Abstract
Objectives
The sphenopalatine ganglion (SPG) plays a pivotal role in cluster headache (CH) pathophysiology as the major efferent parasympathetic relay. We evaluated the long-term effectiveness of SPG stimulation in medically refractory, chronic CH patients.
Methods
Thirty-three patients were enrolled in an open-label follow-up study of the original Pathway CH-1 study, and participated through 24 months post-insertion of a microstimulator. Response to therapy was defined as acute effectiveness in ≥ 50% of attacks or a ≥ 50% reduction in attack frequency versus baseline.
Results
In total, 5956 attacks (180.5 ± 344.8, range 2–1581 per patient) were evaluated. At 24 months, 45% (n = 15) of patients were acute responders. Among acute responders, a total of 4340 attacks had been treated, and in 78% of these, effective therapy was achieved using only SPG stimulation (relief from moderate or greater pain or freedom from mild pain or greater). A frequency response was observed in 33% (n = 11) of patients with a mean reduction of attack frequency of 83% versus baseline. In total, 61% (20/33) of all patients were either acute or frequency responders or both. The majority maintained their therapeutic response through the 24-month evaluation.
Conclusions
In the population of disabled, medically refractory chronic CH patients treated in this study, SPG stimulation is an effective acute therapy in 45% of patients, offering sustained effectiveness over 24 months of observation. In addition, a maintained, clinically relevant reduction of attack frequency was observed in a third of patients. These long-term data provide support for the use of SPG stimulation for disabled patients and should be considered after medical treatments fail, are not tolerated or are inconvenient for the patients.
Keywords: Cluster headache, sphenopalatine ganglion, neurostimulation, neuromodulation, long-term effectiveness
Introduction
Cluster headache (CH), one of the most excruciating primary headaches, has unilateral pain occurring up to eight times daily and lasting 15–180 minutes (1). Both disability and impairment are high and lead to reduced quality of life (2,3). Abortive (subcutaneous and nasal triptans or oxygen) and preventative treatments (verapamil, lithium, topiramate, corticosteroids or greater occipital nerve injections) (4,5) tend to be less effective in the considerable proportion of patients with the chronic subtype. Treatment in this subpopulation may also be complicated by systemic side effects limiting the efficacy of these therapies. This can result in continued high disability and substantial direct and indirect costs (6,7). Additional therapies are needed for this difficult-to-treat condition.
Sphenopalatine ganglion (SPG) neuromodulation offers a minimally invasive, targeted option without dose restrictions or systemic side effects. Typical adverse effects are mild and transient and include sensory effects, pain and swelling associated with the microstimulator insertion procedure (8). The SPG is part of the neural reflex responsible for CH pain pathophysiology. For over 100 years, it has been targeted via pharmacological blocks (9), alcohol injection (10), corticosteroid and/or local anaesthetic injections (11) and radiofrequency ablation (12). Acute electrical SPG neuromodulation has shown promising results for treating CH (13), and an implantable, on-demand SPG microstimulator was developed and tested in a randomised controlled multicentre study in chronic CH (cCH) (the Pathway CH-1 study) (14). Significant acute pain relief or freedom at 15 minutes was achieved in 67.1% of attacks treated with full stimulation, compared to only 7.1% in sham stimulation (p < 0.0001). A total of 68% experienced a clinically significant improvement, achieving pain relief in ≥50% of full stimulation-treated attacks and/or experiencing a ≥ 50% reduction in attack frequency in the double-blinded experimental period (ending approximately 6 months following microstimulator insertion) versus baseline (14). The objective of this analysis is to characterise the long-term outcomes in cCH patients who agreed to be followed for 24 months after microstimulator insertion. Outcomes include the acute and frequency response to SPG stimulation, improvements in headache disability and preventative medication usage.
Methods
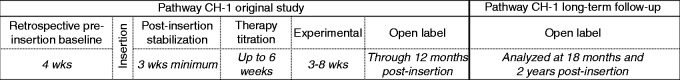
In the original Pathway CH-1 study, the initial inclusion criterion was cCH and dissatisfaction with current headache treatments (14). However, all study sites included only patients who were medically refractory according to the consensus criteria published before (15). Patients underwent trans-oral insertion of a microstimulator such that the stimulating electrodes were placed proximate to the SPG. Additional insertion details have previously been published (14,16). In the Pathway CH-1 study, patients were followed for 12 months after microstimulator insertion. Initially, the efficacy of SPG stimulation was evaluated in a randomised controlled experimental period (14). During the remaining part of the first 12 months, patients had the opportunity to use the therapy in an open-label period. A separate long-term follow-up study was initiated so that these patients could be followed beyond the first 12 months (Figure 1). Open-label results, including both data from open-label use during the first 12 months (Pathway CH-1) and data from the long-term follow-up through to 24 months post-insertion, are presented for all patients enrolled in the long-term follow-up study.
Figure 1.
Pathway CH-1 study chart.
Patient selection
Patient selection criteria for the long-term follow-up study included participation in the Pathway CH-1 study, continued microstimulator implantation, previous compliance with study protocol and written informed consent. Reasons for not being enrolled included non-compliance to the Pathway CH-1 protocol, unwillingness to follow the protocol or a lack of signed informed consent.
