Abstract
Background:
Researchers report difficulties in conducting research with children and young people with life-limiting conditions or life-threatening illnesses and their families. Recruitment is challenged by barriers including ethical, logistical and clinical considerations.
Aim:
To explore how children and young people (aged 0–25 years) with life-limiting conditions or life-threatening illnesses and their families were identified, invited and consented to research published in the last 5 years.
Design:
Systematic review.
Data sources:
MEDLINE, PsycINFO, Web of Science, Sciences Citation Index and SCOPUS were searched for original English language research published between 2009 and 2014, recruiting children and young people with life-limiting conditions or life-threatening illness and their families.
Results:
A total of 215 studies – 152 qualitative, 54 quantitative and 9 mixed methods – were included. Limited recruitment information but a range of strategies and difficulties were provided. The proportion of eligible participants from those screened could not be calculated in 80% of studies. Recruitment rates could not be calculated in 77%. A total of 31% of studies recruited less than 50% of eligible participants. Reasons given for non-invitation included missing clinical or contact data, or clinician judgements of participant unsuitability. Reasons for non-participation included lack of interest and participants’ perceptions of potential burdens.
Conclusion:
All stages of recruitment were under reported. Transparency in reporting of participant identification, invitation and consent is needed to enable researchers to understand research implications, bias risk and to whom results apply. Research is needed to explore why consenting participants decide to take part or not and their experiences of research recruitment.
Keywords: Child, palliative care, research design, research report, patient selection, review
What is already known about this topic?
Recruitment to research with children and young people with life-limiting conditions or life-limiting illnesses is challenged by factors including limited researcher access to participants, ethical considerations and characteristics of the population.
What this paper adds?
Recruitment strategies are not consistently reported in current research with this population.
Inadequate reporting of recruitment practices limits our capacity to judge study quality, risk of bias, representativeness of samples, generalizability of results and ultimately the applicability of findings.
Greater and clearer reporting of participant identification, invitation, screening, eligibility and consenting practices is needed for research recruiting children and young people (CYP) with life-limiting conditions (LLC)/life-threatening illnesses (LTI) and their families.
Implications for practice, theory or policy
Consistent use of reporting guidelines and online supplements should be encouraged by all journals.
Introduction
There is an international need for research of all types involving children and young people aged 0–25 years (CYP) with life-limiting conditions (LLC; conditions for which there is no reasonable hope of cure and from which children or young people will die1) or life-threatening illnesses (LTI; conditions for which curative treatment may be feasible but can fail2) and their families.3 Research is required to explore pain and symptom management, decision making about care and treatment, illness experience, and service development and delivery.
A number of challenges to recruitment for research with this group have been reported in the literature.4 These include clinical considerations such as the often unpredictable course of the illnesses,5 limited access to potential participants as a result of both logistical factors and paternalistic attitudes,6 the perceived potential burden on participants7 and difficulties securing ethical approval.8 As a result, recruitment to research may be slow and selective.7 This may affect the quality of research, risk of bias and the generalizability of findings.9,10
There is debate about when and how, and even if, CYP with LLC/LTI and their families might be invited to take part in research.11 At the same time, there is also growing evidence that CYP with LLC/LTI and their families value opportunities to participate in research and that this can be a positive experience for them.11–13
Internationally, the culture of research is changing14,15 as the importance of establishing robust evidence for care is increasingly understood. In the United Kingdom, the National Institute for Health Research (NIHR) has established a Clinical Research Network for Children, and CYP and their families are being encouraged to share their views and participate in the design, review and conduct of research to ensure that it is valid, feasible and acceptable to potential participants.16–19
CYP with LLC/LTI are likely to receive a palliative approach to care, often alongside active treatments.20 Interest in research on both adult and children’s palliative care is gaining momentum and guidance has been developed for its design and conduct. The MORECare (Methods of Researching End of Life Care) statement21 provides best practice solutions specifically for planning and conducting palliative care research, as well as the reporting of attrition data. However, it appears that such guidance is not routinely adhered to and a lack of reporting of methodological information has been identified in research with CYP with LLC/LTI and their families.14–16,22
In this systematic review, we explored the reported methods of participant identification, invitation and recruitment of CYP with LLC/LTI and their families to research, conducted internationally and published over the last 5 years (September 2009 to September 2014). We wished to understand the challenges to recruitment, how these differ between types of studies, identify areas of good practice and provide evidence for areas in which improvements might be made.
Aims and objectives
We aimed to explore methods of recruitment of CYP with LLC/LTI and their families to qualitative, quantitative and mixed-methods research reported in peer-reviewed journal articles.
Our objectives were as follows:
To document the procedures for identifying, inviting and consenting eligible CYP with LLC/LTI and their families to research;
To document recruitment rates;
To identify reasons given for non-enrolment, both reasons given by researchers and clinicians for not approaching eligible participants, and those given by potential participants for deciding not to take part;
To explore whether recruitment differs between types of studies;
To explore what barriers and facilitators to research recruitment were highlighted by the authors of reviewed studies.
Methods
Inclusion criteria
Primary studies of all methodologies (quantitative, qualitative and mixed-method design) were included. Eligible studies reported research recruiting CYP with LLC/LTI (and/or their family members), were written in English and published between September 2009 and September 2014. Conference abstracts were not included. We applied the United Nations definition of ‘young people’, extending from birth to 25 years. Studies recruiting parents, grandparents or siblings of CYP with LLC/LTI were also included. The Richard Hain Directory23 of International Classification of Diseases, 10th Revision (ICD-10) diagnoses was used to ascertain whether diagnoses could be considered life-limiting or life-threatening; where definitions were unclear, the opinion of a clinical expert in paediatric palliative care (M.C.) was sought.
Search strategy
A systematic search of the literature was performed in the following: MEDLINE, PsycINFO, Web of Science, Sciences Citation Index and SCOPUS in September 2014. A combination of indexed and free-text terms was used to reflect the three components forming the search strategy (CYP; LLC/LTI recruitment).
Descriptive terms that have been used previously by other systematic reviews24,25 relating to all stages of youth were used. Terms relating to palliative care, death, bereavement and recruitment practices were also included. The MEDLINE search strategy is shown in Appendix 1.
Data screening
Two reviewers (B.F.H. and L.J.M.O.) screened citations against the inclusion criteria. Disagreements regarding eligibility were resolved through reading full text articles and discussion.
Data extraction
The data extraction tool was piloted by B.F.H. and L.J.M.O., and minor adjustments were made. We sought to extract the following from each study: research design, recruitment location and setting, funding source and type of body providing ethical review, sample characteristics, and numbers of participants screened, identified as eligible, invited to participate and consented. We also extracted reasons for the non-invitation of eligible participants and reasons given by eligible participants for non-participation. Data were extracted independently by one of four reviewers (B.F.H., L.J.M.O., V.V. or B.C.). A sample of data extracted by each reviewer was checked for accuracy and consistency by another reviewer.
Where data that we wished to extract could not be found in the article, we categorized this as ‘not reported’. No attempts were made to obtain this information by contacting the authors. We did not assess, alongside data extraction, the overall quality of each study using recommended checklists as we wished to explore the quality of reporting of recruitment information, rather than quality of the research itself.
If provided, descriptions of researchers’ experiences of recruiting participants were also extracted. We were interested in strategies employed by authors that aimed to facilitate recruitment and any issues encountered which hindered the recruitment processes. This information was usually found in the discussion section of the included papers. These data were not available in all reviewed studies.
Data analysis
Quantitative analysis
We used descriptive statistics to summarize the type of studies included, the recruitment strategies employed and the recruitment rates achieved. If we had found sufficient levels of reporting of items of interest, we planned to conduct comparative statistical analyses to assess differences in recruitment rates between (1) studies with different types of aims, such as intervention studies and psychosocial studies and (2) studies using different participant invitation methods, for example, an invitation letter in comparison to inviting participants in person.
Qualitative analysis
Any text specifically on researchers’ experiences of recruiting their sample was identified and analysed. We applied thematic synthesis as outlined by Thomas and Harden26 and described in Langford et al.27 to these informal data. Thematic synthesis is an adaptation of thematic analysis and can be used to pool qualitative data across different studies. Three stages of the recruitment process were used as a coding (grouping) framework: (1) identifying or screening participants, (2) inviting or approaching them to participate and (3) obtaining consent. Codes relating to the barriers and facilitators experienced in relation to each of these stages emerged from the data. Coding was completed in NVivo 10 by one reviewer (B.F.H.) and reviewed by two further reviewers (B.C. and L.J.M.O.), disagreements were resolved through discussion.
Results
Literature search
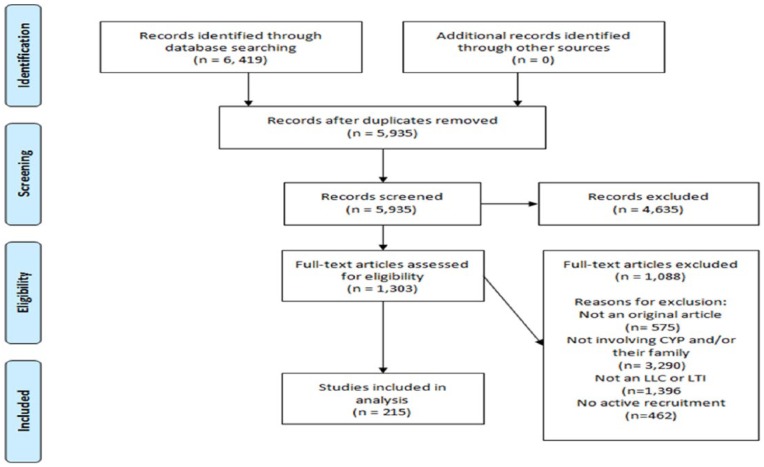
Figure 1 presents a Preferred Reporting items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram of the inclusion process. Our initial search yielded 6419 citations, 215 of these met all criteria and were included in this review. Appendix 2 lists references for all included studies.
Figure 1.
PRISMA flow diagram.
Study types
Table 1 summarizes the research aims of reviewed studies.
Table 1.
Research aims of reviewed studies.
| Research aim | Number of studies (%) | Definition |
|---|---|---|
| Evaluating an intervention | 71 (33) | Studies aiming to test the efficacy or safety of a treatment, medical intervention (n = 59) or non-medical intervention (n = 12), including randomized and non-randomized trials |
| Assessing quality of life | 22 (10) | Assessments of quality of life of CYP with LLC/LTI and/or their family members |
| Observing the course of illness | 36 (17) | Studies monitoring the course of an illness without providing additional interventions other than standard care |
| Exploring views and perspectives | 60 (28) | Qualitative studies exploring experiences, views or perspectives of CYP with LLC/LTI and/or their family members |
| Evaluating current practice | 26 (12) | Evaluations of current non-medical practice or research-related practice, from the perspective of CYP with LLC/LTI and/or their family members |
CYP: children and young people; LLC: life-limiting conditions; LTI: life-threatening illnesses.
Context and setting
Nearly half of reviewed studies were conducted in North America (46%), a quarter in Europe (26%), 10% in Asia, 7% in Australia and New Zealand, 6% in Africa, 1% in South America and a minority across multiple countries (4%). The majority of research was conducted within hospitals (83%).
Sample
Over half of studies recruited only CYP (56%), over a third recruited only family members (35%) and the remaining 11% recruited both CYP and family members. Young people aged 12–25 years were the least represented with 7% of studies recruiting this age group exclusively. The ages of CYP were not reported in a consistent manner, making descriptions of the ages of participants difficult. Ages of CYP with LLC/LTI were reported in 76% of all studies. Mean ages reported ranged from 19 min of life to 21.1 years.
CYP with malignant and non-malignant conditions were roughly equally represented (malignant: 45%; non-malignant: 44%; and both conditions: 11%). The majority of studies recruited families whose child was alive at the start of the study (79%). In almost half of the 45 studies (47%), recruiting bereaved families, recruitment occurred more than 1 year after bereavement (Table 2).
Table 2.
