Abstract
Syntaxin 11 (STX11) controls vesicular trafficking and is a key player in exocytosis. Since Stx11 mutations are causally associated with a Familial Hemophagocytic Lymphohistiocytosis (FHL-4), we wanted to clarify whether STX11 is functionally important for key immune cell populations. This was studied in primary cells obtained from newly generated Stx11-/- mice. Our data revealed that STX11 is not only widely expressed in different immune cells, but also induced upon LPS or IFN-γ treatment. However, Stx11 deficiency does not affect macrophage phagocytic function and cytokine secretion, mast cell activation, or antigen presentation by DC. Instead, STX11 selectively controls lymphocyte cytotoxicity in NK and activated CD8+ T cells and degranulation in neutrophils. Stx11-/- NK cells and CTL show impaired degranulation, despite a comparable activation, maturation and expression of the complex-forming partners MUNC18-2 and VTI1B. In addition, Stx11-/- CTL and NK cells produce abnormal levels of IFN-γ. Since functional reconstitution rescues the defective phenotype of Stx11-/- CTL we suggest a direct, specific and key role of STX11 in controlling lymphocyte cytotoxicity, cytokine production and secretion. Finally, we show that these mice are a very useful tool for dissecting the role of STX11 in vesicular trafficking and secretion.
Keywords: SNARE, cytokines, exocytosis, CTL
Introduction
STX11 belongs to the family of SNARE proteins and is involved in intracellular membrane trafficking events by interacting with other family members or by associating with membranes by palmitoylation of cysteine residues [1–3]. STX11 is expressed in a wide variety of cells including human monocytes/macrophages, neutrophils, B cells, NK cells and CD8+ T cells [2–9]. STX11 is enriched in immune tissues such as thymus, spleen and lymph nodes, but lower levels of protein are detectable also in heart, kidney and liver. In lung tissue the molecular mass of the protein is lower than that reported in other tissues [2]. STX11 localizes mainly in the vesicular tubular clusters, an organelle complex between the endoplasmic reticulum and the Golgi, and displays, in addition, a spotted staining pattern throughout the cell periphery [2, 9].
While STX11 has been recognized to act as a negative regulator of both phagocytosis and intracellular trafficking between endosomes, lysosomes and the outer membrane in macrophages [6, 9], in human NK and CTL cells, STX11 has been suggested to regulate granule exocytosis and cytotoxicity [4, 5].
Several disease-causing mutations have been identified in genes encoding for various components of the cytolytic machinery, including the pore-forming protein perforin, the proteins belonging to the exocytic machinery (e.g. Munc13-4 and Rab27a), and STX11 [10–13]. The underlying pathology is called Familial Hemophagocytic Lymphohistiocytosis, a rare autosomal recessive disorder characterized by an increased inflammatory response with abnormal secretion of proinflammatory cytokines (IFN-γ, TNF, IL-6 and IL-18). Patients show impaired NK-cell and CTL function, as well as a lymphohistiocytic infiltration of visceral organs associated with macrophage activation. Long term cure can only be achieved by hematopoietic stem cell transplantation [14].
Recently two groups have shown a direct interaction of STX11 and MUNC18-2 in regulating granule exocytosis, by demonstrating that FHL type 5 is caused by mutations in MUNC18-2 (STXBP2 encoding syntaxin binding protein 2) that affect its binding to STX11, mutated in FHL-4 [15, 16]. MUNC18 proteins are involved in the regulation of intracellular trafficking and in the control of SNARE complex assembly and disassembly [17]. Recently VTI1B, a target-membrane SNARE protein which controls CTL degranulation, has also been described as interacting with STX11 in macrophages [9, 18].
Taking advantage of the newly generated Stx11-/- mice we investigated the role of STX11 in controlling selective activities in BMMs, BMDCs, BMMCs, neutrophils, NK cells and CTLs. Here we could demonstrate that, while the cytokine secretion, degranulation, phagocytosis and antigen presentation are not altered in Stx11-/- BMM, BMDC and BMMC, neutrophils display an impaired degranulation, and NK and CTL cells show an impaired cytotoxic activity, a decreased degranulation and a higher production and secretion of IFN-γ compared with WT cells. Our results demonstrate the essential and selective role of STX11 in controlling granule exocytosis in neutrophils, NK and CTL while its function is dispensable or complemented in other STX11 expressing cell types.
Results
Loss of STX11 expression does not affect immune system development
STX11 has been recognized to regulate vesicle trafficking events [19]. However, its mechanism of action remains ill defined. To address the role of STX11 in vivo, we generated Stx11-/- mice. A targeting vector was constructed to disrupt the murine Stx11 gene (Supporting Information Fig. 1A). Homologous recombined RW-4 ES cell clones were used to generate chimeras that transmitted the introduced mutation to their offsprings. Excision of the targeted region of Stx11 was confirmed by Southern blot analysis (Supporting Information Fig. 1B), and STX11 protein was undetectable in membrane enriched fractions of peripheral blood mononuclear cells from Stx11-/- mice (Supporting Information Fig. 1C). Stx11-/- mice were born at the expected Mendelian frequency, developed normally to adulthood, were fertile and did not manifest overt phenotypic abnormalities. Compared with WT mice, Stx11-/- mice exhibited no differences either in cell numbers, cell population percentages, expression of specific cell surface molecules, or in activation markers in spleen and lymph nodes (Table 1, Supporting Information Table and data not shown). B- and T-cell differentiation in the bone marrow and thymus were also comparable between WT and Stx11-/- mice (data not shown). Additionally, a histologic examination of lymphoid tissues such as spleen, lymph nodes and thymus, from WT and Stx11-/- mice showed similar structural features (Supporting Information Fig. 1D). Thus, deletion of Stx11 has no overt effect on the phenotype of the mice in relation to immune system development and immune cell populations in lymphatic tissues.
Table I.
Phenotype of Stx11-/- mice compared to WT micea.
| Cell type | Surface marker | WT (%) | Stx11-/- (%) | |
|---|---|---|---|---|
| Spleen | CD4+ T cells | CD3+ CD4+ | 17.78 ± 0.49 | 19.22 ± 1.13 |
| CD8+ T cells | CD3+ CD8+ | 12.40 ± 0.97 | 12.94 ± 0.68 | |
| B cells | B220+ | 49.30 ± 4.16 | 51.16 ± 3.24 | |
| Immature B cells | B220+IgMhighIgDlow | 12.07 ± 2.44 | 15.12 ± 4.72 | |
| Mature B cells | B220+IgMlowIgDhigh | 33.46 ± 4.59 | 40.39 ± 1.90 | |
| Granulocytes | CD11b+ Gr-1high | 2.92 ± 0.23 | 2.95 ± 0.24 | |
| NK cells | CD3- DX5+ | 2.21 ± 0.36 | 1.99 ± 0.31 | |
| DC | CD11c+ | 3.77 ± 0.19 | 3.55 ± 0.25 | |
| Macrophages | Gr-1-CD11b+ | 4.17 ± 0.21 | 4.38 ± 0.39 | |
| Lymph node | CD4+ T cells | CD3+ CD4+ | 24.69 ± 3.23 | 25.91 ± 1.26 |
| CD8+ T cells | CD3+ CD8+ | 15.22 ± 1.58 | 15.51 ± 1.75 | |
| B cells | B220+ | 50.90 ± 3.51 | 52.14 ± 2.15 | |
| NK cells | CD3- DX5+ | 0.91 ± 0.12 | 0.93 ± 0.13 |
Splenocytes and lymph node cells from WT and Stx11-/- mice were analyzed by flow cytometry.
Values represent the mean ± SEM percentage of cell subsets in the spleen (n=8) and lymph nodes (WT n=7; Stx11-/- n=6) and are the summary of 3 experiments performed.
Effector functions of macrophages and dendritic cells are not affected by STX11 deficiency
STX11 expression is upregulated in the murine macrophage cell line RAW264.7 upon LPS stimulation [9]. In human monocytes STX11 is upregulated after IFN-γ stimulation and acts as a negative regulator of macrophage phagocytosis [6]. We therefore examined the expression of STX11, its regulation, as well as its role in macrophage phagocytosis and cytokine secretion. Semi-quantitative PCR analysis revealed that the expression of Stx11 is up-regulated in BMM stimulated for 4h with 1 µg/ml LPS or 500 U/ml IFN-γ (Fig. 1A). In order to confirm the induced upregulation of Stx11, lysates from enriched membrane fractions of LPS-stimulated BMM were subjected to Western blot analysis. LPS induced an approximately two fold increase in STX11 protein levels (Fig. 1B), consistent with up-regulated mRNA levels. Interestingly, BMDC also showed stx11 expression at both the mRNA and protein levels upon LPS or IFN-γ stimulation (Fig. 1A,B).
