Abstract
Background:
In cost-effectiveness analyses in healthcare, Quality-Adjusted Life Years are often used as outcome measure of effectiveness. However, there is an ongoing debate concerning the appropriateness of its use for decision-making in palliative care.
Aim:
To systematically map pros and cons of using the Quality-Adjusted Life Year to inform decisions on resource allocation among palliative care interventions, as brought forward in the debate, and to discuss the Quality-Adjusted Life Year’s value for palliative care.
Design:
The integrative review method of Whittemore and Knafl was followed. Theoretical arguments and empirical findings were mapped.
Data sources:
A literature search was conducted in PubMed, EMBASE, and CINAHL, in which MeSH (Medical Subject Headings) terms were Palliative Care, Cost-Benefit Analysis, Quality of Life, and Quality-Adjusted Life Years.
Findings:
Three themes regarding the pros and cons were identified: (1) restrictions in life years gained, (2) conceptualization of quality of life and its measurement, including suggestions to adapt this, and (3) valuation and additivity of time, referring to changing valuation of time. The debate is recognized in empirical studies, but alternatives not yet applied.
Conclusion:
The Quality-Adjusted Life Year might be more valuable for palliative care if specific issues are taken into account. Despite restrictions in life years gained, Quality-Adjusted Life Years can be achieved in palliative care. However, in measuring quality of life, we recommend to—in addition to the EQ-5D— make use of quality of life or capability instruments specifically for palliative care. Also, we suggest exploring the possibility of integrating valuation of time in a non-linear way in the Quality-Adjusted Life Year.
Keywords: Quality-Adjusted Life Year, debate, cost-effectiveness analysis, palliative care
What is already known about the topic?
Medical (technological) progress and resulting competing alternatives increasingly raise the question “must everything that can be done, be done?” The Quality-Adjusted Life Year (QALY) is widely used as outcome measure for cost-effectiveness analyses in healthcare. However, there is an ongoing debate concerning the appropriateness of its use to inform decisions on resource allocation among palliative care interventions.
What this paper adds?
This paper offers the first systematic overview of pros and cons of using QALYs to inform decisions on resource allocation among palliative care interventions. It provides a critical appraisal of the arguments and discusses the QALYs’ value for palliative care. Furthermore, it explores whether difficulties are experienced in research practice and how they are dealt with, for example, are alternative approaches or outcome measures used?
Implications for practice, theory, or policy
Our review concludes that, despite criticisms, the QALY might be of value in informing decisions on resource allocation among palliative care interventions if specific issues are taken into account. Since standard quality-of-life measurement instruments (such as the EQ-5D) lack dimensions that are essential to palliative care, we recommend to add quality-of-life or capability instruments for economic evaluations in palliative care. Also, we suggest exploring the possibility of integrating valuation of time in a non-linear way in the QALY framework. However, to appropriately allocate scarce resources across healthcare, a common metric is needed. Therefore, the issues suggested should not remain restricted to palliative care, but be considered in the QALY conceptualization throughout healthcare.
Background
Patients are entitled to receive timely, acceptable, and affordable care of appropriate quality.1 Due to new (expensive) drugs and treatments, and the fact that people live longer, the duration of intensive and costly care has increased. This puts pressure on the collective affordability of our healthcare. The question “must everything that can be done, be done?” is being asked more frequently, particularly in end-of-life care (EoLC). Palliative care also competes for limited healthcare resources. Since the number of patients in advanced stages of incurable conditions is increasing,2,3 expenditures in this field are likely to increasingly represent a bigger share of total spending.
Because of this, economic evaluations used when allocating resources are becoming increasingly important.4 Insight into the effectiveness, its costs, and their incremental ratio (incremental cost-effectiveness) is important when allocating resources. It is frequently argued that the evaluation of palliative care interventions should also include cost-effectiveness.5–8 The Quality-Adjusted Life Year (QALY) is the predominant outcome measure for cost-effectiveness analyses (CEAs) in healthcare, and its use is recommended by both the National Institute for Health and Clinical Excellence (NICE) and the Dutch guideline for economic evaluation in healthcare.9–11 However, in the (scientific) palliative care community, a debate concerning the appropriateness of the QALY’s use as part of the efficiency decision rule in palliative care is taking place.6,7,12–14
The QALY takes into account two factors: the quality (of life; “Q”) and the quantity (life years gained; “LY”) generated by healthcare interventions. In the QALY, the length of time spent in a certain health state is weighed by the utility score given to that health state.15 For instance, 1 year of perfect health is worth one QALY, a year of less than perfect health is worth less than one QALY, and death is considered to be equivalent to zero QALYs. Some health states may be considered worse than death and have negative scores.15 By integrating Q and LY, the QALY provides a common metric to measure the added values from a variety of interventions, making it useful for budget allocation. In principle, deciding to allocate resources toward a specific intervention depends on the value for money question in terms of societal willingness to pay for a QALY gained.
This general application of the QALY, however, also contains a major objection.16 Some think that the nature of palliative care makes it more difficult to provide evidence on efficiency, which puts palliative care in a disadvantaged position when competing for resources with other healthcare services that have better evidence.17–19 It is argued that other approaches, such as the capability approach, in which capabilities are considered rather than functioning,14 might provide a richer evaluative space. The aim of this review is to systematically map pros and cons of using the QALY to inform decisions on resource allocation among palliative care interventions as brought forward in the debate and to discuss the QALY’s value for palliative care.
Methods
Rationale
In order to unfold the coherent body of knowledge, insights generated from separate studies were integrated using Whittemore and Knafl’s20 methodology for integrative reviews. Both non-empirical (theoretical) and empirical (CEAs) literature was searched. Theoretical literature was analyzed from bottom-up to find and compare arguments regarding the appropriateness of using the QALY to inform decisions on resource allocation among palliative care interventions. All the pro- and con arguments were presented in their original form regardless of their strength. In the discussion, the various arguments were critically appraised and the value of QALYs for palliative care was discussed. Analysis of the CEAs focused on identifying whether the perceived difficulties are described in research practice.
Literature search and data extraction
A literature search was conducted in the electronic databases PubMed, EMBASE, and CINAHL (Table 1). MeSH (Medical Subject Headings) terms in the search strategy were Palliative Care, Cost-Benefit Analysis, Quality of Life, and Quality-Adjusted Life Years. The search was limited to English-language articles published between 2000 and May 2015. In March 2016, a search update was done. Reference lists were scanned iteratively for supplementary publications.
Table 1.
Electronic databases for search strategies.
| PubMed | EMBASE | CINAHL |
|---|---|---|
| (((((((Quality Adjusted Life Year[tiab] OR Quality Adjusted Life Years[tiab] OR QALY[tiab] OR QALYs[tiab]))) OR “Quality-Adjusted Life Years”[Mesh])) OR (((((“Quality of Life”[Mesh]) OR quality of life[tiab]) OR life quality[tiab])) AND (((“Cost-Benefit Analysis”[Mesh]) OR ((Cost Benefit[tiab] OR Cost Effectiveness[tiab] OR Cost Utility[tiab] OR Costs and Benefits[tiab] OR Benefits and Costs[tiab]))))))) AND (((((“Hospice Care”[Mesh]) OR “Terminal Care”[Mesh:noexp])) OR “Palliative Care”[Mesh]) OR ((Palliative[tiab] OR Terminal care[tiab] OR End of life care[tiab] OR EOLC[tiab] OR EOL care[tiab] OR hospice care[tiab] OR Hospice Programs[tiab] OR Hospice Program[tiab]))) | (terminal care/or hospice care/OR palliative therapy/OR (Palliative or Terminal care or End of life care or EOLC or EOL care or hospice care or Hospice Programs or Hospice Program).ti,ab.) AND ((Cost Benefit or Cost Effectiveness or Cost Utility or (Costs and Benefits) or (Benefits and Costs)).ti,ab. OR cost benefit analysis/or cost effectiveness analysis/) AND (exp “quality of life”/OR (quality of life or life quality).ti,ab OR (Quality Adjusted Life Year or Quality Adjusted Life Years or QALY or QALYs).ti,ab. OR quality adjusted life year/) | ((MH “Hospice Care”) OR (MH “Palliative Care”) OR (MH “Terminal Care”) OR (TI Palliative OR Terminal care OR End of life care OR EOLC OR EOL care OR hospice care OR Hospice Programs OR Hospice Program) OR (AB Palliative OR Terminal care OR End of life care OR EOLC OR EOL care OR hospice care OR Hospice Programs OR Hospice Program)) AND ((MH “Cost Benefit Analysis”) OR (TI Cost Benefit OR Cost Effectiveness OR Cost Utility or (Costs and Benefits) or (Benefits and Costs)) OR (AB Cost Benefit OR Cost Effectiveness OR Cost Utility or (Costs and Benefits) or (Benefits and Costs))) AND ((MH “Quality of Life”) OR (MH “Comfort”) OR (TI quality of life OR life quality) OR (AB quality of life OR life quality) OR (MH “Quality-Adjusted Life Years”) OR (TI Quality Adjusted Life Year or Quality Adjusted Life Years or QALY or QALYs) OR (AB Quality Adjusted Life Year or Quality Adjusted Life Years or QALY or QALYs)) |
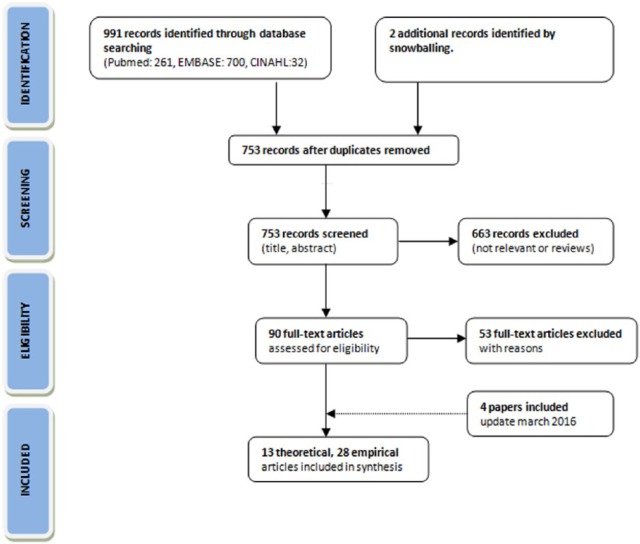
In assessing the records identified by the database search, the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flowchart was used (Figure 1).21 After identification of the records and the removal of duplications, titles and abstracts were screened for their relevance. If the article did not concern our research question, for example, when the title or abstract not indicated the study concerned cost-effectiveness/utility analysis within the palliative care field, it was excluded. Then, full-text articles were read to evaluate their eligibility. Inclusion and exclusion (Tables 2 and 3) in both the screening and the eligibility rounds were independently done by two researchers (A.B.W. and S.K.). Since the emphasis was on finding various pros and cons of using the QALY in palliative care, an inclusive sampling approach was used. That is, all titles that seemed to be of interest were included. Primary sources were not assessed on their individual quality.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram.22
Table 2.
