Abstract
Xerostomia, or chronic dry mouth, is a common syndrome caused by a lack of saliva that can lead to severe eating difficulties, dental caries and oral candida infections. The prevalence of xerostomia increases with age and affects approximately 30% of people aged 65 or older. Given the large numbers of sufferers, and the potential increase in incidence given our aging population, it is important to understand the complex mechanisms that drive hyposalivation and the consequences for the dentition and oral mucosa. From this study we propose the Fgf10 +/- mouse as a model to investigate xerostomia. By following embryonic salivary gland development, in vivo and in vitro, we show that a reduction in Fgf10 causes a delay in branching of salivary glands. This leads to hypoplasia of the glands, a phenotype that is not rescued postnatally or by adulthood in both male and female Fgf10 +/- mice. Histological analysis of the glands showed no obvious defect in cellular differentiation or acini/ductal arrangements, however there was a significant reduction in their size and weight. Analysis of saliva secretion showed that hypoplasia of the glands led to a significant reduction in saliva production in Fgf10 +/- adults, giving rise to a reduced saliva pellicle in the oral cavity of these mice. Mature mice were shown to drink more and in many cases had severe tooth wear. The Fgf10 +/- mouse is therefore a useful model to explore the causes and effects of xerostomia.
Keywords: Branching morphogenesis, Fgf10, saliva, salivary gland dysfunction, tooth wear, xerostomia
Introduction
Mice and humans have three pairs of major salivary glands that secrete into the oral cavity: the parotid (PG), submandibular (SMG) and sublingual glands (SLG), as well as numerous minor salivary glands which are located in the tongue, palate, cheeks and lips [1]. The major salivary glands are derived from the oral ectoderm and neural crest derived mesenchyme [2, 3]. The saliva they produce is essential for the well being of the oral cavity, producing lubricants, digestive enzymes, anti-microbials, and buffering the pH of the mouth [4]. A reduction in saliva leads to xerostomia, or dry mouth, an oral symptom that can seriously impinge on the quality of life in human patients. This hyposalivation leads to problems such as mastication difficulties, halitosis, deficiency of taste and a high incidence of caries and candida infections. Xerostomia occurs in approximately 30% in people over the age of 65 and other underlying causes include prescribed medications and diseases that target the exocrine glands such as Sjögren’s Syndrome (OMIM 270150) [5]. Additionally, patients exposed to radiation therapy for the treatment of head and neck cancers develop xerostomia due to severe damage of the salivary glands [6]. Due to this high prevalence it is important to understand the underlying mechanisms that lead to dry mouth and the consequences this has on the oral mucosa.
Animal models are indispensable tools for delineating the underlying aetiology of diseases and their contribution to biomedical discoveries has been invaluable. Our advanced knowledge of the mouse genome and the physiological similarities shared with humans makes the mouse an important mammalian model in uncovering the pathogenesis of many diseases. Murine models of xerostomia, such as the non-obese diabetic mouse (NOD), have been generated and extensively researched, however the dry mouth phenotype in these mice is a result of autoimmune exocrinopathy and therefore the xerostomia is a secondary effect of the underlying autoimmune disease [7-9]. While these autoimmune models are beneficial, an animal model of dry mouth without any underlying disease is necessary to examine the effect of dry mouth in otherwise healthy individuals.
In humans, mutations in FGF10 or its receptor FGFR2b, lead to Lacrimo Auriculo Dento Digital (LADD) syndrome (OMIM 149730) and Aplasia of Lacrimal and Salivary Glands (ALSG)(OMIM 180920), both syndromes being characterized by aplasia or hypoplasia of the lacrimal and salivary glands and hence severe xerostomia [10, 11]. In the mouse, knockout of Fgf10 or its receptor leads to complete aplasia of the major salivary glands, which arrest at the epithelial thickening stage [12]. These mice however are perinatal lethal as they also lack lungs [13]. Heterozygous mutants (Fgf10 +/-) are viable and the adult SMG and SLGs have been reported as being normal in histology, but with atrophy of the parotid gland, and reduced size of the sublingual and submandibular glands [14] (N=2), while a second report described a reduction of ducts and terminal end buds in the SMG at postnatal day (P) 0 [12].
To further our knowledge of salivary gland defects, we investigated the SMG and SLGs in Fgf10 +/- (heterozygous) mice to assess when the defect first becomes apparent, the adult phenotype and importantly the consequence of loss of one copy of Fgf10 on the function of the glands. Our results support the use of Fgf10 +/- adult mice as a new model of xerostomia.
Materials and Methods
Animals
Fgf10 deficient mice have been previously described [15, 16]. Genotypes of Fgf10 +/- animals were determined by PCR using DNA isolated from mouse ear clips. All procedures and culling methods were performed under a project licence approved by the United Kingdom’s Home Office and in accordance with the Animal (Scientific Procedures) Act of 1986, UK.