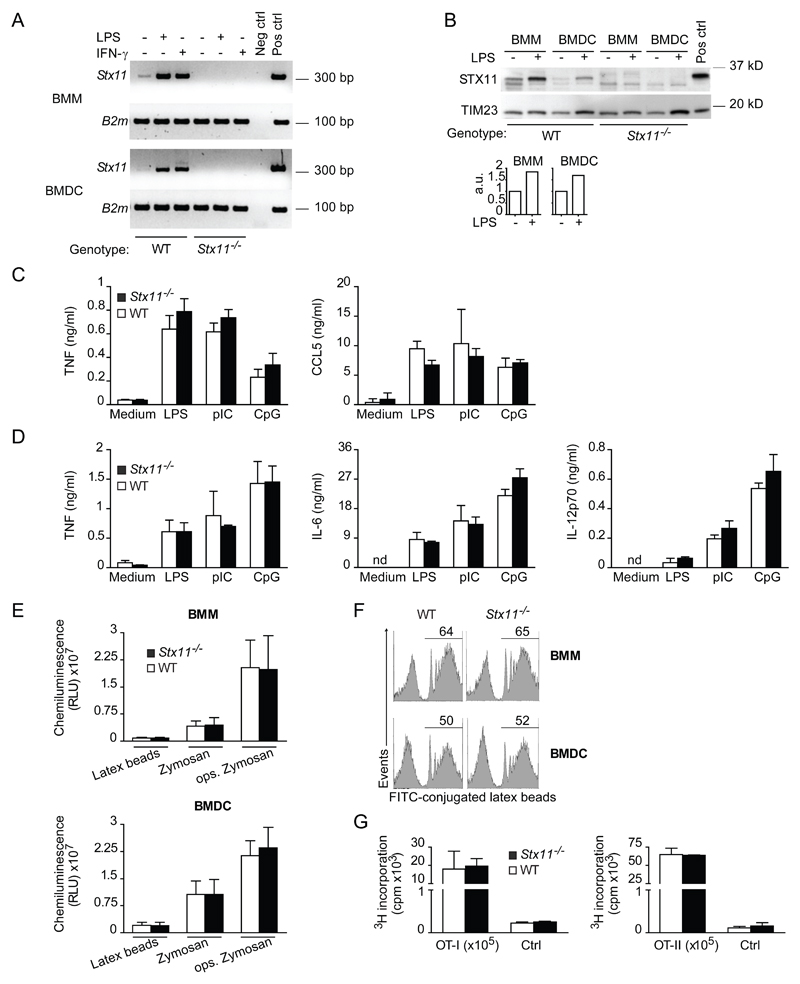
Figure 1. STX11 expression is regulated upon cell activation, but its deficiency does not impair BMM and BMDC functions.
(A) RNA and cDNA were prepared from WT or Stx11-/- BMMs and BMDCs. PCR was performed with gene-specific primers as indicated. Neg ctrl, negative control, omission of cDNA in PCR reaction; Pos ctrl, positive control, cDNA from WT LPS-stimulated BMMs. Results shown are representative of two independent experiments. (B) STX11 expression was measured by immunoblotting enriched membrane fractions obtained from BMMs or BMDCs stimulated 24h with 100 ng/ml LPS or left untreated. Pos ctrl, membrane fractions of HEK cells transfected with a construct expressing murine STX11. Quantification is shown as values of arbitrary units (a.u.) relative to TIM23 expression levels as measured by ImageJ Software. Image shown is representative of two experiments. (C) BMM and (D) BMDC supernatants were collected and levels of indicated cytokines were measured by ELISA. Data are expressed as mean + SD of cultures from three different mice of different genotype and are representative of three to four independent experiments. nd, not detectable. (E) BMMs or BMDCs were stimulated as indicated and ROS formation was quantified by chemiluminescence in the presence of lucigenin and recorded for 60 min. Data were integrated over 60 min and shown as mean + SD of 5-6 individual mice. Data are representative of three experiments performed. RLU, relative light units. (F) Cells were stimulated with fluorochrome-labelled latex beads and phagocytosis was analyzed by flow cytometry. The values in the histograms represent the percentage of cells phagocytosing FITC-labelled latex beads. One experiment is shown (n=5), representative of three performed. (G) CD8+ T cells (OT-I) and CD4+ T cells (OT-II) were stimulated for proliferation with peptide-loaded BMDCs from WT or Stx11-/- mice. T-cell proliferation was evaluated by incorporation of 3H-thymidine and shown as mean + SD of two to three samples. Data are representative of three independent experiments. Ctrl, proliferation of T cells cultured in the absence of peptide.
To determine whether STX11 is required for BMM and DC function, we compared cytokine secretion and phagocytosis in WT and Stx11-/- BMM and BMDC. Across a panel of selected cytokines, TNF, IL-6 and IL-12p70 were found to be secreted by BMDC in response to LPS, pIC or CpG treatment for 24h or 48h, and TNF and CCL5 by BMM. However, secretion of these cytokines and chemokines was not significantly different in Stx11-/- BMM and BMDC compared with the WT controls (Fig. 1C,D and data not shown). Importantly, the induction of a respiratory burst and the phagocytic capacity were not impaired in BMM and BMDC from Stx11-/- mice (Fig. 1E,F). The production of free radicals after cell treatment with latex beads, zymosan, or opsonized zymosan particles, measured by chemiluminescence (Fig. 1E), and the phagocytic uptake, evaluated as the percentage of cells capable to phagocytose FITC-labelled latex beads (Fig. 1F), showed a similar phagocytic activity in both WT and Stx11-/- BMDC as well as BMM.
Altogether, these results suggest that Stx11 deletion does not affect BMM and BMDC activities such as phagocytosis, formation of oxygen radicals and TLR-induced secretion of selected cytokines.
Furthermore, we have evaluated the ability of Stx11-/- BMDC to induce antigen-specific T cell proliferation. To this purpose, we cultured purified TCR-transgenic CD8+ OT-I or CD4+ OT-II T cells with OVA257-264- or OVA323-339-loaded BMDC, respectively. Stx11-/- BMDC induced CD8+ and CD4+ T cell proliferation comparable with that mediated by WT BMDC (Fig. 1G). Similar results were obtained with the use of the OVA protein (data not shown). Therefore, STX11 is not essential for DC antigen processing and DC-mediated induction of CD8+ and CD4+ T cell proliferation.
Stx11-/- BMMC exhibit a normal antigen-induced degranulation while neutrophils show a defective exocytosis
Granule exocytosis in mast cells requires SNARE expression [19]. Therefore, we investigated the expression and the requirement of STX11 for mast cell degranulation. In primary BMMC, Stx11 transcripts were upregulated after LPS and IgE+Ag stimulation (Fig. 2A). STX11 protein was detected in primary BMMC (Fig. 2B). Antigen stimulation of IgE-sensitized Stx11-/- BMMC induced rapid degranulation, the extent of which was comparable with that detected in WT BMMC (Fig. 2C). Thus even though STX11 expression is regulated upon cell activation, it is dispensable for BMMC degranulation.
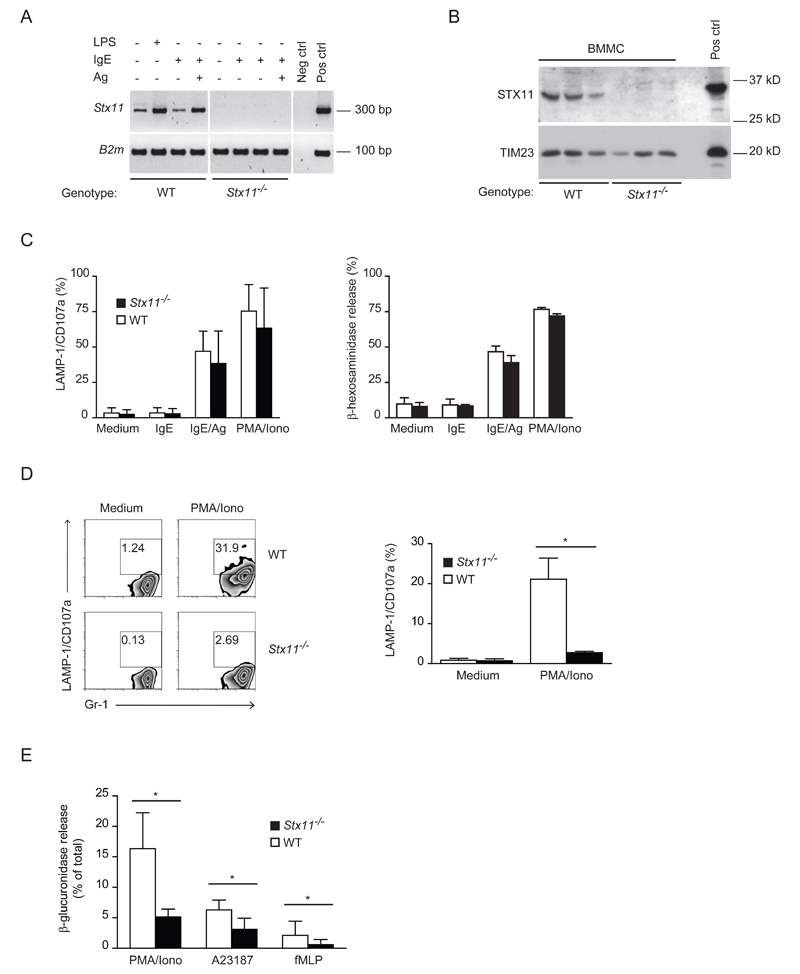
Figure 2. STX11 deficiency impairs neutrophil, but not BMMC, degranulation.
