Abstract
A key transformation in mammalian ear evolution was incorporation of the primary jaw joint of premammalian synapsids into the definitive mammalian middle ear of living mammals. This evolutionary transition occurred in two-steps, starting with a partial or “transitional” mammalian middle ear in which the ectotympanic and malleus were still connected to the mandible by an ossified Meckel’s Cartilage (MC), as observed in many Mesozoic mammals. This was followed by MC breakdown, freeing the ectotympanic and the malleus from the mandible and creating the definitive mammalian middle ear. Here we report novel findings on the role of chondroclasts in MC breakdown, shedding light on how therian mammals lost MC connecting the ear to the jaw. Genetic or pharmacological loss of clast cells in mice and opossums leads to persistence of embryonic MC beyond juvenile stages, with MC ossification in mutant mice. The persistent MC causes a distinctive postnatal groove on the mouse dentary. This morphology phenocopies the ossified MC and Meckelian groove observed in Mesozoic mammals. Clast cell recruitment to MC is not observed in reptiles, where MC persists as a cartilaginous structure. We hypothesize that ossification of MC is an ancestral feature of mammaliaforms, and that a shift in the timing of clast cell recruitment to MC prior to its ossification is a key developmental mechanism for the evolution of the definitive mammalian middle ear in extant therians.
A key transformation in mammalian ear evolution was incorporation of the primary jaw joint of premammalian synapsids into the definitive mammalian middle ear (DMME) of living mammals 1–5. The quadrate and articular in the jaw joint of reptiles and the incus and malleus of the middle ear of mammals are homologs 6–12. These structures are formed by a Type II Collagen expressing condensation continuous with the proximal part of Meckel’s Cartilage (MC)11,13,14. The evolutionary transition from jaw joint to middle ear occurred in two-steps, first with a partial or “transitional” mammalian middle ear (PMME) in which the ectotympanic and malleus were still connected to the mandible by an ossified Meckel’s Cartilage (MC) as observed in many Mesozoic mammals (Fig. 1A) 6–8,15,16. This was followed by MC breakdown, freeing the ectotympanic and the malleus from the mandible and creating the definitive mammalian middle ear (DMME). The majority of MC is transient in mammalian development (Fig. 1B,D,E) but remains cartilaginous through to adulthood in other amniotes, such as extant squamates (Fig. 2F, Supplementary Fig. S1) and crocodiles17 . The part of Meckel’s connected to the malleus and ectotympanic breaks down at postnatal day 2 (P2) in mice11, and around P20 in the marsupial opossum, Monodelphis domestica 18. An understanding of the cellular processes involved in MC breakdown in extant placentals (mice) and marsupials (opossum) has important implications for the mechanisms underlying the transformation of the mammalian middle ear during this evolutionary transition 6,9–11,18,19.
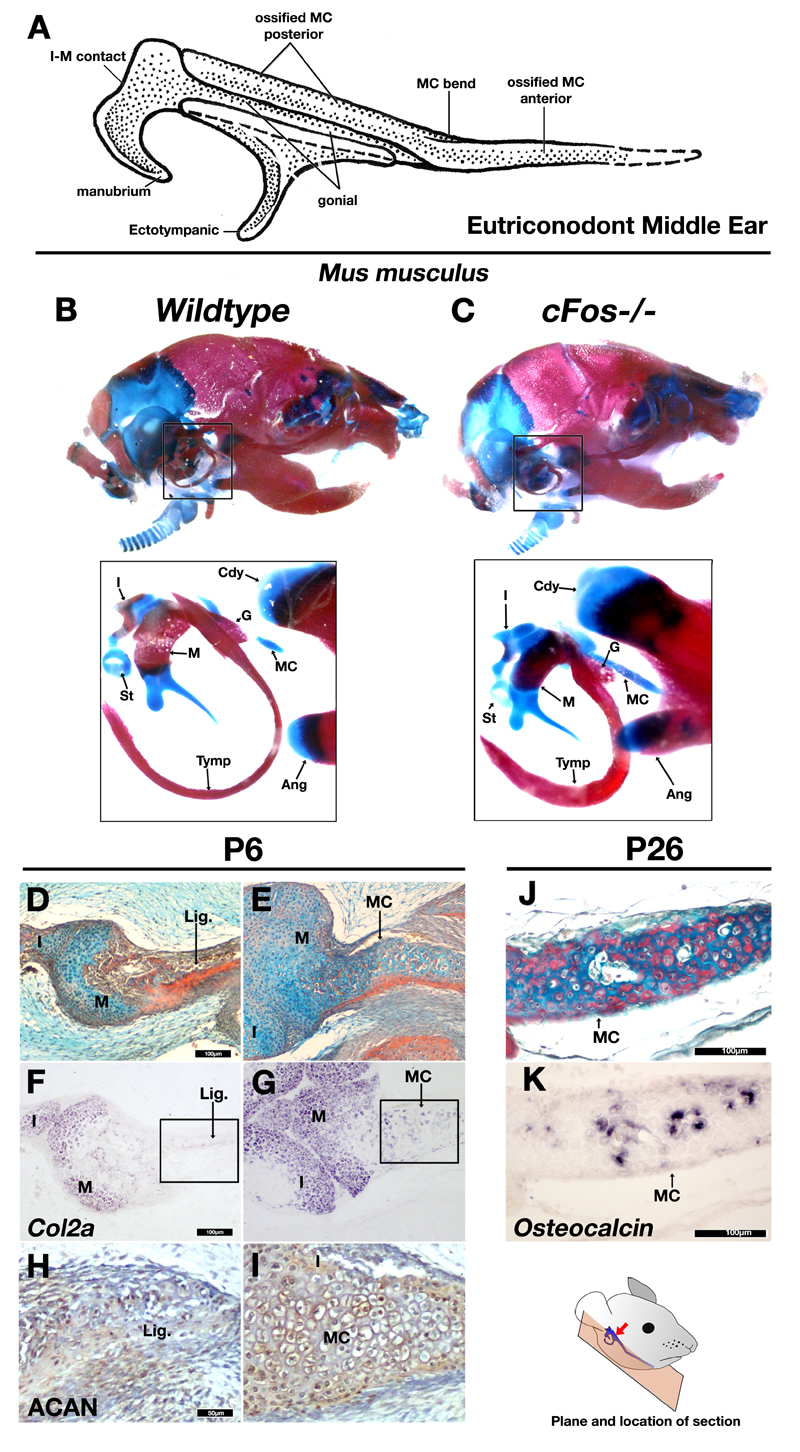
Figure 1. cFos mutant mice mimic morphologies of PMME (“TMME”) in fossil mammaliaforms.