P14 and Adult Salivary Gland Weight Analysis
At postnatal day (P) 14 wildtype (WT) and Fgf10 +/- littermates were culled by exposure to rising levels of CO2 gas. Animals of this age were chosen as sexual dimorphism in salivary glands does not arise until P15 [17]. Mice were weighed and the SMG and SLGs were dissected together. Parotid glands were not weighed as they are more difficult to dissect and therefore weight analysis is less accurate. Excess fat surrounding the glands was removed. Glands were dried using an air stream and weighed immediately. Glands were then fixed in 4% paraformaldehyde in PBS (PFA) at 4°C overnight and processed for histological analysis. The same process was adopted for measuring the weight of adult (7-10 weeks) salivary glands from WT and Fgf10 +/- littermates. There was no significant difference between left and right gland weights of WT animals. The same was seen for heterozygous mice therefore, right gland weights were chosen for statistical analysis. Differences in gland weight were analyzed using a two-tail unpaired Student’s t-test.
Embryonic Gland Cultures
Mice were set up for matings at approximately midnight. Salivary glands at embryonice day (E)12.5 were dissected out of embryos and cultured as explants on transparent nucleopore filters (VWR) supported on metal grids on the surface of the medium using a modified Trowel culture method. Explants were cultured at 37°C/5% CO2 up to 4 days in D-MEM/F12 plus penicillin/streptomycin and 1% Glutamax (Invitrogen), changing the medium every 2 days. Glands were photographed every 24 hours with a Leica DFC300 camera.
Adult Salivary Gland Function Tests
Saliva Collection
Fgf10 WT and +/- adult littermates were given anaesthetic (ketamine 80mg/kg; xylazine 16mg/kg) by peritoneal injection. Mice were weighed and positioned under a light microscope. An incision was made and a thin tube inserted into the trachea to facilitate breathing. Mice were then subcutaneously injected with a low dose of pilocarpine (0.54-0.64 μg/g body weight, Sigma Aldrich), individual SMG and PG ducts were cut and after 10 minutes saliva was collected at the opening of ducts at room temperature and put into preweighed 1.5ml eppendorf tubes and kept on ice. Saliva secretion volume was calculated where 1mg = 1μl saliva. Animals were humanely killed by one lethal dose of anaesthetic. Saliva secretion was calculated as volume of saliva per minute (μl/min). Specimens used for saliva secretion tests were littermates from a range of adult ages (13-54 weeks). Data from these experiments were pooled and statistical significance was calculated using a Wilcoxon’s signed rank test. Due to the variation of ages used in secretion analysis, Fgf10 +/- saliva secretion was expressed as a ratio of secretion from a matched WT littermate, where WT secretions equal to 1. Graphs were generated using GraphPad Prism 6 software.
Drinking Experiments
A fixed amount of water was provided for 5 days in drinking bottles to Fgf10+/+ and Fgf10+/- male mice, which were then left undisturbed for the duration of the experiment. Mice for this experiment were at least 50 weeks old, representing old age. At day 5 the total amount of water intake was measured as weight of the bottle at the end of the experiment minus the weight at the start. To remove any bias due to animal size, water intake was adjusted for body weight by dividing the total amount of water intake with the body weight of each animal [18]. Data was analysed with Prism using the Mann–Whitney test.
Enamel Mineral Content Analysis and 3D Reconstruction
Heads from old (over 50 weeks) Fgf10+/+ or Fgf10+/- male mice were scanned using a GE Locus SPmicroCT scanner (GE Healthcare, London, ON, Canada). The specimens were immobilized using cotton gauze and scanned to produce 1µm voxel size volumes, using a X-ray tube voltage of 80kVp and a tube current 80µA. An aluminum filter (0.05mm) was used to adjust the energy distribution of the X-ray source. CT attenuation values were calibrated to hydroxyapatite standards. Regions of interest were reconstructed using the Microview 2.2 software package (GE Healthcare) to create a 3D reconstruction of the lower molars and to analyse the enamel mineral content [19]. The enamel mineral content was measured for both the upper and lower molars of each mouse using the advanced bone module of Microview 2.2. The enamel was selected from the teeth by adjusting the image threshold. Data was analysed with Prism using the Mann–Whitney test.
Histological Analysis
Following fixation in 4% PFA, SMG and SLG were put through two 30 minute washes in PBS and underwent dehydration washes in increasing methanol concentrations (30%, 50%, 70%, 80%, 90%, 95%, 100%). Glands were then washed in Isopropanol (Sigma Aldrich) followed by 1,2,3,4 Tetrahydronaptha-lene (Sigma Aldrich) at room temperature (RT). Tissue was transferred to paraffin wax at 65°C and embedded and cooled into wax blocks. Serial 9μm sections were cut and collected and mounted on slides (Solmedia Colourslides). Gland sections were stained with Haematoxylin and Eosin with Alcian Blue. Sections were then observed under a Nikon Eclipse 80i light microscope and images taken with a Nikon Digital Sight DS-Fi1 camera. Images were then analysed on an iMAC computer and any editing was carried out using Adobe Photoshop CS5.1.