Inclusion and exclusion criteria of theoretical papers.
| Inclusion | Exclusion |
|---|---|
| Years 2000–2016 | Reviews |
| Non-empirical papers (articles, editorial letters, etc.) | Non-English language studies |
| Studies about pros or cons of using QALYs in palliative care | |
| Studies about quantifying quality of EoLC |
QALY: Quality-Adjusted Life Year; EoLC: end-of-life care.
Table 3.
Inclusion and exclusion criteria of empirical (CE) papers.
| Inclusion | Exclusion |
|---|---|
| Years 2000–2016 | Reviews |
| Journal articles | Non-English language studies |
| Cost-effectiveness/utility studies | Conference abstracts |
| Studies in advanced, mortally ill patients (EOL period during which a person’s condition is actively deteriorating and when death is expected) | Study protocols |
| Studies written from palliative care paradigm | BSC studies |
| Studies in non-human or children | |
| Broader palliative care studies | |
| Broader health economic studies (without CE analyses) | |
| Chronic/allergic/non-EOL diseases | |
| First-line/primary treatments | |
| Critical/intensive care studies | |
| Studies about predictive testing/prevention/screening | |
| Studies on developing/evaluating new interventions |
CE: cost-effectiveness; EOL: end of life; BSC: best supportive care.
Data analysis
In order to provide a comprehensive understanding of pros and cons of using QALYs to inform decisions on resource allocation among palliative care interventions, the storyline of the coherent body of knowledge was unfolded. Heterogeneous literature (Tables 4 and 5 in Appendix 1) was explored and analyzed. The research strategy for integrative reviews was used for data analysis.20 Two subgroups consisting of theoretical and empirical literature were analyzed separately (see the following subsections). Subsequently, the theoretical and empirical subgroups were integrated.23
Theoretical literature
Pros and cons of using the QALY in palliative care, as well as alternative outcome measures and approaches, were inferred from the theoretical literature. All arguments from primary sources were coded, ordered, and clustered to identify patterns that could be translated into themes (Table 6 in Appendix 1). To meaningfully analyze the arguments, it was done in chronological order.
Empirical literature
Empirical CEAs were studied to find out whether perceived difficulties are experienced in research practice in the theoretical literature and how they are being dealt with, for example, are alternative approaches or outcome measures used? A data extraction form was used to systematize all findings. The empirical studies were ordered alphabetically, by author name, and on methodological characteristics (Table 5 in Appendix 1).
Findings
Of the 993 publications initially identified through database searching and snowballing, 753 studies of potential interest were left after removal of duplications (Figure 1). A total of 13 theoretical and 30 empirical CEAs were included. The theoretical literature encompassed clinical, economic, policy/management, and philosophical studies. Tables 4 and 5 in Appendix 1 show the characteristics of the included studies.
Interpretation and integration
After ordering all arguments regarding the use of the QALY to inform decisions on resource allocation among palliative care interventions—as they were originally brought forward in the literature—three themes were identified from bottom-up (Table 6 in Appendix 1). These themes concern groups of arguments about the “life years gained,” “conceptualization of quality of life (QoL) and its measurement,” and the “valuation and additivity of time” elements of the QALY. Some of these arguments could also be found in the empirical literature (Table 7 in Appendix 1), and some were supported by alternative outcome measures or approaches such as the “PalY” and the “Peak End Rule.” In the discussion, we critically appraise the main arguments and the value of QALYs for palliative care.
Theme 1. Life years gained
Low life expectancy is considered to be a problem (con)
Some authors view the LY component of the QALY as problematic. It is argued that the main objective of palliative care is to improve QoL and enable people to opt for a dignified death14 and not (necessarily) prolonging survival.5,14,18 Egan,5 for example, argues that the QALY has the implicit and flawed assumption that interventions should prolong survival to be valuable. He states that since patients eligible for palliative care have a relatively low life expectancy, any life-saving therapy will result in potentially higher QALY gains.5 A consequence of this assumption, it is posed, is that even when costs are quite modest, palliative care interventions cannot prove themselves to be cost-effective, as there is no enough time for them to generate QALYs.5,12 This difficulty is also encountered in empirical studies.24,25,26 For instance, Stevenson et al.24 state that “any survival advantage has a marked effect on the cost-effectiveness, which reflects the frequent issue that it can be more cost-effective to let patients die rather than to use relatively costly treatments.”
Gains in QALYs are possible even if life expectancy is low (pro)
Hughes6 contends against this argument by citing Keynes’ “in the long run we are all dead.” This fact, he argues, does not make QALY analysis inapplicable across the board. After all, our QoL matters while we are alive, and this is what the QALY seeks to capture, too.6 This view is supported by Round,7 who by means of an illustration shows that increases in QALYs are possible even if life cannot be lengthened. This is also backed by empirical studies that found that their results were most sensitive to changes in utilities,27 and that palliative therapies began to gain very high QALY values with only modest decreases in QoL.28 This implies that the Q weight significantly influences the QALY, and that survival advantage even seems to be undermined by declines in QoL. Moreover, Hughes6 poses the objective of improving QoL (or limit its potential loss, red.) rather than increasing life expectancy is true for other non-life prolonging interventions which can be measured in QALYs, such as hip operations. In other words, gains in QALYs are possible even if one of its components does not change significantly, since improvements can be made in the other component.7
Theme 2. Conceptualization of QoL and its measurement
Health-related domains are less relevant in palliative care (con)
Other arguments concern the Q weight of the QALY. One of the main arguments is about its conceptualization and measurement. The instrument predominantly used to measure the Q weight is the EuroQol instrument (EQ-5D). However, the health-related QoL (HR-QoL) dimensions—pain/discomfort, anxiety/depression, mobility, self care, and usual activities—that are covered by this instrument are often seen as less relevant in the context of palliative care, in which values such as patient dignity, spiritual and psychosocial well-being, and bereavement support are central. These rather broad, multidimensional, complex, and holistic intentions of palliative care are said to be lacking in HR-QoL measurement instruments such as the EQ-5D.12,29 In other words, the authors argue that the EQ-5D is mainly concerned with health and the recovery of health, and not with the quality of end of life (EoL) or dying.
Do not dismiss the framework: develop valid measurement instruments (pro)
Yang and Mahon,30 however, argue that palliative care and the QALY are compatible. They state that “like QALY and cost-utility calculations, palliative care involves a benefit-burden analysis for optimal treatment recommendations.” However, they argue that palliative care must be optimally integrated in QALY calculations.8 Other authors are also convinced that, despite current difficulties, the champagne should not be thrown out with the cork. Improper measurement (as of yet) of the Q weight of the QALY should not result in a relinquishment of the whole outcome measure.6,7,31 Hughes6 argues, what should be done is develop the best ways for estimating QoL. According to Chochinov,29 in palliative care, this means measurement should embrace a perspective as broad as the notion of QoL itself. With further refinements of analytical instruments, the conviction is that palliative care can be optimally integrated in the QALY calculation.30 Coast,14 with the capability approach, argues for a richer evaluative space when measuring QoL.
