Abstract
Background:
Refractory breathlessness in advanced chronic disease leads to high levels of disability, anxiety and social isolation. These result in high health-resource use, although this is not quantified.
Aims:
To measure the cost of care for patients with advanced disease and refractory breathlessness and to identify factors associated with high costs.
Design:
A cross-sectional secondary analysis of data from a randomised controlled trial.
Setting/participants:
Patients with advanced chronic disease and refractory breathlessness recruited from three National Health Service hospitals and via general practitioners in South London.
Results:
Of 105 patients recruited, the mean cost of formal care was £3253 (standard deviation £3652) for 3 months. The largest contributions to formal-care cost were hospital admissions (>60%), and palliative care contributed <1%. When informal care was included, the total cost increased by >250% to £11,507 (standard deviation £9911). Increased patient disability resulting from breathlessness was associated with high cost (£629 per unit increase in disability score; p = 0.006). Increased breathlessness on exertion and the presence of an informal carer were also significantly associated with high cost. Patients with chronic obstructive pulmonary disease tended to have higher healthcare costs than other patients.
Conclusion:
Informal carers contribute significantly to the care of patients with advanced disease and refractory breathlessness. Disability resulting from breathlessness is an important clinical cost driver. It is important for policy makers to support and acknowledge the contributions of informal carers. Further research is required to assess the clinical- and cost-effectiveness of palliative care interventions in reducing disability resulting from breathlessness in this patient group.
Keywords: Healthcare costs, palliative care, terminal care, end-of-life care, chronic disease, breathlessness, dyspnoea, cancer, chronic obstructive pulmonary disease, heart failure, interstitial lung diseases
What is already known about the topic?
Refractory breathlessness in advanced chronic disease leads to high levels of disability, anxiety and social isolation.
These result in high health-resource use, although this is not quantified.
We also do not know what factors are associated with high cost in these patients.
What this paper adds?
The largest contributions to formal-care cost were hospital admissions (>60%), while palliative care contributed <1%
The total cost of care increased by >250% when informal-care costs were included.
Increased patient disability resulting from breathlessness was the main clinical factor associated with increased cost.
Implications for practice, theory or policy
Our findings highlight the need for health and social care policy makers to support and acknowledge the contributions of informal carers.
Further research is required to assess the clinical- and cost-effectiveness of palliative care interventions in reducing disability resulting from breathlessness in patients with advanced disease and refractory breathlessness.
Introduction
Breathlessness is a common and distressing symptom affecting many people with advanced chronic disease. It causes substantial disability, anxiety and social isolation and is difficult to treat.1–4 Globally, over 75 million people have breathlessness annually, including over 58 million people with severe lung disease,5 more than 5 million people with incurable cancer and more than 12 million people with heart failure.6,7
The high symptom burden and progressive nature of refractory breathlessness in advanced disease suggest that patients will require increasing help from professionals and family members. As the underlying disease progresses, breathlessness increases and is accompanied by panic.8,9 This is terrifying for patients and their families and often results in emergency hospital admissions.9 Despite the concerns about the large resource inputs for these patients, there is no evidence on the costs of care for them. Thus, it is important to establish the costs of caring for people with advanced disease and refractory breathlessness. Such information is vital to understand the likely ‘burden’ of the condition and to provide a baseline to assess the relative economic impact of different treatments. It is conceivable that costs will arise as a result of direct healthcare input and care provided by other agencies, such as social care services. Some unpaid care will also frequently be provided by family members and/or friends (informal care), and this has never been quantified.
The aims of this study were to measure the formal and informal costs of care for patients with advanced disease and refractory breathlessness and to identify patient and clinical factors associated with high costs.
Materials and methods
Design
This study is a cross-sectional secondary analysis of data from a randomised controlled parallel-group, pragmatic, single-blind fast-track trial (ClinicalTrials.gov, number NCT01165034).4 The main study, and also this study, had ethical approval from the King’s College Hospital Ethics Committee (Ref. 10/H0808/17). Participants provided their written consent to participate in the main study. The data set used for this cross-sectional secondary analysis was anonymised and de-identified prior to analysis. This procedure was approved by the Ethics Committee.
Setting and sample
Study participants were included according to a standard proforma completed by the identifying clinician. Details of the trial have been published elsewhere.4 But in summary, participants were adult patients (⩾18 years) with advanced disease (e.g. cancer, chronic obstructive pulmonary disease (COPD), chronic heart failure, interstitial lung disease (ILD) and motor neuron disease) and refractory breathlessness on exertion or rest (Medical Research Council (MRC) dyspnoea scale score ⩾2), despite optimum treatment of the underlying disease, as deemed by the identifying clinician. Participants were recruited from three large teaching hospitals and via general practitioners (GPs) in South London.
