Abstract
Acetylcholine (ACh) is a key transmitter in the mesocorticolimbic circuit. By interacting with muscarinic ACh receptors (mAChR) enriched in the circuit, ACh actively regulates various neuronal and synaptic activities. The extracellular signal-regulated kinase (ERK) is one of members of the mitogen-activated protein kinase family and is subject to the regulation by dopamine receptors, although the regulation of ERKs by limbic mAChRs is poorly understood. In this study, we investigated the role of mAChRs in the regulation of ERK phosphorylation (activation) in the mesocorticolimbic system of adult rat brains in vivo. We targeted a sub-pool of ERKs at synaptic sites. We found that a systemic injection of the mAChR antagonist scopolamine increased phosphorylation of synaptic ERKs in the striatum (caudate putamen and nucleus accumbens) and medial prefrontal cortex (mPFC). Increases in ERK phosphorylation in both forebrain regions were rapid and transient. Notably, pretreatment with a dopamine D1 receptor (D1R) antagonist SCH23390 blocked the scopolamine-stimulated ERK phosphorylation in these brain regions, while a dopamine D2 receptor antagonist eticlopride did not. Scopolamine and SCH23390 did not change the amount of total ERK proteins. These results demonstrate that mAChRs inhibit synaptic ERK phosphorylation in striatal and mPFC neurons under normal conditions. Blockade of this inhibitory mAChR tone leads to the upregulation of ERK phosphorylation likely through a mechanism involving the level of D1R activity.
Keywords: Acetylcholine, Dopamine, ERK, Striatum, Nucleus accumbens, Prefrontal cortex, Scopolamine, SCH23390
Introduction
Mitogen-activated protein kinases (MAPK) are a family of serine/threonine protein kinases [1, 2]. One of three subfamilies of MAPKs is extracellular signal-regulated kinases (ERK). ERKs are activated by a phosphorylation-dependent mechanism. After phosphorylation, cytosolic ERKs translocate into the nucleus to activate a discrete set of transcription factors to regulate gene expression [3]. In addition, a specific sub-pool of ERKs resides at peripheral synaptic sites and phosphorylates local substrates to modulate synaptic transmission [4–7]. As a kinase that is abundantly expressed in postmitotic neurons in widespread regions of adult mammalian brains [8] and is sensitive to changing cellular and synaptic input, ERKs are actively involved in the transcriptional and local regulation of cellular and synaptic activities and are linked to a variety of neurological and neuropsychiatric disorders [9–11].
Acetylcholine (ACh) is a principal transmitter in the central nervous system. The transmitter interacts with nicotinic and muscarinic receptors to achieve its action. Muscarinic ACh receptors (mAChR) are G protein-coupled receptors and five subtypes of mAChRs have been identified, named M1–M5 [12]. M1, M3, and M5 receptors are coupled with Gq proteins, whereas M2 and M4 receptors are coupled with Gi/o proteins [13]. Thus, activation of M1, M3, and M5 receptors stimulates phospholipase Cβ1 to hydrolyze phosphoinositide into diacylglycerol (DAG) and inositol-1,4,5-triphosphate (IP3). DAG in turn activates protein kinase C, while IP3 triggers release of intracellular Ca2+. As to M2 and M4 receptors, activation of them inhibits adenylyl cyclase, thereby reducing the level of cAMP and cAMP activation of protein kinase A (PKA). Since mAChRs are widely expressed in the brain, ACh through activating mAChRs is believed to regulate various neural activities.
ACh in the dopamine responsive regions, i.e., the striatum and medial prefrontal cortex (mPFC), is of particular importance. In these regions, ACh and dopamine are thought to form a dynamic balance to maintain local homeostasis. Indeed, activating mAChRs, predominant cholinergic receptors in the striatum and mPFC [14, 15], suppressed motor responses to dopamine stimulation, while blocking mAChRs augmented them [16–18]. Similarly, non-selective mAChR antagonists (scopolamine and atropine) potentiated the efficacy of dopamine in stimulating gene expression [16–21]. However, while the dopamine-mediated regulation of ERK phosphorylation has been thoroughly investigated [7, 22–25], the ACh regulation of ERK phosphorylation via mAChRs is poorly understood in both the striatum and mPFC.
We thus conducted experiments to explore the role of mAChRs in regulating ERKs in adult rats in vivo. By targeting synaptic ERKs, we first carried out a time-course study to investigate the time-dependent effect of the mAChR antagonist scopolamine on phosphorylation and expression of ERKs in the two striatal subdivisions, caudate putamen (CPu) and nucleus accumbens (NAc), and mPFC. We then investigated the role of dopamine D1 receptors (D1R) in the scopolamine-stimulated ERK phosphorylation by testing the effect of a D1R antagonist SCH233909 on the scopolamine-stimulated ERK phosphorylation in the three brain regions. Finally, the effect of a dopamine D2 receptor (D2R) antagonist eticlopride on the scopolamine-stimulated ERK phosphorylation was tested.
Materials and Methods
Animals
Male Wistar rats weighting 240–410 g from Charles River (New York, NY) were used in this study. All animals were housed at 23 °C and humidity of 50 ± 10% with food and water available ad libitum. The animal room was on a 12/12 h light/dark cycle with lights on at 0700. Animal use procedures were in strict accordance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee.
Systemic Drug Injection and Experimental Arrangements
All drugs were given via an intraperitoneal (i.p.) injection at a volume of ~0.5 ml. Three experiments were carried out. The first experiment was a time-course study, in which time-dependent effects of scopolamine on ERK phosphorylation and expression of total ERK proteins were investigated. In details, rats were divided into four groups (n = 5 per group). Rats received a single dose of scopolamine (5 mg/kg, i.p.) and were then sacrificed at different time points (7.5, 15, or 30 min) after scopolamine injection. One group of rats were given an injection of saline and sacrificed immediately after saline injection to serve as controls (0 min). Brain regions of interest were collected for following Western blot analysis. The second experiment aimed to investigate whether D1R activity is required for the effect of scopolamine on ERK phosphorylation. A total of four group of rats (n = 5 per group) were used and administered with saline + saline, SCH23390 + saline, saline + scopolamine, and SCH23390 + scopolamine. SCH23390 (0.2 mg/kg, i.p.) was given 15 min prior to scopolamine (5 mg/kg, i.p.). Rats were sacrificed 10 min after scopolamine injection for Western blot analysis. In the third experiment, four groups of rats (n = 4 per group) received different drug treatments: saline + saline, eticlopride + saline, saline + scopolamine, and eticlopride + scopolamine. Eticlopride was given at a dose of 0.5 mg/kg (i.p.) 15 min prior to scopolamine (5 mg/kg, i.p.). Rats were sacrificed 10 min after scopolamine administration. The dose of scopolamine, SCH23390 and eticlopride was calculated as the salt and was chosen due to their established effectiveness after a systemic injection based on a number of our previous studies [20, 26, 27].
