Abstract
Purpose
Pharmacokinetic parameters derived from plasma sampling are used as a surrogate of tumour pharmacokinetics. However, pharmacokinetics modulating strategies do not always result in increased therapeutic efficacy. Non-surrogacy of plasma kinetics may be due to tissue-specific factors such as tumour perfusion.
Experimental design
To assess the impact of tumour perfusion and plasma drug exposure on tumour pharmacokinetics, positron emission tomography (PET) studies were performed with oxygen-15 radiolabelled water in twelve patients, with six patients undergoing PET studies with carbon-11 radiolabelled N-[2-(dimethylamino)ethyl]acridine-4-carboxamide and the other six with fluorine-18 radiolabelled 5-fluorouracil.
Results
We found tumour blood flow (ml blood/ml tissue/min) was significantly correlated to early tumour radiotracer uptake between 4 and 6 minutes (SUV4-6; ρ= 0.79; p = 0.002), tumour radiotracer exposure over 10 minutes, (AUC0-10; predominantly parent drug; ρ= 0.86; p < 0.001) and tumour radiotracer exposure over 60 minutes, (AUC0-60; predominantly radiolabelled metabolites; ρ= 0.80; p = 0.002). Similarly, fractional volume of distribution of radiolablled water in tumour (Vd) was significantly correlated with SUV4-6 (ρ= 0.80; p = 0.002), AUC0-10 (ρ= 0.85; p < 0.001) and AUC0-60 (ρ= 0.66; p = 0.02). In contrast, no correlation was observed between plasma drug or total radiotracer exposure over 60 min and tumour drug uptake or exposure. Tumour blood flow was significantly correlated to Vd (ρ= 0.69; p = 0.014), underlying the interdependence of tumour perfusion and Vd.
Conclusions
Tumour perfusion is a key factor that influences tumour drug uptake/exposure. Tumour vasculature-targeting strategies may thus result in improved tumour drug exposure and therefore drug efficacy.
Introduction
Clinical anti-cancer drug development begins with dose-escalating phase I clinical trials of the investigational agent and leads on to phase III/IV trials, before a drug is accepted for clinical use. Although the primary objectives of phase I clinical trials are to establish the recommended phase II dose and assess drug toxicity, most phase I trials also aim to evaluate drug pharmacology (pharmacokinetics and pharmacodynamics). Clinical pharmacology plays an ever increasing role in drug development and can potentially aid in dose-schedule selection, therapy response monitoring and in the development of targeted strategies. In addition to providing information on drug pharmacology, plasma pharmacokinetic parameters such as plasma drug exposure (area under the concentration-time curve; AUC), peak plasma concentrations (Cmax) and steady-state plasma concentrations (Css) have been used as pharmacodynamic markers of drug efficacy and utilised to optimise therapy with anti-cancer agents such as methotrexate [1] and carboplatin [2]. However, the limited utility of plasma pharmacokinetic parameters to select and optimise anti-cancer therapy so far may be due to non-translation of parameters obtained from plasma samples as surrogates of tumour pharmacokinetics.
Functional imaging modalities such as positron emission tomography (PET) and magnetic resonance imaging (MRI) allow non-invasive evaluation of tissue and tumour drug uptake and exposure in vivo, thus providing invaluable tissue pharmacokinetic data [3, 4]. We have previously reported from PET studies of radiolabelled anti-cancer agent’s that tumour drug exposure is several-fold less than in most normal tissues [4, 5]. This differential in drug exposure may be a result of several factors that may be either drug-specific (drug pharmacokinetics, ionisation status, lipophilicity) or tissue-specific (tissue vasculature/permeability, drug efflux mechanisms, saturation in uptake). Tumour vasculature is widely recognised as a potential therapeutic target in order to improve tumour drug delivery and clinical efficacy [6]. However, in vivo clinical data on the influence of tumour perfusion on tumour drug delivery and intra-tumoural drug distribution has so far been limited.
In this paper, we report our findings obtained from PET perfusion studies with oxygen-15 radiolabelled water and tumour drug uptake and exposure of two radiolabelled drugs- N-[2-(dimethylamino)ethyl]acridine-4-carboxamide (DACA), which is lipophilic [7] and 5-fluorouracil (5-FU), a hydrophilic agent [8]. The highly sensitive and quantitative nature of PET allowed in vivo evaluation of both tumour blood perfusion and the tumour pharmacokinetics of anti-cancer agents after ‘microdoses’ of the radiolabelled anti-cancer agents. We hypothesised that tumour blood flow was an important determinant of tumour drug uptake and exposure and therefore evaluated the influence of tumour perfusion on tumour drug pharmacokinetics. In addition, we also evaluated the influence of plasma drug exposure on tumour drug uptake and exposure.