(A) cDNA was prepared from WT (n=3) or Stx11-/- (n=3) BMMCs. PCR was performed with gene-specific primers as indicated. Neg ctrl, negative control, omission of cDNA in PCR reaction; Pos ctrl, positive control, WT LPS stimulated BMM cDNA. Shown is one representative experiment out of two performed. (B) STX11 protein expression was measured by immunoblotting enriched membrane fractions obtained from different WT or Stx11-/- BMMC cultures. Pos ctrl, membrane fractions of HEK cells transfected with a construct expressing murine STX11. Shown is one representative experiment out of three performed. (C) BMMC surface expression of CD107a/LAMP-1 was analyzed by flow cytometry (left); data are shown as mean + SD of cultures from different mice of different genotype pooled from two degranulation experiments performed (WT: n=6, Stx11-/-: n=5). Enzymatic activity of secreted β-hexosaminidase was assessed (right); data are shown as mean + SD of three samples and are representative of three independent experiments. (D) Neutrophil degranulation was measured by LAMP-1/CD107a surface expression on CD11b+Gr-1+ cells. Values in the plots indicate the percentage of gated cells. Graph reports results pooled from two degranulation experiments performed (n=5); data are expressed as mean + SD. *= p<0.05. Mann Whitney test was performed. (E) Release of β-glucuronidase from primary granules of WT or Stx11-/- neutrophils was measured using the activity of neutrophil-specific β-glucuronidase in the supernatant of leukocytes. Data are shown as mean + SD of samples pooled from three independent experiments (WT: n=5-6, Stx11-/-: n=5-8). *= p<0.05 Mann Whitney test was performed.
Since STX11 has been described to be highly expressed in human neutrophils [7], the role of STX11 deficiency in the murine counterparts was investigated. To this purpose, the degranulation activity of Stx11-/- neutrophils was compared with that of WT cells by measuring LAMP-1/CD107a translocation and release of neutrophil-specific β-glucuronidase upon stimulation with PMA/ionomycin or fMLP. As shown in Figure 2D and E, Stx11-/- neutrophils show a reduced LAMP-1/CD107a staining and a reduced β-glucuronidase release compared with that of WT cells. Thus, STX11 controls neutrophils degranulation.
Cytotoxicity and degranulation are impaired in Stx11-/- CTL and NK cells
Defective NK cell degranulation and cytotoxicity have been identified in patients with FHL-4, which correlates with a deficiency in STX11 expression [5, 13]. Therefore we investigated whether a constitutive deficiency of STX11 in NK cells and CTL results in dysregulated degranulation and granule exocytosis in both cell types and whether this altered effector function correlates with a specific NK or CD8+ T cell phenotype. We first evaluated STX11 expression in primary murine NK and CD8+ T cells. Freshly isolated NK and CD8+ T cells both expressed Stx11 mRNA (Fig. 3A). In addition, STX11 protein could be detected in freshly isolated CD8+ T cells as well as in naive NK cells (Fig. 3B).
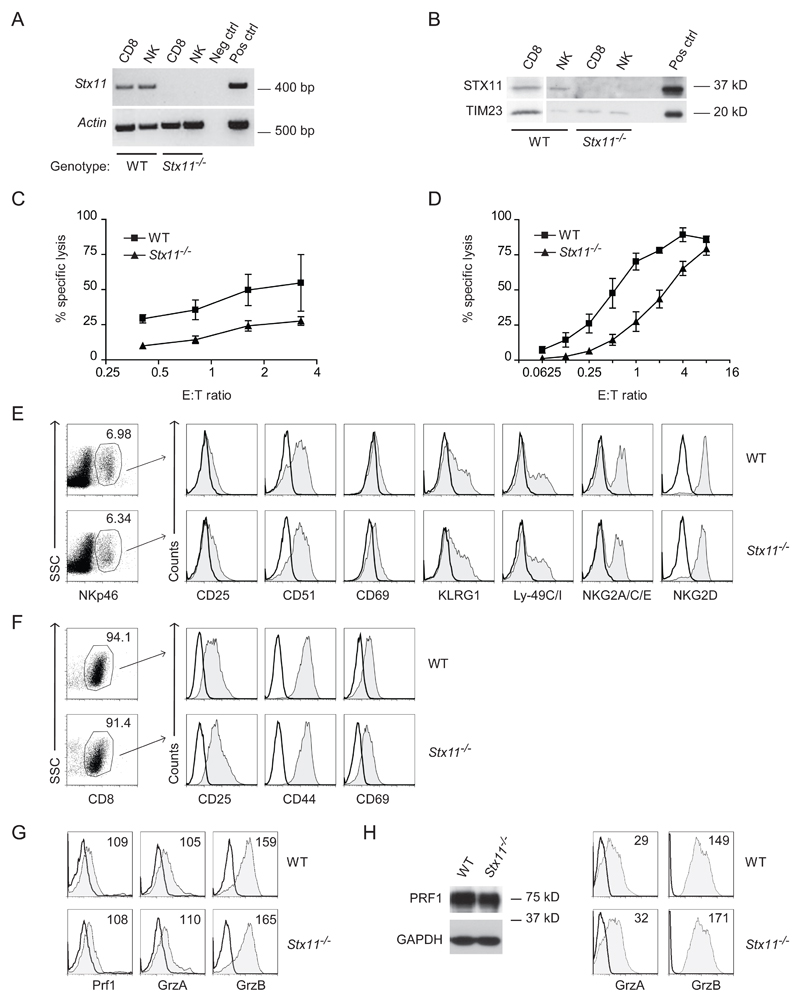
Figure 3. Cytotoxicity is impaired in Stx11-/- CTLs and NK cells, which display a phenotype comparable with that of WT counterparts.
(A) cDNA was prepared from ex vivo-sorted DX5+CD3− or CD8+ T cells from WT or Stx11-/- splenocytes and PCR was performed using gene-specific primers for Stx11 or Actin. Neg ctrl, negative control, omission of cDNA in PCR reaction; Pos ctrl, positive control, WT LPS stimulated BMM cDNA. Shown is one representative experiment out of two performed. (B) Western blot analysis of stx11 protein expression in naive NK and CD8+ T cells. Pos ctrl, membrane fractions of HEK cells transfected with a construct expressing murine STX11. Shown is one representative of three independent experiments. (C) NK cell specific lysis was assessed by release of cytoplasmic lactate dehydrogenase in vitro from YAC-1 cells co-incubated with IL-15 treated WT or Stx11-/- splenocytes, at various effector/target ratios (E:T). Prior to the assay, an aliquot of effector cells was analyzed by flow cytometry to determine the relative percentages of NK cells, thus a splenocyte:target ratio of 50:1 corresponds to an NK cell:target ratio of 3.25:1. Results are expressed as mean + SD of three samples and are representative of three independent experiments. (D) CTL mediated cytotoxicity was assessed against P815 cells, in the presence of anti-CD3ε antibody, co-incubated with WT or Stx11-/- CD8+ T cells stimulated for 2 rounds with anti-allo stimulation, at various effector/target ratios (E:T). Results are expressed as mean + SD of three samples and are representative of two independent experiments. (E, F) Flow cytometry analysis of WT or Stx11-/- IL-15 treated NK cells and CTL stimulated for 2 rounds with anti-allo stimulation. Plots are gated on (E) SSC and NKp46+ or (F) SSC and CD8+ T cells. Values in plots indicate percent of positive cells. Shaded histograms show marker expression as indicated, bold lines show the corresponding isotype control. (E) Plots are representative of three independent experiments (n=8-9 mice in total). (F) Plots are representative of two independent experiments (n=5 mice in total). (G) Intracellular staining for Granzyme A (GrzA), -B (GrzB) or perforin of WT or Stx11-/- NK cells treated with IL-15 (gated on NK1.1+CD3- cells). Shaded histograms show marker expression as indicated (mean fluorescence intensity displayed), bold lines show the corresponding isotype control. Data shown are representative of three to four independent experiments. (H) Western blot for perforin and intracellular staining for GrzA and GrzB of WT or Stx11-/- CD8+ T cells stimulated for 3 rounds of anti-allo stimulation (gated on CD8+ T cells). Shaded histograms show marker expression as indicated (mean fluorescence intensity displayed), bold lines show the corresponding isotype control. Data shown are representative of three to four independent experiments.