Synaptic Protein Extraction
Synaptosomal proteins were prepared as described previously [7, 28]. Briefly, rats were anesthetized with sodium pentobarbital (65 mg/kg, i.p.) and sacrificed by decapitation. Rat brains were removed and cut into coronal sections, from which the brain regions of interest, including the CPu, NAc and mPFC, were dissected on an ice-cold dissection plate. Brain tissue was homogenized in isotonic sucrose homogenization buffer containing 0.32 M sucrose, 10 mM HEPES, pH 7.4, 2 mM EDTA, and a protease/phosphatase inhibitor cocktail (Thermo Scientific, Rochester, NY). After centrifugation at 800×g for 10 min (4 °C), the supernatant was collected and centrifuged at 10,000×g for 30 min (4 °C). Crude synaptosomal plasma membranes were collected in the pellet (P2). The pellet was washed once and centrifuged at 10,000×g for 15 min. The pellet was then resuspended and solubilized in the sucrose homogenization buffer containing 0.5% Triton X-100, 1% sodium dodecyl sulfate (SDS), 1% deoxycholic acid, 1 mM dithiothreitol, and the protease/phosphatase inhibitor cocktail, with gentle rotation (1 h at 4 °C). Concentrations of solubilized proteins were determined. Samples were stored at −80 °C until use.
Western Blot Analysis
Western blot analysis was conducted by following our previous procedures [29, 30]. We separated proteins on SDS NuPAGE 4–12% Bis-Tris gels (Invitrogen, Carlsbad, CA). Gel proteins were then transferred to polyvinylidene fluoride membranes. To immunoblot proteins on membranes, we incubated membranes with primary antibodies overnight at 4 °C. Membranes next day were incubated with a secondary antibody (1:2000). Immunoblots were visualized with the enhanced chemiluminescence reagent (GE Healthcare Life Sciences, Piscataway, NJ). Immunoblots on films were measured using NIH ImageJ gel analysis software. β-Actin was used as a loading control. The optical density of all proteins measured was normalized to β-actin in Western blot analysis.
Antibodies and Pharmacological Agents
Primary antibodies used in the current study include rabbit antibodies against phosphorylated ERK1/2 (pERK1/2) at Thr202/Tyr204 (Cell Signaling Technology, Beverly, MA), ERK1/2 (Cell Signaling), or β-actin (Sigma-Aldrich, St. Louis, MO). Pharmacological agents, including (−)-scopolamine hydrobromide, R(+)-SCH23390 hydrochloride and S-(−)-eticlopride hydrochloride, were purchased from Sigma-Aldrich (St. Louis, MO). Scopolamine, SCH23390 and eticlopride were dissolved in physiological saline. All agents were freshly prepared at the day of experiments.
Statistics
Data in this study are presented as means ± SEM. Data were evaluated using one-way analysis of variance followed by a Bonferroni (Dunn) comparison of groups. Probability levels of <0.05 were considered statistically significant.
Results
Scopolamine Increases ERK Phosphorylation in the Striatum
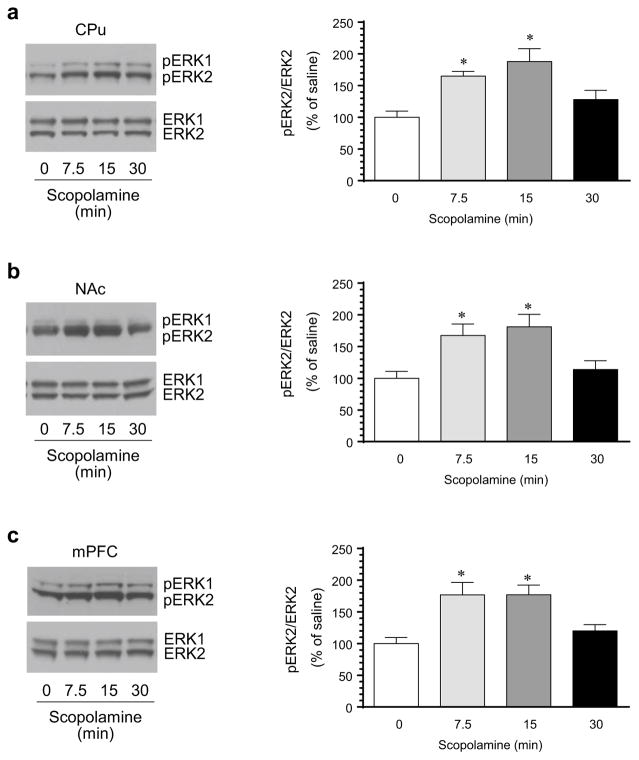
To determine the regulatory role of mAChRs in synaptic ERK phosphorylation, we examined the effect of a mAChR antagonist scopolamine widely used in experimental animals on basal ERK phosphorylation. The antagonist was administered at a dose of 5 mg/kg (i.p.) [20, 21]. Rats were sacrificed at different time points (7.5, 15, or 30 min) after drug injection. Changes in phosphorylation of ERK and expression of total ERK proteins in the striatum (both CPu and NAc subdivisions) were analyzed with Western blot. We found that scopolamine induced a time-dependent increase in ERK phosphorylation in the CPu. As shown in Fig. 1a, pERK1/2 levels were elevated in the CPu 7.5 min after scopolamine injection. The elevation remained at 15 min in scopolamine-treated rats. At 30 min, the elevation in pERK1/2 declined to an insignificant level. Quantification analysis of pERK2 immunoreactive signals confirmed the time-dependent elevation of ERK2 phosphorylation in the CPu. In the NAc, similar results were observed. A rapid and transient increase in pERK1/2 levels was seen in this region (Fig. 1b). In contrast to pERK1/2, cellular levels of total ERK1/2 proteins in the CPu and NAc were not significantly altered by scopolamine at all time points surveyed. These data demonstrate that pharmacological blockade of mAChRs results in the upregulation of ERK phosphorylation in the striatum.