Summary of characteristics of reviewed studies, n (%).
| All studies (n = 215) | Evaluating an intervention (n = 71, 33%) | Assessing quality of life (n = 22, 10%) | Observing course of illness (n = 36, 17%) | Exploring views or perspectives (n = 60, 28%) | Evaluating current practice (n = 26, 12%) | |
|---|---|---|---|---|---|---|
| Sample, n (%) | ||||||
| CYP only | 116 (56) | 66 (93) | 6 (27) | 34 (95) | 9 (16) | 1 (4) |
| Family members only | 75 (35) | 2 (3) | 11 (50) | 2 (5) | 43 (71) | 17 (65) |
| Both CYP and family members | 24 (11) | 3 (4) | 5 (23) | – | 8 (13) | 8 (31) |
| CYP alive at recruitment | 170 (79) | 70 (98) | 17 (77) | 33 (92) | 37 (62) | 13 (50) |
| Diagnosis, n (%) | ||||||
| Malignant | 96 (45) | 33 (47) | 8 (36) | 12 (33) | 27 (45) | 16 (62) |
| Non-malignant | 95 (44) | 38 (53) | 9 (41) | 23 (64) | 23 (38) | 2 (8) |
| Mixed malignant or non-malignant | 24 (11) | – | 5 (23) | 1 (3) | 10 (17) | 8 (30) |
| Design | ||||||
| Qualitative | 54 (25) | 2 (3) | 3 (14) | 1 (3) | 41 (68) | 7 (27) |
| Quantitative | 152 (71) | 69 (97) | 16 (73) | 35 (97) | 16 (27) | 16 (62) |
| Mixed methods | 9 (4) | – | 3 (14) | – | 3 (5) | 3 (11) |
| Methodology, n (%) | ||||||
| Interviews | 57 (27) | – | 7 (32) | 3 (8) | 39 (65) | 8 (31) |
| Questionnaires | 49 (23) | – | 15 (68) | 2 (6) | 17 (28) | 15 (58) |
| Physiological measures | 40 (19) | 10 (14) | – | 29 (80) | – | 1 (4) |
| Experimental (randomized) | 29 (12) | 29 (41) | – | – | – | – |
| Experimental (non-randomized) | 33 (15) | 32 (45) | – | 1 (3) | – | 1 (4) |
| Other | 8 (4) | – | – | 1 (3) | 4 (7) | 1(4) |
CYP: children and young people.
Study design
Most studies were quantitative (71%), 25% were qualitative, while mixed-methods studies were uncommon (4%). Over a quarter of studies (27%) used interviews, 27% used experimental methods, 23% used questionnaires, 19% recorded physiological measures and 4% of studies used other methods.
Recruitment strategies – procedures used for identifying, inviting and consenting eligible patients and their families to research
Information concerning the methods of participant recruitment was not often reported (Table 3).
Table 3.
Methods used for the identification and invitation of participants and details of who provided consent for participation.
| All studies, n (%) | Evaluating an intervention (n = 71, 33%) | Assessing quality of life (n = 22, 10%) | Observing course of illness (n = 36, 17%) | Exploring views or perspectives (n = 60, 28%) | Evaluating current practice (n = 26, 12%) | |
|---|---|---|---|---|---|---|
| Identification of participants – method | ||||||
| Medical records | 25 (12) | 5 (7) | 2 (9) | 5 (14) | 9 (15) | 4 (15) |
| Clinic attendance | 20 (9) | 2 (3) | 2 (9) | 3 (8) | 10 (17) | 3 (12) |
| Database/registry | 19 (9) | 3 (4) | 4 (18) | 2 (6) | 5 (8) | 5 (19) |
| Participation in previous research | 7 (3) | – | 1 (5) | 3 (8) | 2 (3) | 1 (4) |
| Other | 7 (3) | 2 (2) | 1 (5) | – | 3 (5) | 1 (4) |
| Not reported | 137 (64) | 59 (83) | 12 (55) | 23 (64) | 31 (52) | 12 (46) |
| Identification of participants – person | ||||||
| Physician | 26 (12) | – | 3 (14) | 3 (8) | 17 (28) | 3 (12) |
| Researcher | 10 (5) | 1 (1) | 1 (5) | 1 (3) | 1 (2) | 6 (23) |
| Nurse | 5 (2) | – | 1 (5) | – | 3 (5) | 1 (4) |
| Other | 11 (5) | 2 (2) | 3 (14) | – | 5 (8) | 1 (4) |
| Not reported | 163 (76) | 68 (96) | 14 (64) | 32 (89) | 34 (57) | 15(58) |
| Invitation of participants – method | ||||||
| Letter | 37 (17) | – | 8 (36) | – | 20 (33) | 9 (35) |
| In person | 22 (10) | 2 (3) | 2 (9) | 2 (6) | 11 (18) | 5 (19) |
| 1 (1) | – | – | – | 1 (2) | – | |
| Telephone | 2 (1) | – | – | – | 2 (4) | – |
| Other | 7 (4) | – | 1 (5) | 1 (3) | 3 (5) | 2 (8) |
| Not reported | 146 (68) | 69 (97) | 11 (50) | 33 (92) | 23 (38) | 10 (39) |
| Invitation of participants – person | ||||||
| Researcher | 20 (9) | 3 (4) | 1 (5) | 1 (3) | 10 (17) | 5 (19) |
| Physician | 10 (5) | – | – | – | 7 (12) | 3 (12) |
| Nurse | 8 (4) | – | 1 (5) | – | 6 (10) | 1 (4) |
| Other | 12 (6) | 2 (2) | 2 (9) | 1 (3) | 5 (8) | 2 (8) |
| Not reported | 165 (77) | 66 (93) | 18 (82) | 34 (94) | 32 (53) | 15 (58) |
| Consenting of participants | ||||||
| Parents only | 109 (50) | 34 (48) | 10 (46) | 18 (50) | 37 (62) | 10 (39) |
| CYP only | 4 (2) | 1 (1) | 1 (5) | – | 2 (3) | – |
| Parents and CYP | 52 (24) | 14 (20) | 2 (9) | 11 (31) | 5 (8) | 7 (27) |
| Parents and siblings | 2 (1) | – | 1 (5) | – | 1 (2) | 1 (4) |
| Siblings | 2 (1) | – | – | – | 1 (2) | 1 (4) |
| Parents, siblings and CYP | 1 (1) | – | – | – | 1 (2) | – |
| Not reported | 48 (22) | 19 (27) | 6 (27) | 6 (17) | 10 (17) | 7 (27) |
CYP: children and young people.
Identification of potential participants
Over half of the studies did not report how potential participants were identified (64%). In the 78 studies reporting this, nearly one-third identified potential participants using medical records (29%) and a quarter used clinic attendance (26%).
Less than a quarter of studies reported the person responsible for identifying potential participants (24%). Physicians identified potential participants in nearly half of the studies providing this information (48%). Studies evaluating an intervention or observing the course of an illness were least likely to report how potential participants were identified, whereas those evaluating current practice were most likely to report their methods for participant identification.
Invitation of participants
Over three quarters of studies did not report who invited potential participants (76%). In research evaluating an intervention, including treatment for disease, 96% of studies did not provide this information. In studies reporting this, it was most often a researcher (40%) or a physician (20%). Across all studies, 68% did not report the methods used for participant invitation, over half of those reporting this invited participants by letter (54%) and around a third invited participants in person (32%).
Consenting of participants
All studies required informed consent prior to participation; however, specifically who provided consent was not reported in 22% of studies. The majority of studies recruiting both CYP and their family (that provided this information) obtained CYP consent/assent in additional to parental consent (78%).
Recruitment rates and completeness of reporting
Numbers of potential participants screened and the proportion found to be eligible
The numbers of participants screened for eligibility were not consistently reported; over three quarters of studies did not report this (77%) (Table 4).
Table 4.
Recruitment rates, numbers of participants screened, found to be eligible, approached and consented in all studies.
| All studies, n (%) | Evaluating an intervention (n = 71, 33%) | Assessing quality of life (n = 22, 10%) | Observing course of illness (n = 36, 17%) | Exploring views or perspectives (n = 60, 28%) | Evaluating current practice (n = 26, 12%) | |
|---|---|---|---|---|---|---|
| Numbers of potential participants screened | ||||||
| <50 | 7 (3) | 4 (6) | – | 1 (3) | 1 (2) | 1 (4) |
| 50–100 | 8 (4) | 2 (3) | 1 (5) | 2 (6) | 1 (2) | 2 (8) |
| 101–1000 | 29 (14) | 10 (14) | 4 (18) | 5 (15) | 7 (12) | 3 (12) |
| >1000 | 5 (2) | 2 (3) | – | 2 (6) | – | 1 (4) |
| Not reported | 166 (77) | 53 (75) | 17 (77) | 26 (72) | 51 (85) | 19 (73) |
| Percentage of eligible participants out of those screened | ||||||
| 0–50 | 10 (5) | 5 (7) | 1 (5) | 2 (6) | 1 (2) | 2 (8) |
| 51–100 | 31 (15) | 10 (16) | 3 (14) | 6 (17) | 4 (7) | 7 (27) |
| Not reported | 173 (80) | 55 (77) | 18 (81) | 16 (44) | 55 (92) | 17 (65) |
| Proportion approached out of those eligible (%) | ||||||
| 0–50 | – | – | – | – | – | – |
| 51–100 | 55 (26) | 18 (25) | 5 (23) | 8 (22) | 16 (26) | 8 (31) |
| Not reported | 160 (74) | 53 (75) | 17 (77) | 28 (78) | 44 (73) | 18 (69) |
| Proportion consented out of those approached (%) | ||||||
| 0–50 | 13 (6) | 3 (4) | 1 (5) | – | 5 (9) | 4 (16) |
| 51–100 | 69 (32) | 24 (34) | 5 (23) | 13 (72) | 18 (29) | 8 (34) |
| Not reported | 133 (62) | 44 (62) | 16 (73) | 21 (58) | 37 (62) | 14 (54) |
| Recruitment rate (percentage recruited out of those eligible) | ||||||
| 0–50 | 15 (7) | 8 (11) | 2 (10) | 2 (6) | 1 (2) | 2 (8) |
| 51–100 | 34 (16) | 12 (17) | 4 (18) | 7 (19) | 6 (10) | 5 (19) |
| Not reported | 166 (77) | 51 (72) | 16 (73) | 27 (75) | 53 (88) | 19 (73) |
It was not possible to calculate the proportion of eligible participants out of those screened in 80% of studies. Where this proportion could be calculated, 24% found less than half of those screened to be eligible and 76% found over half of screened to be eligible for participation.
Proportion approached out of those eligible
The proportion of eligible participants approached was not reported in nearly three quarters of studies (74%). Of the minority of studies providing this information (n = 55), all approached over 50% and the majority approached between 75% and 100% of individuals eligible for participation (93%).
Proportion consented out of those approached
The proportion of eligible participants consented out of those approached could not be calculated in over half of the studies (62%). Of the 82 studies providing this information, the majority consented more than half of approached participants (84%), while 16% recruited less than half those approached.
Recruitment rate (proportion recruited out of those eligible)
Just over 10% of studies reported recruitment targets; of these, 71% met their target. Over three quarters (77%) of studies did not provide the necessary information to calculate recruitment rates. In the 49 studies where this was possible, around a third recruited less than 50% of eligible participants (31%).
Reasons for non-enrolment
A total of 19% of studies approached all eligible participants. In 7% of studies, all invited participants decided to take part. Despite the majority of studies not approaching all eligible participants, 66% did not report reasons for this and 80% did not report reasons given by potential participants for deciding not to participate.
Reasons for not approaching eligible participants (non-invitation)
Of the 20% of studies reporting reasons for not contacting eligible participants, the most commonly cited were missing participant data (either clinical or contact, n = 25), judgements from clinicians of participant unsuitability (n = 14), unanticipated death (n = 13) or caregivers denying approach (n = 9). Other reasons included logistical considerations (e.g. distance participants lived from hospital, n = 7), researchers’ perception of participant unsuitability (n = 2) and communication difficulties (n = 2).