A: A generalized Partial (“Transitional”) Mammalian Middle Ear of eutriconodonts showing middle ear with ossified Meckel’s cartilage (reconstructed from refs3,6). B-C: Stained and cleared DMME middle ear from P3 wildtype (B) and cFos-/- mutant mice showing a persistence of Meckel’s cartilage, persistent cartilage in endochondrally ossifying elements such as the malleus and incus, and thickening of the tympanic ring and gonial bones. D-E: Picro-sirius red / alcian blue trichrome staining of horizontal section through malleus of postnatal day 6 (P6) wildtype (D) and cFos-/- (E) littermates. In wildtype mice the Meckel’s cartilage has undergone resorption and transformation to ligament connective tissue, whereas in the cFos-/- mutant mouse the Meckel’s cartilage is maintained (arrowhead in E). F-G: Col2a expression by in situ hybridization. Collagen type 2 (encoded by Col2a mRNA) is lost in Meckel’s derived ligament in wildtype mice (F), but continues to express during the prolonged retention of Meckel’s in cFos mutant. H-I Immunohistochemistry staining for the extracellular matrix protein Aggrecan (ACAN) shows weak expression in the ligament replacing the MC of the wildtype mouse (H) and strong expression MC of the cFos null (I) confirming a persistent cartilage (boxes in F and G show location of H and I). J-K: Picro-sirius red / alcian blue trichrome staining of horizontal sections of cFos mutant mouse at P26 (J) demonstrates that Meckel’s persists at this stage, and that calcified tissue is present. Furthermore, in situ hybridization for Osteocalcin (K) suggests that ossification occurs in the absence of Meckel’s breakdown. MC – Meckel’s Cartilage; M – Malleus; I – Incus; St – Stapes; Tymp – ectotympanic ring; G – Gonial; Cdy – Condylar process of dentary; Ang – Angular process of dentary; lig. – ligament. Scale bar in D-G,J and K = 100µm. Scale in H and I =50µm
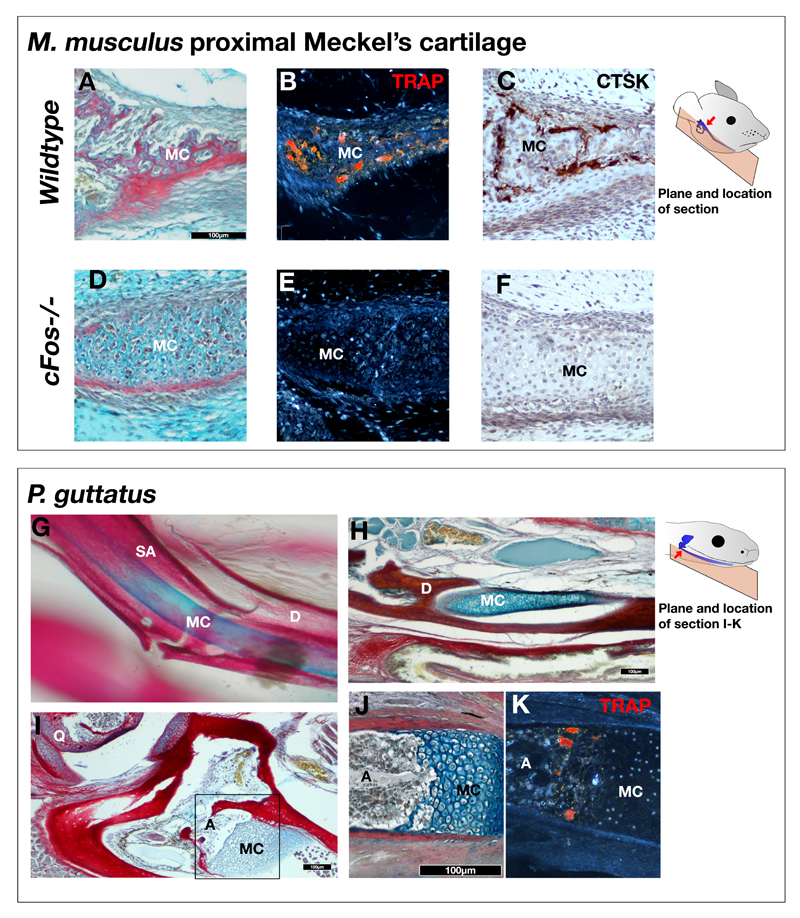
Figure 2. Detachment of the malleus in mammals is due to recruitment c-Fos dependent chondroclasts,
A-D: Picro-sirius red / alcian blue trichome (A,D) tartrate-resistant acid phosphatase (TRAP) (B,E) , and Cathespsin K (CTSK) immunohistochemistry (C,F) staining of proximal Meckel’s cartilage in P4 wildtype (A-C) and cFos-/- (C-F) mice. TRAP and CTSK staining reveals chondroclast activity around the Meckel’s cartilage of wildtype mice, but not in cFos mutants. G. Stained and cleared mandible of new born corn snake reveals persistence of Meckel’s cartilage. H-J: Trichome staining of histological sections of 5 week old corn snake (Pantherophis guttatus). The Meckel’s cartilage persists (F), until it meets the ossified articular (I,J). K: TRAP staining in corn snakes demonstrates that chondroclasts and osteoclasts do not act upon Meckel’s cartilage, except where endochondral ossification has occurred at the articular. Box in F indicates approximate location of panels J and K. Meckel’s cartilage. MC – Meckel’s Cartilage; D – Dentary; SA – Surangular; A – Articular; Q – Quadrate Scale bar = 100µm.