Fig. 1.
Effects of blockade of mAChRs on ERK1/2 phosphorylation in the rat striatum and mPFC. a Effects of the mAChR antagonist scopolamine on ERK1/2 phosphorylation in the CPu. b Effects of scopolamine on ERK1/2 phosphorylation in the NAc. c Effects of scopolamine on ERK1/2 phosphorylation in the mPFC. Representative immunoblots are shown to the left of the quantified data. Two bands were typically observed in pERK1/2 and ERK1/2 immunoblots (upper bands: pERK1 and ERK1 at 44 kDa; lower bands: pERK2 and ERK2 at 42 kDa). Note that scopolamine time-dependently increased pERK2 levels in the CPu, NAc, and mPFC. Rats were treated with an i.p. injection of scopolamine (5 mg/kg) and were sacrificed at different time points (7.5, 15, or 30 min) after drug injection for Western blot analysis. Data are presented as means ± SEM (n = 5 per group). *p < 0.05 versus saline (0 min)
Scopolamine Increases ERK Phosphorylation in the mPFC
The mPFC is another dopamine-innervated area where mAChRs are also expressed [14, 15]. We thus examined the role of mAChRs in the regulation of ERK phosphorylation and expression in this region. The mPFC was dissected from the same rats and synaptosomal samples were prepared to target the synaptic pool of ERKs. Like the results we observed in the striatum, scopolamine elevated ERK1/2 phosphorylation in the mPFC (Fig. 1c). This elevation was time-dependent. An evident increase in pERK1/2 levels was seen as early as 7.5 min following scopolamine administration. The increase remained at 15 but not 30 min. Apparently, blockade of mAChRs in the mPFC positively regulates ERK phosphorylation.
SCH23390 Blocks Scopolamine-Stimulated ERK Phosphorylation
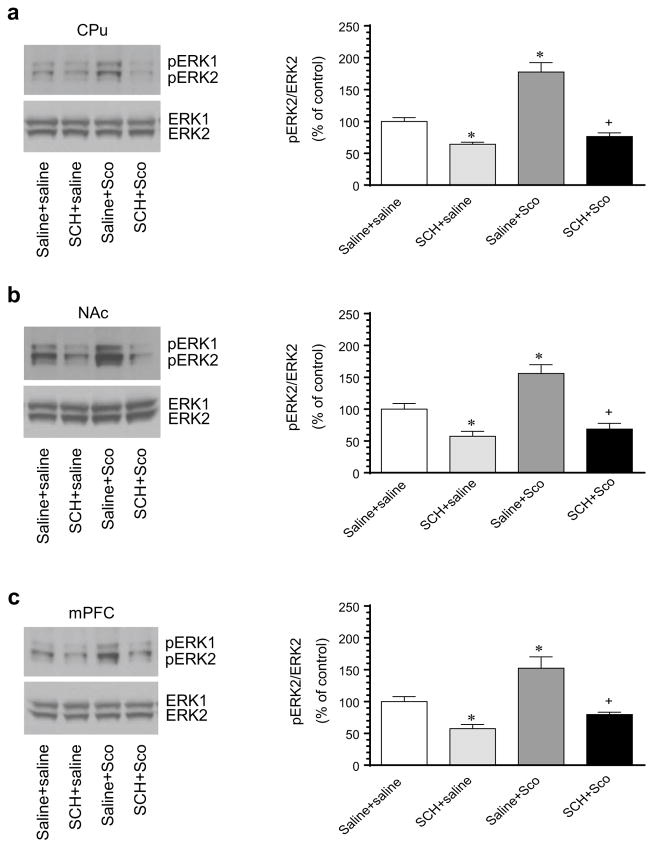
D1Rs, as Gs-coupled receptors [31], are expressed in striatonigral output neurons of the striatum and are positively linked to ERK phosphorylation [22, 23]. To determine the role of D1Rs in maintaining basal ERK phosphorylation and in contributing to the scopolamine-stimulated ERK phosphorylation in the striatum, we investigated the effect of a common D1R antagonist SCH23390 on basal and scopolamine-stimulated ERK phosphorylation in the CPu and NAc. As shown in immunoblots, a systemic injection of SCH23390 (0.2 mg/kg, i.p.) induced a significant decrease in pERK1/2 levels in the CPu (Fig. 2a). The reduction of basal pERK1/2 levels was also seen in the NAc following SCH23390 administration (Fig. 2b). In addition, SCH23390 (15 min prior to scopolamine) completely blocked the scopolamine-stimulated ERK1/2 phosphorylation in the CPu (Fig. 2a) and NAc (Fig. 2b). Quantification of pERK2 and ERK2 signals confirmed the impression of immunoblots. All drug treatments had a minimal impact on total ERK1/2 protein levels in the two striatal regions. These data support that D1R activity is required for the maintenance of basal ERK phosphorylation and for the stimulation of ERK phosphorylation by scopolamine in striatal neurons.
Fig. 2.