Procedures
In the main study, data were collected at study entry by trained interviewers, usually in participants’ homes. The data set used for this cross-sectional secondary analysis was collected during the main trial. This data set was anonymised and de-identified prior to analysis and comprises demographic, clinical outcome assessments and use of healthcare services including the Chronic Respiratory Disease Questionnaire (CRQ);10,11 Numerical Rating Scale (NRS), average, at rest and on exertion;12 London Chest Activity of Daily Living (LCADL), a questionnaire of the level of disability induced by breathlessness;12 EuroQol five dimensions questionnaire (EQ-5D) (three levels);12 Palliative care Outcome Scale (POS), a 10-item measure for advanced disease widely validated in cancer and non-cancer;13 Hospital Anxiety and Depression Scale (HADS);14 and the breathlessness version of the Client Services Receipt Inventory15 (CSRI) which has been provided in Supplementary File S1. Details of other procedures conducted in the main trial have been published elsewhere.4
Clinical outcomes
Study outcomes included breathlessness mastery (using a sub-domain of the CRQ); severity of breathlessness on exertion in the previous 24 h, disability (LCADL); health-related quality of life (EQ-5D); palliative needs (POS); breathlessness, fatigue and emotional function (other domains of the CRQ); anxiety and depression (HADS) and lung function (spirometer).
Service use and cost
A broad costing perspective was taken with services including those provided by health and social care agencies and also informal carers. However, societal costs were not calculated as this analysis did not include lost productivity. In this analysis, formal care comprised both direct health care and social care. Service use was measured at study entry using a version of the Client Service Receipt Inventory (CSRI).15 Patients gave details of services used during the 3 months prior to study entry.
Services included hospital care, primary health care, social care, the provision of aids and home adaptations and informal care provided by family members and/or friends. Length of hospital admission was recorded, while the number of contacts and, where relevant, the mean length of these contacts were documented for other services. Information was provided on the number of hours that family/friends spent providing personal care and help in and outside the home and in other tasks, per week.
Costs were calculated by combining resource use data with unit costs obtained from standard sources such as the National Health Service (NHS) reference cost data16 or the Unit Costs of Health and Social Care (Personal Social Services Research Unit (PSSRU)),17 where applicable. We assumed that in the absence of an informal carer, social services would need to provide home care, and therefore, the unit cost of a home care worker was used as proxy for informal care. Missing values for quantities of resource use were imputed using multiple imputation by chained equations (MICE) via the ‘ice’ command in Stata.18,19
Statistical analysis
We compared formal and informal health-resource use and cost across primary diagnosis using descriptive statistics. We used scatter and box plots to graphically examine variations in costs and Spearman’s Rho to assess correlations.
We used generalised linear models (GLM) to determine which factors were associated with cost. The dependent variable was cost, while clinical and demographic variables were used as independent variables. Age and EQ-5D index were centred to means to enable appropriate interpretation of intercepts. Spearman’s rank correlations of the independent variables were used to determine whether any variables were highly correlated and therefore not recommended for inclusion in the same regression model. A high correlation was defined as a correlation coefficient >|0.7|.20 Where two or more independent variables were found to be highly correlated, we conducted univariate analysis with the dependent variable and selected the independent variable with highest adjusted R2. Separate regression models were fitted for total cost, health service cost and informal care cost. We used the modified Park test21 and the modified Hosmer–Lemeshow test22 to determine the appropriate combination of link function and distributional family. The analysis was performed using STATA release 13.23 This study was reported according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement for cross-sectional studies.
Regression equation
where is the linear predictor formed from explanatory variables and coefficients. follows gamma distribution which has an increasing variation with larger mean. , where g is the link function.
Results
In all, 105 patients were recruited. The mean age was 67 years, 61 (58%) were men, over half (54%) had COPD, 20% had cancer, 18% had ILD, 5% had heart failure and 3% had other diseases. Patients had severe disease: forced expiratory volume (FEV1) was 46% predicted, vital capacity (VC) 58% predicted, oxygen saturation at rest 93%, average breathlessness 5.9/10 and on exertion 8.3/10. Their mean EQ-5D index score was 0.35, and their average total Palliative care Outcome Score was 15/40, indicating a disabled group with poor quality of life (see Table 1).
Table 1.