Methods
Patients and procedures
Twelve patients who had undergone PET scanning as part of previously published studies were included in the analysis [4, 5, 9]. PET scans to obtain tumour perfusion and tumour drug kinetics were performed in all twelve patients included in this analysis. Dynamic PET scanning to assess tumour perfusion was performed after the inhalation/injection of oxygen-15 [15O] radiolabelled CO2/H2O, respectively, as described previously [4, 5, 9]. To assess tumour drug pharmacokinetics dynamic PET scanning was performed after the injection of tracer doses of carbon-11 radiolabelled DACA ([11C]DACA; 6 patients) [4, 9] or fluorine-18 radiolabelled 5-FU ([18F]FU; 6 patients) [5]. [11C]DACA and 5-[18F]FU were radiosynthesised as described previously [4, 5]. The mean doses of DACA and 5-FU administered as a bolus injection during the scan were 7 μg/m2 and 1 mg/m2, respectively. As none of the patients were administered therapeutic drug (DACA or 5-FU; cold drug) doses prior to or during PET scanning, tissue drug pharmacokinetic parameters were not confounded by changes in tissue pharmacokinetics due to saturable kinetics and potential drug-related tissue pharmacodynamic (vascular) changes.
All PET scans were performed on the ECAT931 scanner as described previously [4, 5, 9]. Briefly, a perfusion PET scan was performed after the inhalation of [15O] CO2 or infusion of [15O]H2O. The short half-life of oxygen-15 (2 min), allowed drug pharmacokinetic scans to be performed soon thereafter, after a short time interval. Arterial blood sampling was performed throughout the scan duration to obtain plasma time-radioactivity curves. Metabolite analysis was performed as described previously at predetermined times, during the drug scan duration [4, 5, 9]. From this the total radioactivity and the parent drug radioactivity ([11C]DACA or 5-[18F]FU) was calculated. Metabolite analysis was performed at 5, 10, 20, 40 and 60 min in 3 patients [4] and at 5, 10, 20, 45 and 60 min for the other 3 patients [9] with [11C]DACA-PET scans and at 2, 5, 10, 30 and 60 mins with 5-[18F]FU-PET scans [5], as described previously.
Data analysis
Tumour perfusion was calculated using the methods described previously to obtain perfusion parameters [5]. Briefly, kinetic modelling was used to fit the plasma arterial input function derived from calibrated continuous arterial plasma radioactivity data and the tumour time-activity curve (output) to obtain perfusion parameters - Flow (ml blood/ml tissue/min) and the partition co-efficient of water or the volume of distribution of radiolabelled water (units attributed include ml blood per ml tissue, ml blood per cc tissue or ratio - unitless). The ‘volume of distribution’ or Vd is defined as volume of blood that contains the same quantity of radioactivity as unit ml (or cc) of tissue at equilibrium [10]. This definition is equivalent to the ratio of tissue concentration of the tracer (KBq/ml) to the blood concentration (KBq/ml) at steady state, and for water can be thought of as the fractional volume of tissue into which water can pass (i.e. the volume of the tissue excluding lipid deposits such as fat and membranes).
Three tumour pharmacokinetic parameters – standardized uptake value from 4 to 6 min (SUV4-6) reflective of peak drug uptake, area under the time-activity curves from 0 to 10 min (AUC0-10) and area under the time-activity curves from 0 to 60 min (AUC0-60) were used to quantify radiolabelled drug uptake and exposure. It should be noted that the radiolabelled tumour uptake was representative of the total activity i.e. radiolabelled drug and its radiolabelled metabolites and not the radiolabelled parent drug alone. Tumour uptake (SUV4-6) and exposure (AUC0-10 and AUC0-60) parameters were normalised for injected radioactivity and patient body surface areas to obtain parameters with units of m2/ml and m2/ml × sec, respectively.
Plasma exposure to the parent drug and total radiotracer from 0 to 10 min (AUC 0-10) and 0 to 60 min (AUC 0-60) to signify early and total plasma exposure, respectively were calculated from the plasma parent and plasma total radiotracer time-activity curves (TAC) respectively, using the trapezoidal method. Plasma parent drug exposure (Pl_Parent AUC0-10 and Pl_Parent AUC0-60) and total radiotracer exposure (Pl_Total AUC0-10 and Pl_Total AUC0-60) were similarly normalised as tissue data for injected radioactivity and patient body surface area to obtain parametric values in units of m2/ml × sec for comparison with the tumour pharmacokinetic data.
Statistical analysis
Correlations between tumour pharmacokinetic parameters (SUV4-6, AUC0-10 and AUC 0-60), tumour perfusion parameters (F and Vd) and plasma exposure parameters (Pl_Total AUC0-10, Pl_Parent AUC0-10, Pl_Total AUC 0-60 and Pl_Parent AUC 0-60) were sought using Spearman’s correlation analysis. A p value of < 0.05 was considered significant.
Results
Patients
All 12 patients included had both perfusion and drug kinetic PET imaging of tumours. For the DACA scans, 6 patients with 9 tumour metastases were included (Table 1). Similarly, 6 patients with 9 metastases were included in the 5-FU study. A list of patients included, diagnosis and amount of drug and radioactivity injected is given in Table 1. Tumour uptake (SUV4-6) and exposure (AUC0-10, AUC 0-60) to radiolabelled drug and metabolites and tumour perfusion parameters (F and Vd) were available in all patients. All plasma samples were taken at the proposed times in all patients, except in 1 patient (patient 5), who did not have the 15 min plasma sample.