Effects of blockade of D1Rs on basal and scopolamine-stimulated ERK1/2 phosphorylation in the rat striatum and mPFC. a Effects of the D1R antagonist SCH23390 on basal and scopolamine-stimulated ERK1/2 phosphorylation in the CPu. b Effects of SCH23390 on basal and scopolamine-stimulated ERK1/2 phosphorylation in the NAc. c Effects of SCH23390 on basal and scopolamine-stimulated ERK1/2 phosphorylation in the mPFC. Representative immunoblots are shown to the left of the quantified data. Note that SCH23390 reduced and blocked basal and scopolamine-stimulated ERK1/2 phosphorylation, respectively, in the CPu, NAc, and mPFC. Rats were treated with an i.p. injection of saline or SCH23390 (SCH, 0.2 mg/kg) 15 min prior to saline or scopolamine (Sco, 5 mg/kg) and were sacrificed 10 min after final agent administration for Western blot analysis. Data are presented as means ± SEM (n = 5 per group). *p < 0.05 versus control (saline + saline). +p < 0.05 versus saline + scopolamine
D1Rs are expressed in pyramidal output neurons of the mPFC [32, 33]. We thus examined the role of D1Rs in processing basal ERK phosphorylation as well as ERK responses to scopolamine in this region. We observed that SCH23390 alone reduced basal ERK1/2 phosphorylation (Fig. 2c). SCH23390 also blocked the scopolamine-stimulated ERK1/2 phosphorylation (Fig. 2c). Total ERK1/2 levels remained unchanged after all drug treatment groups. Thus, D1R activity is also required for basal and scopolamine-stimulated ERK phosphorylation in mPFC neurons.
Effects of the D2R Antagonist on Scopolamine-Stimulated ERK Phosphorylation
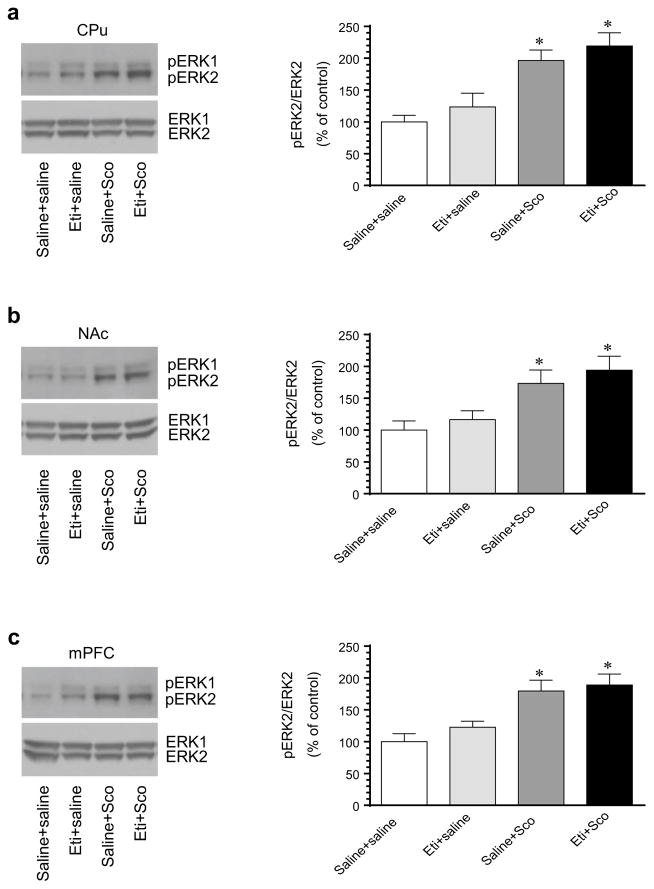
To determine the role of D2Rs in processing the scopolamine-stimulated ERK phosphorylation, we examined the effect of the D2R antagonist eticlopride on ERK responses to scopolamine. In the CPu, eticlopride alone (0.5 mg/kg, i.p.) induced a slight but insignificant increase in pERK1/2 levels (Fig. 3a). Pretreatment with eticlopride (15 min prior to scopolamine) did not alter ERK1/2 responses to scopolamine (5 mg/kg, 10 min prior to tissue collection). The increased level of pERK1/2 in rats treated with rats treated with scopolamine alone. Similar results were observed in the NAc (Fig. 3b). In this region, scopolamine remained its ability to induce a significant increase in ERK1/2 phosphorylation in rats pretreated with eticlopride. These data indicate an insignificant role of D2Rs in processing the scopolamine-stimulated ERK phosphorylation in the two subdivisions of the striatum.
Fig. 3.
Effects of blockade of D2Rs on basal and scopolamine-stimulated ERK1/2 phosphorylation in the rat striatum and mPFC. a Effects of the D2R antagonist eticlopride on basal and scopolamine-stimulated ERK1/2 phosphorylation in the CPu. b Effects of eticlopride on basal and scopolamine-stimulated ERK1/2 phosphorylation in the NAc. c Effects of eticlopride on basal and scopolamine-stimulated ERK1/2 phosphorylation in the mPFC. Representative immunoblots are shown to the left of the quantified data. Rats were treated with an i.p. injection of saline or eticlopride (Eti, 0.5 mg/kg) 15 min prior to saline or scopolamine (Sco, 5 mg/kg) and were sacrificed 10 min after final agent administration for Western blot analysis. Data are presented as means ± SEM (n = 4 per group). *p < 0.05 versus control (saline + saline)
In the mPFC, the effect of eticlopride was also tested. We found that eticlopride had no significant influence over ERK responses to scopolamine. As shown in Fig. 3c, scopolamine induced a similar increase in ERK1/2 phosphorylation in rats pretreated with saline or eticlopride. Thus, D2R activity is seemingly not important for the scopolamine-stimulated ERK phosphorylation in the mPFC.
Discussion
In this study, we investigated the role of mAChRs in the regulation of synaptic ERK phosphorylation in adult rat brains. In three major dopamine responsive forebrain regions, we examined the effect of the mAChR antagonist scopolamine on ERK phosphorylation. We found that a single dose of scopolamine induced marked increases in ERK phosphorylation in the synaptosomal samples prepared from the CPu, NAc, and mPFC. These increases were rapid and transient. Pretreatment with a D1R antagonist SCH23390 but not a D2R antagonist eticlopride blocked these increases. These results indicate that there exists a tonic scopolamine-sensitive mAChR activity in the striatum and mPFC that inhibits constitutive phosphorylation of a synaptic sub-pool of ERKs under normal conditions. Blockade of the inhibitory mAChR drive may upregulate ERK phosphorylation via a D1R-dependent pathway.