Reasons given by participants for non-enrolment (non-participation)
For studies in which not all eligible participants were recruited, 14% reported reasons given by potential participants for non-participation. Lack of interest was the most commonly reported reason (n = 10), followed by participants’ perception of practical (n = 9) and psychological burdens associated with research participation (n = 6). Personal reasons (n = 5), the child’s condition (n = 3), caregivers’ perceptions that their child needed their attention (n = 3) and refusal to consent to medical/surgical protocols (n = 1) or to randomization (n = 1) were also reported.
Researchers’ experiences of recruitment
Text regarding researchers’ experiences of recruitment were extracted from 58 studies (26%). This was coded thematically in relation to stages of participant recruitment.
Identification of potential participants
The unpredictable course and nature of illnesses and differing institutional policies and practices across research sites were reported as barriers to the identification of potentially eligible participants in four studies. Strategies used to facilitate the identification of eligible participants included widening inclusion criteria (e.g. changing the number of years since bereavement28) or seeking support from lay steering groups (‘The parent advisors had particular interest in improving palliative care services and programs and volunteered to assist’29).
Invitation of participants
Perceived barriers to the invitation of potential participants included gatekeeping from both professionals (‘staff … often chose not to approach families where the child was at the end of life’) and parents (‘In most cases the mother received the initial telephone call … This evolved as another layer of gatekeeping’9). Logistical factors such as being unable to contact potential participants were also reported as barriers (‘Many young people were not consistently engaging with medical services and hence did not have the opportunity to be invited to take part in research’).
Strategies used to facilitate invitation and recruitment processes included considerations of the method and timing of invitations (‘6-24 months after bereavement was chosen to facilitate recall whilst being sensitive to the emotional requirements of parents’), outlining participants’ options during the invitation period (‘Providing opportunities for parents or formal carers to be present during meetings was welcomed by both the young people and parents/formal carers’30) or providing monetary incentives.
Consenting of participants
Barriers to obtaining consent included overcoming participants’ attitudes or preconceptions towards research (‘Some parents felt that their son/daughter would be unable to participate as they were either non-verbal or had severe learning disabilities’30), logistical factors and CYP characteristics (‘Symptoms such as fatigue can keep children in critical condition from participating in research’31)
Methods found to facilitate the consenting process included incorporating a degree of flexibility (‘Rather than press for a decision on participation, they [participants] were advised they would be contacted after a week or so to discuss the study further’32), the attributes of the research team (‘Recruitment to the study depended on the appointment of an appropriately qualified and experienced research officer’9) and attitudes of potential participants (‘The majority of families treated in other hospitals … approached the physician themselves to discuss the possibility of an autopsy for research aims’33).
Discussion
This review explored systematically how CYP with LLC/LTI and their families have been identified, invited and consented to research, internationally, in the last 5 years.
Statement of principle findings
The majority of reviewed studies did not describe the methods employed in the identification, invitation or consenting of participants or the numbers considered or approached at each stage of the recruitment process. The lack of reporting observed that the proportion of eligible participants recruited (recruitment rates) could not be calculated in over three quarters of studies. This hindered our exploration of differences in recruitment practices between different types of research and to identify areas of good practice.
Where such information was available, we found that the documented reasons for non-invitation included missing clinical or contact data, or clinician judgements of participant unsuitability. The documented reasons for eligible patients deciding not to take part included lack of interest and participants’ perceptions of potential burdens.
The observed lack of reporting practices has implications for the interpretation and generalizability of the current evidence base underpinning the care and treatment of CYP with LLC/LTI and their families. Research with this population is open to the potential for bias for a number of reasons, whether this is due to characteristics of the population, the environment in which it is conducted or the different parties involved in their care. Without knowing who was considered and ultimately included or excluded from research, we cannot be sure that the results obtained are valid, generalizable and relevant for the populations that we are interested in. Moving forward research needs to be reported transparently in order for readers to be able to draw their own conclusions about how to use the information available.
Relationship with previous research and current reporting standards
Clinicians were most often reported to be responsible for the identification of potentially eligible participants and there is potential for this process to be influenced by clinician gatekeeping. As 80% of reviewed studies did not report how many screened participants were identified as eligible, we cannot expand further on this based on the findings of this review.
Strategies to overcome barriers to the invitation of participants reported included obtaining advice from steering groups and parent advisory committees. This is in line with current guidance advocating for the involvement of patients and the public in research design and conduct.16
Guidance and standards are in place for the conduct and reporting of research34 including the CONSORT (Consolidated Standards of Reporting Trials) statement,35 the TREND (Transparent Reporting of Evaluations with Nonrandomized Designs) guidelines,36 STROBE (STrengthening the Reporting of OBservational studies in Epidemiology) guidelines37 and for research with palliative populations, the MORECare Statement.21,38
While the CONSORT statement asks authors to provide information about the numbers of participants screened, identified as eligible and to provide reasons for excluding participants, not all journals require authors to follow reporting guidelines. Indeed information required by the CONSORT is not consistently reported in paediatric randomized controlled trials.39,40 This lack of adherence to guidelines could go some way to explaining the inconsistencies and inadequacies of reporting evidenced in this review.
The impact of inadequate reporting in research
Much research conducted with CYP with LLC/LTI and their families acknowledges its limited generalizability, but fails to report precisely to whom the research is generalizable to. Without the provision of more transparent information about the recruitment of CYP with LLC/LTI, it will be difficult to develop a sound understanding of the nature of barriers to research and for solutions to be generated and shared.
Our objective to explore what could be learnt from current research regarding recruitment practices was limited by a lack of reporting. Such learning is important to enhance success of future projects, thereby reducing waste and unnecessary exposure of CYP to suboptimal practices. Inadequate reporting of recruitment practices limits our capacity to judge study quality, risk of bias, representativeness of samples, generalizability of results and ultimately the applicability of findings. This has worrying implications for the policies which are underpinned by the current evidence base.
Strengths and limitations
The lack of reporting about recruitment processes meant that while we were able to conduct a narrative synthesis of the data, and a thematic synthesis of text from the discussion section of included papers provided, it was not possible to conduct an analysis of the association between research aim, recruitment strategies and recruitment rates.
We recognize that one potential explanation for the lack of information about recruitment processes may be related to journal constraints and word limits. In addition, for some variables, such as reasons for non-participation, researchers may not have access to this information, or may not have had the ethical approvals to collect and record data on non-participation. Nonetheless, the rate of recruitment reporting is lower than we had anticipated.
Our review was limited to studies published in English over the last 5 years, which may have introduced the potential for a language or publishing bias; however, studies from a range of countries were included. We restricted ourselves to the last 5 years for both practical reasons (limited resources) and in order to focus on the most current reporting practices. We did not include studies with mixed populations (studies recruiting CYP with LLC/LTI plus healthy controls or professionals involved in their care). Given the uniqueness of this population, we wanted to focus exclusively on CYP with LLC/LTI and their families. We are unable to comment on the reporting of recruitment practices in studies recruiting more heterogeneous populations.
The inclusion and analysis of informal data in systematic reviews are not common but have been successfully implemented in a few pioneering studies.27,41 Informal evidence provided a richer, fuller picture of recruitment than formal evidence alone. However, as relevant text was extracted from just over a quarter of reviewed studies (26%), the potential for biases within the data reviewed exists.
A potential criticism of this review could be the grouping of both medical (e.g. drug trials) and psychosocial studies under the research aim ‘evaluating an intervention’. We acknowledge that requirements and methodologies of studies within this category vary, yet these are the only studies for which there is the potential for direct physical participant benefit. The majority of studies within this category evaluated a medical intervention and from the data reported, there appears to be minimal differences between research with different aims in terms of recruitment methodologies used and recruitment rates achieved.
Recommendations for reporting of recruitment
In order to strengthen the evidence base and inform the development of future research and policies to improve the care and treatment of CYP with LLC/LTI and their families, greater clarity is needed in the reporting of research conducted with this population.
Future research should endeavour to provide transparent accounts of participant recruitment. In order to achieve this goal, we would urge authors to report recruitment methods and practices and we would encourage journals to make this part of the manuscript submission requirements. The use of online supplementary material facilities should be used where authors are constrained by word limits.
Implications for future research
Based on the content of recently published papers, we cannot judge the impact of different recruitment strategies or the extent of impact of the barriers reported due to the inadequacies of current reporting practices. Explorations of the effectiveness of different identification, invitation and recruitment strategies and the impact of flexibility in recruitment may serve to strengthen the evidence base and advance the care and treatment of CYP with LLC/LTI and their families. Further reviews of existing literature, including studies with mixed populations (those recruiting CYP with LLC/LTI plus healthy controls or professionals involved in their care), could prove illuminating.
This review has pooled the experiences of researchers’ recruiting CYP with LLC/LTI and their families to research in an attempt to explore recruitment practices employed, barriers encountered and steps taken to overcome them. Expanding this approach and further drawing upon both researchers’ and participants’ experiences and expertise could enable solutions to be generated, developed, implemented and shared among the research community. Researchers’ recommendations for facilitating the recruitment of this population spoke to the importance of listening to the views and preferences of potential participants with regard to research participation, and accommodating these preferences where possible.
We cannot presume to know what constitutes an unacceptable burden to research participation from the perspective of CYP with LLC/LTI and/or their families. Providing consenting participants, in research of all designs, with the opportunity to share their reasons for deciding to participate, and their views on what constitutes a burden or barrier to research could provide meaningful and useful insights into the research experience of this population and could inform the development of future research design and recruitment.
Acknowledgments
The authors acknowledge Margaret Comac (advice regarding whether diagnoses could be considered life-limiting or life-threatening), Lizzie Chambers (advice on interests and concerns of organizations using data about CYP with LLC/LTI and/or their families), Doug Hall (PPI), Kate Hall (PPI), Thines Ganeshamoorthy (PPI) and Grazia Manzotti (assistance with search strategy).
Appendix 1
MEDLINE search terms
1. Children and young people
Neonate* or newborn or new born or infant* or child* or adolescen* or pediatric or paediatric* or baby or babies or toddler* or juvenile* or boy* or girl* OR ‘child’ [MeSH Terms]) OR ‘infant’ [MeSH Terms]) OR ‘adolescent’ [MeSH Terms]) OR ‘pediatrics’ [MeSH Terms].
2. Life-limiting conditions and life-threatening illnesses
palliative) OR life threatening) OR life limiting) OR end of life) OR terminal care) OR terminal illness) OR bereave*) OR death) OR dying) OR ‘palliative care’ [MeSH Terms]) OR ‘terminally ill’ [MeSH Terms]) OR ‘death’ [MeSH Terms]) OR ‘terminal care’ [MeSH Terms]) OR ‘bereavement’ [MeSH Terms].
3. Invitation and recruitment practices
(participat* or recruit* or enrol* or invit* or select* or attitude* to research or accrual or enlist* or non-participat*)) OR (‘patient selection’ [MeSH Terms] OR ‘patient participation’ [MeSH Terms] OR ‘research subjects’ [MeSH Terms] OR ‘researcher-subject relations’ [MeSH Terms] OR ‘refusal to participate’ [MeSH])))))).