SPG Microstimulator System
The SPG Microstimulator System (Pulsante® Microstimulator System, Autonomic Technologies, Inc., Redwood City, CA, USA) is designed to fit the facial anatomy with an integrated lead placed proximate to the SPG. The microstimulator communicates with a handheld remote controller by radio-waves and is inductively powered, and as such contains no battery (14). Using the remote controller, patients apply on-demand SPG stimulation to treat the acute pain of their cluster attacks. Apart from instructions on how to use the remote controller, patients were advised to treat attacks early, defined as ‘as soon as the patient felt a CH attack’.
Data collection
Acute response to SPG stimulation was captured prospectively in an electronic headache diary incorporated into the remote controller. Pain scores are reported using the Categorical Pain Scale (CPS; 0 = none, 1 = mild, 2 = moderate, 3 = severe, 4 = very severe). Headache pain was reported prior to stimulation use and headache pain and acute medication use were reported at either 15 minutes following the start of stimulation (through the first 12 months post-insertion) or immediately following cessation of each SPG stimulation (between 12 and 24 months post-insertion) as appropriate.
Average cluster attack frequency over past 4 weeks, preventative medications and headache disability were captured prior to microstimulator insertion and again at each study visit using case report forms, which were monitored.
Outcomes and analyses
The primary objective of this analysis was to characterise the therapeutic response to SPG stimulation at 24 months. Per the protocol, the therapeutic response was calculated as a responder analysis, or the percentage of patients experiencing an acute abortive effect from SPG stimulation, a preventative effect from SPG stimulation or both. Acute and frequency responses are described in detail below.
Acute effectiveness (referred to as effective therapy) is pain relief (decrease in CPS score from 2 (moderate) or greater to 1 (mild) or 0 (none) without the use of acute medications) or pain freedom (CPS score decreased from 1 (mild) or greater to 0 (none) without the use of acute medications) following SPG stimulation. These analyses include all SPG stimulation attempts during the open-label period (following the randomised controlled period through to the 24-month follow-up visit) with completed electronic headache diary data. Attacks were evaluated at the per-protocol evaluation point (15 minutes following the start of stimulation or immediately following the cessation of stimulation). Acute response was defined as effective therapy in at least 50% of evaluable attacks occurring during the open-label period and through to the 24-month study visit.
The cluster attack frequency was recorded as the average number of attacks per week over the prior 4 weeks. Frequency response was defined as at least a 50% reduction in cluster attack frequency at the 24-month study visit as compared to baseline.
Therapeutic response to SPG stimulation was defined as acute response and/or frequency response (for a detailed definition, see above). As an additional analysis, therapeutic response was evaluated and characterised at the 12- and 18-month study visits.
Additional protocol pre-specified analyses included characterisation of use of preventative medications and headache disability at the 24-month study visit as compared to baseline. Changes in preventative medication use were characterised, identifying those patients who stopped, decreased dose, remained off or added/increased doses of preventative medications.
Headache disability improvements were evaluated using the headache-impact test (HIT-6) questionnaire and were considered clinically meaningful if scores improved by at least 2.3 units relative to baseline (17). A paired t-test was used to account for changes over time, with p < 0.05 regarded as a statistically significant difference between baseline and the 24-month study visit.
Any missing data for frequency analyses, use of preventative medications and headache disability were imputed from the respective preceding study visit.
A post-hoc exploratory analysis was performed in order to evaluate acute medication use for SPG stimulation-treated attacks, with data collected in conjunction with headache pain ratings on the remote controller. An additional post-hoc exploratory analysis was performed in order to evaluate the percentage of patients achieving a therapeutic response for either an acute or preventative effect of >30% and >75%, respectively.
Results
Patient disposition, demographics and clinical characteristics
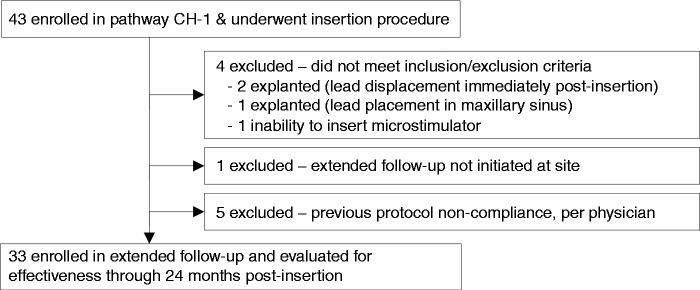
Forty-three medically refractory cCH patients with a minimum of four attacks per week were enrolled into the Pathway CH-1 study from six European centres. Of these, 33 patients were enrolled into the long-term follow-up study at five centres (Figure 2). Of the ten patients not eligible for enrolment into the long-term follow-up study, four did not meet the inclusion/exclusion criteria as they no longer had implanted neurostimulators, one was not eligible because the extended follow-up study was not initiated at their site and five were not eligible for previous protocol non-compliance. Of the five non-compliant patients, four were frequency responders during the open-label period of the Pathway CH-1 study (for further details, please see Figure 2 and Table 1).
Figure 2.
Patient disposition from the Pathway CH-1 and extended follow-up studies.
Table 1.