Characteristics of the study participants at study entry.
| N = 105 | |
|---|---|
| Age (years) | 67 (10) |
| Sex | |
| Women | 44 (42%) |
| Men | 61 (58%) |
| Diagnosis | |
| Chronic obstructive pulmonary disease | 57 (54%) |
| Cancer | 21 (20%) |
| Interstitial lung disease | 19 (18%) |
| Heart failure | 5 (5%) |
| Othera | 3 (3%) |
| Has carer or family member | |
| Yes | 75 (71%) |
| No | 30 (29%) |
| Living situation | |
| Living home | 97 (92%) |
| Living elsewhere | 8 (8%) |
| EQ-5D indexb | 0.35 (0.33) |
| Clinical variables | |
| Saturated O2 (%)c | 93.6 (3.9) |
| POS total score (score range = 0–40)d | 15.1 (6.5) |
| HADS anxiety (score range = 0–21)d | 9.2 (2.7) |
| HADS depression (score range = 0–21)d | 9.9 (3.2) |
| NRS breathlessness worst at rest (0–10)d | 4.9 (2.6) |
| Predicted FEV1 (% predicted)e | 46.2 (23.3) |
| Predicted VC (% predicted)e | 57.9 (25.7) |
| NRS breathlessness on exertion (0–10)1 | 8.3 (1.4) |
| NRS breathlessness average 24 h (0–10)1 | 5.9 (2.0) |
SD: standard deviation; NRS: Numerical Rating Scale; FEV1: forced expiratory volume; VC: vital capacity; HADS: Hospital Anxiety and Depression Scale; POS: Palliative care Outcome Scale; EQ-5D: EuroQol five dimensions questionnaire.
Data are absolute numbers or mean (SD) unless otherwise stated.
Other diagnoses were left lower lobe collapse of unknown aetiology associated with severe symptoms; lupus, shrinking lung syndrome and rheumatoid arthritis; and severe asthma and gastro-oesophageal reflux disease.
EQ-5D index scores based on the standard UK population-based preference weights with the standard scoring algorithm; 0.0 = death and 1.0 = perfect health.
Measured for 13 patients (3 in breathlessness support service group and 10 in control group) while on supplemental oxygen (mean (SD) SaO2 = 91.8 (5.1)) and the remainder on room air (mean (SD) 93.8 (3.6)).
Scale interpretation: high score indicates worse.
Scale interpretation: high score indicates better.
The results show high levels of outpatient visits, GP contacts, inpatient care and accident and emergency attendance. More than 75% received care from family and friends (Table 2). The mean total cost of care per patient was £11,507 (standard deviation (SD) £9911). Informal care accounted for over 70% of this.
Table 2.
Cost of breathlessness for 3 months prior to recruitment into the study.
| Contacts (users) |
Costsa |
|||||||
|---|---|---|---|---|---|---|---|---|
| N | % | Mean | SD | Min | Max | Mean | SD | |
| Health care | ||||||||
| Inpatient care | 43 | 41 | 10.5b | 7.44 | 0 | 11,352 | 1294 | 2131 |
| Critical care | 6 | 6 | 4.2b | 3.13 | 0 | 11,890 | 286 | 1420 |
| A&E | 39 | 38 | 1.7 | 1.14 | 0 | 649 | 67 | 115 |
| Outpatient | 75 | 72 | 2.8 | 2.47 | 0 | 1330 | 236 | 271 |
| Outpatient otherc | 33 | 32 | 3.4 | 4.18 | 0 | 1781.25 | 89 | 234 |
| Other doctor | 4 | 4 | 1.5 | 1.00 | 0 | 222 | 4 | 26 |
| Day hospital | 7 | 7 | 4.4 | 2.44 | 0 | 1120 | 42 | 177 |
| Hospice | 1 | 1 | 1.0 | 0.00 | 0 | 132 | 1 | 13 |
| District nurse | 28 | 27 | 12.0 | 35.41 | 0 | 9450 | 168 | 959 |
| GP | 84 | 81 | 3.2 | 3.22 | 0 | 1039 | 130 | 165 |
| Practice nurse | 15 | 14 | 2.0 | 1.51 | 0 | 61 | 3 | 9 |
| Physiotherapist | 15 | 14 | 7.9 | 7.26 | 0 | 396 | 19 | 64 |
| Occupational therapist | 8 | 8 | 1.6 | 1.04 | 0 | 132 | 4 | 16 |
| Psychologist | 11 | 11 | 8.7 | 17.33 | 0 | 8160 | 126 | 821 |
| Other therapies | 5 | 5 | 2.8 | 1.92 | 0 | 150 | 4 | 23 |
| Rehab (pulmonary) | 12 | 12 | 6.2 | 4.92 | 0 | 1890 | 78 | 300 |
| Rehab (other) | 7 | 7 | 4.6 | 3.82 | 0 | 895 | 23 | 110 |