Appendix 2
References of reviewed studies
Studies aiming to evaluate an intervention
- Adams MS, Khan NZ, Begum SA, et al. Feeding difficulties in children with cerebral palsy: low-cost caregiver training in Dhaka, Bangladesh. Child Care Health Dev 2012; 38(6): 878–888. [DOI] [PubMed] [Google Scholar]
- Adzick NS, Thom EA, Spong CY, et al. A randomized trial of prenatal versus postnatal repair of myelomeningocele. N Engl J Med 2011; 364: 993–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akber A, Portale AA, Johansen KL. Use of pedometers to increase physical activity among children and adolescents with chronic kidney disease. Pediatr Nephrol 2014; 29(8): 1395–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albers E, Donahue BS, Milne G, et al. Perioperative plasma F(2)-Isoprostane levels correlate with markers of impaired ventilation in infants with single-ventricle physiology undergoing stage 2 surgical palliation on the cardiopulmonary bypass. Pediatr Cardiol 2012; 33: 562–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almond CS, Morales DL, Blackstone EH, et al. Berlin Heart EXCOR pediatric ventricular assist device for bridge to heart transplantation in US children. Circulation 2013; 127(16): 1702–1711. [DOI] [PubMed] [Google Scholar]
- Altarabsheh SE, Dearani JA, Burkhart HM, et al. Outcome of septal myectomy for obstructive hypertrophic cardiomyopathy in children and young adults. Ann Thorac Surg 2013; 95: 663–669. [DOI] [PubMed] [Google Scholar]
- Ancora G, Maranella E, Grandi S, et al. Early predictors of short term neurodevelopmental outcome in asphyxiated cooled infants. A combined brain amplitude integrated electroencephalography and near infrared spectroscopy study. Brain Dev 2013; 35(1): 26–31. [DOI] [PubMed] [Google Scholar]
- Azzopardi DV, Strohm B, Edwards AD, et al. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med 2009; 361(14): 1349–1358. [DOI] [PubMed] [Google Scholar]
- Baker DL, Schmidt ML, Cohn SL, et al. Outcome after reduced chemotherapy for intermediate-risk neuroblastoma. N Engl J Med 2010; 363(14): 1313–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black AK, Sadanala UK, Mascio CE, Hornung CA, Keller BB. Challenges in implementing a pediatric cardiovascular home telehealth project. Telemed J E Health 2014; 20: 858–867. [DOI] [PubMed] [Google Scholar]
- Cefalo G, Massimino M, Ruggiero A, et al. Temozolomide is an active agent in children with recurrent medulloblastoma/primitive neuroectodermal tumor: an Italian multi-institutional phase II trial. Neuro Oncol 2014; 16(5): 748–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chagaluka G, Stanley C, Banda K, et al. Kaposi’s sarcoma in children: an open randomised trial of vincristine, oral etoposide and a combination of vincristine and bleomycin. Eur J Cancer 2014; 50: 1472–1481. [DOI] [PubMed] [Google Scholar]
- Cohen KJ, Gibbs IC, Fisher PG, et al. A phase I trial of arsenic trioxide chemoradiotherapy for infiltrating astrocytomas of childhood. Neuro Oncol 2013; 15(6): 783–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen KJ, Heideman RL, Zhou TN, et al. Temozolomide in the treatment of children with newly diagnosed diffuse intrinsic pontine gliomas: a report from the Children’s Oncology Group. Neuro Oncol 2011; 13(4): 410–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotton MF, Violari A, Otwombe K, et al. Early time-limited antiretroviral therapy versus deferred therapy in South African infants infected with HIV: results from the children with HIV early antiretroviral (CHER) randomised trial. Lancet 2013; 382(9904): 1555–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuvelier GD, Baker TJ, Peddie EF, et al. A randomized, double-blind, placebo-controlled clinical trial of megestrol acetate as an appetite stimulant in children with weight loss due to cancer and/or cancer therapy. Pediatr Blood Cancer 2014; 61(4): 672–679. [DOI] [PubMed] [Google Scholar]
- Dilli D, Aydin B, Zenciroglu A, et al. Treatment outcomes of infants with cyanotic congenital heart disease treated with synbiotics. Pediatrics 2013; 132(4): E932–E938. [DOI] [PubMed] [Google Scholar]
- Dingley J, Tooley J, Liu X, et al. Xenon ventilation during therapeutic hypothermia in neonatal encephalopathy: a feasibility study. Pediatrics 2014; 133(5): 809–818. [DOI] [PubMed] [Google Scholar]
- Dworzak MN, Gaipa G, Schumich A, et al. Modulation of antigen expression in B-cell precursor acute lymphoblastic leukemia during induction therapy is partly transient: evidence for a drug-induced regulatory phenomenon. Results of the AIEOP-BFM-ALL-FLOW-MRD-Study Group. Cytometry B Clin Cytom 2010; 78: 147–153. [DOI] [PubMed] [Google Scholar]
- Foster C, McDonald S, Frize G, et al. ‘Payment by Results’ – financial incentives and motivational interviewing, adherence interventions in young adults with perinatally acquired HIV-1 infection: a pilot program. AIDS Patient Care STDS 2014; 28(1): 28–32. [DOI] [PubMed] [Google Scholar]
- Gohar SF, Comito M, Price J, et al. Feasibility and parent satisfaction of a physical therapy intervention program for children with acute lymphoblastic leukemia in the first 6 months of medical treatment. Pediatr Blood Cancer 2011; 56(5): 799–804. [DOI] [PubMed] [Google Scholar]
- Granger M, Grupp SA, Kletzel M, et al. Feasibility of a tandem autologous peripheral blood stem cell transplant regimen for high risk neuroblastoma in a cooperative group setting: a Pediatric Oncology Group study: a report from the Children’s Oncology Group. Pediatr Blood Cancer 2012; 59(5): 902–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser PM, Bernard T, Greub G, et al. Microbiota present in cystic fibrosis lungs as revealed by whole genome sequencing. PLoS ONE 2014; 9(3): e90934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes-Jordan A, Green H, Ludwig J, et al. Toxicity of hyperthermic intraperitoneal chemotherapy (HIPEC) in pediatric patients with sarcomatosis/carcinomatosis: early experience and phase 1 results. Pediatr Blood Cancer 2012; 59(2): 395–397. [DOI] [PubMed] [Google Scholar]
- Hessissen L, Khtar R, Madani A, et al. Improving the prognosis of pediatric hodgkin lymphoma in developing countries: a moroccan society of pediatric hematology and oncology study. Pediatr Blood Cancer 2013; 60(9): 1464–1469. [DOI] [PubMed] [Google Scholar]
- Hickey EJ, Caldarone CA, Blackstone EH, et al. Biventricular strategies for neonatal critical aortic stenosis: high mortality associated with early reintervention. J Thorac Cardiovasc Surg 2012; 144(2): 409–+. [DOI] [PubMed] [Google Scholar]
- Hoving MA, van Raak EPM, Spincemaille G, et al. Safety and one-year efficacy of intrathecal baclofen therapy in children with intractable spastic cerebral palsy. Eur J Paediatr Neurol 2009; 13: 247–256. [DOI] [PubMed] [Google Scholar]
- Jakacki RI, Burger PC, Zhou TN, et al. Outcome of children with metastatic medulloblastoma treated with carboplatin during craniospinal radiotherapy: a children’s oncology group phase I/II study. J Clin Oncol 2012; 30(21): 2648–2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jozwiak M, Harasymczuk P, Koch A, et al. Incidence and risk factors of hip joint pain in children with severe cerebral palsy. Disabil Rehab 2011; 33(15–16): 1367–1372. [DOI] [PubMed] [Google Scholar]
- Karaman S, Vural S, Yildirmak Y, et al. Comparison of piperacillin tazobactam and cefoperazone sulbactam monotherapy in treatment of febrile neutropenia. Pediatr Blood Cancer 2012; 58(4): 579–583. [DOI] [PubMed] [Google Scholar]
- Keating JJ, Simsic JM, Kogon BE, et al. Impact of early fundoplication or gastrostomy tube on midterm outcomes for patients with single ventricle. J Thorac Cardiovasc Surg 2012; 143: 891–895. [DOI] [PubMed] [Google Scholar]
- Kishnani PS, Corzo D, Leslie ND, et al. Early treatment with alglucosidase alpha prolongs long-term survival of infants with Pompe disease. Pediatr Res 2009; 66(3): 329–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Tripathi P, Baranwal M, et al. Randomized, controlled trial of oral ribavirin for Japanese encephalitis in children in Uttar Pradesh, India. Clin Infect Dis 2009; 48: 400–406. [DOI] [PubMed] [Google Scholar]
- Latus H, Binder W, Kerst G, et al. Right ventricular-pulmonary arterial coupling in patients after repair of tetralogy of Fallot. J Thorac Cardiovasc Surg 2013; 146(6): 1366–1372. [DOI] [PubMed] [Google Scholar]
- Lindsey JC, Hughes MD, Violari A, et al. Predictors of virologic and clinical response to nevirapine versus lopinavir/ritonavir-based antiretroviral therapy in young children with and without prior nevirapine exposure for the prevention of mother-to-child HIV transmission. Pediatr Infect Dis J 2014; 33(8): 846–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipshultz SE, Scully RE, Lipsitz SR, et al. Assessment of dexrazoxane as a cardioprotectant in doxorubicin-treated children with high-risk acute lymphoblastic leukaemia: long-term follow-up of a prospective, randomised, multicentre trial. Lancet Oncol 2010; 11(10): 950–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald TJ, Vezina G, Stewart CF, et al. Phase II study of cilengitide in the treatment of refractory or relapsed high-grade gliomas in children: a report from the Children’s Oncology Group. Neuro Oncol 2013; 15(10): 1438–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McJunkin JE, Nahata MC, Reyes E, et al. Safety and pharmacokinetics of ribavirin for the treatment of la crosse encephalitis. Pediatr Infect Dis J 2011; 30(10): 860–865. [DOI] [PubMed] [Google Scholar]
- Meinhardt A, Burkhardt B, Zimmermann M, et al. Phase II window study on rituximab in newly diagnosed pediatric mature B-cell non-Hodgkin’s lymphoma and Burkitt leukemia. J Clin Oncol 2010; 28(19): 3115–3121. [DOI] [PubMed] [Google Scholar]
- Ndeezi G, Tumwine JK, Bolann BJ, et al. Zinc status in HIV infected Ugandan children aged 1–5 years: a cross sectional baseline survey. BMC Pediatr 2010; 10: 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolbris MJ, Ahlstrom BH. Siblings of children with cancer – their experiences of participating in a person-centered support intervention combining education, learning and reflection: pre- and post-intervention interviews. Eur J Oncol Nurs 2014; 18(3): 254–260. [DOI] [PubMed] [Google Scholar]
- Novak I, Cusick A, Lannin N. Occupational therapy home programs for cerebral palsy: double-blind, randomized, controlled trial. Pediatrics 2009; 124(4): e606–e614. [DOI] [PubMed] [Google Scholar]
- Ohman A, Stromvall-Larsson E, Nilsson B, et al. Pulse oximetry home monitoring in infants with single-ventricle physiology and a surgical shunt as the only source of pulmonary blood flow. Cardiol Young 2013; 23: 75–81. [DOI] [PubMed] [Google Scholar]
- Okomo U, Togun T, Oko F, et al. Mortality and loss to programme before antiretroviral therapy among HIV-infected children eligible for treatment in The Gambia, West Africa. AIDS Res Ther 2012; 9: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquali SK, Ohye RG, Lu MM, et al. Variation in perioperative care across centers for infants undergoing the Norwood procedure. J Thorac Cardiovasc Surg 2012; 144(4): 915–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pession A, Masetti R, Rizzari C, et al. Results of the AIEOP AML 2002/01 multicenter prospective trial for the treatment of children with acute myeloid leukemia. Blood 2013; 122: 170–178. [DOI] [PubMed] [Google Scholar]
- Pickering D, Horrocks LM, Visser KS, et al. ‘Every picture tells a story’: interviews and diaries with children with cerebral palsy about adapted cycling. J Paediatr Child Health 2013; 49(12): 1040–1044. [DOI] [PubMed] [Google Scholar]
- Prado JG, Prendergast A, Thobakgale C, et al. Replicative capacity of human immunodeficiency virus type 1 transmitted from mother to child is associated with pediatric disease progression rate. J Virol 2010; 84(1): 492–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radbill AE, Triedman JK, Berul CI, et al. Prospective evaluation of defibrillation threshold and postshock rhythm in young ICD recipients. Pacing Clin Electrophysiol 2012; 35(12): 1487–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridola V, Barone G, Lazzareschi I, et al. Feasibility study of 21-day-on/7-day-off temozolomide in children with brain tumors. J Neurooncol 2011; 103(1): 147–153. [DOI] [PubMed] [Google Scholar]
- Sakzewski L, Carlon S, Shields N, et al. Impact of intensive upper limb rehabilitation on quality of life: a randomized trial in children with unilateral cerebral palsy. Dev Med Child Neurol 2012; 54(5): 415–423. [DOI] [PubMed] [Google Scholar]