Table 1.
| Pt. no. | Diagnosis | Drug | Patient BSA (m 2 ) | Drug dosage | Drug activity (MBq) |
|---|---|---|---|---|---|
| 1 | Lung cancer | [11C]DACA | 2.07 | 4.1 μg/m2 | 483.7 |
| 2 | Lung cancer | [11C]DACA | 1.63 | 7.6 μg/m2 | 495.2 |
| 3 | Leiomyosarcoma | [11C]DACA | 1.79 | 7.5 μg/m2 | 528.8 |
| 4 | Lung metatases (Cervical cancer) * | [11C]DACA | 1.53 | 4.7 μg/m2 | 479.0 |
| 5 | Hepatic cancer * | [11C]DACA | 1.66 | 9.3 μg/m2 | 477.2 |
| 6 | Metastatic ovarian cancer | [11C]DACA | 1.70 | 9.2 μg/m2 | 504.0 |
| 7 | Liver metastases (Colorectal cancer) * | 5-[18F]FU | 1.96 | 1 mg/m2 | 339.2 |
| 8 | Liver metastases (Colorectal cancer) | 5-[18F]FU | 1.90 | 1 mg/m2 | 342.8 |
| 9 | Liver metastases (Colorectal cancer) * | 5-[18F]FU | 1.60 | 1 mg/m2 | 348.2 |
| 10 | Liver metastases (Colorectal cancer) | 5-[18F]FU | 1.74 | 1 mg/m2 | 377.7 |
| 11 | Liver metastases (Gastro-intestinal cancer)* | 5-[18F]FU | 1.84 | 1 mg/m2 | 335.0 |
| 12 | Locally advanced pancreatic cancer | 5-[18F]FU | 1.66 | 1 mg/m2 | 349.7 |
More than one tumour region imaged
Plasma drug exposure
Plasma TAC’s demonstrated a rapid decrease in plasma radioactivity of total tracer (radiolabelled parent drug and metabolites) and the radiolabelled parent drug after tracer doses of both the 5-[18F]FU and [11C]DACA. TAC profiles demonstrated a rapid clearance of radioactivity from plasma with both radiotracers, especially of the parent radiolabelled drug (figures 1(a) and 1(b)), underlining the extent of metabolite contribution with both these agents. In contrast to [11C]DACA, plasma 5-[18F]FU levels were not observed 30 minutes after the injection of tracer doses of 5-[18F]FU, as reported previously [11]. In keeping with the observed plasma profile, plasma AUC for the parent drug (Pl_Parent AUC 0-60) was lesser than the total tracer (Pl_Total AUC 0-60). A strong correlation was observed between Pl_Total AUC0-10 and Pl_Parent AUC0-10 (ρ= 0.88; p < 0.001) and Pl_Total AUC 0-60 and Pl_Parent AUC 0-60 (ρ= 0.99; p < 0.001). Similarly, early and late plasma exposure parameters were significantly correlated (Pl_Total AUC0-10 and Pl_Total AUC0-60, ρ= 0.96; p < 0.001 and Pl_Parent AUC0-10 and Pl_Parent AUC0-60, ρ= 0.92; p < 0.001). A summary of the plasma AUC for parent and total tracer for 5-[18F]FU and [11C]DACA are given in Table 2.
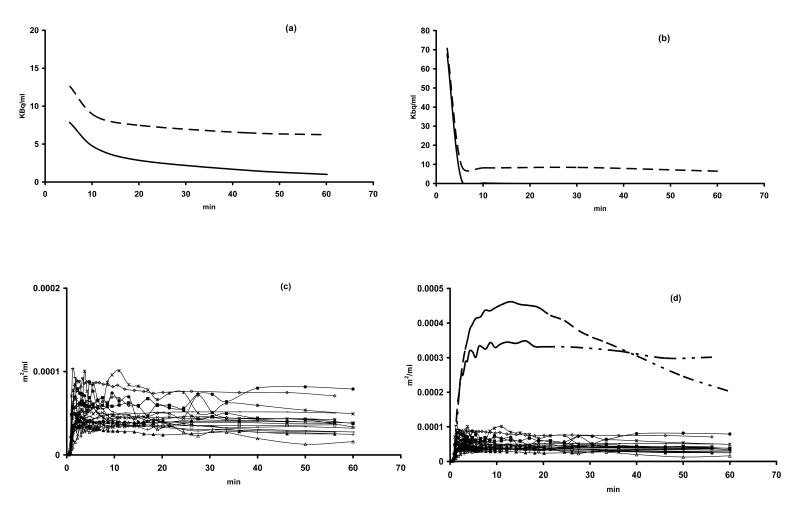
Figure 1.
Mean plasma time-activity curves after injection of (a) [11C]DACA and (b) 5-[18F]FU. The total [11C]- or [18F]- radiolabelled parent and metabolites are shown as broken lines, while [11C]DACA and 5-[18F]FU are shown as unbroken lines. Individual tumour TACs for all 12 patients (18 tumour TACs) are illustrated in (c) and for comparison in (d) all tumour TACs and hepatic TACs (broken lines) for DACA (patient 5) and 5-FU (patient 7) are illustrated. Both 5-FU and DACA undergo hepatic metabolism.