During development, Meckel’s breakdown is associated with the onset of function of the squamosal-dentary jaw joint in the mouse and opossum, although timing differs 18. The most proximal part of MC forms the malleus and incus, which undergo endochondrial ossification, while the slightly more distal part of MC is thought to transdifferentiate to become the anterior ligament of the malleus and the sphenomandibular ligament 10,11, breaking the hard tissue link between the ear and jaw. One general mechanism for remodelling of cartilage matrix is via the action of chondroclasts: large, multinucleated, hematopoietic derived osteoclast-like cells that secrete enzymes that degrade cartilage 20–22. Haematopoietic lineage clast cells are born as mononuclear precursors that travel along blood vessels to the site of activity where they are activated under the control of the transcription factor c-Fos to fuse and to form multinucleated mature clast cells 23,24.
Results
To determine the role of clast activity in MC breakdown and the isolation of the ear from the jaw in mammals, we investigated the role of chondroclasts in the breakdown of MC in transgenic c-Fos null mice in which clast cells fail to differentiate 23. At P3 the proximal MC of wildtype mice has already broken down and the middle ear is separated from the jaw as observed by alcian blue / alizarin red skeletal preparation (Fig. 1B). In contrast, the connection between Meckel’s cartilage in the lower jaw and the middle ear is intact in c-Fos-/- littermates at P3 (Fig. 1C). This connection is still present in these mice at P6, as indicated by histological staining (Fig. 1D,E), in situ hybridization for Col2a mRNA - the gene encoding Type II collagen that makes up a large portion of the collagen content of cartilage (Fig. 1F,G), and immunohistochemistry for aggrecan, a proteoglycan of the extra cellular matrix that is highly expressed in cartilage (Fig. 1H,I). The element further persists in c-Fos-/- mice until at least P26, at which stage the mice are weaned and independently feeding. Histological staining at this late stage shows that the matrix of MC was a mixture of alcian blue stained proteoglycan rich cartilage, and picro-sirius red stained collagen-rich tissue (Fig. 1J), with strong expression of the osteoblast marker Osteocalcin (Fig. 1K), demonstrating that in the absence of chondroclast activity the cartilage has begun to ossify.
The activity of clast cells during Meckel’s breakdown in wildtype mice was confirmed by TRAP staining and Cathespsin K (CTSK) immunohistochemistry (Fig. 2). In P4 wildtype mice TRAP positive and CTSK positive clast cells were present along the borders of MC at the site of breakdown (Fig. 2A-C), and associated with the malleus, which was undergoing endochondrial ossification. In contrast, c-Fos mutant littermates were TRAP and CTSK negative (Fig. 2D-F). To confirm that the persistence of MC in c-Fos null mice was a direct consequence of the loss of clast cells, rather than due to the role of c-Fos in other processes, wildtype CD1 mouse pups were treated at postnatal day 0 with the bisphosphonate Alendronate - a drug that inhibits the activity of clast cells and induces their apoptosis25. P3 alendronate treated mice showed a lack of TRAP positive clast cells around MC in contrast to control littermates, and in keeping with the c-Fos phenotype, treated mice maintained a connection between the malleus and MC, which had broken down in control littermates (Supplementary Fig. S2). Endochondrial ossification in the malleus and incus were also disrupted, as would be expected from a lack of osteoclasts. Some clast cells were scantly observed in the bone of treated pups, possibly due to either an incomplete inhibition of clast cells or recovery of the clast cell population following initial treatment.