… this flexibility is offered in the extra-welfarist framework (pro)
Round7 agrees that instruments could be developed that take account of the domains of relevance to a certain population. This flexibility of preference-based measurement is said to be offered within the extra-welfarism framework. In this (conceptual) approach, non-health domains next to or instead of health-related domains, or capabilities instead of functioning,14 can or should preferably be considered when estimating QALYs. It is argued that accepting some degree of heterogeneity in QoL measurement may be less detrimental than squeezing all evaluation activity into standard instruments.18 This flexibility, however, is said to be rarely applied in research practice,7 and “the fact that researchers have not taken advantage of this flexibility is not a criticism of the framework itself.”7
An extra-welfarist capability approach (con)
Coast and colleagues,14,32 inspired by the work of Amartya Sen,33–35 make the case for the capability approach. In this approach, interventions are not based solely on functioning but are assessed based on their impact on what a person is able to do or be—in terms of capabilities that allow a person to have a good EoL.14 Although there is disagreement on the scope to which these capabilities can differ,36,37 Coast14 advocates for different sets of capabilities in different contexts.
The Palliative Care Yardstick as alternative approach (con)
An alternative approach also using this flexibility is suggested by Normands’12 Palliative Care Yardstick (PalY). By adding items to the QALY, the PalY would incorporate dimensions of palliative care (i.e. caring externalities) that are not considered when calculating QALYs.12 This approach, however, has not yet been studied in practice.
(Availability of) instruments not known (con)
In the empirical literature, mostly (standard) HR-QoL measurement instruments—such as the EQ-5D, EORTC QLQ-LC13/30, and SF-36—were used (Table 5 in Appendix 1). In one of these CEAs, it is noted that standard HR-QoL instruments were used that do not include QoL domains specifically relevant for the valuation of EoLC, due to the assumption that “unfortunately, no valuation instrument exists that incorporates such issues.”38 In the empirical literature, some authors look for intermediate solutions for this problem by taking into account aspects of HR-QoL that have the largest impact on general QoL in palliative patients. Barton et al.39 for example, use response to pain treatment in their utility calculations, since “chronic pain has an enormous effect on QoL of patients with bone metastases.” Pace et al.40 measure rehospitalization, as this is correlated with a lack of symptom control, worsening patient QoL.
Linear continuum and the narrative approach (con)
Another main argument against the use of the QALY in palliative care is its assumption of a mathematical linear continuum between death (0) and excellent health (1).5,12 Authors specifically have a problem with the fixed valuing of zero for death, due to which the benefits of a good or desired death currently cannot be captured.12,41 Normand12 argues that the non-linearity could be “accepted” by putting a value on components of a good death, and that this non-linear valuing would be separate from the days that led up to it (We will come back to the valuation question in theme 3.). The linear continuum assumption is challenged by the narrative approach.6 This theory describes that the manner in which a life ends impacts the overall value of that life. In this approach, the benefit of good EoLC is independent of any particular time-slice and thus cannot be captured by the QALY.6 Hughes6 and Cowley42 are in favor of this approach, as good terminal care adds greater meaning to the past life, and “the increase in rediscovered meaningful years can be measured backwards rather than forwards.” Cowley argues that this increase in rediscovered life years can even constitute new quality life years, since quality is retrospectively added to lived years.
Theme 3. Valuation and additivity of time
Valuation of time increases as time is running out (con)
As briefly mentioned above, the QALY assumes that preferences on time are stable. Therefore, in the QALY methodology, it is common practice to weight each year of added life equally. That is, time for any individual at any point in time is treated as being constant, making it additive.18 By some, this feature of additivity is seen as problematic,5,12 since valuation of time might not be fixed.12,29,43 It would increase as time itself runs out.29,41 Chochinov29 describes it as follows: “Each moment becomes increasingly precious as death draws near, while for the rest of the world, the clock marks time at its usual pace, with its usual indifference.”
Adding the Valuation Index Palliative to the PalY (con)
Normands’ previously introduced PalY not only suggests adding items to the QALY, but also deals with the valuation problem. For example by allowing a value to be put on components of a “good death,” which is separate from the days that led up to it. Chochinov further explores the PalY suggestion by adding the Valuation Index Palliative (VIP). In the VIP, the supposed increasing value of time as death gets closer is taken into account by ascribing a higher value to time in proximity to death.29 Although these concepts did not (yet) reach research practice, Furlan et al., in their empirical study, theorize about this idea:
… patients at the EoL tend to have low QALYs because of very poor health status […] This raises the question of whether economic evaluations […] ought to use some adjustment that would give additional weight to gains to health occurring at the EoL.
Billingham et al.44 may have used the VIP. They state that their CEA takes no account of the diminishing marginal utility, but that “an extra 2 months of good QoL to a life-expectancy of 6 months is potentially more valuable than an extra 2 months to a much better average life-expectancy.”
And: the willingness to pay for it increases (con)
When following this line of reasoning, a QALY gained at the EoL would not be equivalent to a QALY gained earlier in life.41 Moreover, a rising willingness to pay for time gained at the EoL is assumed.29 This line of reasoning can also be found in empirical studies. Arguedas et al.45 state that in their CEA, “a value closer to $100,000 per QALY [instead of the regularly cited threshold of $50,00046] might more accurately reflect societal preferences.” Furlan et al.47 argue that their results “indicate that increased expenditures are needed to impact patients’ QoL for such morbid clinical conditions.” According to Haycox,41 society indeed appears to show a willingness to pay for palliative care that lies above the level that would be considered “rational.”
Or doesn’t it? (pro)
Hughes6 though, objects to this reasoning since, according to him, it is not clear that palliative patients have greater needs than others. So, it cannot be the sole criterion for distribution of resources. He poses it should be combined with some measure of benefit.6 Round7 agrees that equity issues arise when resource allocation decisions are made based on situations that are no more unique to patients at the EoL than they are at any other life stages. Round43 puts forward that, as patients themselves are willing to spend increased sums on their care at the EoL, it may not be that the value of time to the individual increases but that the value of alternative uses of the individual’s resources decreases.
The Peak End Rule as alternative approach (con)
When following the non-linear rationale, periods of time cannot be added up at different points in time. Not even after adjusting for quality, since the value behind different time-slices may differ.6,12 Normand12 even states that when adding up benefits for (different) individuals, theorems in welfare economics are violated. This is why it is argued that Kahneman et al.’s48 Peak End Rule theory is applicable. The idea that there are circumstances where people put more or less value on time is supported by this theory. It describes that the way people evaluate past experiences tends to be based on the most intense points (best or worst) and how they end. Authors using Kahnemans’ theory argue that people caught in the gravity of approaching death encounter a profound distortion of how time is experienced and valued.29
But … in what direction does it change? (pro)
Others, however, argue that the assumption that time spent in the terminal phase of life is valued more highly is currently without empirical support.7 It is stated that even if valuation of time changes throughout life, it is not clear in which direction.43 Furthermore, the valuation of time objection is stated to ignore the option of weighing health gains differently for different populations.7
Discussion
We integrated theoretical and empirical literature on arguments concerning the appropriateness of using QALYs to inform decisions on resource allocation among palliative care interventions.20 A total of 13 theoretical and 30 empirical CEAs were included. The theoretical literature encompassed studies from various theoretical bases and perspectives (Table 4 in Appendix 1), which made the juxtaposition of all arguments challenging. Nonetheless, three themes regarding the pros and cons of using the QALY, as well as difficulties concerning its use in research practice (CEAs), were identified: (1) life years gained, (2) QoL measurement and conceptualization, and (3) valuation and additivity of time. Below, we iterate the main arguments theme by theme, critically appraise them, and discuss the QALY’s value for palliative care and potential implications for practice or policy.
Theme 1. Life years gained
In this theme, the main argument against the use of the QALY is that not enough can be gained in its LY component.5,18 This allegedly results in disadvantageous cost-effectiveness ratios in palliative care compared to other healthcare fields.5 Others object that, since the Q weight significantly influences the QALY outcome,6,7 increases in QALYs are possible even if life is not lengthened. Indeed, QALY gains have been reported in empirical studies.27,28
Appraising the above argument, it is clear that—mathematically—improvements in QoL can and will generate QALYs. However, given the short survival, the scope for this (but; also for rises in costs) is clearly limited. Also, higher thresholds for diseases with a high disease burden can be used. Moreover, we want to emphasize that the discussion on the appropriateness of using the QALY in CEAs in palliative care takes place in the narrow context of economic evaluation, where new interventions are compared to a “best alternative,” mostly standard care.49 This means that in research practice, comparators are faced with the same context and constraints. Research has even shown that early palliative care and symptom control not only improve QoL but also, without the use of aggressive medical care, translate into prolonged survival.50–52 So, when calculating QALYs in palliative care, there is a fair competition between competing interventions. Nevertheless, when using the QALY for the allocation of financial resources on a macro level, other very relevant questions—such as how much may a QALY cost?53—are in play that deserve thorough exploration.