Table 2.
| Pt. no. | AUC0-60 (m 2 /ml × sec) |
AUC0-10 (m 2 /ml × sec) |
SUV 4-6 × 10 3 (m 2 /ml) |
Flow (ml/ml/min) |
V d | Pl_Total AUC 0-60 (m 2 /ml × sec) |
Pl_Parent AUC 0-60 (m 2 /ml × sec) |
|---|---|---|---|---|---|---|---|
| 1 | 0.270 | 0.045 | 0.257 | 0.914 | 1.012 | 0.064 | 0.024 |
| 2 | 0.125 | 0.018 | 0.101 | 0.190 | 0.456 | 0.085 | 0.021 |
| 3 | 0.096 | 0.017 | 0.095 | 0.053 | 0.176 | 0.092 | 0.039 |
| 4 * | 0.146 | 0.024 | 0.129 | 0.216 | 0.509 | 0.125 | 0.039 |
| 5 * | 0.160 | 0.021 | 0.118 | 0.131 | 0.575 | 0.086 | 0.037 |
| 6 | 0.112 | 0.020 | 0.117 | 0.086 | 0.395 | 0.085 | 0.038 |
| 7 * | 0.177 | 0.033 | 0.178 | 0.232 | 0.522 | 0.200 | 0.074 |
| 8 | 0.087 | 0.016 | 0.086 | 0.078 | 0.242 | 0.193 | 0.056 |
| 9 * | 0.193 | 0.034 | 0.191 | 0.265 | 0.566 | 0.240 | 0.069 |
| 10 | 0.245 | 0.033 | 0.218 | 0.182 | 0.554 | 0.144 | 0.037 |
| 11 * | 0.123 | 0.027 | 0.150 | 0.114 | 0.641 | 0.197 | 0.065 |
| 12 | 0.142 | 0.035 | 0.186 | 0.233 | 0.794 | 0.205 | 0.062 |
More than one tumour region imaged. Tumour parameters were averaged in these instances.
Patients 1-6 received [11C]DACA, while 7-12 received [18F]5-FU.
Tumour drug uptake and exposure
Tumour uptake and exposure to radioactivity varied between tumour types in contrast to similar TAC profiles seen in normal tissues, as reported previously [4, 5, 9]. Tumour TACs for all the patients included in this analysis are shown in figure 1 (c) and representative hepatic TACs for 2 patients (patient 3 (DACA) and patient 7 (5-FU)), demonstrating the hepatic metabolism of both DACA and 5-FU are also illustrated for comparison with tumour TACs in figure 1 (d). Early tumour uptake (SUV4-6) was significantly correlated to tumour radiotracer (parent drug and metabolites) exposure over 10 min (AUC0-10) (ρ= 0.96; p < 0.001) and was also significantly correlated to tumour radiotracer (parent drug and metabolites) exposure over 60 min (AUC0-60) (ρ= 0.88; p < 0.001). Early tumour radiotracer exposure (AUC0-10), is reflective of tumour exposure to radiolabelled parent, which is the predominant entity in plasma at early time-points (figures 1(a) and 1(b)), while tumour radiotracer exposure over 60 minutes (AUC0-60) is predominantly due to metabolites for tracer doses of both agents studied, which are extensively metabolised. AUC0-10 and AUC0-60 were also correlated (ρ= 0.81; p = 0.002). Tumour uptake and exposure parameters were averaged, where more than one tumour region was sampled in the same patient. A summary of tumour radiotracer uptake parameters are summarised in Table 2.
Tumour perfusion
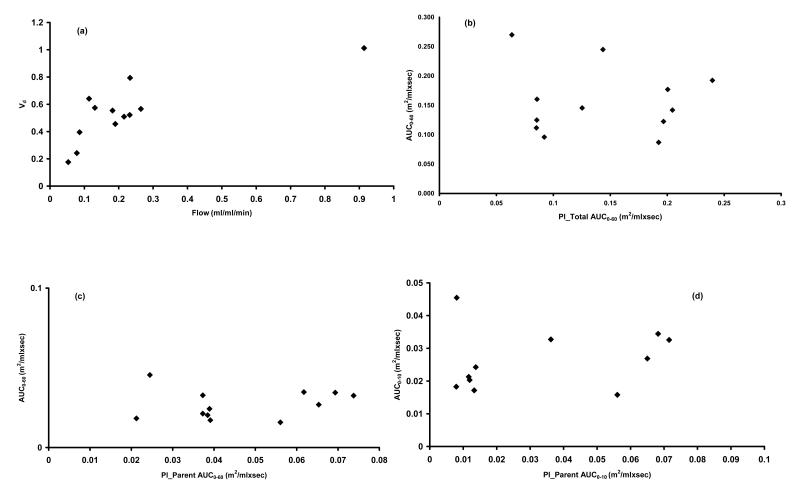
Tumour flow and Vd parameters for the patients sampled are given in Table 2. In instances where more than one tumour region was sampled in the same patient (4 patients), the perfusion parameters were averaged, as in previous studies [12]. Tumour flow varied considerably between patients with a mean (standard error of the mean (SEM)) flow of 0.224 (0.066) ml blood/ml tissue/min. In contrast, the variability in Vd of water was lesser between patients with a mean (SEM) of 0.537 (0.065). A significant correlation was observed between the two tumour perfusion parameters, flow and Vd (ρ= 0.69; p =0.014; figure 2 (a)).
Figure 2.