In contrast to mammals, MC appears to remain as a permanent cartilage in reptiles. Analysis of a range of squamates showed MC was present in adult snakes (Pantherophis guttatus) (Fig. 2G,H), and lizards (chameleon (Chamaeleo calyptratus), gecko (Paroedura pictus), and green anole (Anolis carolinensis)) (Supplementary Fig. S1), confirming that MC breakdown does not occur in squamates. Having established a role for TRAP positive clast cells in MC breakdown in the mouse, clast activity was compared in the corn snake Pantherophis guttatus. Similarly to the mouse malleus, TRAP positive cells were found at the end of MC in the ossifying articular five weeks post hatching (Figure 2I,J,K). In contrast to the mouse, however, TRAP positive cells were absent from the main body of MC (Figure 2K). The absence of clast cells in the reptile MC was confirmed by detection of multinucleated cells in the ossified articular bone, but not in the cartilaginous MC, in haematoxylin and eosin stained sections in an adult gecko (P. pictus) (Supplementary Fig S1).
In c-Fos mutant mice, the persistent MC results in a corresponding Meckelian groove on the dentary through to P21, well beyond the stage (P2) when this groove disappears in wildtypes. The cartilage and its groove mimic the morphology of the ossified Meckel’s cartilage nestled in the wide and deep Meckelian groove in Mesozoic mammals but especially eutriconodonts (Fig. 3A and Supplementary Fig. S6)7,26. µCT analysis of P21 c-Fos null mice revealed a thin Meckelian groove extending proximally from the level of the last molar to below the mandibular foramen, distinct from the mylohyoid muscle attachment ridge (Fig. 3D,E). Such a groove was observed in wildtype mice at P0, prior to the normal onset of Meckel’s cartilage breakdown at P2 (Fig. 3F,G), as well as in other mammalian species (opossum and fruit bat) prior to MC breakdown (Supplementary Fig. S3&S4). Evidently bone remodelling removes the groove in wildtype mice after break down of the cartilage, while this groove is maintained in cFos-/- mice. To confirm if the role of clast cells in breakdown of Meckel’s cartilage extends to marsupials, opossum pups were treated with Alendronate. µCT analysis of alendronate treated pups revealed an inhibition of Meckel’s breakdown, with a reduced gap between MC and the malleus. The MC was also relatively thicker due to the interruption of its resorption, as compared to control littermates (Fig. 4 and Supplementary Fig. S5). Clast cells therefore appear to play a role in disconnecting the ear from the jaw in both mice and opossums.
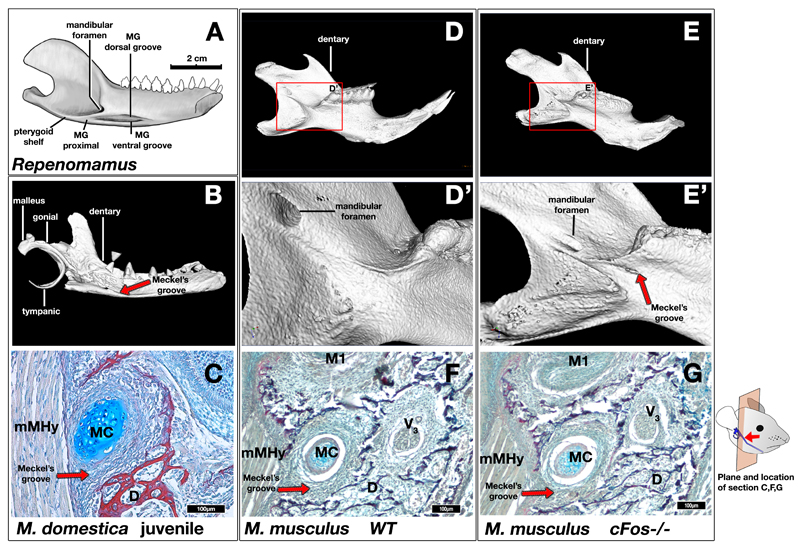
Figure 3. Formation of Meckelian groove as evidence for presence of retained MC in mammaliaforms.
A: Mandible of the eutriconodont Repenomamus showing topographic relationship of Meckelian groove to surrounding structures on the lingual aspect of the dentary. The Meckelian groove (MG) distal section contains two thinner grooves; the Meckelian groove (MG) proximal extending below the mandibular foramen. B: 3D reconstruction of µCT scan of a P17 opossum (Monodelphis domestica). A Meckelian groove is present in the lingual surface of the dentary bone (red arrow). C: Picro-sirius red / alcian blue trichrome histological staining of a frontal section through the dentary Meckelian groove of P16 opossum. Meckel’s cartilage is located within the groove, indicating that the formation of the groove is dependent on the cartilage. D,E,D’,E’: 3D reconstruction of µCT scan of P21 wildtype and cFos-/- mouse mandibles. A groove is observed inferior to the mylohyoid ridge in the cFos-/- mouse (E,E’ red arrow), but not in the wildtype mouse (D,D’). F,G: Picro-sirius red / alcian blue trichrome histological staining of a frontal section through the dentary of P0 wildtype and cFos-/- mice, showing a groove formed by Meckel’s cartilage. MC – Meckel’s Cartilage; D – Dentary; mMHy – Mylohyoid muscle. Images A,B,D-E’ not shown to scale. Scale bar in C,F,G = 100µm.
Figure 4. Clast cell inhibition in the opossum Monodelphis domestica.
µCT analysis of P22 opossum when treated with control carrier (A) or alendronate (B) injections. Breakdown of Meckel’s cartilage is reduced in treated pups when compared to control littermates (n=3 for each treatment). Distance between malleus (arrow x) and the Meckel’s cartilage (arrow y) was compared (n=3) indicating a significantly reduced separation of malleus and Meckel’s cartilage in treated opossums compared to controls (C). Error bars = 1 standard deviation.