Scopolamine (1 mg/kg, i.p.) decreased ERK phosphorylation in the rat hippocampus [34], while the mAChR agonist carbachol increased ERK phosphorylation in rat hippocampal slices, which was blocked by the mAChR antagonist atropine [35]. The carbachol-stimulated ERK phosphorylation in the hippocampus is believed to be mediated by M1 receptors because ERK responses to carbachol were blocked by knocking out M1 but not M2/M3/M4 receptors [36]. In the mouse striatum, the number of pERK immunopositive nuclei was insignificantly altered by an acute injection of scopolamine (1 or 2 mg/kg, i.p., 15 min), although scopolamine increased the number of pERK positive nuclei in the lateral part of the bed nucleus of the stria terminalis and the central nucleus of the amygdala as detected by immunohistochemistry [23]. In this study, we found that scopolamine at a dose of 5 mg/kg induced a significant increase in phosphorylation of synaptic ERKs in the striatum. The upregulation of ERK phosphorylation after scopolamine reveals the existence of a tonic mAChR activity that inhibits striatal synaptic ERK phosphorylation.
With regard to the responsible subtype of mAChRs, M1 and M4 mAChRs are both expressed in the striatum [37] with M4 receptors notably enriched in striatonigral neurons [38, 39]. M1 receptors are known to activate Gq proteins [13], which usually leads to an increase in ERK phosphorylation as seen in the hippocampus (see above). On the contrary, M4 receptors are coupled with Gi/o proteins [13]. As a result, activating M4 receptors inhibits the cAMP-PKA pathway. Since the cAMP-PKA pathway is positively linked to ERK [40–42], scopolamine is likely to stimulate ERK phosphorylation through blocking the M4-mediated inhibition of PKA. Of note, D1Rs like M4 receptors are prominently segregated into striatonigral neurons [43–45] and the dopamine (cocaine)-stimulated striatal ERK phosphorylation exclusively occurred in striatonigral neurons via activating D1Rs [46]. Since D1Rs are coupled with Gs proteins and positively regulate the cAMP-PKA pathway [31], coexpressed D1Rs and M4 receptors in striatonigral neurons could converge on the cAMP-PKA system to antagonistically and dynamically regulate PKA activity. As such, blocking M4 receptors increases PKA activity and thus ERK phosphorylation (this study), while blocking D1Rs with SCH23390 decreased basal ERK phosphorylation [47; this study]. Moreover, loss of the positive D1R drive to ERK in the presence of the D1R antagonist effectuates a state of scopolamine ineffective to stimulate ERK phosphorylation. Indeed, M4 receptors and D1Rs have been frequently demonstrated to antagonize each other in many intracellular events of striatonigral neurons, including immediate early gene expression [16–21].
It should be pointed out that scopolamine used in this study blocks all mAChR subtypes. Thus, whether M4 receptors are preferentially involved in the effect of scopolamine on striatal ERK needs to be proven experimentally in the future. Additionally, phosphatidylinositol (phosphoinositide) 3-kinases (PI3K) mediated ERK activation in striatal and hippocampal neurons [48, 49]. Thus, it will be intriguing to investigate whether D1Rs and mAChRs cooperatively regulate the PI3K signaling pathway in addition to the cAMP-PKA pathway mentioned above to modulate ERK phosphorylation in striatal neurons.
Alternatively, scopolamine could block the M4 receptor- mediated inhibition of dopamine release, leading to an increase in dopamine levels in the striatum. Released dopamine in turn stimulates ERK phosphorylation. In support of this indirect presynaptic mechanism, Tzavara et al. [50] found that basal levels of dopamine in the NAc were elevated in M4 but not M2 knockout mice due to an increase in dopamine synthesis and release as detected using in vivo microdialysis. However, scopolamine (0.01–1 mg/kg) did not increase dopamine release in the striatum [51, 52], while the mAChR agonist carbachol elevated striatal dopamine levels [53]. These data are inconsistent with the notion that scopolamine upregulates ERK phosphorylation via facilitating local dopamine release. Of note, scopolamine increased glutamate levels in the striatum, indicating a tonic inhibition of glutamate release by mAChRs [54]. Thus, there is another possibility that scopolamine increases ERK phosphorylation through stimulating local glutamate release. Future studies need to clarify these possibilities.
Scopolamine at a very low dose (25 μg/kg, i.p.) and at a delayed time point (1 h after injection) did not alter pERK levels in the prefrontal cortex [55]. In this study, we found that scopolamine at 5 mg/kg (7.5 and 15 min after injection) induced a rapid and transient increase in phosphorylation of synaptic ERKs in the mPFC. Thus, the mAChR-mediated cholinergic transmission inhibits ERK phosphorylation in the mPFC under normal conditions. M1, M2, and M4 receptors are major subtypes of mAChRs in mPFC neurons [15]. Among them, M2/M4 receptors may be important for the inhibition of ERK phosphorylation given their negative connections to the cAMP-PKA pathway. Furthermore, SCH23390 reduced basal levels of pERK in the mPFC consistent with an early report [56]. The mAChR-mediated inhibition also relies on D1R activity because in the presence of the D1R antagonist, scopolamine induced an insignificant change in ERK phosphorylation. Thus, as can be seen in the striatum, mAChRs and D1Rs in the mPFC act cooperatively to control ERK phosphorylation.
ERK is known to regulate a discrete set of substrates in the nucleus [3, 57] to transcriptionally modulate cellular activity and synaptic plasticity [3, 9, 10]. At synaptic sites, several local substrates of ERK have also been found [reviewed in Ref. 58]. By phosphorylating these synaptic substrates, ERK is believed to directly modulate their function and thereby determine the strength of synaptic transmission. Of note, more substrates of ERK may exist in synaptic structures. Future studies are needed to identify them and evaluate their roles in regulating local synaptic events.
Acknowledgments
This work was supported by NIH Grants DA10355 (J.Q.W.) and MH61469 (J.Q.W.).
Footnotes
Compliance with Ethical Standards
Conflict of interest The authors declare that they have no conflict of interest.