The cytotoxic potential of both NK cells and CTL from WT and Stx11-/- mice was then examined. To this purpose WT and Stx11-/- splenocytes were treated with IL-15 for 48h in vitro, and cytolytic activity was determined by measuring the lysis of YAC-1 target cells by means of lactate dehydrogenase release. As shown in Figure 3C, the Stx11-/- NK cell-mediated cytotoxicity was lower compared with that of WT NK cells. In addition we looked at the role of rhIL-2 on the cytotoxic effect of NK cells derived from WT and Stx11-/- mice. To this purpose splenocytes from WT and Stx11-/- mice were cultured with 2000 U/ml rhIL-2 for 6 days and then incubated with YAC-1 target cells. Despite the prolonged incubation of Stx11-/- NK cells with high doses of IL-2 the impaired cytolytic defect was maintained (Supporting Information Fig. 2).
To determine whether Stx11-/- CTL were also affected in their effector functions, splenocytes from C57Bl/6 WT and Stx11-/- mice were stimulated two or more rounds in vitro with allogenic mitomycin treated BALB/c splenocytes in order to generate activated CTL. CTL-mediated re-directed cytotoxicity was measured against P815 target cells in the presence of anti-CD3ε mAb, and Stx11-/- CTL cytolytic activity was found to be markedly reduced at all effector:target cell ratios examined (Fig. 3D).
To investigate whether the defective cytotoxicity in Stx11-/- NK cells and CTL may be due to differences in the maturation and activation status, we determined the expression of selected NK and CD8+ T cell receptors. NKp46+ Stx11-/- splenic NK cells display a mature phenotype comparable with WT, characterized by the expression of DX5 and NK1.1, NKG2A/C/E receptors, the stimulatory receptor NKG2D, the inhibitory receptors KLRG1, Ly-49G2 and Ly-49C/I (Supporting Information Fig. 3A). Similarly, Stx11-/- CD8+ T cells were comparable with WT cells in their marker expression, showing a naive mature phenotype. WT and Stx11-/- CD8+ T cells displayed CD45, highly expressed the CD27 receptor, the L-selectin CD62L and CD73; lacked CD25, CD28 and CD69; and expressed CD44 and CD95 at an intermediate degree (Supporting Information Fig. 3B and data not shown). Furthermore, WT and Stx11-/- NK and CD8+ T cells were phenotypically investigated after activation. The expression of inhibitory and activation markers such as CD25, CD51, CD69, KLRG1, Ly-49C/I, NKG2A/C/E, NKG2D in NK and CD25, CD44, CD69 in CTL was found to be comparable between WT and Stx11-/- NK and CTL (Fig. 3E,F). Also the expression of Granzyme A, -B and perforin, as detected by intracellular FACS staining and Western blot, was comparable between WT and Stx11-/- activated NK and CD8+ T cells (Fig. 3G,H).
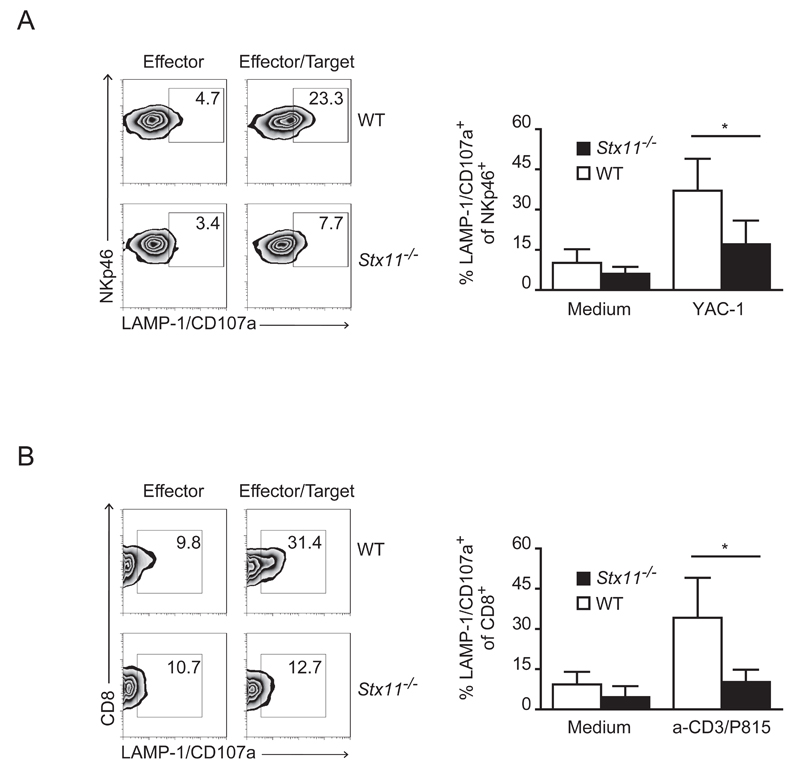
Next we investigated whether the decreased cytotoxicity in Stx11-/- NK cells and CTL correlates with a defect in cell degranulation. Therefore, we assessed the ability of activated NK and CD8+ T cells to degranulate by measuring the translocation of LAMP-1/CD107a to the plasma membrane (Fig. 4A,B). Compared with WT cells, a significant impairment of Stx11-/- NK and CTL exocytosis was observed, as indicated by the lower number of CD8+CD107a+ and NKp46+CD107a+ cells, upon co-incubation with P815 or YAC-1 targets, respectively. This indicates that despite the unaltered phenotype and expression of Granzyme A, -B and perforin, Stx11-/- NK and CD8+ T cells display a defect in their ability to degranulate and consequently in their cytolytic activity.
Figure 4. Stx11-/- NK cells and CTLs display defective degranulation.
(A) rhIL-15 treated splenocytes (NKp46+) or (B) CTL (CD8+) cells were incubated alone or with YAC-1 or anti-CD3 presenting P815 target cells respectively (E/T ratio: 10:1). Target cell-induced degranulation was measured by LAMP-1/CD107a surface expression. Values in plots indicate the percentage of gated cells. One representative result out of five (A) or three (B) independent experiments is shown. Histograms report results pooled from all degranulation experiments performed and are shown as mean + SD of 13-14 (A) or 8 different mice of different genotype (B). *= p<0.005. Mann Whitney test was performed.
Transduction of STX11 into Stx11-/- CTL rescues the defective phenotype
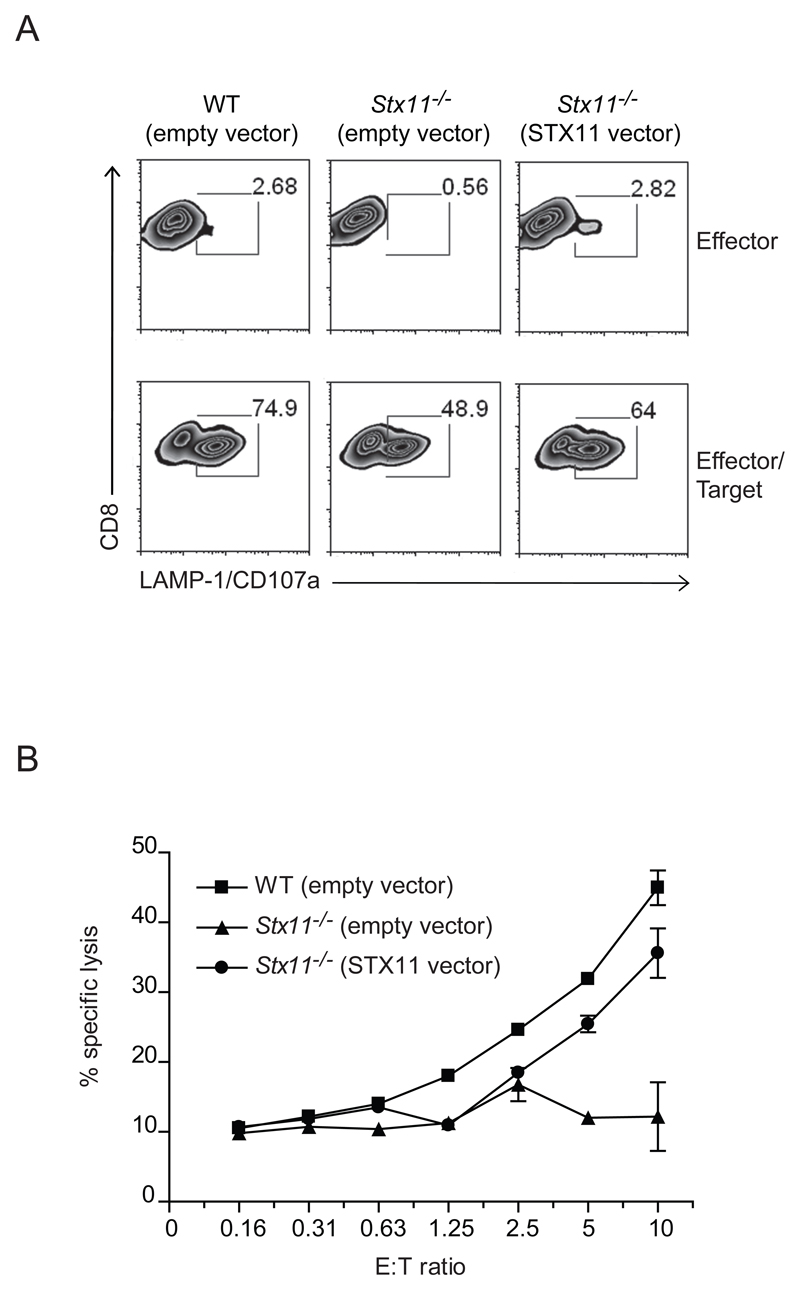
To determine if exogenous STX11 could rescue the defective phenotype in Stx11-/- CTL, we infected allo-stimulated CTL with retroviral particles delivering the vector expressing STX11 or the vector alone. On day 5-6 after infection the cells were stimulated with anti-CD3 and P815 target cells to induce degranulation. Compared with Stx11-/- CTL transduced with the empty vector, Stx11-/- CTL infected with retroviral particles coding for STX11, showed an increased degranulation capacity (Fig. 5A). In addition, CTL infected with STX11 vector partially rescued the defect in cytotoxicity compared with WT CTL infected with the empty vector (Fig. 5B). Transduction of STX11 restores both the degranulation and the killing capacity in Stx11-/- CTL, highlighting an important role of STX11 in CTL’s main activities.