| Home palliative care | 16 | 16 | 3.6 | 4.70 | 0 | 504 | 15 | 62 |
| Dietician | 11 | 11 | 1.7 | 0.71 | 0 | 68 | 3 | 11 |
| Other servicesd | 13 | 13 | 15.6 | 27.40 | 0 | 1500 | 35 | 167 |
| Total healthcare cost (A) | 53 | 22,178 | 2624 | 3456 | ||||
| Social care | ||||||||
| Social worker | 7 | 7 | 1.4 | 0.79 | 0 | 222 | 8 | 32 |
| Home help | 15 | 15 | 24.8 | 29.44 | 0 | 4860 | 94 | 504 |
| Walking aid | 43 | 43 | 1 | N/A | 3 | 8 | ||
| Wheelchair | 26 | 25 | 1 | N/A | 104 | 238 | ||
| Commode | 12 | 12 | 1 | N/A | 3 | 7 | ||
| Special bed | 14 | 13 | 1 | N/A | 327 | 863 | ||
| Bathroom/toilet adaptations | 45 | 43 | 1 | N/A | 15 | 73 | ||
| Other equipment | 51 | 49 | 1.7 | 1.79 | 0 | 1000 | 75 | 240 |
| Total social care cost (B) | 0 | 5793 | 628 | 1132 | ||||
| Total formal service cost (A + B) | 139 | 22,573 | 3253 | 3652 | ||||
| Informal caree | ||||||||
| Hours on call | 51 | 49 | 149 | 48.32 | 0 | 12,479 | 5427 | 6096 |
| Help outside homef | 78 | 75 | 6.6 | 5.55 | 0 | 2228 | 366 | 415 |
| Help with medical procedures | 32 | 31 | 3.5 | 3.04 | 0 | 4200 | 326 | 696 |
| Help inside the homeg | 81 | 78 | 18 | 36.46 | 0 | 20,798 | 1027 | 2438 |
| Personal careh | 36 | 35 | 13 | 25.81 | 0 | 30,240 | 966 | 3400 |
| Other help | 6 | 6 | 33 | 66.17 | 0 | 12,479 | 141 | 1226 |
| Total informal care cost (C) | 0 | 43,536 | 8254 | 8777 | ||||
| Total costs (A + B + C) | 154 | 45,818 | 11,507 | 9911 | ||||
SD: standard deviation; GP: general practitioner; A&E: accident and emergency.
2014 GBP.
Number of days on admission.
Outpatient visits are not directly related with breathlessness or main disease.
Services such as bereavement service, meals, exercise and Red Cross.
Average number of hours used by friends or family members.
For example, shopping or going to the appointments.
For example, cooking or cleaning.
For example, bathing or dressing.
Over 64% of informal care cost was accounted for by the number of hours: on call (45%), providing help inside the home (9%) and providing personal care (8%). Over 60% of direct healthcare cost was accounted for by hospital admissions (inpatient care 49.3% and critical care 11%). Palliative care (home palliative care and hospice) accounted for less than 1% of direct healthcare cost, and rehabilitation and other therapies accounted for less than 5% (Table 2). Over 68% of social care cost was spent on providing special beds (52%) and wheel chairs (17%).
Overall, there was a high variation in cost with more variation in the cost of informal care when compared to formal care, across all diagnoses. The variability in healthcare cost was largest for COPD patients (range: £53–£22,178), whereas cancer patients had the highest variability in informal care cost (range: £0–£43,536) (Figure 1).
Figure 1.
Box plots showing medians and 25th and 75th percentiles of healthcare versus informal care cost by diagnosis (number of observations in brackets).
We found no correlation between direct healthcare and informal care costs (Figure 2).
Figure 2.
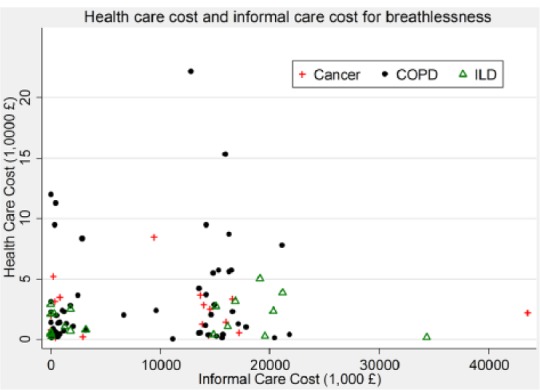
Relationship between healthcare cost and informal care cost.
The following independent variables were found to be highly correlated (Spearman’s Rho >0.78): three variables FEV1 percent predicted, VC percent predicted and CRQ dyspnoea and also between NRS average, NRS breathlessness at rest and NRS on exertion. FEV1 percent predicted and NRS on exertion were selected as they each had higher adjusted R2 values in univariate analysis with cost.