- Salzer WL, Asselin B, Supko JG, et al. Erwinia asparaginase achieves therapeutic activity after pegaspargase allergy: a report from the Children’s Oncology Group. Blood 2013; 122(4): 507–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzer WL, Jones TL, Devidas M, et al. Modifications to induction therapy decrease risk of early death in infants with acute lymphoblastic leukemia treated on Children’s Oncology Group P9407. Pediatr Blood Cancer 2012; 59(5): 834–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandlund JT, Pui CH, Zhou Y, et al. Effective treatment of advanced-stage childhood lymphoblastic lymphoma without prophylactic cranial irradiation: results of St Jude NHL13 study. Leukemia 2009; 23: 1127–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankaran S, Pappas A, McDonald SA, et al. Predictive value of an early amplitude integrated electroencephalogram and neurologic examination. Pediatrics 2011; 128(1): E112–E120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simbruner G, Mittal RA, Rohlmann F, et al. Systemic hypothermia after neonatal encephalopathy: outcomes of neo.nEURO.network RCT. Pediatrics 2010; 126(4): E771–E778. [DOI] [PubMed] [Google Scholar]
- Souid AK, Dubowy RL, Ingle AM, et al. A pediatric phase I trial and pharmacokinetic study of ispinesib: a Children’s Oncology Group phase I consortium study. Pediatr Blood Cancer 2010; 55(7): 1323–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan A, Kasow KA, Cross S, et al. Phase I study of the tolerability and pharmacokinetics of palifermin in children undergoing allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2012; 18: 1309–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan R, Asselin J, Gildengorin G, et al. A prospective study of ventilator-associated pneumonia in children. Pediatrics 2009; 123: 1108–1115. [DOI] [PubMed] [Google Scholar]
- Sugimoto A, Ota N, Ibuki K, et al. Risk factors for adverse neurocognitive outcomes in school-aged patients after the Fontan operation(dagger). Eur J Cardiothorac Surg 2013; 44: 454–461. [DOI] [PubMed] [Google Scholar]
- Sung L, Buxton A, Gamis A, et al. Life-threatening and fatal infections in children with acute myeloid leukemia: a report from the Children’s Oncology Group. J Pediatr Hematol Oncol 2012; 34: e30–e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takenouchi T, Rubens EO, Yap VL, et al. Delayed onset of sleep-wake cycling with favorable outcome in hypothermic-treated neonates with encephalopathy. J Pediatr 2011; 159(2): 232–237. [DOI] [PubMed] [Google Scholar]
- Tomizawa D, Tawa A, Watanabe T, et al. Appropriate dose reduction in induction therapy is essential for the treatment of infants with acute myeloid leukemia: a report from the Japanese Pediatric Leukemia/Lymphoma Study Group. Int J Hematol 2013; 98(5): 578–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzark K, Wang Y, Rudd N, et al. Interstage feeding and weight gain in infants following the Norwood operation: can we change the outcome? Cardiol Young 2012; 22: 520–527. [DOI] [PubMed] [Google Scholar]
- Van Poppel M, Klimo P, Dewire M, et al. Resection of infantile brain tumors after neoadjuvant chemotherapy: the St. Jude experience Clinical article. J Neurosurg Pediatr 2011; 8(3): 251–256. [DOI] [PubMed] [Google Scholar]
- Violari A, Lindsey JC, Hughes MD, et al. Nevirapine versus ritonavir-boosted lopinavir for HIV-infected children. N Engl J Med 2012; 366(25): 2380–2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vora A, Goulden N, Wade R, et al. Treatment reduction for children and young adults with low-risk acute lymphoblastic leukaemia defined by minimal residual disease (UKALL 2003): a randomised controlled trial. Lancet Oncol 2013; 14(3): 199–209. [DOI] [PubMed] [Google Scholar]
- Widemann BC, Arceci RJ, Jayaprakash N, et al. Phase 1 trial and pharmacokinetic study of the farnesyl transferase inhibitor tipifarnib in children and adolescents with refractory leukemias: a report from the Children’s Oncology Group. Pediatr Blood Cancer 2011; 56(2): 226–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaky W, Dhall G, Ji L, et al. Intensive induction chemotherapy followed by myeloablative chemotherapy with autologous hematopoietic progenitor cell rescue for young children newly-diagnosed with central nervous system atypical teratoid/rhabdoid tumors: the Head Start III experience. Pediatr Blood Cancer 2014; 61(1): 95–101. [DOI] [PubMed] [Google Scholar]
- Zhou WH, Cheng GQ, Shao XM, et al. Selective head cooling with mild systemic hypothermia after neonatal hypoxic-ischemic encephalopathy: a multicenter randomized controlled trial in China. J Pediatr 2010; 157(3): 367–372, 372.e1–372.e3. [DOI] [PubMed] [Google Scholar]
- Jones JK, Kamani SA, Bush PJ, et al. Development and evaluation of an educational interactive CD-ROM for teens with cancer. Pediatr Blood Cancer 2010; 55(3): 512–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
Studies assessing quality of life
- Abu-Saad Huijer H, Doumit M, Abboud S, et al. Quality of palliative care. Perspective of Lebanese patients with cancer. J Med Liban 2012; 60(2): 91–98. [PubMed] [Google Scholar]
- Al-Gamal E. Quality of life and anticipatory grieving among parents living with a child with cerebral palsy. Int J Nurs Prac 2013; 19(3): 288–294. [DOI] [PubMed] [Google Scholar]
- Andrinopoulos K, Clum G, Murphy DA, et al. Health related quality of life and psychosocial correlates among HIV-infected adolescent and young adult women in the US. AIDS Educ Prev 2011; 23: 367–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya M, Rajeshwari K, Saxena R. Demographic and clinical features of orphans and nonorphans at a pediatric HIV centre in North India. Indian J Pediatr 2010; 77(6): 627–631. [DOI] [PubMed] [Google Scholar]
- Bradford N, Young J, Armfield NR, et al. A pilot study of the effectiveness of home teleconsultations in paediatric palliative care. J Telemed Telecare 2012; 18(8): 438–442. [DOI] [PubMed] [Google Scholar]
- Byrne MW, Evan E, Goshin LS, et al. Parent self-efficacy for managing pain in seriously ill children and adolescents nearing end of life. Palliat Support Care 2011; 9: 137–147. [DOI] [PubMed] [Google Scholar]
- Cadell S, Kennedy K, Hemsworth D. Informing social work practice through research with parent caregivers of a child with a life-limiting illness. J Soc Work End Life Palliat Care 2012; 8(4): 356–381. [DOI] [PubMed] [Google Scholar]
- Caeymaex L, Jousselme C, Vasilescu C, et al. Perceived role in end-of-life decision making in the NICU affects long-term parental grief response. Arch Dis Child Fetal Neonatal Ed 2013; 98: F26–F31. [DOI] [PubMed] [Google Scholar]
- Ceravolo F, Mascaro I, Sestito S, et al. Home treatment in paediatric patients with Hunter syndrome: the first Italian experience. Italian J Pediatr 2013; 39: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelen V, Koopman HM, Detmar SB, et al. Health-related quality of life after completion of successful treatment for childhood cancer. Pediatr Blood Cancer 2011; 56(4): 646–653. [DOI] [PubMed] [Google Scholar]
- Jalmsell L, Onelov E, Steineck G, et al. Hematopoietic stem cell transplantation in children with cancer and the risk of long-term psychological morbidity in the bereaved parents. Bone Marrow Transplant 2011; 46(8): 1063–1070. [DOI] [PubMed] [Google Scholar]
- Knapp C, Madden V, Revicki D, et al. Health status and health-related quality of life in a pediatric palliative care program. J Palliat Med 2012; 15(7): 790–797. [DOI] [PubMed] [Google Scholar]
- Li HCW, Chung OKJ, Chiu SY. The impact of cancer on children’s physical, emotional, and psychosocial well-being. Cancer Nurs 2010; 33(1): 47–54. [DOI] [PubMed] [Google Scholar]
- O’Byrne ML, Mercer-Rosa L, et al. Morbidity in children and adolescents after surgical correction of truncus arteriosus communis. Am Heart J 2013; 166(3): 512–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly D, Huws J, Hastings R, et al. Life and death of a child with down syndrome and a congenital heart condition: experiences of six couples. Intellect Dev Disabil 2010; 48(6): 403–416. [DOI] [PubMed] [Google Scholar]
- Reisi-Dehkordi N, Baratian H, Zargham-Boroujeni A. Challenges of children with cancer and their mothers: a qualitative research. Iran J Nurs Midwifery Res 2014; 19(4): 334–339. [PMC free article] [PubMed] [Google Scholar]
- Rosenberg AR, Postier A, Osenga K, et al. Long-term psychosocial outcomes among bereaved siblings of children with cancer. J Pain Symptom Manage 2015; 49: 55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenmaker NJ, Haverman L, Tromp WF, et al. Children of non-Western origin with end-stage renal disease in the Netherlands, Belgium and a part of Germany have impaired health-related quality of life compared with Western children. Nephrol Dial Transplant 2014; 29: 448–457. [DOI] [PubMed] [Google Scholar]
- Steele R, Siden H, Cadell S, et al. Charting the territory: symptoms and functional assessment in children with progressive, non-curable conditions. Arch Dis Child 2014; 99: 754–762. [DOI] [PubMed] [Google Scholar]
- Syczewska M, Dembowska-Baginska B, Perek-Polnik M, et al. Gait pathology assessed with Gillette Gait Index in patients after CNS tumour treatment. Gait Posture 2010; 32: 358–362. [DOI] [PubMed] [Google Scholar]
- Tomlinson D, Hinds PS, Bartels U, et al. Parent reports of quality of life for pediatric patients with cancer with no realistic chance of cure. J Clin Oncol 2011; 29(6): 639–645. [DOI] [PubMed] [Google Scholar]
- Whittingham K, Wee D, Sanders MR, et al. Predictors of psychological adjustment, experienced parenting burden and chronic sorrow symptoms in parents of children with cerebral palsy. Child Care Health Dev 2013; 39(3): 366–373. [DOI] [PubMed] [Google Scholar]
Studies observing the course of illness
- Anga G, Barnabas R, Kaminiel O, et al. The aetiology, clinical presentations and outcome of febrile encephalopathy in children in Papua New Guinea. Ann Trop Paediatr 2010; 30(2): 109–118. [DOI] [PubMed] [Google Scholar]
- Arnon R, Kerkar N, Davis MK, et al. Liver transplantation in children with metabolic diseases: the studies of pediatric liver transplantation experience. Pediatr Transplant 2010; 14(6): 796–805. [DOI] [PubMed] [Google Scholar]
- Badiei Z, Khalesi M, Alami MH, et al. Risk factors associated with life-threatening infections in children with febrile neutropenia: a data mining approach. J Pediatr Hematol Oncol 2011; 33(1): E9–E12. [DOI] [PubMed] [Google Scholar]
- Bonkowsky JL, Nelson C, Kingston JL, et al. The burden of inherited leukodystrophies in children. Neurology 2010; 75(8): 718–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucuvalas J, Filipovich L, Yazigi N, et al. Immunophenotype predicts outcome in pediatric acute liver failure. J Pediatr Gastroenterol Nutr 2013; 56: 311–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen I, Khaki L, Lindsey JC, et al. Association of pol diversity with antiretroviral treatment outcomes among HIV-infected African children. Plos ONE 2013; 8(11): e81213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong JLY, Coleman L, Hunt RW, et al. Prognostic utility of magnetic resonance imaging in neonatal hypoxic-ischemic encephalopathy substudy of a randomized trial. Arch Pediatr Adolesc Med 2012; 166: 634–640. [DOI] [PubMed] [Google Scholar]
- Chiu SN, Wu MH, Su MJ, et al. Coexisting mutations/polymorphisms of the long QT syndrome genes in patients with repaired Tetralogy of Fallot are associated with the risks of life-threatening events. Hum Genet 2012; 131(8): 1295–1304. [DOI] [PubMed] [Google Scholar]
- Cluca IM, Pop L, Tamas L, et al. Cystic fibrosis liver disease – from diagnosis to risk factors. Rom J Morphol Embryol 2014; 55: 91–95. [PubMed] [Google Scholar]
- Cortez MAA, Scrideli CA, Yunes JA, et al. mRNA expression profile of multidrug resistance genes in childhood acute lymphoblastic leukemia. Low expression levels associated with a higher risk of toxic death. Pediatr Blood Cancer 2009; 53(6): 996–1004. [DOI] [PubMed] [Google Scholar]
- Elgendi HM, Mekawy MA, Wahab S, et al. AC133 Expression in Egyptian children with acute leukemia: impact on treatment response and disease outcome. J Pediatr Hematol Oncol 2010; 32: 286–293. [DOI] [PubMed] [Google Scholar]