Figure 5. STX11 transduction into Stx11-/- CTLs partially rescues the defect.
At 5 to 6 days post infection, CTL cells infected with viral particles carrying an empty vector or STX11 vector, were incubated alone or with anti-CD3 presenting P815 target cells. (A) Target cell-induced degranulation was measured by LAMP-1/CD107a expression on CD8+ T cells (E:T ratio of 1:1). Values in the gates indicate the percentage of gated cells. The plots are the result of three pooled mice. (B) CTL mediated cytotoxicity was assessed against P815 cells, in the presence of anti-CD3ε antibody, co-incubated with WT or Stx11-/- CD8+ T cell stimulated for 4 rounds with anti-allo stimulation. Graph depicts the mean + SD for various effector/target ratios (E:T). Data shown are of pooled cultures from three different mice of each genotype, and are representative of three independent experiments.
STX11 deficiency does not affect MUNC18-2 and VTI1B expression and intracellular localization
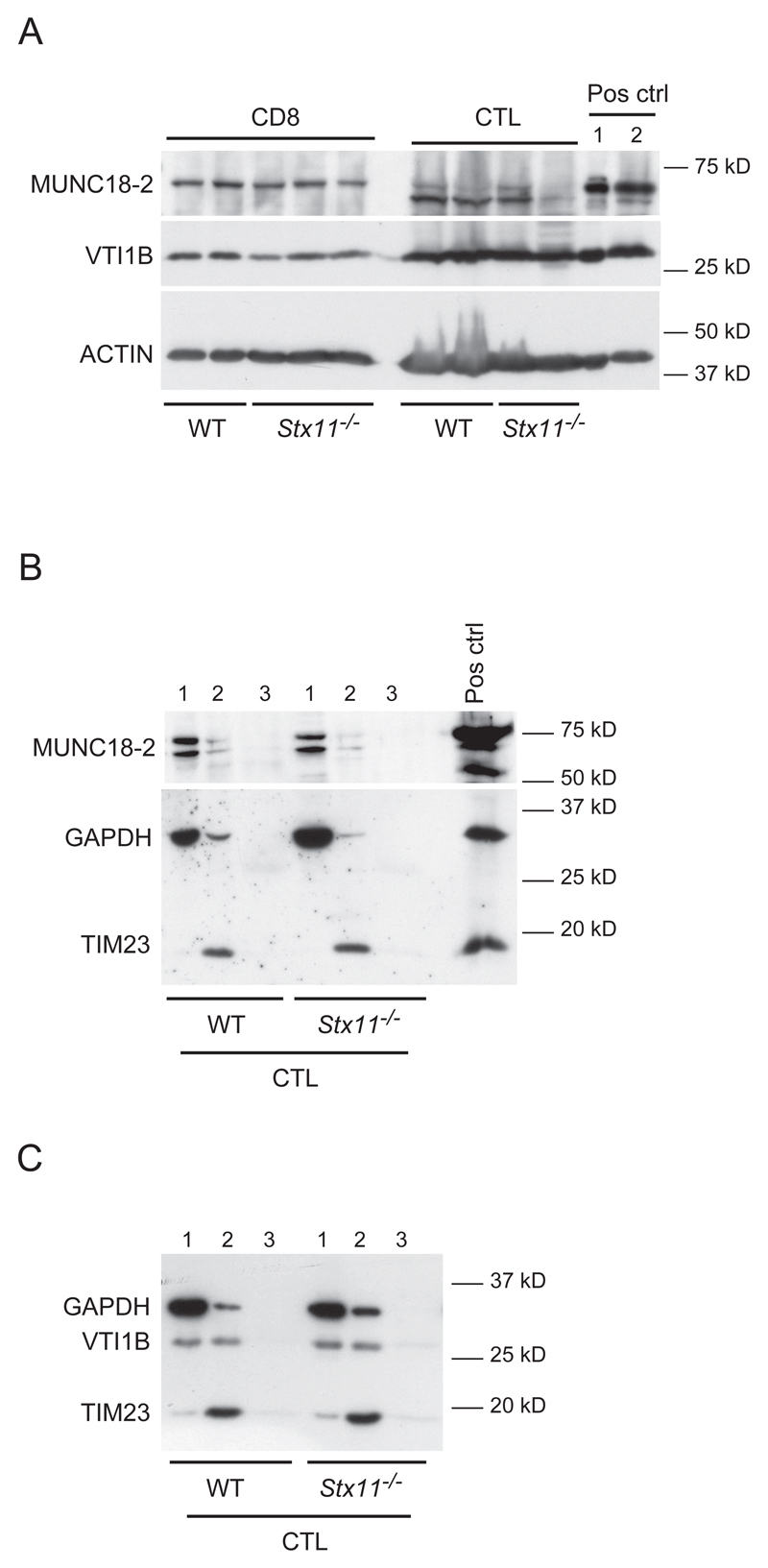
To further explore the role of STX11 in the molecular control of granule exocytosis, we investigated whether the deficiency of STX11 could affect the expression or localization of STX11 co-associating SNARE proteins. To this purpose, the expression of MUNC18-2 and VTI1B was evaluated in whole lysates from naive and activated CD8+ T cells, as well as in the membrane fractions of activated CD8+ T cells. MUNC18-2 and VTI1B protein expression in both naive and activated CD8+ T cell lysates was comparable between WT and Stx11-/- cells (Fig. 6A). In order to determine whether STX11 deficiency might affect the localization of MUNC18-2 after stimulation, activated CD8+ T cells were subjected to subcellular fractionation. As shown in Figure 6B, MUNC18-2 is mainly found in the cytosolic fraction upon stimulation in WT and Stx11-/- CD8+ T cells, while VTI1B is localized in both cytosolic and membrane fractions of CTL cells (Fig. 6C). However, the cellular distribution of VTI1B is comparable between WT and Stx11-/- CD8+ T cells. Thus these data suggest that STX11 does not affect the expression and localization of protein partners found to co-associate in human macrophages, VTI1B, and in lymphocytes, MUNC18-2.
Figure 6. MUNC18-2 and VTI1B expression and localization are not affected by STX11 deficiency.
(A) MUNC18-2 expression was measured by immunoblotting of whole lysates from WT and Stx11-/- purified naive and activated CD8+ T cells. ACTIN expression was used as internal control for the lysates preparation. Pos ctrl, positive control, 1: WT LPS stimulated BMMs pooled lysates and membrane fraction, 2: WT BMMCs lysates. (B, C) Enriched subcellular fractions obtained from allo-stimulated CTL were analyzed by western blot for (B) MUNC18-2 and (C) VTI1B localization. GAPDH is used as internal control for the cytoplasmic fractions; TIM-23 is used as internal control for the membrane fractions. 1, cytosolic fraction; 2, membrane fraction; 3, nuclear fraction; Pos ctrl, positive control, WT BMMCs lysate. Results are representative of two independent experiments.