The results of GLM regression showed that disability resulting from breathlessness was significantly associated with high total cost (£629 per unit increase in LCADL total score; p = 0.006) as well as informal and direct healthcare costs (Table 3). The presence of an informal carer was also significantly associated with high total and informal-care cost. Increased breathlessness on exertion was associated with increased healthcare cost; no other clinical variable was significantly associated with cost. Patients with COPD tended to have higher healthcare costs than patients with cancer, ILD or heart failure (Table 3).
Table 3.
Factors associated with costs of breathlessness (results from generalised linear model regression).
| N = 105 | Total cost (£)a |
Informal care cost (£)a |
Healthcare cost (£)a |
||||||
|---|---|---|---|---|---|---|---|---|---|
| β | CILL | CIUL | β | CILL | CIUL | β | CILL | CIUL | |
| EQ-5D index (centred at mean) | 5923 | −8647 | 20,493 | −6118 | −13,171 | 935 | −3070 | −6951 | 811 |
| HADS depression (=1 if high) | 578 | −8971 | 10,127 | −3741 | −14,085 | 6604 | −1417 | −3821 | 988 |
| HADS anxiety (=1 if high) | −1910 | −12,194 | 8373 | −3788 | −11,859 | 4283 | −1823 | −3703 | 56 |
| LCADL total score | 629** | 180 | 1079 | 411* | 61 | 760 | 635* | 85 | 1186 |
| POS total score | −366 | −1467 | 736 | −24 | −817 | 769 | −82 | −232 | 68 |
| CRQ mastery | −5713 | −11,833 | 407 | −2569 | −5525 | 388 | −226 | −963 | 511 |
| CRQ fatigue | −2062 | −6785 | 2661 | −2148 | −5429 | 1133 | −118 | −751 | 515 |
| Has carer (=1 if yes) | 13,650** | 6793 | 20,507 | 12,345** | 8512 | 16,178 | −492 | −2427 | 1442 |
| FEV1 (% predicted) | −40 | −217 | 136 | 22 | –91 | 135 | −21 | −48 | 5 |
| NRS breathless on exertion | 700 | −2296 | 3696 | 944 | −2029 | 3917 | 699* | 243 | 1155 |
| Age (centred at mean) | −165 | −477 | 147 | −76 | −236 | 85 | −36 | −87 | 16 |
| Female (=1 if yes) | −2602 | −11,376 | 6172 | −1915 | −8627 | 4798 | 496 | −693 | 1685 |
| Living situation (ref: lives at home) | 45,980 | −42,090 | 134,049 | 16,844 | −9906 | 43,595 | 10,771 | −13,911 | 35,452 |
| Cohabitee (ref.: wife/husband) | |||||||||
| Partner | −12,376 | −24,846 | 93 | -8340** | −14,842 | −1837 | −587 | −3205 | 2031 |
| Son/daughter | −2271 | −14,876 | 10,334 | −2287 | −10,334 | 5760 | −223 | −2132 | 1687 |
| Other | −2851 | −11,257 | 5554 | 1260 | −7708 | 10,227 | −689 | −2373 | 994 |
| Diagnosis (ref: COPD) | |||||||||
| Cancer | −3098 | −8845 | 2648 | −1224 | −4848 | 2401 | −1474** | −2397 | −551 |
| Interstitial lung disease | −5508 | −11,221 | 205 | −2480 | −8895 | 3935 | −2256* | −3862 | −650 |
| Heart failure | 37,077 | −1984 | 76,138 | 41,871 | −1352 | 85,094 | −2297** | −3452 | −1143 |
| Other | 19,145 | −3706 | 41,997 | 13,659 | −3734 | 31,051 | −831 | −2731 | 1069 |
| Constant | 1057** | 81 | 13,837 | 1** | 0 | 4076 | 217,597** | 10,523 | 4,499,493 |
| Link function | Log | Log | Log | ||||||
| Family | Gamma | Gaussian | Poisson | ||||||
| Prob. H.L.b | 0.49 | 0.082 | 0.397 | ||||||
EQ-5D: EuroQol five dimensions questionnaire; HADS: Hospital Anxiety and Depression Scale; POS: Palliative care Outcome Scale; FEV1: forced expiratory volume; NRS: Numerical Rating Scale; LCADL: London Chest Activity of Daily Living; CRQ: Chronic Respiratory Disease Questionnaire; COPD: chronic obstructive pulmonary disease; CILL: lower limit of confidence interval; CIUL: upper limit of confidence interval.
2014 GBP.
The p value for the modified Hosmer–Lemeshow test.
p < 0.05; **p < 0.005.
Discussion
This is the first study that examines the cost of care in patients with advanced disease and refractory breathlessness. Over a period of 3 months, hospital admissions (inpatient and critical-care stays) had the largest contribution to direct healthcare cost (>60%), whereas palliative care and rehabilitation accounted for less than 1% and 5%, respectively. High healthcare cost was associated with increasing disability resulting from breathlessness, breathlessness on exertion and a diagnosis of COPD. When informal care was included, the total cost of care increased by >250%. We also found wide variations in cost.