Our observations in mouse and opossum provide a developmental basis for the Meckelian groove of mammaliaforms and many extinct clades of crown mammals 6–8. As such it can be inferred that the presence of a Meckelian groove in the fossil record indicates either a permanent Meckel’s cartilage or one persisting until the dentary was fully ossified, even when an ossified Meckel’s is not preserved (Fig. 3A and Supplementary Fig. S3&S6)6–8,15. The experimentally induced Meckelian groove in the c-Fos null mouse, which persists beyond P21, shows the same topographical relationship to the mandibular foremen as seen in Mesozoic mammaliaforms (Supplementary Fig. S6) and is equivalent to the distal part of the Meckelian groove of Mesozoic mammaliaforms (Supplementary Fig. S6). Among Mesozoic mammals, the distal part of the Meckelian groove can be wide and deep corresponding to a thick and robust MC as seen in Spinolestes 26 and Juchilestes (Supplementary Fig. S6), wide and shallow as seen in Repenomamus and Gobiconodon (Fig. 3A and Supplementary Fig. S6) 7, or relatively thin, corresponding to a gracile Meckel’s cartilage as in Maotherium (Supplementary Fig. S6) and other eutriconodonts8. Variation in clast activity may have influenced these morphologies.
Discussion
Our study demonstrates that chondroclasts play an important role in the breakdown of Meckel’s cartilage, and that persistence of MC leads to the retention of a groove on the dentary. In the absence of these clast cells, the MC, middle ear and dentary of extant mammals phenocopies the morphology of Mesozoic mammals. We have therefore identified part of the mammalian apomorphic programme. Evolutionary developmental biology has long hoped to provide mechanistic insights into the evolution of the DMME 9–11,27. The current study provides fresh evidence of the cellular mechanisms involved in resorption of Meckel’s cartilage crucial for embryogenesis of the mammalian middle ear. Changes in this developmental process can be directly linked to fossil morphology, as seen in early mammalian evolution. Our developmental studies have now corroborated the identity of the ossified Meckel’s cartilage preserved in Mesozoic mammals, and provide evidence that the Meckelian groove in fossil mammaliaformes was likely maintained by MC, as shown in our c-Fos studies (see supplementary data for further historical discussion on other interpretations of this feature).
It is clear that chondroclast activity is required for Meckel’s cartilage breakdown in placental mammals and contributes to breakdown in marsupials. It is likely that clast cell activity alone is not responsible for the complete removal or transformation of Meckel’s cartilage, and that other remodelling mechanisms such as autophagy10, apoptosis (see DJU, NA, ZXL, JA Maier, A Sadier, AST, KET, under review), or chondrocyte expression of MMPs 28 also play an important role. Nevertheless chondroclast recruitment to this part of Meckel’s appears to be a process specific to mammals, and may have been one of the key developmental changes required for disconnection of the DMME during evolution. The ossification of the persistent Meckel’s in c-Fos null mice at P26 suggests that the default state of the cartilage in the absence of breakdown is ossification, likely through the direct differentiation of chondrocytes to osteocytes10,29. This demonstrates that the potential of MC to ossify is retained by living mammal lineages, far beyond their extinct mammaliaform relatives with ossified MC. In extant mammals, as well as in all other osteichthyans, clast cells are normally present during osteogenesis and bone homeostasis30. In the ossified Meckel’s cartilage of early mammals, clast cells would necessarily have been recruited into the Meckel’s as part of the general process of endochondral ossification and bone remodelling. During the earliest evolution of extant placentals and marsupials, a delay in the onset of ossification, or more probably an early recruitment of clast cells would have resulted in resorption of Meckel’s cartilage prior to the initiation of osteogenesis. We therefore hypothesis that a heterochronic shift involving the onset timing of clast activity might have been a key step in the PMME to DMME transition8. At this current time, the mechanism by which clast cell recruitment has changed during this transition is unknown. It would be unlikely that changes in c-Fos expression and activity would be directly involved, as clast cells, the precursors of which are always present in the bone marrow, have key functions throughout the body, including the ear. Therefore changes in c-Fos expression would have profound effects outside MC. Instead changes in the expression domain of the signals that recruit and activate immature clast cells at MC are more likely. One potential signalling pathway is RANK/RANKL/OPG, which has been shown to be expressed in the distal parts of MC in mice31. Alternatively signals upstream of osteogenesis may have changed, either temporally or spatially, resulting in changes in the potential for MC to ossify while still retaining the recruitment of clast cells. Potential candidates include Bone Morphogenetic Protein (BMP) family members, since mice with mutations in Noggin, a BMP antagonist, result in an ossification in the proximal part of MC as a consequence of enhanced BMP signalling32.