STX11 deficiency in NK and CD8+ T cells alters cytokine production
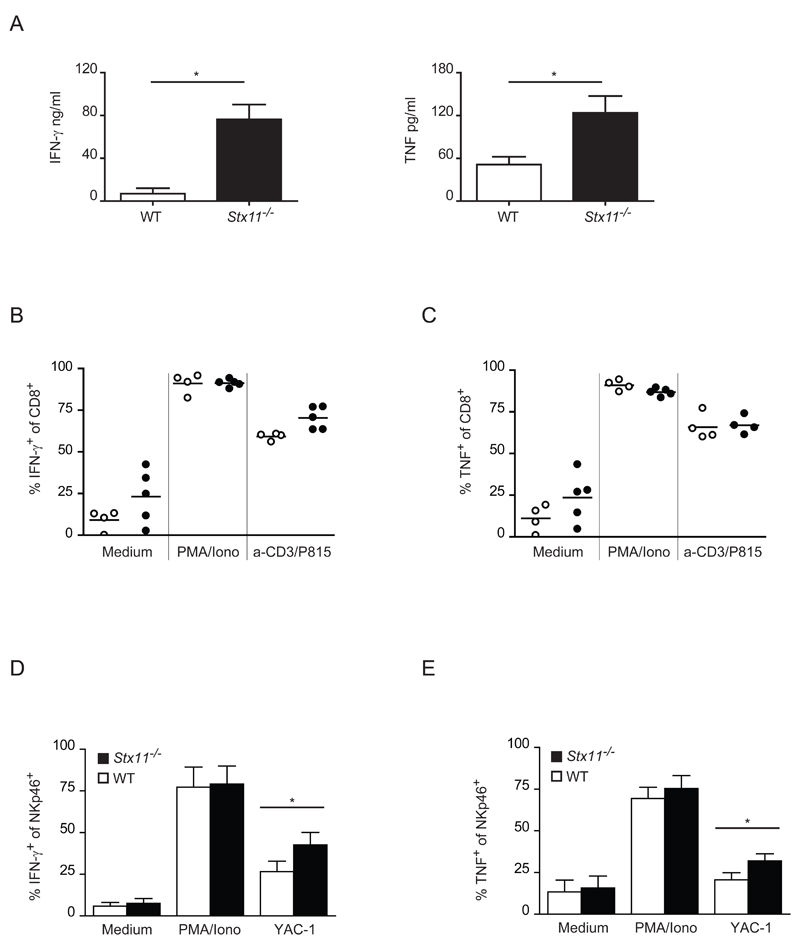
Next, we investigated whether the defect in CD8+ T cell degranulation correlates with a dysregulated cytokine secretion. Supernatants from allo-stimulated CTL cells were examined for IFN-γ and TNF cytokine levels by ELISA. Interestingly, the secretion of both cytokines was found highly increased in supernatants obtained from Stx11-/- CTL compared with WT cells (Fig. 7A). In order to define whether the higher release in Stx11-/- CTL supernatants was due to an increase in the production of these cytokines, IFN-γ and TNF production was measured intracellularly by FACS staining in CTL cells re-stimulated 3 times in vitro with alloantigens and then with either PMA/ionomycin, P815 and anti-CD3ε mAb, or left untreated. While Stx11-/- CTL showed a higher synthesis of IFN-γ compared with WT CTL after re-stimulation with P815 cells and anti-CD3ε, the intracellular expression of TNF was comparable with the WT control not only after re-stimulation with PMA and ionomycin, but also with P815 cells and anti-CD3ε (Fig. 7B,C). Comparable results were obtained as well after 4 rounds of anti-allo stimulation (data not shown). Stx11-/- NK cells cytokine production was also examined. Splenocytes were cultured for 48h in the presence of rhIL-15, harvested and incubated with PMA and ionomycin, YAC-1 target cells or left untreated. The intracellular cytokine content was detected by flow cytometry. As shown in Figure 7D and 7E Stx11-/- NK cells expressed an increased level of IFN-γ and TNF compared with WT controls after incubation with YAC-1 target cells. Therefore these results suggest that STX11 is indirectly involved in the regulation of the constitutive secretory pathway responsible for the secretion of proinflammatory cytokines, such as IFN-γ and TNF, in CTL.
Figure 7. Cytokine production is increased in Stx11-/- CTLs and NK cells.
(A) CTLs were activated upon 4 rounds of stimulation with alloantigenic spleen cells. IFN-γ and TNF levels were measured by ELISA from the cell supernatants collected 5 days after the last stimulation. Results are shown as mean + SD of 6 samples pooled from three different experiments. *= p<0.005. Mann Whitney test was performed. (B, C) CTLs were activated with 3 rounds of anti-allo stimulation and re-stimulated as indicated. Expression of (B) IFN-γ and (C) TNF was assessed by intracellular staining on CD8+ T cells. Open symbols, WT CTLs; filled symbols, Stx11-/- CTLs. Each symbol represents a single sample and data were pooled from three independent experiments. (D, E) Splenocytes activated with rhIL-15 were re-stimulated as indicated. Expression of (D) IFN-γ and (E) TNF was assessed by intracellular staining of NKp46+ cells. Data are shown as mean + SD of 7-9 different mice of different genotype samples pooled from three independent experiments. *= p<0.005. Mann Whitney test was performed.
Discussion
In the present study, we analysed the dynamic of STX11 expression in different cell types, as well as the contribution of this protein to cell-specific functional activities. Our results indicate that STX11 unique activity is the control of degranulation in neutrophils, and cytokine secretion, degranulation and cytotoxicity in activated NK cells and CTL, although, the spectrum of activation and maturation markers investigated, the expression of Granzyme A, -B and perforin in these cells is unaffected. Reconstitution of CTL rescues the ability of these cells to degranulate and to kill demonstrating the direct participation of STX11 in controlling these processes.
The expression of STX11 in macrophages has previously been appreciated [6], here we newly report STX11 expression in BMDC and its regulation upon LPS or IFN-γ treatment. Furthermore, we provide evidence that in the absence of STX11, ROS production in response to mouse BMM and BMDC phagocytosis triggered by latex beads, zymosan or opsonised zymosan occurs normally. The different cell origin (mouse versus human), the different triggering conditions and the fact that we have used a constitutive deficient model in contrast to the short interfering RNA targeting approach used by others [6] may explain the differences between ours and the published findings that showed that STX11 has a role in restricting LAMP-1 trafficking to the cell surface, and serves as a negative regulator of phagocytosis [6,9].
Furthermore, other SNARE family member proteins may compensate for the STX11 deficiency in mouse macrophages. Candidates are for example, STX7 which has been found to reside in late endosomes and to participate in the process of phagocytosis [20]; alternatively or in addition STX18, which has been implicated in endoplasmic reticulum-mediated phagocytosis in J774 macrophages [21]. Furthermore, STX4 and STX6, which are regulated in LPS activated RAW264.7 macrophages, have been described to regulate the exocytosis of TNF [22, 23] in these cells.
We found an increase in Stx11 mRNA also in DC upon activation. Therefore, we investigated the role of STX11 not only in phagocytosis but also in cytokine secretion, antigen processing and BMDC-mediated induction of CD8+ and CD4+ T cell proliferation. However, Stx11-/- BMDC appeared not impaired in any of the activities investigated. Our results partially mirror previous findings which report that the ability of VTI1B and VAMP8 deficient DC to process antigen and stimulate the proliferation of CTL was not reduced [18], although an inhibitory role for VAMP8, VTI1A and VTI1B was found in murine immature DC phagocytosis [24,25].
Next, we have reported that STX11 is constitutively expressed in BMMC at the mRNA level and regulated upon LPS or IgE/Ag treatment. Again despite upregulation, STX11 deficiency does not affect BMMC degranulation activity based on LAMP-1/CD107a and β-hexosaminidase measurements. It has been long appreciated that in both RBL-2H3, a rat mast cell line, and BMMC the complex STX4, SNAP23 and VAMP8 contributes to the regulation of mast cell exocytosis [26–29]. One could speculate that the integrity of this complex compensates the STX11 deficiency. In macrophages STX11 has been located in late endosomes [9], but also on post-Golgi membranes and intermediate compartments [2, 6]. The exact localization of STX11 in mast cells is still unclear. It has been recognized that mast cells have different secretory granule subsets characterized by different SNARE proteins able to selectively lead their release [30]. Interestingly, neutrophils, a cell type with a dominant exocytosis activity and heterogeneous granules, show a diminished degranulation capacity in absence of STX11. Whether exocytosis of one particular subset of granules is predominantly affected by STX11 deficiency and how STX11 deficiency affects mice susceptibility to bacterial or fungal infections and systemic inflammatory response remains to be investigated.
Our results indicate that Stx11-/- activated NK and CTL cells display an impaired degranulation and cytotoxic activity when compared with their WT counterparts, despite displaying a comparable spectrum of activation and maturation markers as well as Granzyme A, -B and perforin expression. This is in concert with previous findings in lymphocytes from healthy donors and FHL type 4 patient samples showing that a deficiency in STX11 leads to an impaired degranulation and cytotoxic activity [4, 5]. Thus, Stx11-/- mice which were found to carry no overt defect in immune system development and immune cell populations in lymphatic tissues could be an ideal in vivo model for further dissecting the HLH pathogenesis, in particular FHL-4 which is caused by a mutated STX11 gene in humans [13]. Nevertheless, Stx11-/- mice would still represent an important tool for investigating the principles of cytotoxic lymphocyte degranulation and vesicular trafficking.