- Finkel RS, McDermott MP, Kaufmann P, et al. Observational study of spinal muscular atrophy type I and implications for clinical trials. Neurology 2014; 83(9): 810–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima H, Fukushima T, Sakai A, et al. Polymorphisms of MTHFR associated with higher relapse/death ratio and delayed weekly MTX administration in pediatric lymphoid malignancies. Leukemia Res Treat 2013; 2013: 238528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamis AS, Alonzo TA, Gerbing RB, et al. Natural history of transient myeloproliferative disorder clinically diagnosed in Down syndrome neonates: a report from the Children’s Oncology Group Study A2971. Blood 2011; 118(26): 6752–6759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavidia R, Fuentes SL, Vasquez R, et al. Low socioeconomic status is associated with prolonged times to assessment and treatment, sepsis and infectious death in pediatric fever in El Salvador. PLoS ONE 2012; 7(8): e43639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnekow AK, Falkenstein F, von Hornstein S, et al. Long-term follow-up of the multicenter, multidisciplinary treatment study HIT-LGG-1996 for low-grade glioma in children and adolescents of the German Speaking Society of Pediatric Oncology and Hematology. Neuro Oncol 2012; 14(10): 1265–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goergen SK, Ang H, Wong F, et al. Early MRI in term infants with perinatal hypoxic-ischaemic brain injury: interobserver agreement and MRI predictors of outcome at 2 years. Clin Radiol 2014; 69(1): 72–81. [DOI] [PubMed] [Google Scholar]
- Horn AR, Swingler GH, Myer L, et al. Early clinical predictors of a severely abnormal amplitude-integrated electroencephalogram at 48 hours in cooled neonates. Acta Paediatrica 2013; 102: e378–e384. [DOI] [PubMed] [Google Scholar]
- Lau DT, Hesson LB, Norris MD, et al. Prognostic significance of promoter DNA methylation in patients with childhood neuroblastoma. Clin Cancer Res 2012; 18(20): 5690–5700. [DOI] [PubMed] [Google Scholar]
- Liu FT, Xiong YY, Zhao Y, et al. Identification of aberrant microRNA expression pattern in pediatric gliomas by microarray. Diagn Pathol 2013; 8: 158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luhi F, Leibundgut K, Niggli FK, et al. Serious medical complications in children with cancer and fever in chemotherapy-induced neutropenia: results of the prospective multicenter SPOG 2003 FN study. Pediatr Blood Cancer 2012; 59: 90–95. [DOI] [PubMed] [Google Scholar]
- Massaro AN, Chang T, Kadom N, et al. Biomarkers of brain injury in neonatal encephalopathy treated with hypothermia. J Pediatr 2012; 161(3): 434–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moodley A, Qin M, Singh KK, et al. Vitamin D-related host genetic variants alter HIV disease progression in children. Pediatr Infect Dis J 2013; 32: 1230–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottosson-Wadlund A, Ceder R, Preta G, et al. Requirement of apoptotic protease-activating factor-1 for bortezomib-induced apoptosis but not for Fas-mediated apoptosis in human leukemic cells. Mol Pharmacol 2013; 83: 245–255. [DOI] [PubMed] [Google Scholar]
- Pietrzyk JJ, Bik-Multanowski M, Balwierz W, et al. Additional genetic risk factor for death in children with acute lymphoblastic leukemia: a common polymorphism of the MTHFR gene. Pediatr Blood Cancer 2009; 52: 364–368. [DOI] [PubMed] [Google Scholar]
- Pisani F, Orsini M, Braibanti S, et al. Development of epilepsy in newborns with moderate hypoxic-ischemic encephalopathy and neonatal seizures. Brain Dev 2009; 31: 64–68. [DOI] [PubMed] [Google Scholar]
- Radman M, Keller RL, Oishi P, et al. Preoperative B-type natriuretic peptide levels are associated with outcome after total cavopulmonary connection (Fontan). J Thorac Cardiovasc Surg 2014; 148: 212–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainwater-Lovett K, Nkamba HC, Mubiana-Mbewe M, et al. Immunologic risk factors for early mortality after starting antiretroviral therapy in HIV-infected Zambian children. AIDS Res Hum Retroviruses 2013; 29(3): 479–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland M, Gallagher CG, O’Laoide R, et al. Outcome in cystic fibrosis liver disease. Am J Gastroenterol 2011; 106(1): 104–109. [DOI] [PubMed] [Google Scholar]
- Sanchez Mejia AA, Simpson KE, Hildebolt CF, et al. Tissue Doppler septal Tei index indicates severity of illness in pediatric patients with congestive heart failure. Pediatr Cardiol 2014; 35: 411–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaram V, Dutta S, Ahluwalia J, et al. Score for neonatal acute physiology II predicts mortality and persistent organ dysfunction in neonates with severe septicemia. Indian Pediatr 2009; 46: 775–780. [PubMed] [Google Scholar]
- Tzanetos DRT, Yu C, Hernanz-Schulman M, et al. Prospective study of the incidence and predictors of thrombus in children undergoing palliative surgery for single ventricle physiology. Intensive Care Med 2012; 38(1): 105–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk JH, Sutcliffe CG, Munsanje B, et al. HIV-infected children in rural Zambia achieve good immunologic and virologic outcomes two years after initiating antiretroviral therapy. Plos ONE 2011; 6(4): e19006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westbom L, Bergstrand L, Wagner P, et al. Survival at 19 years of age in a total population of children and young people with cerebral palsy. Dev Med Child Neurol 2011; 53: 808–814. [DOI] [PubMed] [Google Scholar]
- Wong DTH, George K, Wilson J, et al. Effectiveness of serial increases in amino-terminal pro-B-type natriuretic peptide levels to indicate the need for mechanical circulatory support in children with acute decompensated heart failure. Am J Cardiol 2011; 107(4): 573–578. [DOI] [PubMed] [Google Scholar]
- Wood F, Simpson S, Barnes E, et al. Disease trajectories and ACT/RCPCH categories in paediatric palliative care. Palliat Med 2010; 24(8): 796–806. [DOI] [PubMed] [Google Scholar]
Studies exploring views and perspectives
- Alam R, Barrera M, D’Agostino N, et al. Bereavement experiences of mothers and fathers over time after the death of a child due to cancer. Death Stud 2012; 36(1): 1–22. [DOI] [PubMed] [Google Scholar]
- Anderzen-Carlsson A, Sorlie V, Kihlgren A. Dealing with fear – from the perspective of adolescent girls with cancer. Eur J Oncol Nurs 2012; 16(3): 286–292. [DOI] [PubMed] [Google Scholar]
- Baker JN, Windham JA, Hinds PS, et al. Bereaved parents’ intentions and suggestions about research autopsies in children with lethal brain tumors. J Pediatr 2013; 163(2): 581–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bally JMG, Duggleby W, Holtslander L, et al. Keeping hope possible: a grounded theory study of the hope experience of parental caregivers who have children in treatment for cancer. Cancer Nurs 2014; 37(5): 363–372. [DOI] [PubMed] [Google Scholar]
- Barling JA, Stevens J, Davis KM. Family members’ retrospective stories of the treatment stage of an adolescent or young adult who subsequently died of cancer. Cancer Nurs 2013; 36(5): E39–E48. [DOI] [PubMed] [Google Scholar]
- Bratt EL, Ostman-Smith I, Sparud-Lundin C, et al. Parents’ experiences of having an asymptomatic child diagnosed with hypertrophic cardiomyopathy through family screening. Cardiol Young 2011; 21(1): 8–14. [DOI] [PubMed] [Google Scholar]
- Bratt EL, Sparud-Lundin C, Ostman-Smith I, et al. The experience of being diagnosed with hypertrophic cardiomyopathy through family screening in childhood and adolescence. Cardiol Young 2012; 22(5): 528–535. [DOI] [PubMed] [Google Scholar]
- Brennan C, Hugh-Jones S, Aldridge J. Paediatric life-limiting conditions: coping and adjustment in siblings. J Health Psychol 2013; 18(6): 813–824. [DOI] [PubMed] [Google Scholar]
- Cataudella DA, Zelcer S. Psychological experiences of children with brain tumors at end of life: parental perspectives. J Palliat Med 2012; 15(11): 1191–1197. [DOI] [PubMed] [Google Scholar]
- Cote-Arsenault D, Denney-Koelsch E. ‘My baby is a person’: parents’ experiences with life-threatening fetal diagnosis. J Palliat Med 2011; 14: 1302–1308. [DOI] [PubMed] [Google Scholar]
- Dussel V, Joffe S, Hilden JM, et al. Considerations about hastening death among parents of children who die of cancer. Arch Pediatr Adolesc Med 2010; 164(3): 231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Malla H, Kreicbergs U, Steineck G, et al. Parental trust in health care – a prospective study from the Children’s Cancer Hospital in Egypt. Psychooncology 2013; 22(3): 548–554. [DOI] [PubMed] [Google Scholar]
- Foster TL, Gilmer MJ, Davies B, et al. Comparison of continuing bonds reported by parents and siblings after a child’s death from cancer. Death Stud 2011; 35(5): 420–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaab EM, Owens RG, MacLeod RD. Primary caregivers’ experiences living with children involved in pediatric palliative care in New Zealand. Vulnerable Child Youth Stud 2013; 8(1): 1–9. [Google Scholar]
- Gaab EM, Owens RG, MacLeod RD. The voices of young New Zealanders involved in pediatric palliative care. J Palliat Care 2013; 29: 186–192. [PubMed] [Google Scholar]
- Garvie PA, He J, Wang J, et al. An exploratory survey of end-of-life attitudes, beliefs, and experiences of adolescents with HIV/AIDS and their families. J Pain Symptom Manage 2012; 44(3): 373–385.e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt CA, Fairclough DL, Grossenbacher JC, et al. Peer relationships of bereaved siblings and comparison classmates after a child’s death from cancer. J Pediatr Psychol 2012; 37(2): 209–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granek L, Barrera M, Shaheed J, et al. Trajectory of parental hope when a child has difficult-to-treat cancer: a prospective qualitative study. Psychooncology 2013; 22(11): 2436–2444. [DOI] [PubMed] [Google Scholar]
- Guon J, Wilfond BS, Farlow B, et al. Our children are not a diagnosis: the experience of parents who continue their pregnancy after a prenatal diagnosis of trisomy 13 or 18. Am J Med Genet A 2014; 164(2): 308–318. [DOI] [PubMed] [Google Scholar]
- Hexem KR, Mollen CJ, Carroll K, et al. How parents of children receiving pediatric palliative care use religion, spirituality, or life philosophy in tough times. J Palliat Med 2011; 14: 39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinds PS, Oakes LL, Hicks J, et al. ‘Trying to Be a Good Parent’ As defined by interviews with parents who made phase I, terminal care, and resuscitation decisions for their children. J Clin Oncol 2009; 27(35): 5979–5985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinojosa MS, Knapp CA, Madden VL, et al. Caring for children with life-threatening illnesses: impact on white, African American, and Latino families. J Pediatr Nurs 2012; 27(5): 500–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogwood J, Campbell T, Butler S. I wish I could tell you but I can’t: adolescents with perinatally acquired HIV and their dilemmas around self-disclosure. Clin Child Psychol Psychiatry 2013; 18(1): 44–60. [DOI] [PubMed] [Google Scholar]
- Hoven E, von Essen L, Norberg AL. A longitudinal assessment of work situation, sick leave, and household income of mothers and fathers of children with cancer in Sweden. Acta Oncol 2013; 52(6): 1076–1085. [DOI] [PubMed] [Google Scholar]
- Janvier A, Farlow B, Wilfond BS. The experience of families with children with trisomy 13 and 18 in social networks. Pediatrics 2012; 130(2): 293–298. [DOI] [PubMed] [Google Scholar]
- Kars MC, Grypdonck MH, Beishuizen A, et al. Factors influencing parental readiness to let their child with cancer die. Pediatr Blood Cancer 2010; 54(7): 1000–1008. [DOI] [PubMed] [Google Scholar]
- Lagrange RD, Mitchell SJ, Lewis M, et al. Health protective behaviors among young people lving with HIV/AIDS. J AIDS Clin Res 2012; 13: 7348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lannen P, Wolfe J, Mack J, et al. Absorbing information about a child’s incurable cancer. Oncology 2010; 78(3–4): 259–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen HB, Heilmann C, Johansen C, et al. An analysis of parental roles during haematopoietic stem cell transplantation of their offspring: a qualitative and participant observational study. J Adv Nurs 2011; 67: 1458–1467. [DOI] [PubMed] [Google Scholar]
- Lathrop A, Vandevusse L. Affirming motherhood: validation and invalidation in women’s perinatal hospice narratives. Birth 2011; 38(3): 256–265. [DOI] [PubMed] [Google Scholar]
- Lazarus R, Struthers H, Violari A. Starting HIV-positive babies on antiretroviral treatment: perspectives of mothers in Soweto, South Africa. J Pediatr Health Care 2010; 24(3): 176–183. [DOI] [PubMed] [Google Scholar]
- Lindahl Norberg A, Poder U, von Essen L. Early avoidance of disease- and treatment-related distress predicts post-traumatic stress in parents of children with cancer. Eur J Oncol Nurs 2011; 15(1): 80–84. [DOI] [PubMed] [Google Scholar]