Scanning Electron Microscopy
Female adult littermates (10 weeks old) (n=6) were culled by exposure to CO2 gas. Tongues were dissected and fixed in 2.5% glutaraldehyde in 0.15M cacodylate buffer (pH 7.2) overnight at 4°C. Tongues were washed in cacodylate buffer and postfixed in 1% osmium tetroxide. To remove mucus from the surface of the tongues, the tissue was submersed in 1ml of 3N hydrochloric acid for 20 minutes at 60°C [20]. Tissue was then dehydrated through an ethanol series and dried using a Polaron E3000 critical point dryer. Tongues were mounted and coated with gold (Emitech K550X sputter coater). Using a Hitachi S-3500N scanning electron microscope (SEM) the surface mucosa of the tongue was analyzed. Images were taken of specific temporal regions of different papillae of the tongue. All images were taken with the microscope set at 10kV in high vacuum mode.
Results
A Reduction in Fgf10 Does Not Effect the Gross Morphology of the Gland at the Histological Level
To investigate a sex-independent affect of loss of Fgf10 on salivary gland morphology, we analyzed glands at P14. At this age, sex hormones have not been released and sexual dimorphism is not evident [17, 21]. In agreement with this, no obvious histological differences were seen between male and female WT animals at P14 (Supplementary Data, Fig. S1). No obvious difference in histology was evident between Fgf10 WT and +/- littermates at P14 (Fig. 1). In Fgf10 +/- glands, the numbers of ducts and acini appeared similar to WTs, however the overall size of gland lobes appeared smaller (Fig. 1A-D). In keeping with the lack of a defect in differentiation in juvenile glands, no difference in histology was apparent at E18.5, just before birth (Fig. 1E, F).
Fgf10+/- Animals Show Reduced Salivary Gland Weight at P14
Given the lack of a change in histology we moved to assess whether there was a weight difference between WT and Fgf10 +/- groups at P14. Comparison of WT and Fgf10 +/- gland weight showed a significant difference, with smaller glands observed in heterozygous animals (Fig. 1G). These results indicate that loss of function of one copy of Fgf10 leads to a reduction in size of salivary glands at an early postnatal age with the affect of this phenotype being independent of sex.
Fgf10 +/- Mice Show a Delay in Gland Development
In order to assess when the difference in size occurred we investigated gland development during embryonic stages. At E14.5 the mesenchymal capsule of the submandibular gland appeared similar in size in WT and +/- mice, but the epithelium was less developed in the heterozygous glands, with fewer branches, larger epithelial buds and more extensive areas of mesenchyme not invaded by the epithelium (Fig. 2A, B). To further study the defect we used explant culture to follow branching in vitro. At E12.5 when the submandibular gland has reached the bud stage in WT littermates (Fig. 2C), the Fgf10 +/- glands had smaller epithelial prebud-like invaginations of the epithelium (Fig. 2G, K). In culture this delay in development continued with reduced branching in the heterozygous mice compared to littermate controls. As seen at E14.5, the epithelium failed to invade the most aboral part of the mesenchymal capsule in the cultures (Fig. 2F, J, N). The reduced size of postnatal glands therefore appears to be due to a failure to catch up with the development of the glands in WT littermates, resulting in a final gland with fewer branches and smaller overall size. Variation in the severity of defective epithelial branching was observed between Fgf10 +/- littermate glands in culture, agreeing with the reported variability in severity of phenotype in LADD syndrome patients [22, 23] (Fig. 2G-N).
Adult Fgf10 +/- Animals have Reduced Salivary Gland Weight
We further investigated SMG and SLGs in littermates aged between 7-10 weeks to delineate if glands remained smaller throughout adult life. Smaller glandular lobes were observed in Fgf10 +/- glands compared to WT littermates (Fig. 3A, B). Furthermore, a significant difference in weight of WT and Fgf10 +/- glands was observed in both adult females and males (Fig. 3C). No significant difference was seen in body weight between WT and Fgf10 +/- 10 littermates (males p=0.5, females p=0.2), therefore a reduction in overall body weight was excluded as a factor influencing the salivary gland phenotype.
Although a significant difference was seen in gland weight Fgf10 +/- glands showed no morphological differences to WT littermates at a histological level (Fig. 3D-G). As expected a significant difference was seen between female and male glands in both genotypes, with males having considerably larger glands to females and in both genotypes the granular convoluted tubules (GCT) were larger and more prevalent than those in females (Fig. 3D-G and inset images) (Supplementary Fig. S2).
Adult Salivary Gland Secretion is Severely Reduced in Fgf10 +/- Mice
Human patients with ALSG or LADD syndrome have severe dryness of mouth due to aplasia of the major salivary glands [22-24]. We therefore wanted to test salivary gland function in the Fgf10+/- mice. Fgf10+/- animals showed a significant reduction in saliva flow compared to their WT littermates from both the SMG and PG (Fig. 4). Our results collectively suggest that the hyposalivation observed in Fgf10+/- adults is due to the reduction in size of each gland as opposed to a gross defect in differentiation.