Theme 2. QOL measurement and conceptualization
A major perceived objection regarding the use of the QALY in palliative care is that it takes into account health-related domains, which are considered less relevant than other dimensions of QoL. Therefore, it is argued that alternative QoL measurement instruments should be developed that embrace a perspective as broad as the notion of QoL itself29 and that the flexibility as offered in the extra-welfarist framework even makes it possible to use other QoL concepts broader than HR-QoL—such as the capability approach.14,31,54 This flexibility, however, is seldom used according to some.7,18 Moreover, several authors mentioned that standard HR-QoL instruments were used because of the assumption that no valuation instrument exists that incorporates EoLC issues.
However, to appraise these arguments, there are several instruments taking into account EoLC values such as peace, emotions, and spiritual and psychosocial well-being (e.g. the ICECAP-SCM—measuring capabilities—the POS, and the FACIT-Pal).55,56 Probably, they are hardly used as they are not suggested in CEA guidelines; the EQ-5D is the norm.10,11 But in the QALY framework, deviation from this norm is legitimate with solid arguments. Therefore, we recommend researchers in palliative care to, in the first place in addition to the EQ-5D, use these alternative instruments.
Moreover, a strict weighing of HR-QoL leads to unfavorable QALY results for healthcare domains that do not primarily focus on improving HR-QoL (but, for example, on improving autonomy, social well-being, or capabilities). In these domains, standard HR measurement tools are biased estimators as benefits (other than the EQ-5D dimensions) are missed. Therefore, we suggest to move to a broader concept of QoL. The time for doing this is right, since the new concept of health—in which health no longer refers to a state of complete well-being (WHO definition 1948), but to the ability to adapt and self-manage—more or less closes the gap between HR-QoL and QoL.57
Theme 3. Valuation and additivity of time
In the third theme, it is argued that time episodes throughout life may be valued differently, and that this should be taken into account when making budget allocation decisions. The Peak End Rule theory is invoked to back the argument of varying valuations of time. To deal with the increasing valuation of time as death gets closer, the PaLY and VIP are introduced as alternative approaches. Others pose that it is not clear in which direction time preferences act.43
Appraising these arguments, we note that there is no scientific consensus on the idea of the increasing valuation, and thus additivity, of time. However, more voices are heard on the non-linearity and changing valuation of time in proximity to death,48,58 while in the normative framework of the QALY, valuation of time is considered linear. Other descriptive models on valuation of time—such as the Peak End Rule,48 maximal endurable time,59 and lexicographic preferences, for example, the primacy of the “Q” over the “LY” weight—may be alternatives. We suggest further exploring the possibility of integrating valuation of time might be in a non-linear way in the QALY framework, for example, by operationalizing the VIP. If it is not legitimate to add up quality-adjusted time periods, it might be worthwhile to consider “whole experiences” and determine how these are valued.48,60
Strengths and limitations
Our integrative review offers the first systematic overview of pros and cons for using the QALY to inform decisions on resource allocation among palliative care interventions, adding new insights to the broader topical issue of whether everything that can be done or must be done. In our review, however, we focused on “technical” efficiency, informing allocation decisions among palliative care interventions only. Although this information is of importance, it cannot be used to inform on resource allocation throughout healthcare.61 Moreover, because of controversy about the definition of “palliative care” in the field, we might have missed studies of importance. Furthermore, since we choose to bundle arguments in pros and cons, and analyzed them from bottom-up, our presentation might not have captured every link, making the discussion seem more black-and-white than it actually is. For example, the link between themes 1 and 3 (if you agree that time may not be additive, then the problem of short time horizons is less of an issue) was not reflected in the bottom-up analysis. Finally, although important for the QALY discussion across the entire width, the debate around QALY issues on a macro level, preferences in relation to health, and who should value these preferences was put aside.53,62,63
Conclusion
Three themes regarding the appropriateness of using QALYs to inform decisions on resource allocation among palliative care interventions were identified. The debate as identified in theoretical literature is recognized in the empirical literature. However, alternative outcome measures are not used. Despite criticism, concerning theme 1, the limited gain in LY in palliative care, QALYs can be gained, despite the fact that palliative care itself not primarily aims at this weight. Moreover, in the (narrow) context of economic evaluation, new interventions face the same context and constraints as their competitors, making the limited-scope issue less of an issue. In theme 2, it was argued that standard measurement of the Q weight of the QALY—for example, based on the EQ-5D or measuring functioning (instead of capabilities) at all—does not fit the palliative care context. We recommend making use of the possibility to use additional QoL or capabilities measurement instruments that incorporate important values for palliative care patients. As for theme 3, we suggest exploring whether valuation of time might be integrated into the QALY framework in a non-linear way. In short, the QALY might be more valuable when informing decisions on resource allocation among palliative care interventions, when specific issues related to the above-mentioned themes are taken into account.
Appendix 1
Table 4.
Characteristics of non-empirical “theoretical” papers.
| Author(s) | Title | Journal | Type of article |
|---|---|---|---|
| Egan5 | QALYs or quackery? The quagmire of quantifying the cost of breathing | Journal of Thoracic and Cardiovascular Surgery | Editorial |
| Hughes6 | Palliative care and the QALY problem | Health Care Analysis: Journal of Health Philosophy and Policy | Journal article |
| Normand12 | Measuring outcomes in palliative care: limitations of QALYs and the road to PalYs | Journal of Pain & Symptom Management | Special article |
| Haycox41 | Optimizing decision making and resource allocation in palliative care | Journal of Pain & Symptom Management | Special article |
| Cowley42 | Justifying terminal care by “retrospective quality-adjusted life-years” | Journal of Medical Ethics | Ethics |
| Chochinov29 | Death, time and the theory of relativity | Journal of Pain & Symptom Management | Special article |
| Chochinov70 | Relatively speaking. To the editor. | Journal of Pain & Symptom Management | |
| Yang and Mahon30 | Considerations of quality-adjusted life-year in palliative care for the terminally ill | Journal of Palliative Medicine | Editorial letter |
| Normand12 | Setting priorities in and for end-of-life care: challenges in the application of economic evaluation | Health Economics, Policy and Law | Journal article |
| Round7 | Is a QALY still a QALY at the end of life? | Journal of Health Economics | Journal article |
| Round43 | Death, time, and the theory of relativity: a brief reply? | Journal of Pain & Symptom Management | Editor letter |
| Yang and Mahon8 | Palliative care for the terminally ill in America: the consideration of QALYs, costs, and ethical issues | Medicine, Health Care and Philosophy | Scientific contribution |
| Coast14 | Strategies for the economic evaluation of end-of-life care: making a case for the capability approach | Expert Review of Pharmacoeconomics & Outcomes Research | Perspective |
QALY: quality-adjusted life year; PalY: Palliative Care Yardstick.
Table 5.
Characteristics of empirical (CEAs) papers.