- Malcolm C, Gibson F, Adams S, et al. A relational understanding of sibling experiences of children with rare life-limiting conditions: findings from a qualitative study. J Child Health Care 2014; 18(3): 230–240. [DOI] [PubMed] [Google Scholar]
- Matsuoka M, Narama M. Parents’ thoughts and perceptions on hearing that their child has incurable cancer. J Palliat Med 2012; 15(3): 340–346. [DOI] [PubMed] [Google Scholar]
- McCarthy MC, Clarke NE, Ting CL, et al. Prevalence and predictors of parental grief and depression after the death of a child from cancer. J Palliat Med 2010; 13(11): 1321–1326. [DOI] [PubMed] [Google Scholar]
- Medway M, Tong A, Craig JC, et al. Parental perspectives on the financial impact of caring for a child with CKD. Am J Kidney Dis 2015; 65: 384–393. [DOI] [PubMed] [Google Scholar]
- Menezes A. Moments of realization: life-limiting illness in childhood – perspectives of children, young people and families. Int J Palliat Nurs 2010; 16(1): 41–47. [DOI] [PubMed] [Google Scholar]
- Mitchell W, Sloper P. Making choices in my life: listening to the ideas and experiences of young people in the UK who communicate non-verbally. Child Youth Serv Rev 2011; 33: 521–527. [Google Scholar]
- Montoya-Juárez R, García-Caro MP, Schmidt-Rio-Valle J, et al. Suffering indicators in terminally ill children from the parental perspective. Eur J Oncol Nurs 2013; 17(6): 720–725. [DOI] [PubMed] [Google Scholar]
- Naftel RP, Tubergen E, Shannon CN, et al. Parental recognition of shunt failure: a prospective single-institution study Clinical article. J Neurosurg Pediatr 2012; 9: 363–371. [DOI] [PubMed] [Google Scholar]
- Nasr AS, Rehm RS. Parental live liver donation: a transformational experience. Prog Transplant 2014; 24(1): 69–75. [DOI] [PubMed] [Google Scholar]
- Paisley MA, Kang TI, Insogna IG, et al. Complementary and alternative therapy use in pediatric oncology Patients with failure of frontline chemotherapy. Pediatr Blood Cancer 2011; 56(7): 1088–1091. [DOI] [PubMed] [Google Scholar]
- Pousset G, Bilsen J, De Wilde J, et al. Attitudes of adolescent cancer survivors toward end-of-life decisions for minors. Pediatrics 2009; 124(6): E1142–E1148. [DOI] [PubMed] [Google Scholar]
- Price J, Jordan J, Prior L, et al. Comparing the needs of families of children dying from malignant and non-malignant disease: an in-depth qualitative study. BMJ Support Palliat Care 2012; 2(2): 127–132. [DOI] [PubMed] [Google Scholar]
- Pritchard M, Srivastava DK, Okuma JO, et al. Bereaved parents’ perceptions about when their child’s cancer-related death would occur. J Pain Symptom Manage 2009; 38(4): 561–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman B, Macciocca I, Sahhar M, et al. Adolescents with implantable cardioverter defibrillators: a patient and parent perspective. Pacing Clin Electrophysiol 2012; 35(1): 62–72. [DOI] [PubMed] [Google Scholar]
- Rapoport A, Shaheed J, Newman C, et al. Parental perceptions of forgoing artificial nutrition and hydration during end-of-life care. Pediatrics 2013; 131(5): 861–869. [DOI] [PubMed] [Google Scholar]
- Ravindran VP, Rempel GR. Grandparents and siblings of children with congenital heart disease. J Adv Nurs 2011; 67(1): 169–175. [DOI] [PubMed] [Google Scholar]
- Robert R, Zhukovsky DS, Mauricio R, et al. Bereaved parents’ perspectives on pediatric palliative care. J Soc Work End Life Palliat Care 2012; 8(4): 316–338. [DOI] [PubMed] [Google Scholar]
- Seth A, Gupta R, Chandra J, et al. Adherence to antiretroviral therapy and its determinants in children with HIV infection – experience from Paediatric Centre of Excellence in HIV Care in North India. AIDS Care 2014; 26: 865–871. [DOI] [PubMed] [Google Scholar]
- Tan JS, Docherty SL, Barfield R, et al. Addressing parental bereavement support needs at the end of life for infants with complex chronic conditions. J Palliat Med 2012; 15(5): 579–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson D, Hesser T, Ethier MC, et al. Complementary and alternative medicine use in pediatric cancer reported during palliative phase of disease. Support Care Cancer 2011; 19(11): 1857–1863. [DOI] [PubMed] [Google Scholar]
- Vles GF, Soudant DL, Hoving MA, et al. Long-term follow-up on continuous intrathecal Baclofen therapy in non-ambulant children with intractable spastic Cerebral Palsy. Eur J Paediatr Neurol 2013; 17(6): 639–644. [DOI] [PubMed] [Google Scholar]
- Von Lutzau P, Otto M, Hechler T, et al. Children dying from cancer: parents’ perspectives on symptoms, quality of life, characteristics of death, and end-of-life decisions. J Palliat Care 2012; 28(4): 274–281. [PubMed] [Google Scholar]
- Wells F, Ritchie D, McPherson AC. ‘It is life threatening but I don’t mind’. A qualitative study using photo elicitation interviews to explore adolescents’ experiences of renal replacement therapies. Child Care Health Dev 2013; 39: 602–612. [DOI] [PubMed] [Google Scholar]
- Yoshida S, Amano K, Ohta H, et al. A comprehensive study of the distressing experiences and dupport needs of parents of children with intractable cancer. Jpn J Clin Oncol 2014; 44: 1181–1188. [DOI] [PubMed] [Google Scholar]
- Yuen WY, Duipmans JC, Jonkman MF. The needs of parents with children suffering from lethal epidermolysis bullosa. Br J Dermatol 2012; 167: 613–618. [DOI] [PubMed] [Google Scholar]
- Zahmacioglu O, Yildiz CE, Koca B, et al. Coming from behind to win – a qualitative research about psychological conditions of adolescents who have undergone open-heart surgery for single ventricle between the ages 0–5. J Cardiothorac Surg 2011; 6: 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelcer S, Cataudella D, Cairney AEL, et al. Palliative care of children with brain tumors: a parental perspective. Arch Pediatr Adolesc Med 2010; 164(3): 225–230. [DOI] [PubMed] [Google Scholar]
- Love E, Schneiderman JE, Stephens D, et al. A cross-sectional study of overweight in pediatric survivors of acute lymphoblastic leukemia (ALL). Pediatr Blood Cancer 2011; 57(7): 1204–1209. [DOI] [PubMed] [Google Scholar]
Studies evaluating current practice
- Akard TF, Gilmer MJ, Miller K, et al. Factors affecting recruitment and participation of bereaved parents and siblings in grief research. Prog Palliat Care 2014; 22(2): 75–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akard TF, Wray S, Gilmer MJ. Facebook advertisements recruit parents of children with cancer for an online survey of web-based research preferences. Cancer Nurs 2015; 38: 155–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker JN, Leek AC, Salas HS, et al. Suggestions from adolescents, young adults, and parents for improving informed consent in phase 1 pediatric oncology trials. Cancer 2013; 119(23): 4154–4161. [DOI] [PubMed] [Google Scholar]
- Beslow LA, Ichord RN, Gindville MC, et al. Pediatric intracerebral hemorrhage score: a simple grading scale for intracerebral hemorrhage in children. Stroke 2014; 45(1): 66–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingen K, Kupst MJ, Himelstein B. Development of the palliative care parental self-efficacy measure. J Palliat Med 2011; 14(9): 1009–1016. [DOI] [PubMed] [Google Scholar]
- Bona K, Bates J, Wolfe J. Massachusetts’ Pediatric Palliative Care Network: successful implementation of a novel state-funded pediatric palliative care program. J Palliat Med 2011; 14: 1217–1223. [DOI] [PubMed] [Google Scholar]
- Caretti V, Jansen MH, van Vuurden DG, et al. Implementation of a multi-institutional diffuse intrinsic pontine glioma autopsy protocol and characterization of a primary cell culture. Neuropathol Appl Neurobiol 2013; 39: 426–436. [DOI] [PubMed] [Google Scholar]
- Darbyshire P, Cleghorn A, Downes M, et al. Supporting bereaved parents: a phenomenological study of a telephone intervention programme in a paediatric oncology unit. J Clin Nurs 2013; 22(3–4): 540–549. [DOI] [PubMed] [Google Scholar]
- Davies B, Contro N, Larson J, et al. Culturally-sensitive information-sharing in pediatric palliative care. Pediatrics 2010; 125(4): E859–E865. [DOI] [PubMed] [Google Scholar]
- Dickinson HO, Rapp M, Arnaud C, et al. Predictors of drop-out in a multi-centre longitudinal study of participation and quality of life of children with cerebral palsy. BMC Res Notes 2012; 5: 300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eilegard A, Steineck G, Nyberg T, et al. Bereaved siblings’ perception of participating in research – a nationwide study. Psychooncology 2013; 22(2): 411–416. [DOI] [PubMed] [Google Scholar]
- Gilmer MJ, Foster TL, Bell CJ, et al. Parental perceptions of care of children at end of life. Am J Hosp Palliat Care 2013; 30(1): 53–58. [DOI] [PubMed] [Google Scholar]
- Groh G, Borasio GD, Nickolay C, et al. Specialized pediatric palliative home care: a prospective evaluation. J Palliat Med 2013; 16: 1588–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon ME, Jacobs S, Briggs L, et al. Family-centered advance care planning for teens with cancer. JAMA Pediatr 2013; 167(5): 460–467. [DOI] [PubMed] [Google Scholar]
- Miller KS, Vannatta K, Vasey M, et al. Health literacy variables related to parents’ understanding of their child’s cancer prognosis. Pediatr Blood Cancer 2012; 59(5): 914–918. [DOI] [PubMed] [Google Scholar]
- Miller VA, Baker JN, Leek AC, et al. Patient involvement in informed consent for pediatric phase I cancer research. J Pediatr Hematol Oncol 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley TE, Cataudella D, Fernandez CV, et al. Development of the Pediatric Advanced Care Quality of Life Scale (PAC-QoL): evaluating comprehension of items and response options. Pediatr Blood Cancer 2014; 61(10): 1835–1839. [DOI] [PubMed] [Google Scholar]
- Olcese ME, Mack JW. Research participation experiences of parents of children with cancer who were asked about their child’s prognosis. J Palliat Med 2012; 15(3): 269–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg AR, Dussel V, Orellana L, et al. What’s missing in missing data? Omissions in survey responses among parents of children with advanced cancer. J Palliat Med 2014; 17: 953–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele R, Cadell S, Siden H, et al. Impact of research participation on parents of seriously Ill children. J Palliat Med 2014; 17(7): 788–796. [DOI] [PubMed] [Google Scholar]
- Stevens MM, Lord BA, Proctor M-T, et al. Research with vulnerable families caring for children with life-limiting conditions. Qual Health Res 2010; 20(4): 496–505. [DOI] [PubMed] [Google Scholar]
- Van der Geest IMM, Darlington ASE, Streng IC, et al. Parents’ experiences of pediatric palliative care and the impact on long-term parental grief. J Pain Symptom Manage 2014; 47(6): 1043–1053. [DOI] [PubMed] [Google Scholar]
- Walsh KE, Roblin DW, Weingart SN, et al. Medication errors in the home: a multisite study of children with cancer. Pediatrics 2013; 131(5): E1405–E1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiener L, Sweeney C, Baird K, et al. What do parents want to know when considering autopsy for their child with cancer? J Pediatr Hematol Oncol 2014; 36: 464–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff J, Robert R, Sommerer A, et al. Impact of a pediatric palliative care program. Pediatr Blood Cancer 2010; 54: 279–283. [DOI] [PubMed] [Google Scholar]
Footnotes
Author contribution: B.F.H. designed and ran the search strategy, screened the articles, extracted data, analysed the results, drafted and approved the final manuscript as submitted. L.J.M.O and B.C. designed the search strategy, screened the articles, extracted data, analysed the results, reviewed the manuscript and approved the final manuscript as submitted. V.V. designed the search strategy, extracted data and approved the final manuscript as submitted. L.J., M.L., M.B-L. and P.S. conceptualized the study, reviewed the manuscript and approved the final manuscript as submitted.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: B.F.H.’s post is supported by The Health Foundation (grant code G25 512881 2LAAB), L.J.M.O.’s post is supported by Great Ormond Street Children’s Charity (G25 513947 2LGC), M.B.-L.’s post is supported by funding from The True Colors Trust (grant code G25 511830 2LGA), M.L. was supported by the National Institute for Health Research (NIHR) Collaboration for Leadership in Applied Health Research and Care (CLAHRCs) North Thames at Bart’s Health NHS Trust. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health. B.C., V.V., L.J. and P.S.’ posts are supported by Marie Curie core funding to the Marie Curie Palliative Care Research Department, UCL, grant MCCC-FCO-11-U.