In these 105 patients with advanced disease and refractory breathlessness, we found that the cost of formal care was £3253 (equivalent to US$4593.70: using 2014 Organisation for Economic Co-operation and Development (OECD) purchasing power parities (PPP) conversion rate) over a period of 3 months. This was mainly accounted for by inpatient care, critical-care and outpatient clinics. It is difficult to compare our estimates with other studies (or patients) because no other studies have examined the cost of care in patients with advanced disease and refractory breathlessness. Nevertheless, the cost estimates we observed appear to be higher than the costs observed in studies in people with COPD. In the 2011 systematic review by Bustacchini et al.,24 which explored the economic burden of COPD in those aged 60 years or older, mean healthcare costs at 3 months ranged from £540.98 (US$763.94) in the United States to £2535.69 (US$3580.75) in Australia (inflated for the purpose of this comparison from 2010 US$ to 2014 GBP using standard conversion formulae). Inpatient care was the main driver of healthcare cost in the Bustacchini et al.24 review.
Our study suggests that the cost of formal care for people with advanced disease and refractory breathlessness is very high (more expensive than the cost of COPD). This perhaps is a reflection of the high cost of care in the last year of life; 25% of annual Medicare hospital costs are spent on people in the last year of life in the United States, while in the United Kingdom, up to 29% of NHS hospital expenditures are for people who are in the last year of life.25
This is the first study to quantify the informal care cost of advanced disease and refractory breathlessness. We found that the cost of informal care in our study was high. Including informal care increased the total cost per patient by over 250% from £3253 (US$4593.70) to £11,507 (US$16,249.52). Informal care was mainly accounted for by the time spent supervising a patient (hours on call), and to a lesser extent, bathing, dressing, cooking and cleaning. This highlights the importance of informal care particular in advanced disease. It is essential to account for informal care cost because in the absence of informal carers, possibly the same amount of care would need to be provided by formal carers via health or social care services. For example, certain patients (e.g. patients on medical equipment like tracheostomies or non-invasive ventilation or at risk of falls) will require 24-h supervision. In the United Kingdom, if such patients do not have informal carers, then often they are cared for in care-homes where they are supervised by formal carers, and the health service bears the cost. However, it is conceivable that the time spent on supervision reported in this study for some patients may have been more than what would have been required had such supervision been provided by formal carers. Nevertheless, if the cost of supervising patients was completely excluded from our analysis, informal care would still account for more than 50% of the total cost of care. Conversely, it is possible that informal carers may have underestimated how much time they spent supervising. If cost of supervision was assumed to be at the maximum for all carers (£12,479), then informal care cost would account for more than 82% of the total cost of care per patient.
High healthcare cost was associated with increasing disability resulting from breathlessness and breathlessness on exertion. Also, the healthcare cost for COPD was significantly more than lung cancer, ILD. Our results are supported by evidence from several studies on COPD patients which suggest that increased disability and poor physical performance are both independently associated with increased healthcare utilisation, particularly the risk of hospital re-admission.26–32 Further research is required to assess the effectiveness and cost-effectiveness of palliative care interventions in reducing disability resulting from breathlessness in patients with advanced disease and refractory breathlessness.
The presence of a career was found to be associated with high cost (both total and informal-care costs) as is to be expected. Also, we found a high variation in cost with more variation in the cost of informal care when compared to formal care. We cannot explain this high variation based on our data. However, it is conceivable that such variation is either due to random variation or, more likely, due to specific factors that we have not measured. We believe palliative care may reduce some of this variation. Palliative care has been shown to decrease rates of emergency department attendance33 and length of hospital stay and to increase home-death rates, quality of life and possibly survival in patients with advanced illness.4
Our study has limitations. First, we did not account for drugs in our estimates but assumed that these are included in the cost of care, and so, we may have underestimated the full cost of care. Studies in elderly patients with COPD suggest that pharmaceutical agents may have a moderate to high impact on costs.24 Second, we did not obtain resource use estimates from medical records but rather relied on self-report which may have introduced recall bias. However, self-report of service use has been shown to be reliable.15,34 Third, we had to assume a proxy cost for informal care. In the absence of informal carers, it is unlikely that all patients would have received help from social services, but the cost of a home care worker should nevertheless indicate the value of this informal care. Fourth, the regression model only included data recorded as part of the study. It is likely that other unmeasured patient characteristics could have had some impact on cost. Fifth, our study was also limited by a small sample size which made it difficult to conduct more extensive analyses, particularly for some disease groups (such as cardiac failure where we had only five patients). Finally, the data in our study were highly skewed and there were a number of ‘zero’ resource use data (i.e. instances where costs were ‘£0’ because patients did not use any resources), which both reduce the power to detect a significant difference. It is conceivable that a larger study would highlight other cost drivers in this group.