Summary of the patients (n = 10) who were not eligible to participate in the long-term follow-up study.
| Reason not invited to participate in long-term follow-up study | Therapeutic response during open-label period |
|---|---|
| Study not initiated at site (n = 1) | Frequency (n = 1) |
| Non-compliant: would not show up for clinic visits (n = 3) | Frequency (n = 3) |
| Non-compliant: would not follow site instructions (n = 1) | Frequency (n = 1) |
| Non-compliant: would not apply stimulation as directed (n = 1) | Non-responder (n = 1) |
| Did not meet inclusion criteria – did not have microstimulator still implanted (n = 4) | Not evaluable (n = 4) |
Therapeutic responses during the open-label period of the Pathway CH-1 study are provided.
All 33 patients in this analysis completed the follow-up through to 24 months. The open-label period began at 156 ± 66 days post-insertion. Average time in the open-label period through to the 24-month study visit was 594 ± 81 days. Patient characteristics are provided in Table 2.
Table 2.
Baseline characteristics of 33 patients enrolled in the extended follow-up study.
| Baseline characteristics | |
|---|---|
| Gender | 85% male |
| Age | 41.5 ± 12.0 years (range 19–67 years) |
| Laterality of headache | 58% left |
| Years of cluster headache | 10.5 ± 8.3 (range 1–36) |
| Cluster attack frequency/week | 16.8 ± 13.7 (range 5–70) |
| HIT-6 (headache impact test) disability score | 66.7 ± 6.2 (range 56–78) |
| Preventative medications | 64% (n = 21) used verapamil as monotherapy or as part of polytherapy; 12% (n = 4) used other preventative medicationsa 24% (n = 8) used no preventative medication |
Other medications included lithium, topiramate and valproic acid.
Acute response
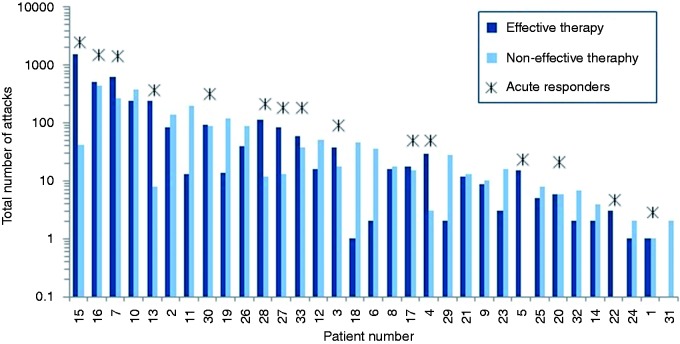
Across all 33 patients, a total of 5956 attacks were treated, of which 65% achieved effective therapy (3849/5956), with a total of 50% (2958/5956) becoming pain free (see Figure 3). On average, each patient treated 181 ± 345 (range 2–1581) CH attacks that were evaluable for the analysis (see Figure 4). The average duration of stimulation was 11.2 ± 8.4 minutes (range 0.1–61.5 minutes). In 4682 of 5956 attacks (79%), patients did not report the use of acute medication. These attacks included both the 3849 that achieved effective therapy and an additional 833 attacks that did not meet the definition of effective therapy as pain was not reduced to mild or none, but in which acute medications were not reported as being used. A total of 45% of patients (15/33) were acute responders, achieving effective therapy in at least 50% of their evaluable attacks. These acute responders each treated, on average, 289 ± 471 (range 2–1581) attacks and obtained effective therapy in 78% (3387/4340) of those attacks. Furthermore, nearly half of all acute responders experienced a very strong response to therapy, achieving the ability to treat at least 75% of their attacks effectively. A total of 73% of all patients (24/33) were able to effectively treat at least 30% of their attacks.
Figure 3.
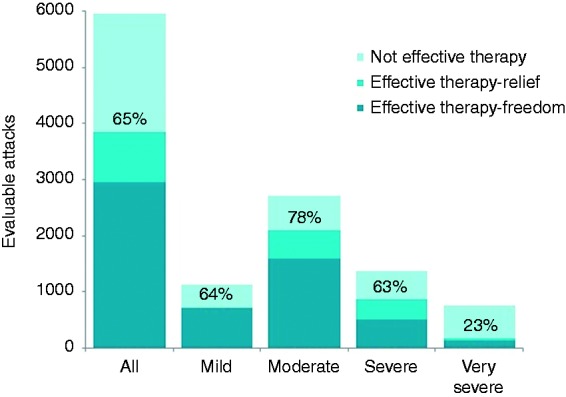
Effective therapy for all patients (n = 33 patients). Acute responses to sphenopalatine ganglion stimulation in 5956 cluster attacks. Percentages of attacks achieving effective therapy at the evaluation point (including both pain freedom and pain relief) are given for all evaluable attacks from the open-label phase through to the 24-month study visit.
Figure 4.
Numbers of attacks treated with sphenopalatine ganglion stimulation and acute effectiveness are shown for each patient. In addition, acute responders are identified with a *.