Reduction in the Salivary Pellicle on the Tongue in Fgf10 +/- Mice
To further investigate the reduction of saliva flow in Fgf10 +/- adults we analyzed the mucosa of adult tongue papillae (10 weeks) using scanning electron microscopy. In order to assess the thickness of the salivary pellicle we used HCl to remove mucus from the oral surface. It was observed that in WT tongues, what appears to be sheets of mucus remained, particularly in the posterior region covering the Circumvallate Papillae (CVP) and fillifom papillae (Fig. 5A, C). In contrast, the Fgf10 +/- tongues showed no mucus on the oral surface. The Fgf10 +/- tongues, did, however, display a healthy lawn of filliform and papillae similar to their WT littermates at this age (Fig. 5B, D). This suggests that HCl treatment is sufficient to remove the amount of saliva on Fgf10 +/- tongues however due to a thicker saliva pellicle in WT animals, sheets of saliva remain.
Increased Drinking and Increased Tooth Wear in Old Fgf10 +/- Mice
A feeling of oral dryness can be compensated for by increased intake of liquids. We therefore analysed the amount of water drunk by aging Fgf10 +/- mice compared to age matched controls. Interestingly, over a five-day period the Fgf10 +/- mice drank significantly more water than the controls (Fig. 6A). The amount drunk, however, was variable between heterozygous mice with some mice drinking the same as controls, this difference agreeing with the earlier observed variation in severity of gland branching pattern (Fig. 2). Saliva due to its buffering capacity and ability to remineralise demineralized enamel, protects the teeth against acids in the mouth that cause dental erosion. In addition, demineralised enamel is more susceptible to attrition and abrasion, with patients with salivary gland dysfunction showing signs of tooth wear [25]. In addition to monitoring the levels of drinking water, we therefore also used microCT to assess the teeth of the old Fgf10 +/- mice for signs of tooth wear. All mice were fed the same diet of hard pellet food throughout their life. The relative amount of enamel coating the crowns of the teeth was assessed by determining the enamel mineral content. The heterozygous mice had significantly less enamel then the age matched controls (Fig. 6B). When the teeth were reconstructed in 3D, many of the Fgf10 +/- mice showed severe tooth wear, with almost complete loss of the cusp pattern in the most severe cases (Fig. 6C, D). Interestingly, the mouse who drank the most water in the drinking experiment also has the most severe tooth wear, linking these two findings together.
Discussion
Loss of Fgf10 Leads to Defective Salivary Gland Function
In our study we show that adult Fgf10 +/- mice have a significant reduction in saliva flow compared to control animals. Previous work has emphasized the important role of Fgf10 in salivary gland development, with the Fgf10 knock out mouse showing absence of the major salivary glands at birth [12, 26-28]. Additionally Fgf10 +/- mice have been reported to have reduced terminal buds and ducts at P0 [12] while another study, albeit using only two samples, reported that adult Fgf10 +/- SMG and SLGs show normal histology [14]. Our histological analysis shows that there are no obvious structural differences between Fgf10 +/- and WT adult major salivary glands. A significant difference in gland weight was observed however; with heterozygous animals showing approximately 23% decrease in gland weight in females and a 15% decrease in males.
Previous reports indicate that FGF10 is not required for initial salivary gland bud formation but is crucial for successional epithelial branching morphogenesis. Fgf10 is expressed in the mesenchyme underlying the presumptive salivary gland epithelium from E11.5 [29]. Ex vivo manipulation of SMGs in organ culture demonstrates that exogenous FGF10 peptide applied at both the Prebud and Pseudglandular Stage gives rise to a significant increase in number of epithelial branches due to an increase in epithelial cell proliferation [12]. Furthermore application of FGF10 to isolated SMG epithelial rudiments induces duct elongation in a dose dependent manner by stimulating proliferation at the tips of ducts [30]. In whole slice organ cultures, application of beads soaked in FGF10 protein leads to the downwards growth of SMG epithelium in the direction of the protein bead [29]. Our studies of Fgf10 +/-embryonic salivary gland culture allowed us to follow development from bud stages and results show that epithelial branches do not extend to their full capacity within the mesenchymal capsule, unlike WT gland cultures. This indicates that a reduced amount of endogenous FGF10 protein leads to defective branching, and thus a smaller gland. This was also seen in vivo at E14.5, with Fgf10 +/- glands showing delayed branching, evident as larger terminal buds. At E18.5 however, the glands appeared similar histologically in both groups, indicating differentiation of these fewer branches proceeds normally in heterozygous animals.
In keeping with this, our analysis of adult gland histology showed no signs of altered differentiation in Fgf10 +/- glands, however smaller salivary gland lobes were observed. This, along with these other studies, indicates that a reduction in epithelial branching may occur in Fgf10 +/- glands leading to shorter branches and thus smaller lobes. Therefore we suggest that the hyposalivation observed in Fgf10 +/- adult glands is due to the reduction in weight and size of the salivary glands caused by a defect in early salivary gland patterning.