The ontogeny of the jaw and middle ear structures of mammals follows a series of developmental events with significant phylogenetic ramifications. These developmental stages include: the migration of the neural crest to form skeletal elements in the branchial arches that occurs in vertebrates but not (with the possible caveats of non-vertebrate chordates) in invertebrates, the formation of the mandibular arch in gnathostomes but not agnathans, the formation of the TMJ and incus-stapes joints present in mammaliaforms but not in non-mammalian amniotes, and finally the separation of the definitive mammalian middle ear by MC breakdown. Each of these stages represents a potential recapitulation of an ancestral state: for example Mesozoic mammals have a TMJ and incus-stapes joint, but have not separated the MC from the middle ear. We have disrupted this final step causing a partial recapitulation of an ancestral form. As such the development of the jaw and middle ear in mammals could be seen as an example of the renovated Von Baer’s law as defined by Abzhanov 33.
In conclusion, we present here experiments highlighting the importance of the developmental programme controlling clast cell activity, and uncover the potential role of alterations in developmental timing in the evolution of the DMME and consequently mammals.
Methods
Sample collection
Experimental mice and reptiles (Pantherophis guttatus, Chamaeleo calyptratus, Paroedura pictus) were cared for and culled at King’s College London (KCL) according to UK Home Office license and regulations in line with the regulations set out under the United Kingdom Animals (Scientific Procedures) Act 1986 and the European Union Directive 2010/63/EU. Opossum (Monodelphis domestica) pups were collected from breeding colonies maintained either at the Crick Institute Mill Hill Laboratories, London in line with regulations stated above, or at the University of Illinois at Urbana-Champaign (UIUC), in accordance with fully approved IACUC procedures.
c-Fos null (Fostm1Wag, MGI:2181817) mice were generated as previously reported34 and biological replicates from different litters were subject to microCT, TRAP staining, in situ hybridisation, and alcian blue – alizarin red staining (total c-Fos mice used: pre-weaning n=8 each genotype, post-weaning n=4 of each genotype).
Following culling the animals were decapitated, the heads were skinned and either fixed in 4% PFA for histological or µCT analysis, or fixed in 95% ethanol for skeletal preparation. Tissue for histological staining was decalcified in EDTA and wax embedded by standard protocols. Bat samples were collected and processes as previously described 35.
Power calculations or previous experience were used to predetermine sample size. In wildtype studies, littermates of the same age were randomly assigned to experimental groups and sex was not considered. In mutant mouse studies, pups were culled at specific ages and then genotyped to assign to groups. All control animals were littermates of the same age.
Alendronate treatment of opossum pups
Opossum pups housed at were treated with the bone resorption inhibitor Alendronate Sodium Trihydrate (Sigma-Aldrich). Alendronate was administered by intraperitoneal injection on postnatal day 16 (20µl of 10mg/ml solution in H20), approximately 4 days before the onset of MC breakdown in opossum (n=3. Control pups from the same litter received injections of 20ul (n=3). Pups were then killed and heads collected for analysis on day 22, after the normal stage of MC breakdown. Dosage of alendronate was based on that administer by viteline vein injection to chick embryos by Ealba et al 36, and adjusted to compensate for both the reduction in efficacy due to I.P. injections compared to intravenous, and for the differences in size between chick embryos and the mammalian pups.
Alendronate treatment of mice pups
CD1 mice housed at KCL were treated with the bone resorption inhibitor Alendronate Sodium Trihydrate (Sigma-Aldrich). Alendronate was administered by intraperitoneal injection on postnatal day 0 (n=3, 10µl of 10mg/ml solution). Control pups from the same litter received injections of 10µl water (n=3). Pups were then culled and heads collected for analysis on day 3, after the normal stage of MC breakdown. Dosage was chosen using the same criteria as opossum pups (see above).
Alcian blue – alizarin red skeletal preparation
Following fixation samples were placed in acetone before being stained with alcian blue 8GS and alizarin red S. The embryos were then washed in dH2O, and macerated in 1% KOH until the stained bone and cartilage was clearly visible. Samples were then cleared in a graded series of glycerol in 1% KOH, from 1:3 through to 100% glycerol. Stained and cleared tissue was imaged with a Leica MZFiii stereoscope and Leica DFC300 camera.
Histological Staining
Slides were prepared by microtomy. For histological examination of bone and cartilage, the slides were then stained with picro-sirius red/alcian blue trichrome stain using standard techniques. When in situ hybridization or TRAP staining was to be carried out, sections were mounted in parallel series, and the first slide from each series used for trichrome staining, and the subsequent slides in each series used for mRNA or TRAP detection so that parallel sections could be compared. Col2a and Osteocalcin mRNAs were detected by in situ hybridisation using digoxigenin (dig)-labeled probes. TRAP staining was carried out on de-waxed and rehydrated tissue by incubating the slides in filtered pH 5.32 acetate buffer containing 1mg/ml Naphthol-AS-TR-phosphate (Sigma), 100mM Sodium Tartrate (Sigma), and 1mg/ml Fast Red TR Salt (Sigma) at 37 C until colour reaction was complete. Time of reaction was empirically determined for each tissue, avoiding longer incubation times to prevent bone matrix staining. For identification of large nucleated cells in archived wax embedded gecko samples, sections were dewaxed, rehydrated and stained with haematoxylin and eosin. All histological stains were duplicated in biological replicates from different litters/matings. All stained sections were imaged on a Nikon Eclipse 80i slide microscope and Nikon Digital Sight camera. TRAP stained slides were imaged under dark-field microscopy in order to identify surrounding tissues.