Attack frequency
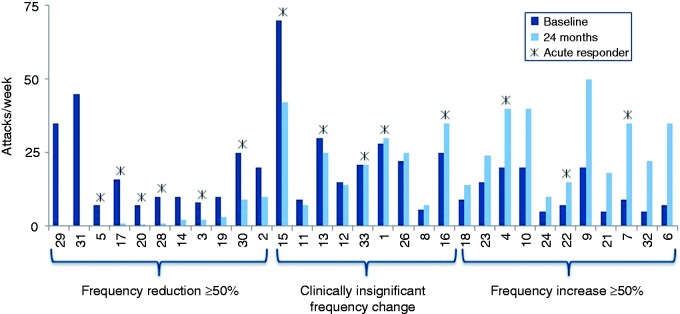
Thirty-one patients had complete data sets at both baseline and 24 months. Two patients had missing frequency data at 24 months that were not able to be imputed. A total of 35% of these patients (11/31) were frequency responders at the 24-month study visit, experiencing at least a 50% reduction in attack frequency relative to baseline. These 11 patients experienced an 83% average reduction in frequency at 24 months compared to baseline (average attack frequencies at baseline and 24 months were 18 ± 13 attacks/week and 3 ± 4 attacks/week, respectively). Six of the 11 frequency responders were also acute responders, achieving effective therapy in greater than 50% of attacks treated. Two frequency responders reported no cluster attacks at the 24-month visit, effectively converting from cCH to episodic CH, and the majority of frequency responders (8/11) experienced a frequency reduction of ≥75%. In addition, 64% (7/11) of frequency responders reduced (n = 3) or stopped (n = 4) their preventative medication.
In the entire population, the average attack frequency at these two time points was unchanged (17 ± 3 (range 5–70) and 17 ± 15 (range 0–50) attacks per week, respectively). In 29% of patients (9/31), attack frequency did not change significantly (< 50% change in either direction); 35% of patients (11/31) experienced an increase in attack frequency of at least 50% (see Figure 5).
Figure 5.
Attack frequencies at baseline and at 24 months are shown for each patient. Eleven patients were attack frequency responders, nine experienced clinically insignificant changes (< 50% change in either direction) and 11 experienced increases in attack frequency of at least 50%. Acute responders are identified with a *.
A total of 40% of the frequency non-responders were acute responders and 65% experienced improvements in preventative medications (stopped all medication (n = 5), stopped some medication (n = 2), remained off (n = 4) and experienced clinical improvement (n = 2)).
Therapeutic response
This study of a novel neuromodulation therapy for severe headache is the first to show both acute effects and a reduction of headache frequency in an open-label cohort. Therefore, therapeutic response (i.e., acute and/or frequency effectiveness) as a novel outcome was considered (14).
At 24 months, 61% of patients experienced a therapeutic response to SPG stimulation (acute effectiveness and/or frequency reduction of at least 50%), and more than 80% of patients had a therapeutic response of at least 30%. Furthermore, the majority of therapeutic responders were considered as very strong (≥75%) responders to SPG stimulation therapy (Figure 6). This group was able to effectively treat at least 75% of their attacks, to experience a reduction in attack frequency of at least 75% compared to baseline, or both.
Figure 6.
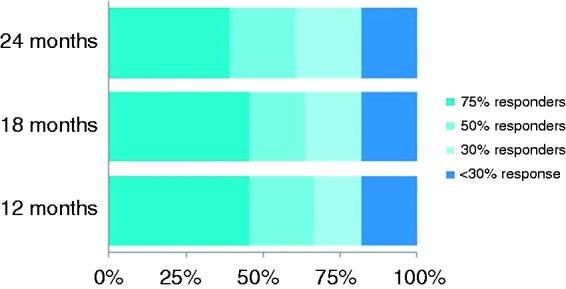
At 24 months, 61% (20/33) had a therapeutic response, defined as either an acute and/or frequency response of at least 50%, and of these, 65% (13/20) had a very strong (≥75%) response to therapy. More than 80% (27/33) of patients experienced a response of at least 30%.
Effectiveness over time
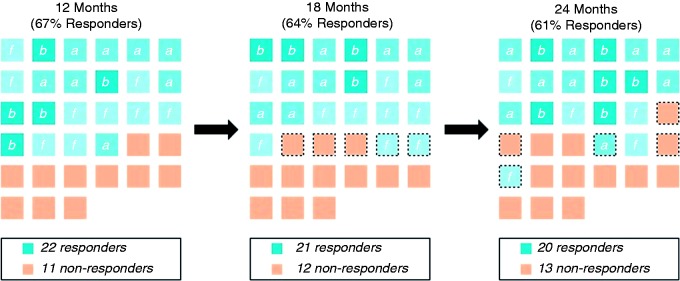
Individual patient responses at each time point are provided, and the majority of patients maintained their response at 18 through to 24 months (Figure 7). Total effectiveness was stable, as three additional patients became responders between these time points, and two became non-responders.
Figure 7.
Therapeutic response to therapy defined as an acute and/or frequency response is consistent over time. At each time point, each square represents an individual patient. Responders are further categorised as acute (a), frequency (f) or both (b). Dotted borders indicate changes in response. The majority of patients maintained a response to therapy, with two patients gaining and three patients losing response between each of the time points evaluated.