Scatter plot (a) illustrating the significant relationship between tumour perfusion parameters flow and Vd (ρ= 0.69; p = 0.014). Scatter plots (b) and (c) illustrate the lack of relationship between tumour drug exposure over 60 minutes (AUC0-60) with plasma exposure to total plasma radiotracer over 60 minutes (ρ= 0.04; p = 0.9) and plasma exposure to parent drug over 60 minutes (ρ= −0.13; p = 0.7 ), respectively. In (d) the lack of relationship between plasma exposure to parent drug over 10 min and tumour exposure over 10 min (AUC0-60) is illustrated (ρ= 0.24; p = 0.47).
Relationship of tumour drug uptake/exposure to plasma exposure and tumour perfusion
Correlation scatter plots for tumour exposure over 60 min with plasma exposure to activity associated with the total radiotracer (parent and metabolites) and parent drug (DACA or 5-FU) over 60 minutes are illustrated in figures 2 (b) and 2 (c), respectively. Plasma exposure to total radiotracer over 60min (Pl_Total AUC0-60) did not correlate to radiotracer tumour SUV4-6 (ρ= 0.25; p = 0.44), tumour AUC0-10 (ρ= 0.32; p = 0.32 ), or tumour AUC0-60 (ρ= 0.04; p = 0.9 ). Similarly, a lack of relationship was observed for plasma exposure to radiolabelled parent drug over 60 min (Pl_Parent AUC0-60) with radiotracer tumour SUV4-6 (ρ= 0.09; p = 0.78), tumour AUC0-10 (ρ= 0.2; p = 0.54), or tumour AUC0-60 (ρ= −0.13; p = 0.7). Additionally, we observed a lack of relationship between plasma exposure to total radiotracer over 10 min (Pl_Total AUC0-10) with radiotracer tumour SUV4-6 (ρ= 0.18; p = 0.59), tumour AUC0-10 (ρ= 0.22; p = 0.51), or tumour AUC0-60 (ρ= −0.03; p = 0.93). No co-relationship was also observed between Pl_Parent AUC 0-10 with radiotracer tumour SUV4-6 (ρ= 0.2; p = 0.56), tumour AUC0-10 (ρ= 0.24; p = 0.47; figure 2 (d)), or tumour AUC0-60 (ρ= 0.009; p = 0.98 ).
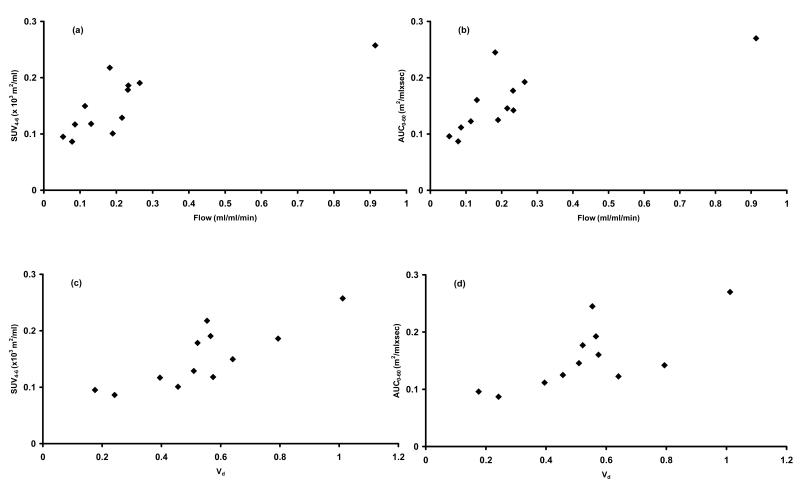
In contrast to lack of correlation between plasma parameters and tumour drug kinetics, early radiotracer uptake (SUV4-6) was significantly correlated to tumour blood flow (ρ= 0.79; p = 0.002; figure 3 (a)). Similarly, tumour perfusion was significantly correlated to was radiotracer tumour AUC0-10 (ρ= 0.86; p < 0.001) and AUC0-60 (ρ= 0.80; p = 0.002 (figure 3 (b)). The volume of distribution of radiolabelled water was also significantly related to SUV4-6 (ρ= 0.80; p = 0.002 (figures 3 (c)), AUC0-10 (ρ= 0.85; p < 0.001) and AUC0-60 (ρ= 0.66; p = 0.02 (figures 3 (d)).
Figure 3.
Scatter plots showing statistically significant relationship between (a) tumour blood and SUV4-6 (ρ= 0.79; p = 0.002), (b) tumour blood and AUC0-60 (ρ= 0.80; p = 0.002), (c) Vd and SUV4-6 (ρ= 0.8; p = 0.002) and (d) Vd and AUC0-60 (ρ= 0.66; p = 0.02).
Discussion
This paper describes the impact of tumour perfusion parameters vis a vis plasma pharmacokinetic parameters in influencing in vivo tumour uptake and exposure of two anti-cancer agents. Conclusions made in this analysis are based on PET perfusion studies with radiolabelled water, tissue pharmacokinetic studies with radiolabelled anti-cancer agents and supplementary plasma pharmacokinetic data obtained during PET scanning in the same patients with cancer. This was made possible by the dynamic nature of PET data acquisition with short half-lived positron emitters, the highly sensitive nature of PET and analysis of plasma data in the same patient during a single scanning session.