Although further experiments are needed to define in detail the molecular mechanisms of STX11 function, our data suggest that the impaired cytolytic phenotype of activated NK and CTL is not indirectly due to the downregulation of the expression of the described complex-forming partners MUNC18-2 and VTI1B. This indeed is not altered upon STX11 deficiency, and our data are perfectly in line with the findings reported from stx11-deficient patients [15].
Notably, Stx11-/- CTL secrete higher levels of IFN-γ and TNF compared with WT CTL. This increased cytokine secretion is possibly due to the ineffective CTL activation and granule exocytosis and a resulting positive feed-back loop on cytokine secretion. Since both IFN-γ and TNF are defectively secreted in Stx11-/- CTL cells we can suggest that a dysregulated cytokine release applies to cytokines described to follow a directed secretion to the immunological synapse, such as IFN-γ [31] or towards phagocytic cups in activated macrophages, such as TNF [32]. Thus, we cannot exclude any specific and unique role of STX11 in regulating distinct secretion of cytokines polarized pathways.
In summary we conclude that STX11 plays a pivotal and essential role in orchestrating granule exocytosis in NK cells, CTL and neutrophils.
Materials and Methods
Cell lines and mice
The human primary embryonal kidney cell line 293T, the mouse lymphoma YAC-1 and the mouse mastocytoma P815 cell lines were purchased from DSMZ (Germany). YAC-1 and P815 cells were cultured in RPMI-1640 including Glu (GIBCO, Invitrogen), 293T in DMEM (PAA) supplemented with 10% FCS (Biochrom) and Penicillin–Streptomycin (PAA). Cells were incubated at 37 °C and 5% CO2.
C57BL/6 WT mice and Stx11-/- were used between 5-12 weeks of age and maintained in specific pathogen-free conditions at the University Medical Center Hamburg Eppendorf animal facilities. BALB/c mice were purchased from Charles River Laboratories (Sulzfeld, Germany). All experiments were performed in compliance with national and institutional guidelines (animal use protocol: #74/04 and #87/07 from the Freie and Hansestadt Hamburg, to Dr. Udo zur Stadt).
Cell isolation, cultivation and stimulation
BMMC, BMDC and BMM were generated by differentiation of murine BM cells in vitro. BMMC were cultivated in 5 ng/ml IL-3 (rmIL-3) and 10 ng/ml rmSCF (both from R&D) supplemented IMDM medium containing 10% FCS, 1% Vitamin solution (GIBCO), 2 mM L-Glu, Penicillin–Streptomycin, 1% MEM non-essential amino acids, 1 mM sodium pyruvate, 0.5 µM 2-Mercaptoethanol (GIBCO), and used after five weeks of culture. BMDC were generated in the presence of GM-CSF 20 ng/ml (Peprotech) as previously described [33] and used at day 8-9. BMM were differentiated for 8 days in RPMI medium supplemented with 10% FCS, Penicillin–Streptomycin, 1 mM sodium pyruvate, 1% MEM non-essential amino acids and 200 ng/ml rhM-CSF (R&D). NK, CD4+ or CD8+ T cells were isolated from spleens or peripheral lymph nodes. Single-cell suspensions were generated by pressing through sterile 40 µm cell strainers (BD Falcon). Erythrocytes were lysed and cells washed in complete RPMI medium (RPMI-1640 with Glu) supplemented with 10% FCS, Penicillin–Streptomycin, 10 mM HEPES (PAA), 1 mM sodium pyruvate and 50 µM 2-Mercaptoethanol. For in vitro cytokine stimulation, 107/ml splenocytes were cultivated with 50 ng/ml rhIL-15 (R&D Systems). Cells were harvested after 48h, except where otherwise indicated, and analyzed by flow cytometry or used as effector cells in in vitro killing assays, degranulation assays or Western blot. For CD8+ anti-allo stimulation splenocytes from Stx11-/- or WT mice were cultured for 5 days in presence of 100 U/ml rhIL-2 (Biotest Pharma). BALB/c splenocytes were treated with 25 µg/ml of Mytomicin C (Sigma-Aldrich) for 30 min at 37°C, washed 3 times in RPMI medium and added to the Stx11-/- or WT splenocytes for allo-stimulation. The cultures were collected on a Histopaque-1083 (Sigma-Aldrich) layer and cultured for 2 more days before another round of anti-allo stimulation and then used for the experiments.
BMM, BMDC and BMMC were seeded (1 × 106 cells/ml) and stimulated with 100 ng/ml LPS (S. friedenau; kindly provided by Dr. H. Brade, Research Center Borstel, Borstel, Germany) or 1 µg/ml LPS (Salmonella minnesota; kindly provided by Dr. K. Brandenburg, Division of Biophysics, Research Center Borstel, Borstel, Germany), 500 U/ml IFN-γ (R&D Systems), IgE (SPE-7, Sigma-Aldrich), 100 ng/ml DNP-HSA (Sigma-Aldrich), 10 µg/ml pIC (Sigma-Aldrich) and 3 µM CpG ODN 1826 (Invivogen) or left unstimulated.
Degranulation assay
Degranulation of BMMC in vitro was quantified by the determination of β-hexosaminidase in BMMC as previously described [34] and via surface LAMP-1 staining. BMMC were incubated with 0.2 µg/ml of DNP-specific IgE (SPE-7) over night, degranulation was induced by addition of 20 ng/ml of DNP-HSA or 100 ng/ml PMA and 1 μM Ionomycin. The LAMP-1 staining was performed after 20 min of stimulation, the cells were collected, washed and stained for FcεR1 (eBioscience) and LAMP-1/CD107a (1D4B) mAb (DSHB, University of Iowa). Cells were subsequently stained with PI before flow cytometric analysis. For neutrophils degranulation assay, peripheral blood cells, removed from erythrocytes, were prewarmed with complete RPMI medium for 30 min at 37°C and then stimulated for 10 min with 100 ng/ml PMA plus 1 µM Ionomycin or left untreated, washed with ice-cold FACS buffer and stained with CD11b, Gr-1 and LAMP-1 specific antibody before flow cytometric analysis. For NK and CTL degranulation assay WT or Stx11-/- cells were cultured with or without tumour cells (YAC-1 and P815, respectively) in the presence of LAMP-1 antibody for 3h at 37°C with 5% CO2. Purified anti-mouse CD3ε was added during the incubation in the CTL degranulation assay. Cells were subsequently stained with NKp46 or CD8α antibodies before flow cytometric analysis.
In vitro killing assay
WT or Stx11-/- IL-15 treated splenocytes were used to measure NK cell cytotoxicity. To measure the cytotoxicity of anti-allo stimulated CD8+ T cells, a re-directed cytotoxicity assay was performed with 1 µg/ml of anti-CD3ε. Target cells were plated at a density of 104 cells/well and effector cells were 'titrated' in duplicates or triplicates. 5h later culture supernatants were analyzed for release of cytoplasmic lactate dehydrogenase as a measure of cytotoxicity with the CytoTox Non-Radioactive Cytotoxicity Assay kit (Promega). Before the assay, an aliquot of effector cells was analyzed by flow cytometry to determine the relative percentages of NK and/or CD8+ T cells in WT and Stx11-/- cell cultures. Cytotoxicity was calculated as suggested by the manufacturer according to the following formula: percent specific lysis = 100 x (experimental lysis – effector spontaneous lysis – target spontaneous lysis) / (target maximum lysis – target spontaneous lysis).
Retroviral transduction of CTL
The pCL-Eco retrovirus packaging vector was purchased from IMGENEX. The pLXSN retroviral vector was purchased from Clontech. cDNA encoding the human STX11 gene was cloned into the pLXSN retroviral expression vector. Each construct was purified for virus production by NucleoBond® Xtra Midi kit (MACHEREY-NAGEL). One day prior to transfection, 0.5 × 106 293T cells where seeded in 6 well plates, the medium was replaced 24h later with Opti-MEM® I Reduced-Serum Medium (Invitrogen), and 2.5 µg DNA, either pLXSN or pLXSN -STX11 vector, plus 1.5 µg pCL-Eco vector were cotransfected in 293T cells using Lipofectamine™ 2000 Transfection Reagent (Invitrogen) following the manufacturer’s instructions. 293T cell culture complete medium was added after 4h and the cells were incubated an additional 16–20h at 32°C, 5% CO2. 24h post transfection the medium was replaced with 293T complete medium and the cells were cultured for 24-72h. The viral supernatant was collected, filtered through 0.45 µm pore size filter (Sarstedt), supplemented with 5 µg/ml Protamine sulfate salt from salmon (Sigma) and used immediately or frozen at −80 °C. One day prior to infection, CD8+ T cell stimulated for 3 to 4 rounds with anti-allo stimulation, were stimulated with mitomycin treated BALB/c splenocytes at 1:1 ratio and seeded at a concentration of 3 × 106 cells/ml in 6 well plates. 24h later, 3 ml viral supernatant was added dropwise in each well containing CTL cells in 1 ml medium. The plate was spinfected at RT for 30min at 1250xg. 24h post infection the cells were centrifuged, the viral supernatant removed and replaced with CTL complete medium supplemented with 100 U/ml IL-2. At day 5 post stimulation the cultures were collected on a Histopaque-1083 layer and cultured for 1 to 2 more days before being used for the experiments.