| Author(s) | Research question | Patient characteristics | QoL assessment (for utilities) | QALY | Alternative measures | Outcomes |
|---|---|---|---|---|---|---|
| Arguedas et al.45 | Cost-effectiveness of initial plastic biliary stenting versus initial metallic stent placement for palliation | Patients with unresectable pancreatic carcinoma | Health state utilities by SG | Yes | Via quality-adjusted life months | Plastic stents US $92,578/QALY versus metal stents US $88,205/QALY |
| Barton et al.39 | Cost-utility of palliative RT in bone metastases. | Patients with bone metastases in advanced cancer | Utilities on pain relief. | Yes | Adjusted to utility adjusted survival | AUD 1200/utility-adjusted life year |
| Billingham et al.44 | Cost-effectiveness of MIC chemotherapy and/or palliative care | Patients with advanced NSCLC | EORTC QLQ-LC13 | No | Incremental cost gain of a single full year of survival | Palliative care ICER of £14,620 (95% CI: £6168–£21 612)/life year gained |
| Coy et al. (2000)71 | Cost-effectiveness of high-dose palliative RT treatment versus BSC | Patients with advanced NSCLC | Question 30 QLQ-C30 questionnaire (“rate your global QoL on an integer scale of 1–7”). | Yes | Via QALD | Estimated in-clinical and societal costs palliative RT 12,836 CAD and 17,012 CAD/QALY, respectively |
| Dooms et al.27 | CU single-agent gemcitabine versus second-generation cisplatin-based chemotherapy | Patients with advanced NSCLC | “How would you rate the quality of your life today?” question from LCSS, measured on a VAS | Yes | Single-agent gemcitabine cost–utility ratio of €13,836/QALY | |
| Furlan et al.26 | Cost-utility of combined use of surgery and RT | Metastatic cancer patients with epidural spinal cord compression | Utilities from Harvard University Catalogue and Health Outcomes Data Repository Data–Health Utility list | Yes | ICER of US$250,307/QALY | |
| Goldfeld et al. (2013)72 | Cost-effectiveness of not having a DNH order and hospitalization of suspected pneumonia | Nursing home residents with advanced dementia | The EOLD-SM and CAD-EOLD to reach utilities via the Health Utility Index Mark 2 | Yes | Via QALD | Estimated ICER no DNH order US $589,130/QALY; pneumonia hospitalization incremental increase of US $3697 and an incremental reduction in quality-adjusted survival of 9.7 QALD |
| Jeurnink et al. (2010)73 | Cost-effectiveness of GJJ versus stent placement as palliative treatments | Malignant GOO | EORT CQLQ-C30, EQ-5D, the EQ-VAS, and the EORTC QLQ-PAN26; pain and nausea scores by self-developed questionnaires | No | Only ICER calculated | Total costs per patient GJJ versus stent placement: €12,433 versus €8819; ICER GJJ versus stent placement: €164 per extra day |
| Johnson et al. (2015)74 | Cost-effectiveness of three breathing training sessions versus one | Patients with intra-thoracic malignancy | EQ-5D | Yes | Probability cost-effectiveness single session at a threshold value of £20,000/QALY >80% | |
| Kim et al.64 | Cost-effectiveness of single-fraction SBRT versus single-fraction EBRT in palliative treatment | Patients with back pain due to vertebral bone metastases | Utilities on pain relief | Yes | SBRT ICER of US $124,552/QALY | |
| Konski (2004)75 | Cost-effectiveness of pain medication only, chemotherapy, and single- and multi-fraction RT as palliative treatments | Patients with hormone-refractory prostate cancer with bone metastases | EQ-5D | Yes | Via quality-adjusted life months | Single-fraction RT US $6857/QALY, multi-fraction RT US $36,000/QALY. Chemotherapy dominated by pain medication |
| Konski et al. (2009)76 | Cost-effectiveness multiple-fraction treatment by preventing further retreatment | Patients with bone metastases | Utilities on pain relief | Yes | ICER of US $6973/QALY | |
| Ljungman et al.25 | Cost-utility estimations of palliative care in patients with pancreatic adenocarcinoma | Patients with unresectable pancreatic adenocarcinoma tumors who experienced palliative care | SF-6D derived from SF-36 items | Yes | Palliative care: €118,418/QALY; surgical resection: €106,146/QALY | |
| Lowery et al. (2013)77 | Cost-effectiveness of EPC | Patients with recurrent, platinum-resistant ovarian cancer | HR-QoL (VAS and TTO) of health state valuation | Yes | ICER <US $50,000/QALY, assuming no clinical benefit other than QoL, ICER: US $37,440/QALY | |
| Miller et al.65 | Cost-effectiveness of therapeutic options surgical resection, diagnostic or palliative surgery, non-operatively treated | Patients with locally recurrent rectal carcinoma | Utilities obtained from convenience samples by SG | Yes | Surgical resection versus non-operative management US $109,777/QALY. Reduced to US $56,698/QALY using mean patient utilities | |
| Olden et al. (2010)78 | Cost-effectiveness of treating MPEs with talc pleurodesis versus placement of Pleurx catheter | Patients with recurrent MPE with any type of cancer | Utilities obtained from literature | Yes | Talc US $29,077/QALY; Pleurx: US $32,650/QALY Pleurx is more cost-effective (<US $100K = QALY) when life expectancy is 6 weeks or less |
|
| Pace et al.40 | Cost-effectiveness of palliative homecare versus no homecare assistance at the EoL | Last-stage BT patients | Utilities from convenience samples using SG | No | Only hospital readmissions and costs. (not integrated in outcome measure, red.) | Hospitalization in homecare group lower (16.7%) than in non-homecare group (38%). Costs of hospitalization differed substantially (€517 vs €24,076 relatively). |
| Phippen66 | Cost-effectiveness of four management strategies (AO supportive care intervention through palliative care) | Recurrent cervix cancer patients | Administrative data on rehospitalization rate in the last 2 months of life | Yes | Extra ICER standard doublet chemotherapy versus selective chemotherapy of US $276,000/QALY. Selective chemotherapy more cost-effective than single-agent chemotherapy with home hospice with ICER of US $78,000/QALY | |
| Roberts et al. (2015)79 | Cost-effectiveness of surgery compared with non-operative treatment for patients with CRLMs | Patients with colorectal liver metastases | Parameterized model with QoL data from secondary data sources | Yes | Operative strategy and optimal strategy across all willingness-to-pay values for a QALY | |
| Roth et al. (2012)80 | Cost-effectiveness of the addition of cisplatin to gemcitabine | Patients with advanced biliary tract cancer | EQ-5D | Yes | Relative to gemcitabine monotherapy, gemcitabine + cisplatin ICER of US $59,480/QALY | |
| Sahlen et al. (2016)81 | Cost-effectiveness of PREFER intervention versus standard care | Patients with chronic and severe heart failure | EQ-5D | Yes | PREFER group: +0.006 QALYs; standard care group: −0.024 QALYs. | |
| Shafiq et al. (2015)82 | Cost-utility of five therapeutic alternatives for MPE | Patients with MPE from any cancer type | EQ-5D | ICER of rapid pleurodesis protocol over repeated thoracentesis at a permanent success estimate of 85% 65,091/QALY. | ||
| Shenfine et al.67 | Cost-effectiveness of SEMS versus rigid, plastic stents and non-stent therapies | Patients with inoperable esophageal cancer | EQ-5D | Yes | No numbers for extra QALY mentioned, only total costs and QALY differences | Difference in total costs and QALYs bootstrapped: non-SEMS treatment greater QALYs than SEMS, costs equivalent between groups; SEMS unlikely to be more cost-effective than non-SEMS |
| Stevenson et al.24 | Cost-effectiveness bosentan or no active intervention, in addition to palliative care, as first-line treatment | Patients with idiopathic pulmonary arterial hypertension | SF-36 | Yes | Bosentan compared with palliative care alone £30,000/QALY | |
| Teerawattananon et al. (2007)83 | To assess the value for money of providing PD or HD versus palliative care | End-stage renal disease patients | Study literature employing utility measurements, for example, HUI and EQ-5D | Yes | ICER initial treatment with PD and ICER initial treatment HD: 672,000 and 806,000 Baht/QALY gained (52,000 and 63,000 US$/QALY) compared with palliative care |
|
| Van den Hout et al.38 | Which RT (single or multiple) schedule provides better value for money? | Poor prognosis NSCLC | EQ-5D | Yes | Quality-adjusted weeks. No numbers for extra QALYs mentioned | Estimated QALYs and societal costs both favored the single-fraction schedule, providing an additional 1.7 quality-adjusted weeks and saving US $1753 relative to the multiple-fraction schedule |
| Van den Hout et al.68 | Which RT schedule (10 fractions of 3 Gy vs 2 fractions of 8 Gy) provides better value for the money? | Patients with painful bone metastases | EQ-5D | Yes | 10 × 3-Gy schedule versus 2 × 8-Gy schedule was estimated at US $40 900/QALY | |
| Xinopoulos et al. (2004)84 | Cost-effectiveness analysis comparing esophageal stenting with laser therapy | Patients with primary esophageal carcinoma | QLQ-C30 | No | Overall costs, changes QLQ-C30 | Mean survival and costs similar. Small difference of €156 noted (€3103 and €2947 for each group, respectively. |
QALY: quality-adjusted life year; QALD: quality-adjusted life days; MIC: mitomycin, ifosfamide, cisplatin; NSCLC: non-small-cell lung cancer; SG: standard gamble; HR-QoL: health-related quality of life; VAS: visual analogue scale; TTO: time trade-off; HUI: health utility index; EORTC: European Organisation for Research and Treatment of Cancer; ICER: incremental cost-effectiveness ratio; DNH: do-not-hospitalize; GJJ: gastrojejunostomy; GOO: gastric outlet obstruction; SBRT: stereotactic body radiation therapy; EBRT: external beam radiation therapy; RT: radiotherapy; SF-36: 36-Item Short Form Health Survey; MPE: malignant pleural effusion; BT: brain tumor; PREFER: Palliative advanced home caRE and heart FailurE care; BSC: best supportive care; EPC: early palliative care; EoL: end of life; SEMS: self-expandable metal stents; PD: peritoneal dialysis; HD: hemodialysis; EQ-5D: EuroQol Five-Dimensional Questionnaire; EOLD-SM: End-of-life in Dementia Scale - Symptom Management; CAD-EOLD: End-of-life in Dementia Scale - Comfort Assessment in Dying; EORTC QLQ-C30: European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Cancer; EORTC QLQ PAN26: European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Pancreatic Cancer; LCSS: Lung Cancer Symptom Score.
Table 6.