We have clearly demonstrated that early tumour uptake and tumour exposure to the parent drug and metabolites (total radiotracer) is neither related to plasma exposure to parent drug or metabolites. This PET study confirms similar findings of a lack of relationship between plasma and tumour drug levels with other anti-cancer agents demonstrated using non-invasive [3, 13] or invasive methods [14]. Despite our inability to make a generalisation based on the limited data, it would be reasonable to conclude that this lack of surrogacy between plasma and tumour kinetics may partly explain the limited clinical activity observed for a number of anti-cancer agents, in spite of tumouricidal plasma pharmacokinetics. It is therefore important that tissue-specific pharmacodynamic information should be accrued wherever possible, in addition to plasma pharmacokinetics during the early phases of anti cancer drug development [15].
In contrast, we observed that both tumour blood flow and volume of distribution of radiolabelled water played a significant role in influencing tumour drug uptake and exposure to the total radiotracer. Although, we have previously reported on the impact of tumour flow on tumour drug exposure and the influence of tumour perfusion on changes in drug uptake [4, 12, 16], this is the first report that has additionally evaluated the influence of tumour volume of distribution of radiolabelled water and plasma pharmacokinetics on tumour drug uptake and exposure in vivo clinically. In addition, to a significant relationship between tumour flow and tumour exposure with both 5-[18F]FU and [11C]DACA [4, 12, 16], we also found that Vd of radiolabelled water significantly correlated with tumour drug uptake/exposure. These findings not only confirm the importance of tumour blood flow but also highlight the role of exchangeable fractional tumour volume (Vd) on drug uptake. The significant positive correlation observed in our study between tumour blood flow and Vd highlights an interdependence of flow and fractional volume of distribution in tumours and underlies the importance of water exchangeable tumour tissue on tumour blood flow. However, the relationship between flow and volume of distribution in normal tissues was not evaluated in this analysis. Our group is also assessing the relationship between the perfusion parameters and the effects of vascular targeting agents on perfusion in normal tissue and tumours. Although not reported by Wilson et al in their manuscript evaluating flow and volume of distribution in normal breast and breast tumours [17], analysis of the individual patient data provided in their paper suggests that flow and volume of distribution of water are related in tumours (ρ= 0.55; p = 0.012) but not in normal breast (ρ= 0.29; p =0.27). Therefore the relationship between flow and Vd seen in tumours is due to physiological changes and not due to parameter estimation artefacts.
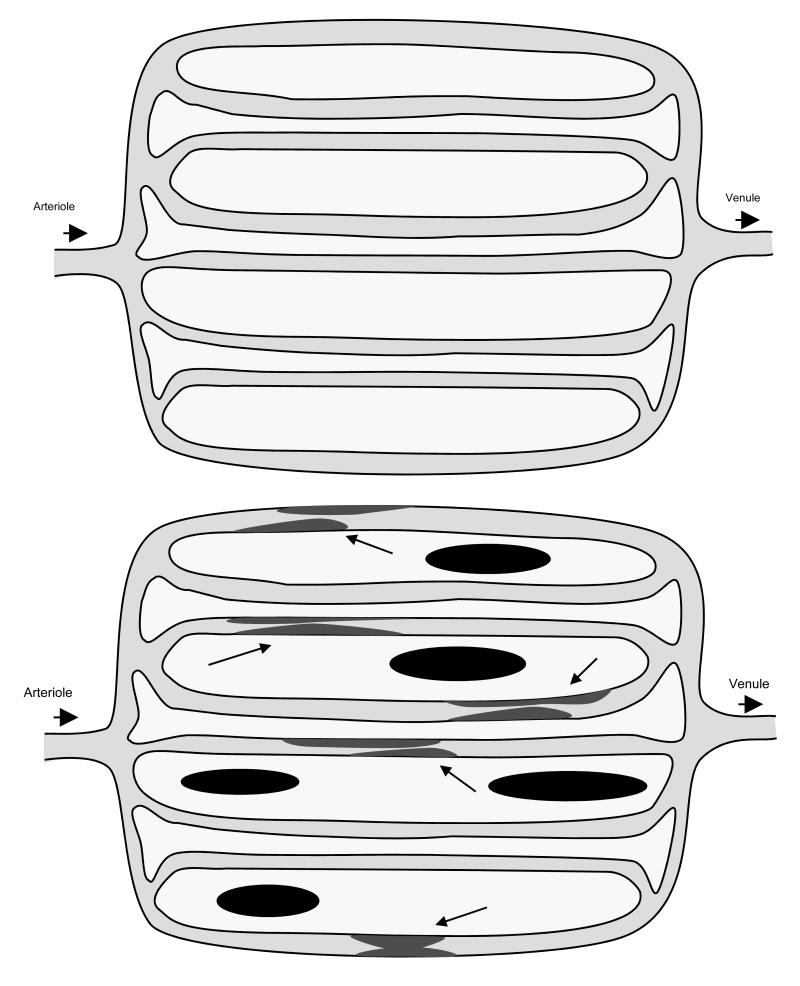
Our study evaluated the importance of two perfusion parameters – blood flow and volume of distribution of water in tumours. Although both these parameters indicate tissue perfusion characteristics, they signify different features of tissue perfusion (figure 4). Whereas tumour flow provides a quantitative indication of the volume of blood perfused per unit volume of tumour, Vd, signifies the exchangeable tumour volume at steady state or the volume of tumour with which radiolabelled water from plasma equilibrates. As a measure of the concentration of radiolabelled water in a fraction of tissue, Vd is dependent on the amount of viable tissue per unit volume measured, fatty content [17], extent of tissue necrosis and fibrosis. Although we do not have a direct measure of tumour interstitial fluid pressure (IFP), we hypothesise (figure 4) that Vd may also represent and reflect changes in interstitial fluid pressure, which is considered an important barrier to the effective delivery of molecules into tumor tissue [18]. This raises interesting possibilities in the sequencing of tumour IFP reducing agents when combining with other cytotoxic agents, vascular disrupting agents or radiotherapy [19-21].