References
- 1.Pearson G, Robinson F, Gibson BT, Xu BE, Karandikar M, Berman K, Cobb MH. Mitogen-activated protein (MAP) kinase pathways: regulation of physiological functions. Endo Rev. 2001;22:153–183. doi: 10.1210/edrv.22.2.0428. [DOI] [PubMed] [Google Scholar]
- 2.Volmat V, Pouyssegur J. Spatiotemporal regulation of the p42/p44 MAPK pathway. Biol Cell. 2001;93:71–79. doi: 10.1016/s0248-4900(01)01129-7. [DOI] [PubMed] [Google Scholar]
- 3.Treisman R. Regulation of transcription by MAP kinase cascades. Curr Opin Cell Biol. 1996;8:205–215. doi: 10.1016/s0955-0674(96)80067-6. [DOI] [PubMed] [Google Scholar]
- 4.Suzuki T, Mitake S, Murata S. Presence of up-stream and downstream components of a mitogen-activated protein kinase pathway in the PSD of the rat forebrain. Brain Res. 1999;840:36–44. doi: 10.1016/s0006-8993(99)01762-x. [DOI] [PubMed] [Google Scholar]
- 5.Suzuki T, Okumura-Noji K, Nishida E. ERK2-type mitogen-activated protein kinase (MAPK) and its substrates in postsynaptic density fractions from the rat brain. Neurosci Res. 1995;22:277–285. doi: 10.1016/0168-0102(95)00902-6. [DOI] [PubMed] [Google Scholar]
- 6.Boggio EM, Putignano E, Sassoe-Pognetto M, Pizzorusso T, Glustetto M. Visual stimulation activates ERK in synaptic and somatic compartments of rat cortical neurons with parallel kinetics. PLoS ONE. 2007;2(7):e604. doi: 10.1371/journal.pone.0000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mao LM, Reusch JM, Fibuch EE, Liu Z, Wang JQ. Amphetamine increases phosphorylation of MAPK/ERK at synaptic sites in the rat striatum and medial prefrontal cortex. Brain Res. 2013;1494:101–108. doi: 10.1016/j.brainres.2012.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nozaki K, Nishimura M, Hashimoto N. Mitogen-activated protein kinases and cerebral ischemia. Mol Neurobiol. 2001;23:1–19. doi: 10.1385/MN:23:1:01. [DOI] [PubMed] [Google Scholar]
- 9.Sweatt JD. Mitogen-activated protein kinases in synaptic plasticity and memory. Curr Opin Neurobiol. 2004;14:311–317. doi: 10.1016/j.conb.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 10.Thomas GM, Huganir RL. MAPK cascade signaling and synaptic plasticity. Nat Rev Neurosci. 2004;5:173–183. doi: 10.1038/nrn1346. [DOI] [PubMed] [Google Scholar]
- 11.Wang JQ, Fibuch EE, Mao LM. Regulation of mitogen-activated protein kinases by glutamate receptors. J Neurochem. 2007;100:1–11. doi: 10.1111/j.1471-4159.2006.04208.x. [DOI] [PubMed] [Google Scholar]
- 12.Caulfield MP, Birdsall NJ. International union of pharmacology. XVII. Classification of muscarinic acethycholine receptors. Pharmacol Rev. 1998;50:279–290. [PubMed] [Google Scholar]
- 13.Wess J. Molecular biology of muscarinic acetylcholine receptors. Crit Rev Neurobiol. 1996;10:69–99. doi: 10.1615/critrevneurobiol.v10.i1.40. [DOI] [PubMed] [Google Scholar]
- 14.Levey AI, Kitt CA, Simonds WF, Price DL, Brann MR. Identification and localization of muscarinic acetylcholine receptor proteins in brain with subtype-specific antibodies. J Neurosci. 1991;11:3218–3226. doi: 10.1523/JNEUROSCI.11-10-03218.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Volpicelli LA, Levey AI. Muscarinic acetylcholine receptor subtypes in cerebral cortex and hippocampus. Prog Brain Res. 2004;145:59–66. doi: 10.1016/S0079-6123(03)45003-6. [DOI] [PubMed] [Google Scholar]
- 16.Chou H, Ogawa N, Asanuma M, Hirata H, Mori A. Muscarinic cholinergic receptor-mediated modulation on striatal c-fos mRNA expression induced by levodopa in rat brain. J Neural Transm. 1992;90:171–181. doi: 10.1007/BF01250959. [DOI] [PubMed] [Google Scholar]
- 17.Morelli M, Fenu S, Cozzolino A, Pinna A, Carta A, Di Chiara G. Blockade of muscarinic receptors potentiates D1 dependent turning behavior and c-fos expression in 6-hydroxydopamine-lesioned rats but does not influence D2 mediated response. Neuroscience. 1993;53:673–678. doi: 10.1016/0306-4522(93)90615-m. [DOI] [PubMed] [Google Scholar]
- 18.Wang JQ, McGinty JF. Intrastriatal injection of a muscarinic receptor agonist and antagonist regulates striatal neuropeptide mRNA expression in normal and amphetamine-treated rats. Brain Res. 1997;748:62–70. doi: 10.1016/s0006-8993(96)01244-9. [DOI] [PubMed] [Google Scholar]
- 19.Bernard V, Dumartin B, Lamy E, Bloch B. Fos immunoreactivity after stimulation or inhibition of muscarinic receptors indicates anatomical specificity for cholinergic control of striatal efferent neurons and cortical neurons in the rat. Eur J Neurosci. 1993;5:1218–1225. doi: 10.1111/j.1460-9568.1993.tb00976.x. [DOI] [PubMed] [Google Scholar]
- 20.Wang JQ, McGinty JF. Scopolamine augments c-fos and zif/268 messenger RNA expression induced by the full D1 dopamine receptor agonist SKF-82958 in the intact rat striatum. Neuroscience. 1996;72:601–616. doi: 10.1016/0306-4522(95)00597-8. [DOI] [PubMed] [Google Scholar]
- 21.Wang JQ, McGinty JF. Muscarinic receptors regulate striatal neuropeptide gene expression in normal and amphetamine-treated rats. Neuroscience. 1996;75:43–56. doi: 10.1016/0306-4522(96)00277-1. [DOI] [PubMed] [Google Scholar]