µCT
µ Computerized tomography analysis (µCT) was carried out at KCL (Fig. 3, Supplementary Fig. S3) using a GE Locus SP micro scanner (GE Pre-clinical Imaging, London, Ontario, Canada), and visualized with MicroView software (Parallax Innovations Inc, Ilderton, Canada), and at UIUC (Fig. 4 Supplementary Fig. S5) using an Xradia Bio MicroCT (MicroXCT-400, Zeiss) and visualized using Amira 5.6.0 software (FEI Visualization Sciences Group, Bordeaux, France). The gap between the distal edge of the malleus and the proximal edge of the Meckel’s cartilage was measured in pixels using Amira 5.6.0 (n=3). The same zoom factor was applied to each sample before measurement. The measurement of each sample was carried out three times by an operator who was blind to the treatment, and the average of the three measurements taken to generate three biological replicates for each condition. The significance of the effect of Alendonrate treatment was tested by students t-test in Prism 6 (Graphpad).
Supplementary Material
Acknowledgments
The authors would like to thank Christopher Healy (KCL) and Leilei Yin (UIUC) for support with µCT, Elena Popa (KCL) for assistance in processing tissue, Juan Fons Romero (KCL) for injection of newborn mice, April Neander (UofC) for graphics assistance, Agi Grigoriadis (KCL) for supplying the c-Fos mutant mice and in situ probes, and James Turner and Fanny Decarpentrie (Francis Crick Institute, UK) for supplying opossum pups. For this project NA was supported by the Leverhulme Trust (RPG-2013-070) and the Wellcome Trust (102889/Z/13/Z). AST is funded by the Wellcome Trust (102889/Z/13/Z). NA additionally gained support for this project from the NSF/EDEN for a Research Exchange Grant (IOS # 0955517). DJU was supported by a NSF GRF (2013136301) and KES by DDIG (1406802).
Footnotes
Author contributions
NA and AST conceived and designed the project. NA carried out mouse and reptile experimental work, KES and DJU carried out opossum experimental work. ZXL carried out fossil analysis. NA wrote the manuscript with AST. AST, ZXL, KES and DJU critically appraised and edited the manuscript. All authors read and approved the manuscript before submission.
Author information
The authors declare no competing financial interests.
Reference List
- 1.Allin EF, Hopson JA. In: The Evolutionary Biology Of Hearing. Webster DB, Fay RR, Popper AN, editors. Springer-Verlag; 1992. pp. 587–614. [Google Scholar]
- 2.Rowe T. Coevolution of the mammalian middle ear and neocortex. Science. 1996;273:651–654. doi: 10.1126/science.273.5275.651. [DOI] [PubMed] [Google Scholar]
- 3.Luo Z-X. Transformation and diversification in early mammal evolution. Nature. 2007;450:1011–9. doi: 10.1038/nature06277. [DOI] [PubMed] [Google Scholar]
- 4.Anthwal N, Tucker AS. In: From Clone To Bone: The Synergy Of Morphological And Molecular Tools In Palaeobiology. Asher RJ, Müller J, editors. Cambridge University Press; 2012. pp. 207–229. [Google Scholar]
- 5.Maier W, Ruf I. Evolution of the mammalian middle: ear a historical review. Journal of Anatomy. 2016;228:270–83. doi: 10.1111/joa.12379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luo Z-X. Developmental Patterns in Mesozoic Evolution of Mammal Ears. Annual Review of Ecology, Evolution, and Systematics. 2011;42:355–380. [Google Scholar]
- 7.Meng J, Hu Y, Wang Y, Li C. The ossified Meckel’s cartilage and internal groove in Mesozoic mammaliaforms: implications to origin of the definitive mammalian middle ear. Zoological Journal of the Linnean Society. 2003;138:431–448. [Google Scholar]
- 8.Ji Q, Luo Z-X, Zhang X, Yuan C-X, Xu L. Evolutionary Development of the Middle Ear in Mesozoic Therian Mammals. Science. 2009;326:278–281. doi: 10.1126/science.1178501. [DOI] [PubMed] [Google Scholar]
- 9.Takechi M, Kuratani S. History of studies on mammalian middle ear evolution: a comparative morphological and developmental biology perspective. Journal of Experimental Zoology Part B, Molecular and Developmental Evolution. 2010;314:417–33. doi: 10.1002/jez.b.21347. [DOI] [PubMed] [Google Scholar]
- 10.Amano O, et al. Meckel’s Cartilage: Discovery, Embryology and Evolution. Journal of Oral Biosciences. 2010;52:125–135. [Google Scholar]
- 11.Anthwal N, Joshi L, Tucker AS. Evolution of the mammalian middle ear and jaw: adaptations and novel structures. Journal of Anatomy. 2013;222:147–60. doi: 10.1111/j.1469-7580.2012.01526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sánchez-Villagra MR, Gemballa S, Nummela S, Smith KK, Maier W. Ontogenetic and phylogenetic transformations of the ear ossicles in marsupial mammals. Journal of Morphology. 2002;251:219–38. doi: 10.1002/jmor.1085. [DOI] [PubMed] [Google Scholar]
- 13.Amin S, Tucker AS. Joint formation in the middle ear: lessons from the mouse and guinea pig. Developmental Dynamics. 2006;235:1326–33. doi: 10.1002/dvdy.20666. [DOI] [PubMed] [Google Scholar]