Figure 4.
Schematic diagram illustrating normally (top) and abnormally (bottom) perfused tissue. Radiolabelled water, a freely diffusible and inert radioligand enters tissue via arterioles, diffusing to and back from the tissue (white background) and leaving it via the venous system. With PET, tissue perfusion is quantified as tissue blood flow, which is volume of blood perfused through unit volume of tissue per min (ml blood/ml tissue/min) and Vd, which is the fractional volume of tissue with which radiolabelled water exchanges at equilibrium. In tumours, necrotic tissue (dark blocks) are likely to result in a reduction in Vd. Similarly, an increase in interstitial fluid pressure would result in a decrease in Vd and result in a further compromise of tumour vasculature (arrows), which is inherently unstable and poor, resulting in reduction in tissue flow.
Despite drug lipophilicity being an important determinant of tissue drug uptake, the significant relationship observed between tumour perfusion and drug uptake of both lipophilic (DACA) [7] and hydrophilic (5-FU) [8] agents (and their metabolites) in our study underlines the influence of tumour perfusion on tumour drug delivery. It is therefore important to take into consideration the poor tumour vascular supply that may limit tumour drug uptake with all anti-cancer agents, irrespective of their lipophilicity. On the basis of direct influence of perfusion on early tumour drug uptake (SUV4-6) and exposure (AUC0-10) it is likely that tumour perfusion may play an especially influential role on therapy where anti-cancer drug efficacy based on preclinical models is dependent on tumour Cmax levels which usually occur during the early period of bolus infusions. However, it is not possible to extrapolate that similar improvements in perfusion would lead to improvements in tumour exposure, as the tumour exposure data in our analysis is limited to 60 min (AUC0-60) only. Moreover, in our study with tracer quantities of drugs, the predominant plasma contribution at latter time points is mainly due to radiolabelled metabolites unlike early uptake (SUV4-6), when radioactivity is predominantly associated with the parent drug. We would like to point out that all patients included in this analysis were administered tracer amount of drugs (microdoses) and not therapeutic concentrations. It is likely that the relationship between perfusion parameters and tumour drug uptake may not hold true at higher drug concentrations. However, it is not possible to speculate on this relationship from data obtained in this analysis.
In conclusion, we have demonstrated that tumour perfusion is more important than plasma drug exposure in determining early tumour drug uptake and exposure. The importance of tumour perfusion justifies the need for adapting anti-cancer treatment strategies that target tumour vasculature. If tumour vasculature is targeted judiciously with a clear understanding of biological and chronological tumour perfusion patterns, there is tremendous potential to improve the therapeutic benefit.
Statement of Clinical Relevance.
This work adds to our current knowledge on the influence of tumour vasculature on tumour drug pharmacokinetics and highlights the importance of tumour perfusion on tumour delivery and uptake of anti-cancer agents. We have found that both blood flow and fractional volume of distribution of water play an important role in tumour drug pharmacokinetics. On the basis of study findings, we anticipate that vascular-targeted strategies will also improve therapeutic benefit. Such strategies include drug scheduling to improve tumour perfusion prior to chemotherapy, radiotherapy or other treatments, selecting the right drug combination and monitoring response to targeted therapies. Integrating functional imaging generically during anti-cancer drug development process and in clinical practice will help to achieve these aims.
Acknowledgements
We would like to thank Professor Terry Jones for valuable suggestions in preparation of this manuscript and for providing and permitting modification of figure 4. We would also like to thank Professor Herbie Newell for initial discussions and Mr James Cullen for his help in modifying figure 4.
Work carried out was supported Medical Research Council and Cancer Research UK grants C153/A4331, C153/A1797 and C153/A1802. This research was also supported by EC FP6 funding (Contract no. LSHC-CT-2004-505785). This publication does not necessarily reflect the views of the EC. The Community is not liable for any use that may be made of the information contained herein.