Main pros and cons of using the QALY in palliative care and suggested alternatives/approaches from theoretical literature mapped thematically.
| Cons | Pros | Alternatives | |
|---|---|---|---|
| Theme 1 | Objective palliative care is to improve QoL, not (necessarily) life expectancy.5,18 | Fact that “we are all dead in the long run” (Keynes), does not make the QALY inapplicable across the board.6 | |
| QALYs implicit assumption that interventions must increase life expectancy flawed.5 | Our QoL matters to us while we are alive, and this is what the QALY seeks to capture, too.6 | ||
| Because of low life expectancy, in palliative care effects enjoyed over short time, life-saving therapy will result in higher QALY gains.5,18 | QALY enables comparisons between competing demands by combining both quality and quantity of life in a single metric.7 | ||
| Even when costs are modest, palliative interventions cannot prove themselves cost-effective as no enough time for them to generate QALYs.18 | Increases in QALYs are possible; even if one of the weighing factors does not change significantly (i.e. if life cannot be lengthened), improvements can be made in the other.7 | ||
| Developing more accurate QoL instruments (link theme 2, red.) would not solve QALY problem; limiting factor short life expectancy.6 | Other non-life prolonging interventions, only increasing QoL (or limiting its potential loss, red.) can be measured in QALYs (e.g. hip operations).6,8 | ||
| Theme 2 | Analysis of outcomes needs to embrace complex and multidimensional objectives of palliative care, as broad as notion of QoL itself.12,29 | Palliative care and QALY are not incompatible. Like QALY and cost-utility calculations, palliative care involves a benefit-burden analysis.30 | Narrative theory |
| Limitations and standard outcome measures (like the EQ-5D) make comparisons inappropriate.12 | Palliative care can be optimally integrated into the calculation of the QALY.8,30 | ||
| Even if refinement analytical tools lead to increased assessment QoL, limiting factor still shortens life expectancy.6 | QALYs’ ability to rate changes in morbidity and mortality in a single measure and to enable comparison between competing demands for resources are as applicable in this population as in any other.7 | ||
| Resources tend to be biased away from services received at the EoL because they are hard to evaluate.12,18 | Scoring badly on measure of outcome is not a good reason to reject that measure.6 | ||
| Therapeutic nihilism undermines ability to see value beyond cure-oriented disease modification.29 | If aspects are missed or if there is a lack of precision in QALY analysis, this is a shortcoming of ways of measuring rather than failing of QALY approach.6,7 | ||
| Interventions could be assessed based on their impact on what a person is able to do or be (capabilities) and not solely on functioning.14 | In the capability approach, in which capabilities are taken into account instead of functioning, QoL is measured in a richer evaluative space.14 | Capability approach | |
| Dimensions of palliative care that are not considered when calculating QALYs can be added when using the PalY.12 | Instruments could be developed that take account of the domains of relevance to a certain population.7 | ||
| Assumption that there is a mathematical continuum between death and excellent health is a fundamental problem.5 | Non-HR domains can be considered in the QALY, but to date, they are not. Fact that researchers have not taken advantage of the flexibility (as offered in extra welfarism) is not a criticism of the framework itself.7 | ||
| Bad death can destroy much of value of total life,6 allowing a value to be put on components of good death.6,12,29,41 | Terminal care can be justified in QALY terms when refinement of definition of “quality” and “life.”42 | ||
| Assumption that there is a mathematical continuum between death and excellent health is a fundamental problem.5 | Living with heterogeneity in evidence used for policy choices is less serious than fitting all evaluation activity into systematically flawed frameworks.18 | ||
| If EoL patients are treated inequitably, an equity weight could be derived and applied as required.7 | |||
| Theme 3 | Valuation of time not fixed; it increases as time itself is running out.29,30 | Relative simplicity: time for any individual at any point in time has a constant value, which has useful properties (such as being additive).12 | Peak End Rule |
| A value can be put on components of a “good death,” which is separate from the days that led up to it (PalY).12 | Value of time changes throughout life, but not clear in which direction variable preference acts.43 | PalY | |
| Since valuation of time is not fixed, QALYs’ feature of additivity is problematic.5,12 | Valuing time spent in terminal phase is more high than time during other stages without empirical support.7,43 | VIP | |
| Periods of time cannot be added up at different points in time for individuals.12,18 | Assumption valuation of time should be determined by patients, while accepted practice that values placed on health states are determined by general population.43 | ||
| A QALY gained at the EoL is not equivalent to a QALY gained earlier in life.41 | It is not clear that palliative patients have greater needs than others.6 | ||
| Way in which life ends impacts overall value of that life.42 | Objection valuation of time ignores option of weighing health gains differently for different populations.7 | ||
| Benefit EoLC is an addition of value to life as whole, independent of any particular time-slice, which is not captured by QALY.6 | The need-principle cannot be the sole criterion for distribution of resources. It should be combined with some measure of benefit.6 | ||
| As time itself is running out, willingness to pay for it appears to increase.29 | Equity issues arise when resource allocation decisions are made based on situations no more unique to patients at the EoL than they are at any other life stage.7 | ||
| Economic principles suggest that value of time to individuals does not increase, but that value of alternative uses of individual resources decreases.43 |
QoL: quality of life; EoL: end of life; QALY: quality-adjusted life year; VIP: Valuation Index Palliative Care; PalY: Palliative Care Yardstick.
Table 7.
Main pros and cons of using the QALY in palliative care and suggested alternatives/approaches from CEAs mapped thematically.
| Cons | Pros | Alternatives | |
|---|---|---|---|
| Theme 1 | Any survival advantage has a marked effect on the cost-effectiveness, which reflects the frequent issue that it can be more cost-effective to let patients die rather than to use relatively costly treatments.24 | The results of this analysis are sensitive to changes in costs, but even more so to changes in utilities.27 | |
| … the results also highlight that palliative care interventions are likely to generate high ICERs. This is because patients have short remaining life spans over which to benefit from any treatment.47 | Phippen66 palliative therapies began to gain very high QALY values with only modest decreases in QoL. | ||
| If median survival ⩾18 months, SBRT costs $50,000/QALY or less, which is commonly cited as a benchmark of a ‘good buy’ for medical interventions […] most economically feasible approach would involve the judicious use of SBRT for spine metastases in patients with relatively long predicted survival.64 | With only modest decreases in QoL, both selective chemotherapy and single-agent chemotherapy with home hospice strategies began to exceed ICERs of $100,000/QALY. This finding suggests that any survival advantage gained in the chemotherapy-containing treatment arms may be blunted by the associated treatment toxicities, quickly making them cost-prohibitive.66 | ||
| These findings illustrate again that survival is by far the most important factor to target when striving to improve cost-effectiveness in cancer treatment of pancreatic carcinoma.25 | Survival after palliative therapy is an area that demands further research and may become a more central issue in palliation when treatments are combined.67 | ||
| If patients survived longer than 6 months, we would expect greater cost savings from the intervention.69 | |||
| The present findings illustrate that prolonged survival is a key factor to increasing cost-effectiveness, although it becomes necessary to calculate cost-utility over limited periods when it is to enable comparisons among severely ill patients.25 | |||
| Theme 2 | Besides these general attributes, there are other issues that are also specifically relevant in the valuation of EoLC. For example, psychosocial outcomes such as relieving the burden of care and strengthening relationships with loved ones are not included in the EQ-5D. Unfortunately, however, no valuation instrument exists that incorporates these specific end-of-life issues.38,68 | With only modest decreases in QoL, both selective chemotherapy and single-agent chemotherapy with home hospice strategies began to exceed ICERs of $100,000/QALY. This finding suggests that any survival advantage gained in the chemotherapy-containing treatment arms may be blunted by the associated treatment toxicities, quickly making them cost-prohibitive.66 | Rehospitalization as indicator for QoL |
| QoL is an important dimension, particularly in the palliation of terminal illness. Unfortunately, information about the QoL weight (utilities) in patients with unresectable pancreatic cancer is limited.45 | The results of this analysis are sensitive to changes in costs, but even more so to changes in utilities.27 | Utilities on pain, since pain is the single most important factor affecting QoL | |
| Duration of survival is not a meaningful endpoint in palliative care […] Chronic pain has enormous effect on QoL of patients with bone metastases. Hence, the duration of survival, adjusted for the degree of response to pain treatment, is a more appropriate endpoint.39 | |||
| No standardized method available for utility collection. […] May be possible QoL item may not cover all different domains QoL.27 | |||
| We observed a high incidence of distressing symptoms that may influence the QoL during the course of disease and the process of dying.40 | |||
| The main goals of palliative care and EoLC in brain tumor patients are to offer adequate symptom control, relief of suffering, avoiding inappropriate prolongation of dying, and to support psychological and spiritual needs of patients and families. The lack of control of symptoms in patients not included in palliative home-care programs often lead to rehospitalization with an increase in health system economic cost and worsening of patient QoL.40 | |||
| Theme 3 | Patients were more willing to gamble the risks associated with surgery and the possibility of developing pain or complications to have an opportunity to prolong life than were healthcare providers.65 | ||
| Study takes no account of diminishing marginal utility (extra 2 months of good QoL to life-expectancy of 6 months potentially more valuable than extra 2 months to much better average life- expectancy).44 | |||
| A recent meta-analysis […] suggests that a value closer to $100,000/QALY might more accurately reflect societal preferences.45 | |||
| A value closer to $100,000 per QALY might more accurately reflect societal preferences.45 | |||
| Our results indicate that increased expenditures are needed to impact patients’ QoL for such morbid clinical conditions.47 | |||
| Patients at the EoL tend to have low QALYs because of very poor health status […] this raises the question of whether economic evaluations […] ought to use some adjustment that would give additional weight to gains to health occurring at the EoL.47 |
QALY: quality-adjusted life year; QoL: quality of life; EoL: end of life; EoLC: end-of-life care; ICER: incremental cost-effectiveness ratio; PalY: Palliative Care Yardstick; EQ-5D: EuroQol Five-Dimensional Questionnaire.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the EU 7th Framework Programme, grant agreement 6031111.