Headache disability improvements and patient satisfaction
HIT-6 headache disability was clinically and significantly reduced by 4.8 points compared to baseline disability (n = 29; p = 0.0048). Four patients did not complete the HIT-6 survey at their 24-month clinic visit. Of these, one patient missing a HIT-6 survey was an acute responder. A total of 55% of patients (16/29) who completed headache disability surveys were considered HIT-6 responders (clinically meaningful change is a reduction of at least 2.3 points). Frequency responders experienced a reduction in headache disability of 12.6 ± 6.7, patients with both an acute and frequency response experienced a reduction in headache disability of 11.2 ± 8.8, acute responders experienced a reduction in headache disability of 1.9 ± 4.8 and non-responders experienced an increase in headache disability of 0.4 ± 6.9. The only groups to experience clinically meaningful changes in headache disability relative to baseline were the group of patients with only frequency responses and those with both acute and frequency responses. Non-responders remained effectively unchanged in terms of headache disability relative to baseline.
Regarding patient satisfaction, 69% of those that responded to the survey (18/26) indicated that they found SPG stimulation useful for treating their headaches at the 24-month follow-up.
Preventative medication changes from baseline
A total of 64% (21/33) of patients experienced clinical improvements in their preventative medication use at 24 months compared to baseline. Specifically, nine stopped all preventative medications. Six stopped or decreased the doses of their medications (one stopped two medications, one stopped two medications and reduced the dose of a third, two stopped one medication, one stopped one medication and reduced the dose of a second and one decreased the dose of two medications). Furthermore, two had clinically beneficial medication adjustments (change in type of preventative medication that was assessed by the clinician as an improvement or reduction from the baseline mediation regimen), and four who were not using any preventative medications at baseline remained free of preventative medication at the 24-month study visit. Of these 21 patients with clinical improvements in preventative medication use, 67% (14/21) experienced at least a 50% acute or frequency response or indicated that they found SPG stimulation useful. Further details on changes in preventative medication, including therapeutic responses at 24 months, are provided in Table 3.
Table 3.
Preventative medication use at baseline and at the 24-month study visit.
| Preventive medication use at: |
||||
|---|---|---|---|---|
| Patient number | Baseline (dose per day) | 24 months (dose per day) | Therapeutic response at 24 months | |
| Improvement | 2 | Verapamil (360 mg), valproic acid (900 mg) | None | Frequency |
| 29 | Topiramate (200 mg) | None | Frequency | |
| 31 | Valproic acid (800 mg) | None | Frequency | |
| 20 | Verapamil (480 mg), lithium (800 mg) | None | Acute and frequency | |
| 4 | Verapamil (500 mg) | None | Acute | |
| 16 | Lithium (1350 mg) | None | Acute | |
| 22 | Verapamil (120 mg), lithium (400 mg) | None | Acute | |
| 12 | Verapamil (360 mg), lithium (450 mg) | None | Neither | |
| 32 | Verapamil (360 mg), topiramate (100 mg) | None | Neither | |
| 28 | Verapamil (240 mg), topiramate (150 mg) | Verapamil (240 mg) | Acute and frequency | |
| 8 | Verapamil (800 mg), melatonin (12 mg) | Melatonin (12 mg) | Neither | |
| 17 | Verapamil (180 mg), lithium (450 mg), gabapentin (1200 mg) | Gabapentin (600 mg) | Acute and frequency | |
| 30 | Verapamil (720 mg), topiramate (200 mg), melatonin (6 mg) | Verapamil (240 mg), melatonin (6 mg) | Acute and frequency | |
| 11 | Verapamil (400 mg), topiramate (200 mg) | Verapamil (300 mg), topiramate (100 mg) | Neither | |
| 15 | Verapamil (600 mg), topiramate (400 mg), melatonin (6 mg) | Verapamil (480 mg) | Acute | |
| 9 | Verapamil (760 mg), gabapentin (2700 mg), lithium (900 mg) | Verapamil (280 mg), gabapentin (1800 mg), lithium (900 mg) | Neither | |
| 21 | Verapamil (720 mg), lithium (1250 mg) | Topiramate (50 mg) | Neither | |
| 13 | None | None | Acute | |
| 6 | None | None | Neither | |
| 18 | None | None | Neither | |
| 24 | None | None | Neither | |
| No change | 5 | Candesartan (16 mg) | Candesartan (16 mg) | Acute and frequency |
| 1 | Verapamil (720 mg) | Verapamil (720 mg) | Acute | |
| 26 | Verapamil (720 mg), lithium (400 mg) | Verapamil (720 mg), lithium (800 mg) | Neither | |
| Increased dose and/or added | 33 | None | Verapamil (360 mg) | Acute |
| 23 | Verapamil (720 mg) | Verapamil (720 mg), lithium (∼570 mg) | Neither | |
| 3 | Verapamil (960 mg) | Verapamil (960 mg), gabapentin (1800 mg) | Acute and frequency | |
| 7 | None | Prednisone bolus (∼55 mg) | Acute | |
| 19 | Verapamil (360 mg) | Verapamil (1080 mg) | Frequency | |
| 14 | Verapamil (120 mg), lithium (1125 mg) | Verapamil (600 mg) | Frequency | |
| 10 | Verapamil (800 mg), candesartan (32 mg) | Verapamil (900 mg), indomethacin (200 mg) | Neither | |
| Not evaluable | 27 | None | Data missing | Acute |
| 25 | None | Data missing | Neither | |
Therapeutic responses at 24 months are provided for each patient.