References
- 1.Stoller RG, et al. Use of plasma pharmacokinetics to predict and prevent methotrexate toxicity. N Engl J Med. 1977;297(12):630–4. doi: 10.1056/NEJM197709222971203. [DOI] [PubMed] [Google Scholar]
- 2.Calvert AH, et al. Carboplatin dosage: prospective evaluation of a simple formula based on renal function. J Clin Oncol. 1989;7(11):1748–56. doi: 10.1200/JCO.1989.7.11.1748. [DOI] [PubMed] [Google Scholar]
- 3.Findlay MP, et al. Measurement of plasma 5-fluorouracil by high-performance liquid chromatography with comparison of results to tissue drug levels observed using in vivo 19F magnetic resonance spectroscopy in patients on a protracted venous infusion with or without interferon-alpha. Ann Oncol. 1996;7(1):47–53. doi: 10.1093/oxfordjournals.annonc.a010476. [DOI] [PubMed] [Google Scholar]
- 4.Saleem A, et al. Pharmacokinetic evaluation of N-[2-(dimethylamino)ethyl]acridine-4-carboxamide in patients by positron emission tomography. J Clin Oncol. 2001;19(5):1421–9. doi: 10.1200/JCO.2001.19.5.1421. [DOI] [PubMed] [Google Scholar]
- 5.Saleem A, et al. Modulation of fluorouracil tissue pharmacokinetics by eniluracil: in-vivo imaging of drug action. Lancet. 2000;355(9221):2125–31. doi: 10.1016/s0140-6736(00)02380-1. [DOI] [PubMed] [Google Scholar]
- 6.Fukumura D, Jain RK. Tumor microvasculature and microenvironment: targets for anti-angiogenesis and normalization. Microvasc Res. 2007;74(2-3):72–84. doi: 10.1016/j.mvr.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Atwell GJ, et al. Potential antitumor agents. 50. In vivo solid-tumor activity of derivatives of N-[2-(dimethylamino)ethyl]acridine-4-carboxamide. J Med Chem. 1987;30(4):664–9. doi: 10.1021/jm00387a014. [DOI] [PubMed] [Google Scholar]
- 8.Copovi A, et al. Enhancing effect of alpha-hydroxyacids on “in vitro” permeation across the human skin of compounds with different lipophilicity. Int J Pharm. 2006;314(1):31–6. doi: 10.1016/j.ijpharm.2006.01.033. [DOI] [PubMed] [Google Scholar]
- 9.Propper DJ, et al. Use of positron emission tomography in pharmacokinetic studies to investigate therapeutic advantage in a phase I study of 120-hour intravenous infusion XR5000. J Clin Oncol. 2003;21(2):203–10. doi: 10.1200/JCO.2003.02.008. [DOI] [PubMed] [Google Scholar]
- 10.Carson R. Tracer kinetic modeling in PET. In: Valk P, et al., editors. Positron emission tomography - basic science and clinical practice. Springer; London: 2003. pp. 147–179. [Google Scholar]
- 11.Saleem A, et al. Plasma pharmacokinetic evaluation of cytotoxic agents radiolabelled with positron emitting radioisotopes. Cancer Chemother Pharmacol. 2007 doi: 10.1007/s00280-007-0552-2. [DOI] [PubMed] [Google Scholar]
- 12.Harte RJ, et al. Tumor, normal tissue, and plasma pharmacokinetic studies of fluorouracil biomodulation with N-phosphonacetyl-L-aspartate, folinic acid, and interferon alfa. J Clin Oncol. 1999;17(5):1580–8. doi: 10.1200/JCO.1999.17.5.1580. [DOI] [PubMed] [Google Scholar]
- 13.Front D, et al. Human lung tumors: SPECT quantitation of differences in Co-57 bleomycin uptake. Radiology. 1987;165(1):129–33. doi: 10.1148/radiology.165.1.2442794. [DOI] [PubMed] [Google Scholar]
- 14.Pujol JL, et al. Tumor-tissue and plasma concentrations of platinum during chemotherapy of non-small-cell lung cancer patients. Cancer Chemother Pharmacol. 1990;27(1):72–5. doi: 10.1007/BF00689280. [DOI] [PubMed] [Google Scholar]
- 15.Ratain MJ, et al. Pharmacodynamics in cancer therapy. J Clin Oncol. 1990;8(10):1739–53. doi: 10.1200/JCO.1990.8.10.1739. [DOI] [PubMed] [Google Scholar]
- 16.Gupta N, et al. Carbogen and nicotinamide increase blood flow and 5-fluorouracil delivery but not 5-fluorouracil retention in colorectal cancer metastases in patients. Clin Cancer Res. 2006;12(10):3115–23. doi: 10.1158/1078-0432.CCR-05-0513. [DOI] [PubMed] [Google Scholar]
- 17.Wilson CB, et al. Measurements of blood flow and exchanging water space in breast tumors using positron emission tomography: a rapid and noninvasive dynamic method. Cancer Res. 1992;52(6):1592–7. [PubMed] [Google Scholar]
- 18.Jain RK, Tong RT, Munn LL. Effect of vascular normalization by antiangiogenic therapy on interstitial hypertension, peritumor edema, and lymphatic metastasis: insights from a mathematical model. Cancer Res. 2007;67(6):2729–35. doi: 10.1158/0008-5472.CAN-06-4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Willett CG, et al. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med. 2004;10(2):145–7. doi: 10.1038/nm988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Senan S, Smit EF. Design of clinical trials of radiation combined with antiangiogenic therapy. Oncologist. 2007;12(4):465–77. doi: 10.1634/theoncologist.12-4-465. [DOI] [PubMed] [Google Scholar]
- 21.Jackson C, Cunningham D. Where to position monoclonal antibodies in first-line treatment of advanced colorectal cancer. Eur J Cancer. 2008;44(5):652–62. doi: 10.1016/j.ejca.2008.01.021. [DOI] [PubMed] [Google Scholar]