This study provides the first evidence on the costs of caring for people with advanced disease and refractory breathlessness; it highlights the likely ‘burden’ of the condition and provides a baseline for assessing the relative economic impact of different treatments in future studies. Further research is required to assess the effectiveness and cost-effectiveness of palliative care interventions in reducing disability resulting from breathlessness in patients with advanced disease and refractory breathlessness.
Acknowledgments
The authors thank all the patients who participated in this research; everybody who identified and screened patients for this study, especially the Palliative Care, Respiratory Medicine and Physiotherapy Departments at King’s College Hospital (London, UK) and the Community Palliative Care teams across Guy’s and St Thomas’ Hospitals (London, UK); H Bellas (physiotherapist), E Brink (social worker), J Kelly (clinical nurse specialist) and C Pannell and S de Wolf-Linder (research nurses) for their interviews with patients; members of our project advisory group for their advice during the course of the study; and J Fuller and J Davies for providing administrative support during this project. I.J.H., C.B., C.J., P.M.C. and J.M. conceived the idea of the study and secured funding. I.J.H., C.B., C.J., C.C.R. and J.M. set up the study. C.B. and C.C.R. oversaw the study. C.C.R. and M.D. checked and cleaned the data. M.D., D.Y. and P.M. analysed the data. M.D. produced the first draft of the paper. All authors commented on and contributed to the final draft. I.J.H. is the guarantor. All authors had full access to all of the data of the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: This study was primarily funded by the NIHR under its Research for Patient Benefit (RfPB) programme (PB-PG-0808-17311) and the NIHR CLAHRC funding scheme, through the Collaboration for Leadership in Applied Health Research and Care (CLAHRC) in South London. CLAHRC South London is part of the NIHR and is a partnership between King’s Health Partners, St George’s, University of London, and St George’s Healthcare NHS Trust. Additional support for the work was from an NIHR senior investigator award and the Cicely Saunders International BuildCARE programme. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, the Department of Health, or Cicely Saunders International. The funders of the study had no role in protocol design, collection or analysis of the data or interpretation or writing of the results.
References
- 1. Currow DC, Abernethy AP, Ko DN. The active identification and management of chronic refractory breathlessness is a human right. Thorax 2014; 69: 393–394. [DOI] [PubMed] [Google Scholar]
- 2. Gysels MH, Higginson IJ. The lived experience of breathlessness and its implications for care: a qualitative comparison in cancer, COPD, heart failure and MND. BMC Palliat Care 2011; 10: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Parshall MB, Schwartzstein RM, Adams L, et al. An official American Thoracic Society statement: update on the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care Med 2012; 185: 435–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Higginson IJ, Bausewein C, Reilly CC, et al. An integrated palliative and respiratory care service for patients with advanced disease and refractory breathlessness: a randomised controlled trial. Lancet Respir Med 2014; 2: 979–987. [DOI] [PubMed] [Google Scholar]
- 5. Sorenson HM. Palliative care for lung disease: start early, stay late. Lancet Respir Med 2013; 1: 279–280. [DOI] [PubMed] [Google Scholar]
- 6. Solano JP, Gomes B, Higginson IJ, et al. A comparison of symptom prevalence in far advanced cancer, AIDS, heart disease, chronic obstructive pulmonary disease and renal disease. J Pain Symptom Manage 2006; 31: 58–69. [DOI] [PubMed] [Google Scholar]
- 7. Austin J, Williams R, Ross L, et al. Randomised controlled trial of cardiac rehabilitation in elderly patients with heart failure. Eur J Heart Fail 2005; 7: 411–417. [DOI] [PubMed] [Google Scholar]
- 8. Seow H, Barbera L, Sutradhar R, et al. Trajectory of performance status and symptom scores for patients with cancer during the last six months of life. J Clin Oncol 2011; 29: 1151–1158. [DOI] [PubMed] [Google Scholar]
- 9. Barbera L, Taylor C, Dudgeon D. Why do patients with cancer visit the emergency department near the end of life? CMAJ 2010; 182: 563–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Guyatt GH, Berman LB, Townsend M, et al. A measure of quality of life for clinical trials in chronic lung disease. Thorax 1987; 42: 773–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Puhan MA, Guyatt GH, Goldstein R, et al. Relative responsiveness of the Chronic Respiratory Questionnaire, St. Georges Respiratory Questionnaire and four other health-related quality of life instruments for patients with chronic lung disease. Respir Med 2007; 101: 308–316. [DOI] [PubMed] [Google Scholar]