Complications
The side effects, which are described in detail in a prior publication (8), are mainly surgical sequelae occurring during the peri-operative period (within 30 days post-operation) in 81% patients, and typically include sensory disturbances, post-operative pain and swelling. These post-insertion side effects were typically mild to moderate, and resolved within 2–3 months (68 days on average, range 0–312 days) (8).
Contralateral attacks
During the course of the 24-month study, 11 out of 33 patients (33%) reported the occurrence of cluster attacks contralateral to the side of their inserted microstimulator. Five of these reported a history of contralateral attacks in the 6-month period before the insertion. Of the six patients with no history in the immediate 6 months prior to microstimulator insertion, one experienced a nominal number of contralateral attacks (∼1% of all attacks), two experienced contralateral attacks for 1–1.5 months only, two experienced a combination of ipsilateral and contralateral attacks for 3.5 and 6 months only and one continued to experience 5–10% of their attacks contralaterally through to the 24-month study visit.
Discussion
In a population of 33 medically refractory cCH patients followed for 24 months while receiving on-demand SPG stimulation, we found sustained effectiveness for acute attack pain relief and attack frequency reductions in a subgroup of patients. At 24 months, 45% of patients were acute responders and 35% were frequency responders. As this study was the first neuromodulation study in CH in which both acute and frequency effects were observed, a novel endpoint (therapeutic response) was used. According to this novel endpoint, 61% of patients experienced a therapeutic response. A total of 64% of patients reduced, stopped or remained off all preventative medications. Mean headache disability improved, with 55% of patients considered to be HIT-6 responders and 69% of the 26 patients responding to the survey indicating that they found SPG stimulation useful for treating their headaches.
Previously published data from the sham-controlled experimental period in the Pathway CH-1 study showed a 68% therapeutic responder rate (acute and/or frequency) for SPG stimulation (14). Compared to these results with similar proportions of acute and frequency responders, the present study shows a maintained effectiveness in the majority of patients over time, indicating that the acute and/or attack frequency reduction benefit is robust, stable and is likely not attributable to the implant procedure itself (i.e., a surgical response).
An at least 50% improvement is routinely used in the headache literature to define a responder (18). However, a 30% threshold for the definition of frequency response has been suggested to be appropriate for trials in chronic migraine and in chronic tension-type headache (19,20). As cCH patients experience very high levels of disability and impairment (2,3), and particularly as only medically refractory patients were included in this study, this threshold may be appropriate here as well. Although not predefined in the protocol, if such criteria were applied to our dataset, more than 80% of the included patients achieved clinical benefit at the 30% responder level in the specified period. Further support includes the fact that 64% of the patients had a meaningful reduction in use of preventative medication use and/or minimised acute therapy, despite some of these patients not falling into the 50% or 75% responder categories. SPG stimulation is the only form of neurostimulation that elicits both an acute and a preventative effect (21). These therapeutic effects of SPG stimulation were quite dramatic, with the majority of responders being able to treat at least 75% of their attacks and/or experiencing a reduction in attack frequency of at least 75%. Furthermore, two of these cCH patients were no longer having cluster attacks at the 24-month study visit, and effectively converted from cCH to episodic CH.
The field of neurostimulation is highly dynamic and evolving, and yet there are no official guidelines for the study design. At the time of conception of the original Pathway CH-1 study, preventative effects of SPG stimulation were not anticipated, and thus the design was not ideally tuned to capture this information. Furthermore, the question of how to interpret both acute and preventative responses is open to discussion. The authors believe that a meaningful therapeutic response that is not exclusively focused on an acute or preventative effect should be considered as an additional outcome in future studies.
SPG stimulation may offer some unique benefits. For example, in contrast to triptans, there are no quantitative limits to SPG stimulation therapy, and no reported cardiovascular or systemic side effects thus far. Furthermore, the stigmatisation and logistical hassle of using and transporting oxygen canisters can be avoided.
However, SPG stimulation itself is not without side effects, and whenever patients are subjected to surgical procedures, due caution must be exercised. As expected, the majority of reported side effects have been associated with the insertion procedure itself. These are mostly mild to moderate, and most resolve within 2–3 months. The sequelae are comparable to oral cavity interventions (22,23). These data have been published separately (8).
It is nevertheless important to note that at the 24-month visit, a third (11/33) of patients experienced an increase in attack frequency of >50% compared to the 1-month baseline period. In the design of future studies, it will be necessary to take the frequency component of the response into consideration and record frequency continuously during the entire study so that both increases and decreases in frequency can be better evaluated.
Furthermore, the issue of contralateral attacks is often raised with regards to neurostimulation, but the underlying mechanisms are as yet unknown. However, peripheral intervention in CH is likely to change the side of the attack if the intervention is positive (i.e., effective) (24). Reports on the prevalence of contralateral attacks in CH are sparse and based on retrospective analyses (25). Furthermore, single contralateral attacks probably occur more frequently than permanent side-shifts. In our population, 11 patients reported contralateral attacks in the 24-month period. We note that of these patients, nearly half of them had a history of contralateral attacks prior to the insertion of the SPG microstimulator.