References
- 1. Fraser LK, Miller M, Hain R, et al. Rising national prevalence of life-limiting conditions in children in England. Pediatrics 2012; 129(4): e923–e929. [DOI] [PubMed] [Google Scholar]
- 2. Baum D. A guide to the development of children’s palliative care services (Report of a Joint Working Party of the Association for Children with Life-Threatening or Terminal Conditions and Their Families and the Royal College of Paediatrics and Child Health). Bristol: Association for Children with Life-Threatening or Terminal Conditions and their Families, 1997. [Google Scholar]
- 3. Keeley PW. Improving the evidence base in palliative medicine: a moral imperative. J Med Ethics 2008; 34(10): 757–760. [DOI] [PubMed] [Google Scholar]
- 4. Duke S, Bennett H. Review: a narrative review of the published ethical debates in palliative care research and an assessment of their adequacy to inform research governance. Palliat Med 2010; 24(2): 111–126. [DOI] [PubMed] [Google Scholar]
- 5. Addington-Hall J. Research sensitivities to palliative care patients. Eur J Cancer Care 2002; 11(3): 220–224. [DOI] [PubMed] [Google Scholar]
- 6. Steele R, Cadell S, Siden H, et al. Impact of research participation on parents of seriously ill children. J Palliat Med 2014; 17(7): 788–796. [DOI] [PubMed] [Google Scholar]
- 7. Hinds PS, Burghen EA, Pritchard M. Conducting end-of-life studies in pediatric oncology. Western J Nurs Res 2007; 29(4): 448–465. [DOI] [PubMed] [Google Scholar]
- 8. Ewing G, Rogers M, Barclay S, et al. Recruiting patients into a primary care based study of palliative care: why is it so difficult? Palliat Med 2004; 18(5): 452–459. [DOI] [PubMed] [Google Scholar]
- 9. Stevens MM, Lord BA, Proctor M-T, et al. Research with vulnerable families caring for children with life-limiting conditions. Qual Health Res 2010; 20(4): 496–505. [DOI] [PubMed] [Google Scholar]
- 10. Tomlinson D, Bartels U, Hendershot E, et al. Challenges to participation in paediatric palliative care research: a review of the literature. Palliat Med 2007; 21(5): 435–440. [DOI] [PubMed] [Google Scholar]
- 11. Hynson JL, Aroni R, Bauld C, et al. Research with bereaved parents: a question of how not why. Palliat Med 2006; 20(8): 805–811. [DOI] [PubMed] [Google Scholar]
- 12. Scott DA, Valery PC, Boyle FM, et al. Does research into sensitive areas do harm? Experiences of research participation after a child’s diagnosis with Ewing’s sarcoma. Med J Aust 2002; 177(9): 507–510. [DOI] [PubMed] [Google Scholar]
- 13. Olcese ME, Mack JW. Research participation experiences of parents of children with cancer who were asked about their child’s prognosis. J Palliat Med 2012; 15(3): 269–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hellems MA, Gurka KK, Hayden GF. A review of the Journal of Pediatrics: the first 75 years. J Pediatr 2009; 155(1): 16–20. [DOI] [PubMed] [Google Scholar]
- 15. Kumar SP. Reporting of pediatric palliative care: a systematic review and quantitative analysis of research publications in palliative care journals. Indian J Palliat Care 2011; 17(3): 202–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Modi N, Vohra J, Preston J, et al. Guidance on clinical research involving infants, children and young people: an update for researchers and research ethics committees. Arch Dis Child 2014; 99: 887–891. [DOI] [PubMed] [Google Scholar]
- 17. Nuffield Council on Bioethics. Children and clinical research: ethical issues. Nuffield Council on Bioethics: London, 2015. [Google Scholar]
- 18. Medical Research Council (MRC). MRC ethics guide. London: MRC, 2004. [Google Scholar]
- 19. Royal College of Paediatrics, Child Health: Ethics Advisory Committee. Guidelines for the ethical conduct of medical research involving children. Arch Dis Child 2000; 82(2): 177–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bluebond-Langner M, Belasco JB, Goldman A, et al. Understanding parents’ approaches to care and treatment of children with cancer when standard therapy has failed. J Clin Oncol 2007; 25(17): 2414–2419. [DOI] [PubMed] [Google Scholar]
- 21. Higginson I, Evans C, Grande G, et al. Evaluating complex interventions in end of life care: the MORECare Statement on good practice generated by a synthesis of transparent expert consultations and systematic reviews. BMC Med 2013; 11(1): 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gross CP, Mallory R, Heiat A, et al. Reporting the recruitment process in clinical trials: who are these patients and how did they get there? Ann Intern Med 2002; 137(1): 10–16. [DOI] [PubMed] [Google Scholar]
- 23. Hain R, Devins M, Hastings R, et al. Paediatric palliative care: development and pilot study of a ‘Directory’ of life-limiting conditions. BMC Palliat Care 2013; 12(1): 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Beecham E, Candy B, Howard R, et al. Pharmacological interventions for pain in children and adolescents with life-limiting conditions. Cochrane Database Syst Rev 2015; 3, http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD010750.pub2/abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bluebond-Langner M, Beecham E, Candy B, et al. Preferred place of death for children and young people with life-limiting and life-threatening conditions: a systematic review of the literature and recommendations for future inquiry and policy. Palliat Med 2013; 27(8): 705–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Thomas J, Harden A. Methods for the thematic synthesis of qualitative research in systematic reviews. BMC Med Res Methodol 2008; 8: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Langford R, Bonell C, Jones H, et al. Obesity prevention and the Health promoting Schools framework: essential components and barriers to success. Int J Behav Nutr Phys Act 2015; 12(1): 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Eilegard A, Steineck G, Nyberg T, et al. Bereaved siblings’ perception of participating in research – a nationwide study. Psychooncology 2013; 22(2): 411–416. [DOI] [PubMed] [Google Scholar]
- 29. Robert R, Zhukovsky DS, Mauricio R, et al. Bereaved parents’ perspectives on pediatric palliative care. J Soc Work End Life Palliat Care 2012; 8(4): 316–338. [DOI] [PubMed] [Google Scholar]
- 30. Mitchell W, Sloper P. Making choices in my life: listening to the ideas and experiences of young people in the UK who communicate non-verbally. Children Youth Serv Rev 2011; 33: 521–527. [Google Scholar]
- 31. Gaab EM, Owens RG, MacLeod RD. The voices of young New Zealanders involved in pediatric palliative care. J Palliat Care 2013; 29: 186–192. [PubMed] [Google Scholar]
- 32. Bradford N, Young J, Armfield NR, et al. A pilot study of the effectiveness of home teleconsultations in paediatric palliative care. J Telemed Telecare 2012; 18(8): 438–442. [DOI] [PubMed] [Google Scholar]
- 33. Caretti V, Jansen MH, van Vuurden DG, et al. Implementation of a multi-institutional diffuse intrinsic pontine glioma autopsy protocol and characterization of a primary cell culture. Neuropathol Appl Neurobiol 2013; 39: 426–436. [DOI] [PubMed] [Google Scholar]
- 34. APA Publications and Communications Board Working Group on Journal Article Reporting Standards. Reporting standards for research in psychology: why do we need them? What might they be? Am Psychol 2008; 63(9): 839–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Altman DG, Schulz KF, Moher D, et al. The revised CONSORT statement for reporting randomized trials: explanation and elaboration. Ann Intern Med 2001; 134(8): 663–694. [DOI] [PubMed] [Google Scholar]
- 36. Des Jarlais DC, Lyles C, Crepaz N. Improving the reporting quality of nonrandomized evaluations of behavioral and public health interventions: the TREND statement. Am J Public Health 2004; 94(3): 361–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 2007; 45(4): 247–251. [DOI] [PubMed] [Google Scholar]
- 38. Gysels M, Evans CJ, Lewis P, et al. MORECare research methods guidance development: recommendations for ethical issues in palliative and end-of-life care research. Palliat Med 2013; 27(10): 908–917. [DOI] [PubMed] [Google Scholar]
- 39. Brooks R, Higgins G, Webster A. Systematic review of randomized controlled trial quality in pediatric kidney transplantation. Pediatr Nephrol 2010; 25(12): 2383–2392. [DOI] [PubMed] [Google Scholar]
- 40. DeMauro SB, Giaccone A, Kirpalani H, et al. Quality of reporting of neonatal and infant trials in high-impact journals. Pediatrics 2011; 128(3): e639–e644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sutcliffe K, O’Mara A, Caird J, et al. Paediatric medication error: a systematic review of the extent and nature of the problem in the UK and international interventions to address it. London: EPPI-Centre, Social Science Research Unit, UCL Institute of Education, University of London, 2014. [Google Scholar]