- 22.Valjent E, Corvol JC, Pages C, Besson MJ, Maldonado R, Caboche J. Involvement of the extracellular signal-regulated kinase cascade for cocaine-rewarding properties. J Neurosci. 2000;20:8701–8709. doi: 10.1523/JNEUROSCI.20-23-08701.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Valjent E, Pages C, Herve D, Girault JA, Caboche J. Addictive and non-addictive drugs induce distinct and specific patterns of ERK activation in mouse brain. Eur J Neurosci. 2004;19:1826–1836. doi: 10.1111/j.1460-9568.2004.03278.x. [DOI] [PubMed] [Google Scholar]
- 24.Choe ES, Chung KT, Mao L, Wang JQ. Amphetamine increases phosphorylation of extracellular signal-regulated kinase and transcription factors in the rat striatum via group I metabotropic glutamate receptors. Neuropsychopharmacology. 2002;27:565–575. doi: 10.1016/S0893-133X(02)00341-X. [DOI] [PubMed] [Google Scholar]
- 25.Zhang L, Lou D, Jiao H, Zhang D, Wang X, Xia Y, Zhang J, Xu M. Cocaine-induced intracellular signaling and gene expression are oppositely regulated by the dopamine D1 and D3 receptors. J Neurosci. 2004;24:3344–3354. doi: 10.1523/JNEUROSCI.0060-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang JQ, McGinty JF. Differential effects of D1 and D2 dopamine receptor antagonists on acute amphetamine- or methamphetamine-induced up-regulation of zif/268 mRNA expression in rat forebrain. J Neurochem. 1995;65:2706–2715. doi: 10.1046/j.1471-4159.1995.65062706.x. [DOI] [PubMed] [Google Scholar]
- 27.Xue B, Chen EC, He N, Jin DZ, Mao LM, Wang JQ. Integrated regulation of AMPA glutamate receptor phosphorylation in the striatum by dopamine and acetylcholine. Neuropharmacology. 2017;112:57–65. doi: 10.1016/j.neuropharm.2016.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xue B, Mao LM, Jin DZ, Wang JQ. Regulation of synaptic MAPK/ERK phosphorylation in the rat striatum and medial prefrontal cortex by dopamine and muscarinic acetylcholine receptors. J Neurosci Res. 2015;93:1592–1599. doi: 10.1002/jnr.23622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mao LM, Wang W, Chu XP, Zhang GC, Liu XY, Yang YJ, Haines M, Papasian CJ, Fibuch EE, Buch S, Chen JG, Wang JQ. Stability of surface NMDA receptors controls synaptic and behavioral adaptations to amphetamine. Nat Neurosci. 2009;12:602–610. doi: 10.1038/nn.2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Dolah DK, Mao LM, Shaffer C, Guo ML, Fibuch EE, Chu XP, Buch S, Wang JQ. Reversible palmitoylation regulates surface stability of AMPA receptors in the nucleus accumbens in response to cocaine in vivo. Biol Psychiatry. 2011;69:1035–1042. doi: 10.1016/j.biopsych.2010.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neve KA, Seamans JK, Trantham-Davidson H. Dopamine receptor signaling. J Recept Signal Transduct Res. 2004;24:165–205. doi: 10.1081/rrs-200029981. [DOI] [PubMed] [Google Scholar]
- 32.Bergson C, Mrzljak L, Smiley JF, Pappy M, Levenson R, Goldman-Rakic PS. Regional, cellular, and subcellular variations in the distribution of D1 and D5 dopamine receptors in primate brain. J Neurosci. 1995;15:7821–7836. doi: 10.1523/JNEUROSCI.15-12-07821.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Almeida J, Palacios JM, Mengod G. Distribution of 5-HT and DA receptors in primate prefrontal cortex: implications for pathophysiology and treatment. Prog Brain Res. 2008;172:101–115. doi: 10.1016/S0079-6123(08)00905-9. [DOI] [PubMed] [Google Scholar]
- 34.Moosavi M, Khales GY, Abbasi L, Zarifkar A, Rastegar K. Agmatine protects against scopolamine-induced water maze performance impairment and hippocampal ERK and Akt inactivation. Neuropharmacology. 2012;62:2018–2023. doi: 10.1016/j.neuropharm.2011.12.031. [DOI] [PubMed] [Google Scholar]
- 35.Takagi N, Miyake-Takagi K, Takagi K, Tamura H, Takeo S. Altered extracellular signal-regulated kinase signal transduction by the muscarinic acetylcholine and metabotropic glutamate receptors after cerebral ischemia. J Biol Chem. 2002;277:6382–6390. doi: 10.1074/jbc.M108081200. [DOI] [PubMed] [Google Scholar]
- 36.Berkeley JL, Gomeza J, Wess J, Hamilton SE, Nathanson NM, Levey AI. M1 muscarinic acetylcholine receptors activate extracellular signal-regulated kinase in CA1 pyramidal neurons in mouse hippocampal slices. Mol Cell Neurosci. 2001;18:512–524. doi: 10.1006/mcne.2001.1042. [DOI] [PubMed] [Google Scholar]
- 37.Bernard V, Normand E, Bloch B. Phenotypical characterization of the rat striatal neurons expressing muscarinic receptor genes. J Neurosci. 1992;12:3591–3600. doi: 10.1523/JNEUROSCI.12-09-03591.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ince E, Ciliax BJ, Levey AI. Differential expression of D1 and D2 dopamine and m4 muscarinic acetylcholine receptor proteins in identified striatonigral neurons. Synapse. 1997;27:357–366. doi: 10.1002/(SICI)1098-2396(199712)27:4<357::AID-SYN9>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 39.Santiago MP, Potter LT. Biotinylated m4-toxin demonstrates more M4 muscarinic receptor protein on direct than indirect striatal projection neurons. Brain Res. 2001;894:12–20. doi: 10.1016/s0006-8993(00)03170-x. [DOI] [PubMed] [Google Scholar]
