic diseases” within the Operational Program Development of Eastern Poland 2007–2013, Priority Axis I Modern Economy, Operations I.3 Innovation Promotion
References
- 1.Baker N, Boyette LB, Tuan RS. Characterization of bone marrow-derived mesenchymal stem cells in aging. Bone. 2015;70:37–47. doi: 10.1016/j.bone.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 2.Busser H, Najar M, Raicevic G, et al. Isolation and characterization of human mesenchymal stromal cell subpopulations: Comparison of bone marrow and adipose tissue. Stem Cells Dev. 2015;24:2142–57. doi: 10.1089/scd.2015.0172. [DOI] [PubMed] [Google Scholar]
- 3.Trivanović D, Kocić J, Mojsilović S, et al. Mesenchymal stem cells isolated from peripheral blood and umbilical cord Wharton’s jelly. Srp Arh Celok Lek. 2013;144:178–86. doi: 10.2298/sarh1304178t. [DOI] [PubMed] [Google Scholar]
- 4.Najar M, Rodrigues RM, Buyl K, et al. Proliferative and phenotypical characteristics of human adipose tissue-derived stem cells: Comparison of Ficoll gradient centrifugation and red blood cell lysis buffer treatment purification methods. Cytotherapy. 2014;9:1220–28. doi: 10.1016/j.jcyt.2014.05.021. [DOI] [PubMed] [Google Scholar]
- 5.Mimeault M, Batra SK. Recent insights into the molecular mechanisms involved in aging and the malignant transformation of adult stem/progenitor cells and their therapeutic implications. Ageing Res Rev. 2009;8:94–112. doi: 10.1016/j.arr.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aronin PA, George TM. Commentary on ‘quality of life and myelomeningocele: An ethical and evidence-based analysis of the Groningen Protocol’ by Sean Barry. Pediatr Neurosurg. 2010;46:409–14. doi: 10.1159/000322895. [DOI] [PubMed] [Google Scholar]
- 7.Allickson JG, Sanchez A, Yefimenko N, et al. Recent studies assessing the proliferative capability of a novel adult stem cell identified in menstrual blood. Open Stem Cell J. 2011;3:4–10. doi: 10.2174/1876893801103010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sani MA, Separovic F. Progression of NMR studies of membrane-active peptides from lipid bilayers to live cells. J Magn Reson. 2015;253:138–42. doi: 10.1016/j.jmr.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 9.Gala K, Burdzińska A, Pączek L. Mezenchymalne komórki macierzyste szpiku kostnego a starzenie. Post Biol Kom. 2010;1:89–106. [in Polish] [Google Scholar]
- 10.Burkhalter MD, Rudolph LK, Sperka T. Genome instability of ageing stem cells – Induction and defence mechanisms. Ageing Res Rev. 2015;23:29–36. doi: 10.1016/j.arr.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;11:2739–49. doi: 10.1634/stemcells.2007-0197. [DOI] [PubMed] [Google Scholar]
- 12.Tárnok A, Urlich H, Bocsi J. Phenotypes of stem cells from diverse origin. Cytometry A. 2010;77:6–10. doi: 10.1002/cyto.a.20844. [DOI] [PubMed] [Google Scholar]
- 13.Lin CS, Ning H, Lin G, Lue TF. Is CD34 truly a negative marker for mesenchymal stem cells? Cytotherapy. 2012;10:1159–63. doi: 10.3109/14653249.2012.729817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sidney LE, Branch MJ, Dunphy SE, et al. Concise review: Evidence for CD34 as a common marker for diverse progenitors. Stem Cells. 2014;32:1380–89. doi: 10.1002/stem.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Szala S, Wiśniewska E, Matuszczak S, Czapla J. Mezenchymalne komórki zrębu. Postepy Hig Med Dosw. 2014;68:12870–98. doi: 10.5604/17322693.1128671. [in Polish] [DOI] [PubMed] [Google Scholar]
- 16.Bianco P, Gehron Robey P, Simmons PJ. Mesenchymal stem cells: Revisiting history, concepts, and assays. Cell Stem Cell. 2008;2:313–19. doi: 10.1016/j.stem.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sottocornola R, Lo Celso C. Dormancy in the stem cell niche. Stem Cell Res Ther. 2012;3:10. doi: 10.1186/scrt101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harasymiak-Krzyżanowska I, Niedojadło A, Karwat J, et al. Adipose tissue-derived stem cells show considerable promise for regenerative medicine applications. Cell Mol Biol Lett. 2013;4:479–93. doi: 10.2478/s11658-013-0101-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Skalska U, Kontny E. Właściwości regeneracyjne i immunomodulacyjne mezenchymalnych komórek macierzystych z tkanki tłuszczowej. Post Biol Kom. 2011;3:63–378. [in Polish] [Google Scholar]
- 20.Can A, Karahuseyinoglu S. Concise review: Human umbilical cord stroma with regard to the source of fetus-derived stem cells. Stem Cells. 2007;11:2886–95. doi: 10.1634/stemcells.2007-0417. [DOI] [PubMed] [Google Scholar]