Therefore, further studies are needed in order to better understand the occurrence of contralateral attacks, as well as the predictors of acute and frequency responses.
Strengths and limitations
These data represent the largest currently published prospective cohort of patients and attacks in CH. The data set is substantial and unique, encompassing nearly 6000 consecutively recorded attacks in medically refractory and highly disabled patients. It is exceptional in that all patients remained in the study through to 24 months.
As data on attack frequency were collected retrospectively, a recall bias cannot be excluded. In the study’s second year, frequency data were not collected continuously and thus required imputation. Nevertheless, the data suggest that a subset of patients experienced significant reductions in attack frequency. An even larger proportion of these severely affected patients demonstrated acute responses, the majority of which achieved pain freedom. A longer baseline period of at least 2–3 months could have reflected the undulating natural course of the headaches and the side locations more adequately. However, a baseline of 4 weeks is commonly used (26,27), and our included patients had long histories of enduring, chronic and medically refractory CH.
The mean attack frequencies at baseline and after 24 months were similar. However, it must be recognised that there is a dilemma of skewed ratings: improvements cannot fall below 0% (corresponding to a maximum improvement of 100%), yet an increased attack frequency may exceed 100%. Thus, a few patients with large increases in attack frequency can severely skew the analysis, and data should be evaluated on an individual basis. In light of these observations and the fact that the study investigated a highly selected, refractory patient population that was dissatisfied with previous treatment attempts, the authors believe that the acute and preventative results found in a subgroup of patients in this study represent a valid, positive signal.
Conclusion
In an open-label, 24-month follow-up study in 33 medically refractory cCH patients, we found that SPG stimulation may be an effective treatment. The long-term effects on frequency response and the occurrence of contralateral attacks should be investigated further. Overall, the effect of SPG stimulation is maintained in the long term, and preventative medications can be tapered or stopped.
Clinical implications
A sustained effect of sphenopalatine ganglion (SPG) stimulation at 24-month follow-up is demonstrated in a subset of patients.
A total of 45% of medically refractory chronic cluster headache (cCH) patients have an acute effect in 50% or more of their attacks.
A total of 35% of medically refractory cCH patients have a 50% or higher reduction in their attack frequency.
A total of 61% are therapeutic responders to SPG stimulation therapy.
SPG stimulation is a fairly safe treatment alternative for patients with medically refractory cCH.
Acknowledgements
The authors would like to acknowledge the study steering committee, all sub-investigators and site coordinators and our surgical colleagues for their contributions to the Pathway CH-1 studies. The authors would also like to acknowledge the contributions of Tami Crabtree (a consultant for ATI) for her assistance with the statistical analyses. TPJ was involved in the conduct of the study, the analysis and interpretation of the data and drafting and revising the manuscript. MB was involved in the conduct of the study, the analysis and interpretation of the data and drafting and revising the manuscript. AM was involved in the design and conceptualisation of the study, the conduct of the study, the analysis and interpretation of the data and revising the manuscript. JML was involved in the conduct of the study and the design and conceptualisation of the study. JS was involved in the design and conceptualisation of the study, the conduct of the study and revising the manuscript. CG was involved in the conduct of the study and revising the manuscript. AMG was involved in the design and conceptualisation of the study, the analysis and interpretation of the data and revising the manuscript. AC was involved in the design and conceptualisation of the study, the analysis and interpretation of the data and revising the manuscript. RHJ was involved in the conduct of the study, the design and conceptualisation of the study, the analysis and interpretation of the data and revising the manuscript. Both studies were approved by the appropriate competent national, regional and/or institutional review boards at all participating centres. Written informed consent was obtained from all participants. Studies were registered on ClinicalTrials.gov (NCT01255813 and NCT01616511).
Declaration of conflicting interests
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: TPJ, JML, JS, CG and RHJ are or have been (AM) consultants for ATI and have been paid for their services; MB has been a speaker for ATI; AMG and AC are employees of ATI; all investigators apart from TPJ and AM have received per-patient honoraria for the Pathway CH-1 trial.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: the study was funded by Autonomic Technologies, Inc., Redwood City, CA, USA.