Fgf10 Function is Independent of Sex
It has been well established that there is a significant sexual dimorphism in mature rodent salivary glands. Structural differences are most prominently seen in the granular convoluted tubule (GCT) cells, which appear larger and more numerous in adult male mice, and the intercalated duct cells which appear more granulated in adult female mice [31, 32]. These cellular differences are androgen-dependant and begin to take shape at P15, with the development of GCT cells, and complete sexual dimorphism is evident at P20 [17, 31]. To rule out any influence of sex on the Fgf10 phenotype, we analysed weight and histology of glands at P14 in male and female mice. Our results support previous reports of no weight or structural differences seen between the sexes in WT mice. While histology of Fgf10 +/- salivary glands at P14 appeared normal, weights of mutant glands were significantly reduced.
Adult female glands are significantly smaller than male glands by age 7 weeks and GCT cells are less prominent. In both sexes, Fgf10 +/- glands were significantly smaller than WT littermates, with decrease in size being more significant in females. As both WT and Fgf10 +/- male glands develop to be much larger, and a secondary influence of androgens on salivary gland size is occurring, a decrease in weight may be less notable less notable compared to that seen in seen in female heterozygous glands.
This data supports the hypothesis that Fgf10 heterozygosity causes defective embryological salivary gland development, giving rise to a phenotype that is not rescued during postnatal development and adulthood.
Adult Fgf10 +/- Mice as a Model for Xerostomia
When 10 week old adult tongues were treated with hydrochloric acid to remove saliva from the surface mucosa, WT tongues retained what appeared to be sheets of mucus covering their tongue papillae while no mucus remained on Fgf10 +/- tongues, suggesting that a thicker saliva pellicle was produced in WT animals. To investigate the consequences of reduced saliva production we analysed the oral cavity for evidence of a dry mouth in aging mice. Fgf10 +/- mice were shown to drink significantly more then controls, and had significantly less enamel covering their teeth, which in severe cases led to extreme tooth wear. Considering the salivary gland phenotype and evidence of a dry mouth, we propose Fgf10 +/- adult mice as a new mouse model of xerostomia. In addition to a reduction in salivary output, xerostomia can be caused by a change in saliva composition produced by the salivary glands. Our next step therefore is to investigate the composition of the saliva in the Fgf10 +/- mice to understand whether the composition as well as volume produced is altered in these mice.
Fig. (1).
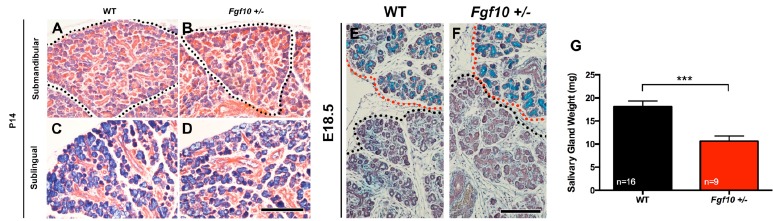
Comparison of WT and Fgf10 +/- salivary glands at P14. General duct and acini arrangements of WT and Fgf10 +/- SMG and SLGs appeared normal at P14 (A-D). A reduction in size was seen in glandular lobes in Fgf10 +/- animal compared to WT littermates (black dotted line) (A, B). At E18.5 no apparent histological differences were observed in WT and Fgf10 +/- littermates (E, F). At P14 combined Fgf10 +/- SMGs and SLGs weighed significantly less compared to WT littermates (G). P14; scale bar = 200I1/4M. A-D shows glands from female specimens. E, F shows E18.5 glands; SMG (black line), SLG (red line), scale bar = 200I1/4M. (Color images available online).
Fig. (2).