- 12. Bausewein C, Jolley C, Reilly C, et al. Development, effectiveness and cost-effectiveness of a new out-patient Breathlessness Support Service: study protocol of a phase III fast-track randomised controlled trial. BMC Pulm Med 2012; 12: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hearn J, Higginson IJ. Development and validation of a core outcome measure for palliative care: the palliative care outcome scale. Palliative Care Core Audit Project Advisory Group. Qual Health Care 1999; 8: 219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bausewein C, Booth S, Gysels M, et al. Understanding breathlessness: cross-sectional comparison of symptom burden and palliative care needs in chronic obstructive pulmonary disease and cancer. J Palliat Med 2010; 13: 1109–1118. [DOI] [PubMed] [Google Scholar]
- 15. Goldberg RW, Seybolt DC, Lehman A. Reliable self-report of health service use by individuals with serious mental illness. Psychiatr Serv 2002; 53: 879–881. [DOI] [PubMed] [Google Scholar]
- 16. Department of Health. National Schedule of Reference Costs 2011–12 for NHS trusts and NHS foundation trusts. London: Department of Health, 2012. [Google Scholar]
- 17. Curtis L. Unit costs of health and social care. Canterbury: PSSRU Canterbury, 2012. [Google Scholar]
- 18. Royston P. Multiple imputation of missing values: further update of ice, with an emphasis on categorical variables. Stata J 2009; 9: 466–477. [Google Scholar]
- 19. Sterne JAC, White IR, Carlin JB, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ 2009; 338: b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tabachnick BG, Fidell LS. Using multivariate statistics. 5th ed. Needham Heights, MA: Allyn & Bacon, Inc, 2006. [Google Scholar]
- 21. Manning WG, Mullahy J. Estimating log models: to transform or not to transform? J Health Econ 2001; 20: 461–494. [DOI] [PubMed] [Google Scholar]
- 22. Malehi AS, Pourmotahari F, Angali KA. Statistical models for the analysis of skewed healthcare cost data: a simulation study. Health Econ Rev 2015; 5: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. StataCorp. Stata statistical software. Release 13. College Station, TX: StataCorp, 2013. [Google Scholar]
- 24. Bustacchini S, Chiatti C, Furneri G, et al. The economic burden of chronic obstructive pulmonary disease in the elderly: results from a systematic review of the literature. Curr Opin Pulm Med 2011; 17: S35–S41. [DOI] [PubMed] [Google Scholar]
- 25. Barnato AE. End-of-life spending: can we rationalise costs? Crit Q 2007; 49: 84–92. [Google Scholar]
- 26. Esteban C, Arostegui I, Aburto M, et al. Influence of changes in physical activity on frequency of hospitalization in chronic obstructive pulmonary disease. Respirology 2014; 19: 330–338. [DOI] [PubMed] [Google Scholar]
- 27. Maddocks M, Kon SS, Singh SJ, et al. Rehabilitation following hospitalization in patients with COPD: can it reduce readmissions? Respirology 2015; 20: 395–404. [DOI] [PubMed] [Google Scholar]
- 28. Emtner MI, Arnardottir HR, Hallin R, et al. Walking distance is a predictor of exacerbations in patients with chronic obstructive pulmonary disease. Respir Med 2007; 101: 1037–1040. [DOI] [PubMed] [Google Scholar]
- 29. Lau AC, Yam LY, Poon E. Hospital re-admission in patients with acute exacerbation of chronic obstructive pulmonary disease. Respir Med 2001; 95: 876–884. [DOI] [PubMed] [Google Scholar]
- 30. Chu CM, Chan VL, Lin AW, et al. Readmission rates and life threatening events in COPD survivors treated with non-invasive ventilation for acute hypercapnic respiratory failure. Thorax 2004; 59: 1020–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Garcia-Aymerich J, Farrero E, Felez MA, et al. Risk factors of readmission to hospital for a COPD exacerbation: a prospective study. Thorax 2003; 58: 100–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nguyen HQ, Chu L, Amy Liu IL, et al. Associations between physical activity and 30-day readmission risk in chronic obstructive pulmonary disease. Ann Am Thorac Soc 2014; 11: 695–705. [DOI] [PubMed] [Google Scholar]
- 33. Henson LA, Gao W, Higginson IJ, et al. Emergency department attendance by patients with cancer in their last month of life: a systematic review and meta-analysis. JCO 2015; 33: 370–376. [DOI] [PubMed] [Google Scholar]
- 34. Calsyn RJ, Allen G, Morse GA, et al. Can you trust self-report data provided by homeless mentally ill individuals? Eval Rev 1993; 17: 353–366. [Google Scholar]