References
- 1.The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia 2013; 33: 629–808. [DOI] [PubMed]
- 2.Jensen RM, Lyngberg A, Jensen RH. Burden of cluster headache. Cephalalgia 2007; 27: 535–541. [DOI] [PubMed] [Google Scholar]
- 3.Jurgens TP, Gaul C, Lindwurm A, et al. Impairment in episodic and chronic cluster headache. Cephalalgia 2011; 31: 671–682. [DOI] [PubMed] [Google Scholar]
- 4.May A, Leone M, Afra J, et al. EFNS guidelines on the treatment of cluster headache and other trigeminal-autonomic cephalalgias. Eur J Neurol 2006; 13: 1066–1077. [DOI] [PubMed] [Google Scholar]
- 5.Leroux E, Valade D, Taifas I, et al. Suboccipital steroid injections for transitional treatment of patients with more than two cluster headache attacks per day: a randomised, double-blind, placebo-controlled trial. Lancet Neurol 2011; 10: 891–897. [DOI] [PubMed] [Google Scholar]
- 6.Gaul C, Finken J, Biermann J, et al. Treatment costs and indirect costs of cluster headache: a health economics analysis. Cephalalgia 2011; 31: 1664–1672. [DOI] [PubMed] [Google Scholar]
- 7.Pietzsch JB, Garner A, Gaul C, et al. Cost-effectiveness of stimulation of the sphenopalatine ganglion (SPG) for the treatment of chronic cluster headache: a model-based analysis based on the Pathway CH-1 study. J Headache Pain 2015; 16: 530–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Assaf AT, Hillerup S, Rostgaard J, et al. Technical and surgical aspects of the sphenopalatine ganglion (SPG) microstimulator insertion procedure. Int J Oral Maxillofac Surg 2016; 45: 245–254. [DOI] [PubMed] [Google Scholar]
- 9.Sluder G. The role of the sphenopalatine (or Meckle’s) ganglion in nasal headaches. New York Med J 1908, pp. 989–990. [Google Scholar]
- 10.Devoghel JC. Cluster headache and sphenopalatine block. Acta Anaesthesiol Belg 1981; 32: 101–107. [PubMed] [Google Scholar]
- 11.Felisati G, Arnone F, Lozza P, et al. Sphenopalatine endoscopic ganglion block: a revision of a traditional technique for cluster headache. Laryngoscope 2006; 116: 1447–1450. [DOI] [PubMed] [Google Scholar]
- 12.Narouze S, Kapural L, Casanova J, et al. Sphenopalatine ganglion radiofrequency ablation for the management of chronic cluster headache. Headache 2009; 49: 571–577. [DOI] [PubMed] [Google Scholar]
- 13.Ansarinia M, Rezai A, Tepper SJ, et al. Electrical stimulation of sphenopalatine ganglion for acute treatment of cluster headaches. Headache 2010; 50: 1164–1174. [DOI] [PubMed] [Google Scholar]
- 14.Schoenen J, Jensen RH, Lanteri-Minet M, et al. Stimulation of the sphenopalatine ganglion (SPG) for cluster headache treatment. Pathway CH-1: a randomized, sham-controlled study. Cephalalgia 2013; 33: 816–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goadsby PJ, Schoenen J, Ferrari MD, et al. Towards a definition of intractable headache for use in clinical practice and trials. Cephalalgia 2006; 26: 1168–1170. [DOI] [PubMed] [Google Scholar]
- 16.Jurgens TP, Schoenen J, Rostgaard J, et al. Stimulation of the sphenopalatine ganglion in intractable cluster headache: expert consensus on patient selection and standards of care. Cephalalgia 2014; 34: 1100–1110. [DOI] [PubMed] [Google Scholar]
- 17.Coeytaux RR, Kaufman JS, Chao R, et al. Four methods of estimating the minimal important difference score were compared to establish a clinically significant change in Headache Impact Test. J Clin Epidemiol 2006; 59: 374–380. [DOI] [PubMed] [Google Scholar]
- 18.Tfelt-Hansen P, Pascual J, Ramadan N, et al. Guidelines for controlled trials of drugs in migraine: third edition. A guide for investigators. Cephalalgia 2012; 32: 6–38. [DOI] [PubMed] [Google Scholar]
- 19.Silberstein S, Tfelt-Hansen P, Dodick DW, et al. Guidelines for controlled trials of prophylactic treatment of chronic migraine in adults. Cephalalgia 2008; 28: 484–495. [DOI] [PubMed] [Google Scholar]
- 20.Dworkin RH, Turk DC, Wyrwich KW, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain 2008; 9: 105–121. [DOI] [PubMed] [Google Scholar]
- 21.Pedersen JL, Barloese M, Jensen RH. Neurostimulation in cluster headache: a review of current progress. Cephalalgia 2013; 33: 1179–1193. [DOI] [PubMed] [Google Scholar]
- 22.Tung TC, Chen YR, Bendor-Samuel R. Surgical complications of the Le Fort I osteotomy – a retrospective review of 146 cases. Changgeng Yi Xue Za Zhi 1995; 18: 102–107. [PubMed] [Google Scholar]
- 23.Al-Din OF, Coghlan KM, Magennis P. Sensory nerve disturbance following Le Fort I osteotomy. Int J Oral Maxillofac Surg 1996; 25: 13–19. [DOI] [PubMed] [Google Scholar]
- 24.Sjaastad O. Cluster Headache Syndrome, London: W. B. Saunders Company, Ltd, 1992. [Google Scholar]
- 25.Meyer EL, Laurell K, Artto V, et al. Lateralization in cluster headache: a Nordic multicenter study. J Headache Pain 2009; 10: 259–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fontaine D, Lazorthes Y, Mertens P, et al. Safety and efficacy of deep brain stimulation in refractory cluster headache: a randomized placebo-controlled double-blind trial followed by a 1-year open extension. J Headache Pain 2010; 11: 23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gaul C, Diener HC, Silver N, et al. Non-invasive vagus nerve stimulation for PREVention and Acute treatment of chronic cluster headache (PREVA): a randomised controlled study. Cephalalgia 2016; 36: 534–546. [DOI] [PMC free article] [PubMed] [Google Scholar]