References
- 1. World Health Organization (WHO). The right to health. Factsheet no. 31. Geneva: WHO, 2015. [Google Scholar]
- 2. National Institutes of Health (NIH). Improving end-of-life care: consensus and state-of-the-science statements. Bethesda, MD: NIH, 2004, pp. 1–28. [Google Scholar]
- 3. Hall S, Petkova H, Tsouros A, et al. Palliative care for older people: better practices. Geneva: World Health Organization (WHO), 2011. [Google Scholar]
- 4. World Health Organization (WHO). The world health report 2006: working together for health. Geneva: WHO, 2006. [Google Scholar]
- 5. Egan TM. QALYs or quackery? The quagmire of quantifying the cost of breathing. J Thorac Cardiovasc Surg 2002; 123: 406–408. [DOI] [PubMed] [Google Scholar]
- 6. Hughes J. Palliative care and the QALY problem. Health Care Anal 2005; 13: 289–301. [DOI] [PubMed] [Google Scholar]
- 7. Round J. Is a QALY still a QALY at the end of life? J Health Econ 2012; 31: 521–527. [DOI] [PubMed] [Google Scholar]
- 8. Yang YT, Mahon MM. Palliative care for the terminally ill in America: the consideration of QALYs, costs, and ethical issues. Med Health Care Philos 2012; 15: 411–416. [DOI] [PubMed] [Google Scholar]
- 9. Dolan P, Lee H, King D, et al. Valuing health directly. BMJ 2009; 339: b2577. [Google Scholar]
- 10. National Institute for Health and Clinical Excellence (NICE). Guide to the methods of technology appraisal 2013: process and methods guides. London: NICE, 2013. [PubMed] [Google Scholar]
- 11. ZINL. Richtlijn voor het uitvoeren van economische evaluaties in de gezondheidszorg. Zorginstituut Nederland 2015. Available at: https://www.zorginstituutnederland.nl/over-ons/werkwijzen-en-procedures/adviseren-over-en-verduidelijken-van-het-basispakket-aan-zorg/beoordeling-van-geneesmiddelen/richtlijnen-voor-economische-evaluatie. (accessed 26 January 2017).
- 12. Normand C. Measuring outcomes in palliative care: limitations of QALYs and the road to PalYs. J Pain Symptom Manage 2009; 38: 27–31. [DOI] [PubMed] [Google Scholar]
- 13. McNamee P, Seymour J. Incorporation of process preferences within the QALY framework: a study of alternative methods. Med Decis Making 2008; 28: 443–452. [DOI] [PubMed] [Google Scholar]
- 14. Coast J. Strategies for the economic evaluation of end-of-life care: making a case for the capability approach. Expert Rev Pharmacoecon Outcomes Res 2014; 14: 473–482. [DOI] [PubMed] [Google Scholar]
- 15. Phillips C, Thompson G. What is QALY? Health Econ 2009; 1: 400–405. [Google Scholar]
- 16. Sculpher MJ, Pang F, Manca A, et al. Generalisability in economic evaluation studies in healthcare: a review and case studies. Health Technol Assess 2004; 8: iii–iv, 1–192. [DOI] [PubMed] [Google Scholar]
- 17. Murtagh FEM, Groeneveld EI, Kaloki YE, et al. Capturing activity, costs, and outcomes: the challenges to be overcome for successful economic evaluation in palliative care. Progr Palliat Care 2013; 21: 232–235. [Google Scholar]
- 18. Normand C. Setting priorities in and for end-of-life care: challenges in the application of economic evaluation. Health Econ Policy Law 2012; 7: 431–439. [DOI] [PubMed] [Google Scholar]
- 19. Kinghorn P, Coast J. Do we have the correct health economics methods to evaluate end of life care? An analysis of stakeholder perspectives. BMJ Support Palliat Care 2014; 4: A4–A5. [Google Scholar]
- 20. Whittemore R, Knafl K. The integrative review: updated methodology. J Adv Nurs 2005; 52: 546–553. [DOI] [PubMed] [Google Scholar]
- 21. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 2010; 8: 336–341. [DOI] [PubMed] [Google Scholar]
- 22. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009; 151: 264–269. [DOI] [PubMed] [Google Scholar]
- 23. O’Cathain A, Murphy E, Nicholl J. Three techniques for integrating data in mixed methods studies. BMJ 2010; 341: c4587. [DOI] [PubMed] [Google Scholar]
- 24. Stevenson MD, Macdonald FC, Langley J, et al. The cost-effectiveness of bosentan in the United Kingdom for patients with pulmonary arterial hypertension of WHO functional class III. Value Health 2009; 12: 1100–1105. [DOI] [PubMed] [Google Scholar]
- 25. Ljungman D, Hyltander A, Lundholm K. Cost-utility estimations of palliative care in patients with pancreatic adenocarcinoma: a retrospective analysis. World J Surg 2013; 37: 1883–1891. [DOI] [PubMed] [Google Scholar]
- 26. Furlan JC, Chan KK, Sandoval GA, et al. The combined use of surgery and radiotherapy to treat patients with epidural cord compression due to metastatic disease: a cost-utility analysis. Neuro Oncol 2012; 14: 631–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dooms CA, Lievens YN, Vansteenkiste JF. Cost-utility analysis of chemotherapy in symptomatic advanced non small cell lung cancer. Eur Respir J 2006; 27: 895–901. [DOI] [PubMed] [Google Scholar]
- 28. Phippen N, Leath IC, Miller C, et al. Is a home-based palliative care treatment strategy preferable to standard chemotherapy in recurrent cervical cancer? Gynecol Oncol 2013; 131(1): 277–278. [DOI] [PubMed] [Google Scholar]
- 29. Chochinov HM. Death, time and the theory of relativity. J Pain Symptom Manage 2011; 42: 460–463. [DOI] [PubMed] [Google Scholar]
- 30. Yang YT, Mahon MM. Considerations of quality-adjusted life-year in palliative care for the terminally ill. J Palliat Med 2011; 14: 1197. [DOI] [PubMed] [Google Scholar]
- 31. Coast J, Smith RD, Lorgelly P. Welfarism, extra-welfarism and capability: the spread of ideas in health economics. Soc Sci Med 2008; 67: 1190–1198. [DOI] [PubMed] [Google Scholar]
- 32. Coast J, Smith R, Lorgelly P. Should the capability approach be applied in health economics? Health Econ 2008; 17: 667–670. [DOI] [PubMed] [Google Scholar]
- 33. Sen A. Inequality reexamined. New York: Russell Sage Foundation; Oxford: Clarendon Press, 1992. [Google Scholar]
- 34. Sen A. Capability and well-being. In: Nussbaum MC, Sen AK. (eds) The quality of life. Oxford: Clarendon Press, 1993, pp.30–66. [Google Scholar]
- 35. Sen A. The idea of justice. New York: JSTOR, 2009. [Google Scholar]
- 36. Nussbaum M. Capabilities as fundamental entitlements: Sen and social justice. Fem Econ 2003; 9: 33–59. [Google Scholar]
- 37. Alkire S. Why the capability approach? J Hum Dev 2005; 6: 115–135. [Google Scholar]
- 38. Van den Hout WB, van der Linden YM, Steenland E, et al. Single- versus multiple-fraction radiotherapy in patients with painful bone metastases: cost-utility analysis based on a randomized trial. J Natl Cancer Inst 2003; 95: 222–229. [DOI] [PubMed] [Google Scholar]
- 39. Barton MB, Jacob SA, Gebsky V. Utility-adjusted analysis of the cost of palliative radiotherapy for bone metastases. Australas Radiol 2003; 47: 274–278. [DOI] [PubMed] [Google Scholar]
- 40. Pace A, Di Lorenzo C, Capon A, et al. Quality of care and rehospitalization rate in the last stage of disease in brain tumor patients assisted at home: a cost effectiveness study. J Palliat Med 2012; 15: 225–227. [DOI] [PubMed] [Google Scholar]
- 41. Haycox A. Optimizing decision making and resource allocation in palliative care. J Pain Symptom Manage 2009; 38: 45–53. [DOI] [PubMed] [Google Scholar]
- 42. Cowley C. Justifying terminalcare by “retrospective quality-adjusted life-years.” J Med Ethics 2010; 36: 290–292. [DOI] [PubMed] [Google Scholar]
- 43. Round J. Death, time, and the theory of relativity: a brief reply? J Pain Symptom Manage 2012; 43: e2–e6. [DOI] [PubMed] [Google Scholar]
- 44. Billingham LJ, Bathers S, Burton A, et al. Patterns, costs and cost-effectiveness of care in a trial of chemotherapy for advanced non-small cell lung cancer. Lung Cancer 2002; 37: 219–225. [DOI] [PubMed] [Google Scholar]
- 45. Arguedas MR, Heudebert GH, Stinnett AA, et al. Biliary stents in malignant obstructive jaundice due to pancreatic carcinoma: a cost-effectiveness analysis. Am J Gastroenterol 2002; 97: 898–904. [DOI] [PubMed] [Google Scholar]
- 46. Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness—the curious resilience of the $50,000-per-QALY threshold. New Eng J Med 2014; 371: 796–797. [DOI] [PubMed] [Google Scholar]
- 47. Furlan JC, Chan KK, Sandoval GA, et al. The combined use of surgery and radiotherapy to treat patients with epidural cord compression due to metastatic disease: a cost-utility analysis. Neuro Oncol 2012; 14: 631–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kahneman D, Diener E, Schwarz N. Well-being: foundations of hedonic psychology. New York: Russell Sage Foundation, 1999. [Google Scholar]
- 49. Drummond M, Sculpher M, Torrance G, et al. Methods for the economic evaluation of health care programmes. New York: Oxford University Press, 2005. [Google Scholar]
- 50. Howie L, Peppercorn J. Early palliative care in cancer treatment: rationale, evidence and clinical implications. Ther Adv Med Oncol 2013; 5: 318–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rowland K, Schumann S-A, Hickner J. Palliative care: earlier is better (Priority Updates to Research Literature (PURLs)) J Fam Pract 2010; 59: 695–698. [PMC free article] [PubMed] [Google Scholar]
- 52. Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non–small-cell lung cancer. New Eng J Med 2010; 363: 733–742. [DOI] [PubMed] [Google Scholar]
- 53. Claxton K, Martin S, Soares M, et al. Methods for the estimation of the National Institute for Health and Care Excellence cost-effectiveness threshold. Health Technol Assess 2015; 19: 1–503, v–vi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Brouwer WB, Culyer AJ, van Exel NJA, et al. Welfarism vs. extra-welfarism. J Health Econ 2008; 27: 325–338. [DOI] [PubMed] [Google Scholar]
- 55. Bailey C, Orlando R, Kinghorn P, et al. Measuring the quality of end of life using ICECAP SCM: feasibility and acceptability. BMJ Support Palliat Care 2014; 4: 112. [Google Scholar]
- 56. Echteld MA. Meetinstrumenten Palliatieve Zorg. VUmc EMGO-Instituut 2016. Available at: https://www.vumc.nl/afdelingen/meetinstr-palliatieve-zorg/ (accessed 27 January 2017).