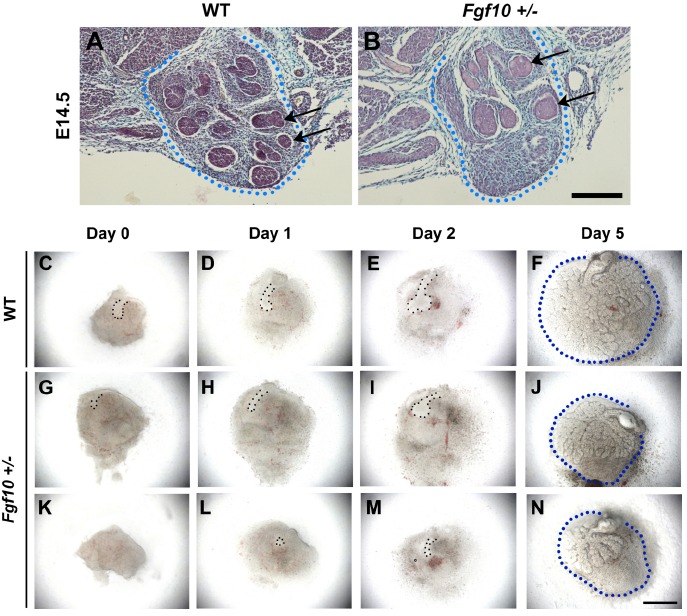
Embryonic Fgf10 +/- salivary gland development. (A, B) At E14.5, in vivo salivary gland capsule size was the same in WT and Fgf10 +/- littermates (dotted line) however epithelial branching was reduced and epithelial endbuds appeared larger in Fgf10 +/- animals (arrows). Scale bar = 200I1/4M. (C-N) In vitro culture of E12.5 salivary glands. At E12.5, WT glands have reached the bud stage (C) while Fgf10 +/- glands show a smaller epithelial bud (G) or an absent bud (K). Delayed bud extension and downgrowth was observed in all Fgf10 +/- glands (H, I; L, M) compared to WT littermates (D, E), which resulted in severely reduced branching of heterozygous glands by 5 days of culture (J, N) compared to WT littermates (F). Fgf10 +/- glands showed failure to fill the mesenchymal capsule compared to WT tissue (F, J, N). Epithelial bud = dotted line (columns 1-3), mesenchymal capsule = dotted line (column 4), scale bar = 500I1/4M.
Fig. (3).
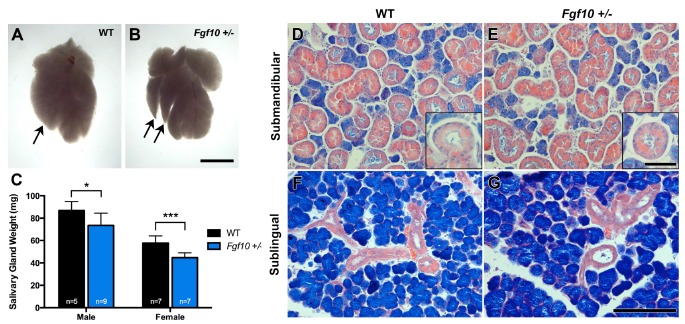
Comparison of adult salivary glands. (A, B) Smaller gland lobes are seen in Fgf10 +/- adult submandibular glands (arrows) (scale bar=3mm). (C) Male and female Fgf10 +/- adult salivary glands show a significant decrease in salivary gland weight compared to WT littermates. (D-G) Male glands; no histological differences are noted between WT and Fgf10 +/- SMGs (D, E) SLGs (F, G), and GCTs (inset images D, E). D-G scale bar=200I1/4m, inset scale bar=50I1/4M.
Fig. (4).

Saliva secretion from the (A) SMG/SLG and (B) parotid glands. Flow rate was significantly reduced in heterozygous adults compared to WT littermates. Saliva secretion was collected from the gland duct openings and was measured as microlitre of saliva produced in 10 minutes after pilocarpine stimulation (A l/min). Graph shows secretion rates of Fgf10 +/- animals normalized against WT secretion rate where WT secretion = 1. Data is expressed as mean A SEM.
Fig. (5).
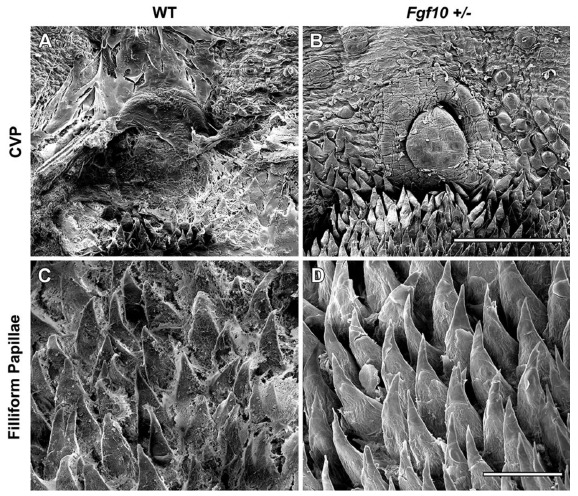
Scanning electron microscopy of 10 week old adult female tongues. WT animals retained what appeared to be sheets of mucus covering the posterior CVP (A) and filliform papillae (C) while no mucus remained on Fgf10 +/- tongues following HCl treatment (B, D). Scale bar in B = 500um (same scale in A). Scale bar in D = 100um (same scale n C).
Fig. (6).

Analysis of signs of xerostomia in aging Fgf10 +/- mice. (A) Water intake/body weight in WT and Fgf10 +/- aged matched mice. Fgf10 +/- mice drank significantly more water over a 5 day period. (B) Enamel mineral content in WT and Fgf10 +/- aged matched mice. Fgf10 +/- have significantly less enamel on their crowns. (C, D) 3D reconstructions from microCT. Fgf10 +/- teeth are flattened compared to controls of the same age fed on the same diet, indicating tooth wear. Double arrowheads indicate tooth height from the level of root bifurcation to top of crown.