- 40.Vossler MR, Yao H, York RD, Pan MG, Rim CS, Stork PJS. cAMP activates MAP kinase and Elk-1 through a B-Raf-and Rap1-dependent pathway. Cell. 1997;89:73–82. doi: 10.1016/s0092-8674(00)80184-1. [DOI] [PubMed] [Google Scholar]
- 41.Yao H, York RD, Misra-Press A, Carr DW, Stork PJ. The cyclic adenosine monophosphate-dependent protein kinase (PKA) is required for the sustained activation of mitogen-activated kinases and gene expression by nerve growth factor. J Biol Chem. 1998;273:8240–8247. doi: 10.1074/jbc.273.14.8240. [DOI] [PubMed] [Google Scholar]
- 42.York RD, Yao H, Dillon T, Ellig CL, Eckert SP, McCleskey EW, Stork PJ. Rap1 mediates sustained MAP kinase activation induced by nerve growth factor. Nature. 1998;392:622–626. doi: 10.1038/33451. [DOI] [PubMed] [Google Scholar]
- 43.Gerfen CR, Engber TM, Mahan LC, Suswl Z, Chase TN, Monsma FJ, Jr, Sibley DR. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science. 1990;250:1429–1432. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- 44.Aubert I, Ghorayeb I, Normand E, Bloch B. Phenotypical characterization of the neurons expressing the D1 and D2 dopamine receptors in the monkey striatum. J Comp Neurol. 2000;418:22–32. [PubMed] [Google Scholar]
- 45.Bertran-Gonzalez J, Herve D, Girault JA, Valjent E. What is the degree of segregation between striatonigral and striatopallidal projections? Front Neuroanat. 2010;4:136. doi: 10.3389/fnana.2010.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bertran-Gonzalez J, Bosch C, Maroteaux M, Matamales M, Herve D, Valjent E, Girault JA. Opposing patterns of signaling activation in dopamine D1 and D2 receptor-expressing striatal neurons in response to cocaine and haloperidol. J Neurosci. 2008;28:5671–5685. doi: 10.1523/JNEUROSCI.1039-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Valjent E, Pages C, Rogard M, Besson MJ, Maldonado R, Caboche J. Delta 9-tetrahydrocannabinol-induced MAPK/ERK and Elk-1 activation in vivo depends on dopaminergic transmission. Eur J Neurosci. 2001;14:342–352. doi: 10.1046/j.0953-816x.2001.01652.x. [DOI] [PubMed] [Google Scholar]
- 48.Perkinton MS, IPJK, Wood GL, Crossthwaite AJ, Williams RJ. Phosphatidylinositol 3-kinase is a central mediator of NMDA receptor signaling to MAP kinase (Erk1/2), Akt/PKB and CREB in striatal neurons. J Neurochem. 2002;80:239–254. doi: 10.1046/j.0022-3042.2001.00699.x. [DOI] [PubMed] [Google Scholar]
- 49.Chen X, Garelick MG, Wang H, Lil V, Athos J, Storm DR. PI3 kinase signaling is required for retrieval and extinction of contexual memory. Nat Neurosci. 2005;8:925–931. doi: 10.1038/nn1482. [DOI] [PubMed] [Google Scholar]
- 50.Tzavara ET, Bymaster FP, Davis RJ, Wade MR, Perry KW, Wess J, McKinzie DL, Felder C, Nomikos GG. M4 muscarinic receptors regulate the dynamics of cholinergic and dopaminergic neurotransmission: relevance to the pathophysiology and treatment of related CNS pathologies. FASEB J. 2004;18:1410–1412. doi: 10.1096/fj.04-1575fje. [DOI] [PubMed] [Google Scholar]
- 51.Meltzer HY, Chai BL, Thompson PA, Yamamoto BK. Effect of scopolamine on the efflux of dopamine and its metabolites after clozapine, haloperidol or thioridazine. J Pharmacol Exp Ther. 1994;268:1452–1461. [PubMed] [Google Scholar]
- 52.Tsukada H, Harada N, Nishiyama S, Ohba H, Kakiuchi T. Cholinergic neuronal modulation alters dopamine D2 receptor availability in vivo by regulating receptor affinity induce by facilitated synaptic dopamine turnover: positron emission tomography studies with microdialysis in the conscious monkey brain. J Neurosci. 2000;20:7067–7073. doi: 10.1523/JNEUROSCI.20-18-07067.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gray AM, Connick JH. Clozapine-induced dopamine levels in the rat striatum and nucleus accumbens are not affected by muscarinic antagonism. Eur J Pharmacol. 1998;362:127–136. doi: 10.1016/s0014-2999(98)00695-5. [DOI] [PubMed] [Google Scholar]
- 54.Rawls SM, McGinty JF. Muscarinic receptors regulate extracellular glutamate levels in the rat striatum: an in vivo microdialysis study. J Pharmacol Exp Ther. 1998;286:91–98. [PubMed] [Google Scholar]
- 55.Voleti B, Navarria A, Liu RJ, Banasr M, Li N, Terwilliger R, Sanacora G, Eid T, Aghajanian G, Duman RS. Scopolamine rapidly increases mTORC1 signaling, synaptogenesis, and antidepressant behavioral responses. Biol Psychiatry. 2013;74:742–749. doi: 10.1016/j.biopsych.2013.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Runyan JD, Dash PK. Intra-medial prefrontal administration of SCH-23390 attenuates ERK phosphorylation and long-term memory for trace fear conditioning in rats. Neurobiol Learn Mem. 2004;82:65–70. doi: 10.1016/j.nlm.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 57.Kosako H, Yamaguchi N, Aranami C, Ushiyama M, Kose S, Imamoto N, Taniguchi H, Nishida E, Hattori S. Phosphoproteomics reveals new ERK MAP kinase targets and links ERK to nucleoporin-mediated nuclear transport. Nat Struct Mol Biol. 2009;16:1026–1035. doi: 10.1038/nsmb.1656. [DOI] [PubMed] [Google Scholar]
- 58.Mao LM, Wang JQ. Synaptically localized mitogen-activated protein kinases: local substrates and regulation. Mol Neurobiol. 2016;53:6309–6315. doi: 10.1007/s12035-015-9535-1. [DOI] [PMC free article] [PubMed] [Google Scholar]