- 57. Huber M, Knottnerus JA, Green L, et al. How should we define health? BMJ 2011; 343: d4163. [DOI] [PubMed] [Google Scholar]
- 58. Groot MM, Derksen EW, Crul BJ, et al. Living on borrowed time: experiences in palliative care. Patient Educ Couns 2007; 65: 381–386. [DOI] [PubMed] [Google Scholar]
- 59. Stalmeier PF, Lamers LM, Busschbach JJ, et al. On the assessment of preferences for health and duration: maximal endurable time and better than dead preferences. Med Care 2007; 45: 835–841. [DOI] [PubMed] [Google Scholar]
- 60. Mehrez A, Gafni A. Quality-adjusted life years, utility theory, and healthy-years equivalents. Med Decis Making 1989; 9: 142–149. [DOI] [PubMed] [Google Scholar]
- 61. Palmer S, Torgerson DJ. Economics notes: definitions of efficiency. BMJ 1999; 318: 1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sprangers MA, Schwartz CE. Integrating response shift into health-related quality of life research: a theoretical model. Soc Sci Med 1999; 48: 1507–1515. [DOI] [PubMed] [Google Scholar]
- 63. Norman G. Hi! How are you? Response shift, implicit theories and differing epistemologies. Qual Life Res 2003; 12: 239–249. [DOI] [PubMed] [Google Scholar]
- 64. Kim H, Rajagopalan MS, Beriwal S, et al. Cost-effectiveness analysis of single fraction of stereotactic body radiation therapy compared with single fraction of external beam radiation therapy for palliation of vertebral bone metastases. Int J Radiat Oncol Biol Phys 2015; 91: 556–563. [DOI] [PubMed] [Google Scholar]
- 65. Miller AR, Cantor SB, Peoples GE, et al. Quality of life and cost effectiveness analysis of therapy for locally recurrent rectal cancer. Dis Colon Rectum 2000; 43: 1695–1703. [DOI] [PubMed] [Google Scholar]
- 66. Phippen NT, Leath CA, 3rd, Miller CR, et al. Are supportive care-based treatment strategies preferable to standard chemotherapy in recurrent cervical cancer? Gynecol Oncol 2013; 130: 317–322. [DOI] [PubMed] [Google Scholar]
- 67. Shenfine J, McNamee P, Steen N, et al. A randomized controlled clinical trial of palliative therapies for patients with inoperable esophageal cancer. Am J Gastroenterol 2009; 104: 1674–1685. [DOI] [PubMed] [Google Scholar]
- 68. Van den Hout WB, Kramer GW, Noordijk EM, et al. Cost-utility analysis of short- versus long-course palliative radiotherapy in patients with non-small-cell lung cancer. J Natl Cancer Inst 2006; 98: 1786–1794. [DOI] [PubMed] [Google Scholar]
- 69. Lowery W, Lowery A, Barnett J, et al. Cost effectiveness of early palliative care intervention in recurrent platinum resistant ovarian cancer. Gynecol Oncol 2013; 131(1): 283–284. [DOI] [PubMed] [Google Scholar]
- 70. Chochinov HM. Relatively speaking. J Pain Symptom Manage 2012; 43: e6–7. [DOI] [PubMed] [Google Scholar]
- 71. Coy P, Schaafsma J, Schofield JA. The cost-effectiveness and cost-utility of high-dose palliative radiotherapy for advanced non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2000; 48: 1025–1033. [DOI] [PubMed] [Google Scholar]
- 72. Goldfeld KS, Hamel MB, Mitchell SL. The cost-effectiveness of the decision to hospitalize nursing home residents with advanced dementia. J Pain Symptom Manage 2013; 46: 640–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Jeurnink SM, Polinder S, Steyerberg EW, et al. Cost comparison of gastrojejunostomy versus duodenal stent placement for malignant gastric outlet obstruction. J Gastroenterol 2010; 45: 537–543. [DOI] [PubMed] [Google Scholar]
- 74. Johnson MJ, Kanaan M, Richardson G, et al. A randomised controlled trial of three or one breathing technique training sessions for breathlessness in people with malignant lung disease. BMC Med 2015; 13: 213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Konski A. Radiotherapy is a cost-effective palliative treatment for patients with bone metastasis from prostate cancer. Int J Radiat Oncol Biol Phys 2004; 60: 1373–1378. [DOI] [PubMed] [Google Scholar]
- 76. Konski A, James J, Hartsell W, et al. Economic analysis of radiation therapy oncology group 97-14: multiple versus single fraction radiation treatment of patients with bone metastases. Am J Clin Oncol 2009; 32: 423–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Lowery WJ, Lowvery AW, Barnett JC, et al. Cost-effectiveness of early palliative care intervention in recurrent platinum-resistant ovarian cancer. Gynecol Oncol 2013; 130: 426–30. [DOI] [PubMed] [Google Scholar]
- 78. Olden AM, Holloway R. Treatment of malignant pleural effusion: PleuRx catheter or talc pleurodesis? A cost-effectiveness analysis. J Palliat Med 2010; 13: 59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Roberts KJ, Sutton AJ, Prasad KR, et al. Cost-utility analysis of operative versus non-operative treatment for colorectal liver metastases. Br J Surg 2015; 102: 388–398. [DOI] [PubMed] [Google Scholar]
- 80. Roth JA, Carlson JJ. Cost-effectiveness of gemcitabine + cisplatin vs. gemcitabine monotherapy in advanced biliary tract cancer. J Gastrointest Cancer 2012; 43: 215–223. [DOI] [PubMed] [Google Scholar]
- 81. Sahlen KG, Boman K, Bränström M. A cost-effectiveness study of person-centered integrated heart failure and palliative home care: Based on a randomized controlled trial. Palliat Med 2016; 30: 296–302. [DOI] [PubMed] [Google Scholar]
- 82. Shafiq M, Frick KD, Lee H, et al. Management of Malignant Pleural Effusion: A Cost-Utility Analysis. J Bronchology Interv Pulmonol 2015; 22: 215–225. [DOI] [PubMed] [Google Scholar]
- 83. Teerawattananon Y, Mugford M, Tangcharoensathien V. Economic evaluation of palliative management versus peritoneal dialysis and hemodialysis for end-stage renal disease: evidence for coverage decisions in Thailand. Value Health 2007; 10: 61–72. [DOI] [PubMed] [Google Scholar]
- 84. Xinopoulos D, Dimitroulopoulos D, Moschandrea I, et al. Natural course of inoperable esophageal cancer treated with metallic expandable stents: quality of life and cost-effectiveness analysis. J Gastroenterol Hepatol 2004; 19: 1397–1402. [DOI] [PubMed] [Google Scholar]