Acknowledgements
We thank Dr. Gema Vizcay-Barrena and Ms. Leanne Glover (Centre for Ultrastructural Imaging, King's College London) for help with the SEM work, and Dr. Chris Healy for help with the microCT. Alison May was funded by the Dental Institute, King’s College London. Lemonia Chatzeli was funded by the Anatomical Society. Abigail Tucker is funded by the MRC and Wellcome Trust.
A.J.M., G.B.P. and A.S.T. designed and planned the research. A.J.M., G.B.P. and A.S.T. carried out the research and collected data. L.C. performed the drinking experiments and analysis of the enamel in aging mice. A.J.M. analysed data. A.J.M. and A.S.T. wrote paper with input from G.B.P.
Abbreviations
- CVP
Circumvallate papilla
- E (14.5)
Embryonic day (14.5)
- Fgf10 +/-
Fgf10 heterozygous
- P (14)
Postnatal day (14)
- PBS
Phosphate buffered saline
- PFA
4% paraformaldehyde in PBS
- PG
Parotid gland
- RT
Room temperature
- SEM
Scanning electron microscopy
- SLG
Sublingual gland
- SMG
Submandibular gland
- WT
Wildtype
Footnotes
The authors confirm that this article content has no conflict of interest.
SUPPLEMENTARY MATERIAL
Supplementary material is available on the publisher’s web site along with the published article.
References
- 1.Hand A.R., Pathmanathan D., Field R.B. Morphological features of the minor salivary glands. Arch. Oral Biol. 1999;44(Suppl. 1):S3–S10. doi: 10.1016/s0003-9969(99)90002-x. [DOI] [PubMed] [Google Scholar]
- 2.Rothova M., Thompson H., Lickert H., Tucker A.S. Lineage tracing of the endoderm during oral development. Dev. Dyn. 2012;241:1183–1191. doi: 10.1002/dvdy.23804. [DOI] [PubMed] [Google Scholar]
- 3.Jaskoll T., Zhou Y.M., Trump G., Melnick M. Ectodysplasin receptor-mediated signaling is essential for embryonic submandibular salivary gland development. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 2003;271:322–331. doi: 10.1002/ar.a.10045. [DOI] [PubMed] [Google Scholar]
- 4.Hall H. Protective and maintenance functions of human saliva. Quintessence Int. 1993;24:813–816. [PubMed] [Google Scholar]
- 5.Ship J.A., Pillemer S.R., Baum B.J. Xerostomia and the geriatric patient. J. Am. Geriatr. Soc. 2002;50:535–543. doi: 10.1046/j.1532-5415.2002.50123.x. [DOI] [PubMed] [Google Scholar]
- 6.Epstein J.B., Emerton S., Kolbinson D.A., et al. Quality of life and oral function following radiotherapy for head and neck cancer. Head Neck. 1999;21:1–11. doi: 10.1002/(sici)1097-0347(199901)21:1<1::aid-hed1>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 7.Humphreys-Beher M.G. Animal models for autoimmune disease-associated xerostomia and xerophthalmia. Adv. Dent. Res. 1996;10:73–75. doi: 10.1177/08959374960100011501. [DOI] [PubMed] [Google Scholar]
- 8.Lavoie TN, Lee BH, Nguyen CQ. 2011.
- 9.Lee B.H., Gauna A.E., Pauley K.M., Park Y-J., Cha S. Animal models in autoimmune diseases: lessons learned from mouse models for Sjögren’s syndrome. Clin. Rev. Allergy Immunol. 2012;42:35–44. doi: 10.1007/s12016-011-8288-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shams I., Rohmann E., Eswarakumar V.P., et al. Lacrimo-auriculo-dento-digital syndrome is caused by reduced activity of the fibroblast growth factor 10 (FGF10)-FGF receptor 2 signaling pathway. Mol. Cell. Biol. 2007;27:6903–6912. doi: 10.1128/MCB.00544-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Milunsky J.M., Zhao G., Maher T.A., Colby R., Everman D.B. LADD syndrome is caused by FGF10 mutations. Clin. Genet. 2006;69:349–354. doi: 10.1111/j.1399-0004.2006.00597.x. [DOI] [PubMed] [Google Scholar]
- 12.Jaskoll T., Abichaker G., Witcher D., et al. FGF10/FGFR2b signaling plays essential roles during in vivo embryonic submandibular salivary gland morphogenesis. BMC Dev. Biol. 2005;5:11. doi: 10.1186/1471-213X-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sekine K., Ohuchi H., Fujiwara M., et al. Fgf10 is essential for limb and lung formation. Nat. Genet. 1999;21:138–141. doi: 10.1038/5096. [DOI] [PubMed] [Google Scholar]
- 14.Entesarian M., Matsson H., Klar J., et al. Mutations in the gene encoding fibroblast growth factor 10 are associated with aplasia of lacrimal and salivary glands. Nat. Genet. 2005;37:125–127. doi: 10.1038/ng1507. [DOI] [PubMed] [Google Scholar]