- 14.Wilson J, Tucker AS. Fgf and Bmp signals repress the expression of Bapx1 in the mandibular mesenchyme and control the position of the developing jaw joint. Developmental Biology. 2004;266:138–150. doi: 10.1016/j.ydbio.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 15.Meng J, Wang Y, Li C. Transitional mammalian middle ear from a new Cretaceous Jehol eutriconodont. Nature. 2011;472:181–5. doi: 10.1038/nature09921. [DOI] [PubMed] [Google Scholar]
- 16.Luo Z-X, Gatesy SM, Jenkins FA, Amaral WW, Shubin NH. Mandibular and dental characteristics of Late Triassic mammaliaform Haramiyavia and their ramifications for basal mammal evolution. Proceedings of the National Academy of Sciences. 2015;112:E7101–E7109. doi: 10.1073/pnas.1519387112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holliday CM, Nesbitt SJ. Morphology and diversity of the mandibular symphysis of archosauriforms. Vol. 379. Geological Society, Special Publications; London: 2013. pp. 555–571. [Google Scholar]
- 18.Smith KK. Craniofacial development in marsupial mammals: developmental origins of evolutionary change. Developmental Dynamics. 2006;235:1181–93. doi: 10.1002/dvdy.20676. [DOI] [PubMed] [Google Scholar]
- 19.Gaupp EWT. Die Reichertsche theorie:(Hammer-, amboss-und kieferfrage) Archiv für Anatomie und Entwickelungsgeschichte. 1913;1912:1–426. [Google Scholar]
- 20.Hall BK. Bones and Cartilage: Developmental and Evolutionary Skeletal Biology. Academic Press; 2015. [Google Scholar]
- 21.Knowles HJ, et al. Chondroclasts are mature osteoclasts which are capable of cartilage matrix resorption. Virchows Archiv. 2012;461:205–210. doi: 10.1007/s00428-012-1274-3. [DOI] [PubMed] [Google Scholar]
- 22.Lewinson D, Silbermann M. Chondroclasts and endothelial cells collaborate in the process of cartilage resorption. The Anatomical Record. 1992;233:504–514. doi: 10.1002/ar.1092330403. [DOI] [PubMed] [Google Scholar]
- 23.Grigoriadis A, et al. c-Fos: a key regulator of osteoclast-macrophage lineage determination and bone remodeling. Science. 1994;266:443–448. doi: 10.1126/science.7939685. [DOI] [PubMed] [Google Scholar]
- 24.Arai A, et al. Fos plays an essential role in the upregulation of RANK expression in osteoclast precursors within the bone microenvironment. Journal of Cell Science. 2012;125:2910–7. doi: 10.1242/jcs.099986. [DOI] [PubMed] [Google Scholar]
- 25.Rogers MJ, Crockett JC, Coxon FP, Mönkkönen J. Biochemical and molecular mechanisms of action of bisphosphonates. Bone. 2011;49:34–41. doi: 10.1016/j.bone.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 26.Martin T, et al. A Cretaceous eutriconodont and integument evolution in early mammals. Nature. 2015;526:380–4. doi: 10.1038/nature14905. [DOI] [PubMed] [Google Scholar]
- 27.Raff RA. Written in stone: fossils, genes and evo–devo. Nature Reviews Genetics. 2007;8:911–920. doi: 10.1038/nrg2225. [DOI] [PubMed] [Google Scholar]
- 28.Sakakura Y. Role of Matrix Metalloproteinases in Extracellular Matrix Disintegration of Meckel’s Cartilage in Mice. Journal of Oral Biosciences. 2010;52:143–149. [Google Scholar]
- 29.Yang L, Tsang KY, Tang HC, Chan D, Cheah KSE. Hypertrophic chondrocytes can become osteoblasts and osteocytes in endochondral bone formation. Proceedings of the National Academy of Sciences. 2014;111:12097–102. doi: 10.1073/pnas.1302703111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boyce BF, et al. New roles for osteoclasts in bone. Annals of the New York Academy of Sciences. 2007;1116:245–54. doi: 10.1196/annals.1402.084. [DOI] [PubMed] [Google Scholar]
- 31.Sakakura Y, et al. Immunolocalization of receptor activator of nuclear factor-kappaB ligand (RANKL) and osteoprotegerin (OPG) in Meckel’s cartilage compared with developing endochondral bones in mice. Journal of Anatomy. 2005;207:325–37. doi: 10.1111/j.1469-7580.2005.00466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Y, Zheng Y, Chen D, Chen Y. Enhanced BMP signaling prevents degeneration and leads to endochondral ossification of Meckelks cartilage in mice. Developmental Biology. 2013;381:301–311. doi: 10.1016/j.ydbio.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abzhanov A. von Baer’s law for the ages: lost and found principles of developmental evolution. Trends in Genetics. 2013;29:712–22. doi: 10.1016/j.tig.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 34.Wang ZQ, et al. Bone and haematopoietic defects in mice lacking c-fos. Nature. 1992;360:741–5. doi: 10.1038/360741a0. [DOI] [PubMed] [Google Scholar]
- 35.Popa EM, Anthwal N, Tucker AS. Complex patterns of tooth replacement revealed in the fruit bat (Eidolon helvum) Journal of Anatomy. 2016 doi: 10.1111/joa.12522. E-pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ealba EL, et al. Neural crest-mediated bone resorption is a determinant of species-specific jaw length. Developmental Biology. 2015;408:151–63. doi: 10.1016/j.ydbio.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.