- 21.Troyer DL, Weiss ML. Concise review: Wharton’s jelly-derived cells are a primitive stromal cell population. Stem Cells. 2008;26:591–99. doi: 10.1634/stemcells.2007-0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fong Ch-Y, Chak L-L, Biswas A, et al. Human Wharton’s jelly stem cells have unique transcriptome profiles compared to human embryonic stem cells and other mesenchymal stem cells. Stem Cell Rev Rep. 2010;7:1–16. doi: 10.1007/s12015-010-9166-x. [DOI] [PubMed] [Google Scholar]
- 23.Murphy MB, Moncivais K, Caplan A. Mesenchymal stem cells: Environmentally responsive therapeutics for regenerative medicine. Exp Mol Med. 2013;45:e54. doi: 10.1038/emm.2013.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jezierska-Woźniak K, Nosarzewska D, Tutas A, et al. Wykorzystanie tkanki tłuszczowej jako źródła mezenchymalnych komórek macierzystych. Post Hig Med Dosw. 2010;64:326–32. [in Polish] [PubMed] [Google Scholar]
- 25.Bajek A, Olkowska J, Drewa T. Mezenchymalne komórki macierzyste narzędziem terapeutycznym w regeneracji tkanek i narządów. Post Hig Med Dosw. 2011;65:124–32. doi: 10.5604/17322693.933878. [in Polish] [DOI] [PubMed] [Google Scholar]
- 26.Ratajczak MZ, Zuba-Surma E, Ratajczak J. Komórki macierzyste – blaski i cienie. Acta Haematol Polon. 2009;40:289–303. [in Polish] [Google Scholar]
- 27.Hass R, Kasper C, Böhm S, Jacobs R. Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell Commun Signal. 2011;9:9–12. doi: 10.1186/1478-811X-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;4:315–17. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 29.Varma MJ, Breuls RG, Schouten TE, et al. Phenotypical and functional characterization of freshly isolated adipose tissue-derived stem cells. Stem Cells Dev. 2007;1:91–104. doi: 10.1089/scd.2006.0026. [DOI] [PubMed] [Google Scholar]
- 30.Baer PC, Kuçi S, Krause M, et al. Comprehensive phenotypic characterization of human adipose-derived stromal/stem cells and their subsets by a high throughput technology. Stem Cells Dev. 2013;22:330–39. doi: 10.1089/scd.2012.0346. [DOI] [PubMed] [Google Scholar]
- 31.Suga H, Matsumoto D, Eto H, et al. Functional implications of CD34 expression in human adipose–derived stem/progenitor cells. Stem Cells Dev. 2009;8:1201–9. doi: 10.1089/scd.2009.0003. [DOI] [PubMed] [Google Scholar]
- 32.Kaiser S, Hackanson B, Follo M, et al. BM cells giving rise to MSC in culture have a heterogeneous CD34 and CD45 phenotype. Cytotherapy. 2007;5:439–50. doi: 10.1080/14653240701358445. [DOI] [PubMed] [Google Scholar]
- 33.Kim DW, Staples M, Shinozuka K, et al. Wharton’s jelly-derived mesenchymal stem cells: Phenotypic characterization and optimizing their therapeutic potential for clinical applications. Int J Mol Sci. 2013;6:11692–712. doi: 10.3390/ijms140611692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Christodoulou I, Kolisis FN, Papaevangeliou D, Zoumpourlis V. Comparative evaluation of human mesenchymal stem cells of fetal (Wharton’s jelly) and adult (adipose tissue) origin during prolonged in vitro expansion: Considerations for cytotherapy. Stem Cells Int. 2013;2013:246134. doi: 10.1155/2013/246134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anker PS, Noort WA, Scherjon SA, et al. Mesenchymal stem cells in human second-trimester bone marrow, liver, lung, and spleen exhibit a similar immunophenotype but a heterogeneous multilineage differentiation potential. Haematologica. 2013;8:845–52. [PubMed] [Google Scholar]
- 36.Patel J, Shafieea A, Wanga W, et al. Novel isolation strategy to deliver pure fetal-origin and maternal-origin mesenchymal stem cell (MSC) populations from human term placenta. Placenta. 2014;11:969–71. doi: 10.1016/j.placenta.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 37.Parant O, Dubernard G, Challier JC, et al. CD34+ cells in maternal placental blood are mainly fetal in origin and express endothelial markers. Lab Invest. 2009;8:915–23. doi: 10.1038/labinvest.2009.55. [DOI] [PubMed] [Google Scholar]
- 38.Trivanović D, Nikolic S, Krstic J, et al. Characteristics of human adipose mesenchymal stem cells isolated from healthy and cancer affected people and their interactions with human breast cancer cell line MCF-7 in vitro. Cell Biol Int. 2014;38:254–65. doi: 10.1002/cbin.10198. [DOI] [PubMed] [Google Scholar]