- 15.Min H., Danilenko D.M., Scully S.A., et al. Fgf-10 is required for both limb and lung development and exhibits striking functional similarity to Drosophila branchless. Genes Dev. 1998;12:3156–3161. doi: 10.1101/gad.12.20.3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rice R., Spencer-dene B., Connor E.C., et al. Disruption of Fgf10 / Fgfr2b -coordinated epithelial-mesenchymal interactions causes cleft palate. J. Clin. Invest. 2004;113:1692–1700. doi: 10.1172/JCI20384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gresik E.W. The postnatal development of the sexually dimorphic duct system and of amylase activity in the submandibular glands of mice. Cell Tissue Res. 1975;157:411–422. doi: 10.1007/BF00225529. [DOI] [PubMed] [Google Scholar]
- 18.Radke A.K., Holtz N.A., Gewirtz J.C., Carroll M.E. Reduced emotional signs of opiate withdrawal in rats selectively bred for low (LoS) versus high (HiS) saccharin intake. Psychopharmacology (Berl.) 2013;227:117–126. doi: 10.1007/s00213-012-2945-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matalova E., Vanden Berghe T., Svandova E., et al. Caspase 7 in molar tooth development. Arch. Oral Biol. 2012;57:1474–1481. doi: 10.1016/j.archoralbio.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 20.Iwasaki S., Yoshizawa H., Kawahara I. Study by scanning electron microscopy of the morphogenesis of three types of lingual papilla in the rat. Anat. Rec. 1997;247:528–541. doi: 10.1002/(SICI)1097-0185(199704)247:4<528::AID-AR12>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 21.Gresik E.W. The granular convoluted tubule (GCT) cell of rodent submandibular glands. Microsc. Res. Tech. 1994;27:1–24. doi: 10.1002/jemt.1070270102. [DOI] [PubMed] [Google Scholar]
- 22.Hollister D.W., Klein S.H., De Jager H.J., Lachman R.S., Rimoin D.L. The lacrimo-auriculo-dento-digital syndrome. J. Pediatr. 1973;83:438–444. doi: 10.1016/s0022-3476(73)80268-9. [DOI] [PubMed] [Google Scholar]
- 23.Thompson E., Pembrey M., Graham J.M. Phenotypic variation in LADD syndrome. J. Med. Genet. 1985;22:382–385. doi: 10.1136/jmg.22.5.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wiedemann H.R., Drescher J. LADD syndrome: report of new cases and review of the clinical spectrum. Eur. J. Pediatr. 1986;144:579–582. doi: 10.1007/BF00496040. [DOI] [PubMed] [Google Scholar]
- 25.Young W., Khan F., Brandt R., Savage N., Abdul Razek A., Huang Q. Syndromes with salivary dysfunction predispose to tooth wear. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2001;92:38–48. doi: 10.1067/moe.2001.113549. [DOI] [PubMed] [Google Scholar]
- 26.Rohmann E., Brunner H.G., Kayserili H., et al. Mutations in different components of FGF signaling in LADD syndrome. Nat. Genet. 2006;38:414–417. doi: 10.1038/ng1757. [DOI] [PubMed] [Google Scholar]
- 27.De Moerlooze L., Spencer-Dene B., Revest J., Hajihosseini M., Rosewell I., Dickson C. An important role for the IIIb isoform of fibroblast growth factor receptor 2 (FGFR2) in mesenchymal-epithelial signalling during mouse organogenesis. Development. 2000;127:483–492. doi: 10.1242/dev.127.3.483. [DOI] [PubMed] [Google Scholar]
- 28.Ohuchi H., Hori Y., Yamasaki M., et al. FGF10 acts as a major ligand for FGF receptor 2 IIIb in mouse multi-organ development. Biochem. Biophys. Res. Commun. 2000;277:643–649. doi: 10.1006/bbrc.2000.3721. [DOI] [PubMed] [Google Scholar]
- 29.Wells K.L., Gaete M., Matalova E., Deutsch D., Rice D., Tucker A.S. Dynamic relationship of the epithelium and mesenchyme during salivary gland initiation: the role of Fgf10. Biol. Open. 2013;2:981–989. doi: 10.1242/bio.20135306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steinberg Z., Myers C., Heim V.M., et al. FGFR2b signaling regulates ex vivo submandibular gland epithelial cell proliferation and branching morphogenesis. Development. 2005;132:1223–1234. doi: 10.1242/dev.01690. [DOI] [PubMed] [Google Scholar]
- 31.Lacassagne A. Dimorphisme sexuel de la glande sous-maxillaire chez la souris. C R Soc Biol (Paris) 1940;133:180–181. [Google Scholar]
- 32.Gresik E.W. A previously unreported cell type in female mouse submandibular glands. J. Cell Biol. 1966;31:144a. [Abstr]. [1]. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material is available on the publisher’s web site along with the published article.