Statistical analysis
Data are represented as mean + standard deviation (SD) if not described otherwise. Statistical significance was tested by Mann Whitney test. A p value of <0.05 was considered as statistically significant.
Supplementary Material
ACKNOWLEDGMENTS
We thank: Ann-Kathrin Brückner, Hanno Ewers, Katrin Westphal, Katrin Streeck, Gesine Rode, Frauke Koops for excellent technical assistance; Julia Strauß for the mice assistance; Dr. Rytis Prekeris for the rabbit anti-human stx11 antibody; Rajia Bahri for critically examining results and experiments; Julia Polansky for critical reading of the manuscript.
This work was supported by the European Union (FP7 CURE HLH grant agreement n. 201461).
Abbreviations
- SNARE
N-ethylmaleimide-sensitive factor attachment protein receptor
- STX11
Syntaxin 11
- FHL
Familial Hemophagocytic Lymphohistiocytosis
- BMM
Bone Marrow Macrophages
- BMDC
Bone Marrow Dendritic Cells
- BMMC
Bone Marrow Mast Cells
- STXBP2
syntaxin binding protein 2
- LDH
Lactate Dehydrogenase
- RLU
relative light units
- LCMV
lymphocytic choriomeningitic virus
Footnotes
Conflict of interest: The authors declare no financial or commercial conflict of interest.
References
- 1.Advani RJ, Bae HR, Bock JB, Chao DS, Doung YC, Prekeris R, Yoo JS, Scheller RH. Seven novel mammalian SNARE proteins localize to distinct membrane compartments. J Biol Chem. 1998;273:10317–10324. doi: 10.1074/jbc.273.17.10317. [DOI] [PubMed] [Google Scholar]
- 2.Prekeris R, Klumperman J, Scheller RH. Syntaxin 11 is an atypical SNARE abundant in the immune system. Eur J Cell Biol. 2000;79:771–780. doi: 10.1078/0171-9335-00109. [DOI] [PubMed] [Google Scholar]
- 3.Valdez AC, Cabaniols JP, Brown MJ, Roche PA. Syntaxin 11 is associated with SNAP-23 on late endosomes and the trans-Golgi network. J Cell Sci. 1999;112(Pt 6):845–854. doi: 10.1242/jcs.112.6.845. [DOI] [PubMed] [Google Scholar]
- 4.Arneson LN, Brickshawana A, Segovis CM, Schoon RA, Dick CJ, Leibson PJ. Cutting edge: syntaxin 11 regulates lymphocyte-mediated secretion and cytotoxicity. J Immunol. 2007;179:3397–3401. doi: 10.4049/jimmunol.179.6.3397. [DOI] [PubMed] [Google Scholar]
- 5.Bryceson YT, Rudd E, Zheng C, Edner J, Ma D, Wood SM, Bechensteen G, et al. Defective cytotoxic lymphocyte degranulation in syntaxin-11 deficient familial hemophagocytic lymphohistiocytosis 4 (FHL4) patients. Blood. 2007;110:1906–1915. doi: 10.1182/blood-2007-02-074468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang S, Ma D, Wang X, Celkan T, Nordenskjold M, Henter JI, Fadeel B, Zheng C. Syntaxin-11 is expressed in primary human monocytes/macrophages and acts as a negative regulator of macrophage engulfment of apoptotic cells and IgG-opsonized target cells. Br J Haematol. 2008;142:469–479. doi: 10.1111/j.1365-2141.2008.07191.x. [DOI] [PubMed] [Google Scholar]
- 7.Xie LX, de la Iglesia-Vicente J, Fang YX, Mollinedo F. Expression and subcellular localization of syntaxin 11 in human neutrophils. Inflamm Res. 2009 doi: 10.1007/s00011-009-0006-x. [DOI] [PubMed] [Google Scholar]
- 8.Dabrazhynetskaya A, Ma J, Guerreiro-Cacais AO, Arany Z, Rudd E, Henter JI, Karre K, et al. Syntaxin 11 marks a distinct intracellular compartment recruited to the immunological synapse of NK cells to co-localize with cytotoxic granules. J Cell Mol Med. 2011 doi: 10.1111/j.1582-4934.2011.01280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Offenhauser C, Lei N, Roy S, Collins BM, Stow JL, Murray RZ. Syntaxin 11 binds Vti1b and regulates late endosome to lysosome fusion in macrophages. Traffic. 2011;12:762–773. doi: 10.1111/j.1600-0854.2011.01189.x. [DOI] [PubMed] [Google Scholar]
- 10.Feldmann J, Callebaut I, Raposo G, Certain S, Bacq D, Dumont C, Lambert N, et al. Munc13-4 is essential for cytolytic granules fusion and is mutated in a form of familial hemophagocytic lymphohistiocytosis (FHL3) Cell. 2003;115:461–473. doi: 10.1016/s0092-8674(03)00855-9. [DOI] [PubMed] [Google Scholar]
- 11.Menasche G, Pastural E, Feldmann J, Certain S, Ersoy F, Dupuis S, Wulffraat N, et al. Mutations in RAB27A cause Griscelli syndrome associated with haemophagocytic syndrome. Nat Genet. 2000;25:173–176. doi: 10.1038/76024. [DOI] [PubMed] [Google Scholar]
- 12.Stepp SE, Dufourcq-Lagelouse R, Le Deist F, Bhawan S, Certain S, Mathew PA, Henter JI, et al. Perforin gene defects in familial hemophagocytic lymphohistiocytosis. Science. 1999;286:1957–1959. doi: 10.1126/science.286.5446.1957. [DOI] [PubMed] [Google Scholar]
- 13.zur Stadt U, Schmidt S, Kasper B, Beutel K, Diler AS, Henter JI, Kabisch H, et al. Linkage of familial hemophagocytic lymphohistiocytosis (FHL) type-4 to chromosome 6q24 and identification of mutations in syntaxin 11. Hum Mol Genet. 2005;14:827–834. doi: 10.1093/hmg/ddi076. [DOI] [PubMed] [Google Scholar]
- 14.Janka GE. Hemophagocytic syndromes. Blood Rev. 2007;21:245–253. doi: 10.1016/j.blre.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 15.Cote M, Menager MM, Burgess A, Mahlaoui N, Picard C, Schaffner C, Al-Manjomi F, et al. Munc18-2 deficiency causes familial hemophagocytic lymphohistiocytosis type 5 and impairs cytotoxic granule exocytosis in patient NK cells. J Clin Invest. 2009;119:3765–3773. doi: 10.1172/JCI40732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.zur Stadt U, Rohr J, Seifert W, Koch F, Grieve S, Pagel J, Strauss J, et al. Familial hemophagocytic lymphohistiocytosis type 5 (FHL-5) is caused by mutations in Munc18-2 and impaired binding to syntaxin 11. Am J Hum Genet. 2009;85:482–492. doi: 10.1016/j.ajhg.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hata Y, Sudhof TC. A novel ubiquitous form of Munc-18 interacts with multiple syntaxins. Use of the yeast two-hybrid system to study interactions between proteins involved in membrane traffic. J Biol Chem. 1995;270:13022–13028. doi: 10.1074/jbc.270.22.13022. [DOI] [PubMed] [Google Scholar]
- 18.Dressel R, Elsner L, Novota P, Kanwar N, Fischer von Mollard G. The exocytosis of lytic granules is impaired in Vti1b- or Vamp8-deficient CTL leading to a reduced cytotoxic activity following antigen-specific activation. J Immunol. 2010;185:1005–1014. doi: 10.4049/jimmunol.1000770. [DOI] [PubMed] [Google Scholar]
- 19.Stow JL, Manderson AP, Murray RZ. SNAREing immunity: the role of SNAREs in the immune system. Nat Rev Immunol. 2006;6:919–929. doi: 10.1038/nri1980. [DOI] [PubMed] [Google Scholar]
- 20.Collins RF, Schreiber AD, Grinstein S, Trimble WS. Syntaxins 13 and 7 function at distinct steps during phagocytosis. J Immunol. 2002;169:3250–3256. doi: 10.4049/jimmunol.169.6.3250. [DOI] [PubMed] [Google Scholar]
- 21.Hatsuzawa K, Tamura T, Hashimoto H, Yokoya S, Miura M, Nagaya H, Wada I. Involvement of syntaxin 18, an endoplasmic reticulum (ER)-localized SNARE protein, in ER-mediated phagocytosis. Mol Biol Cell. 2006;17:3964–3977. doi: 10.1091/mbc.E05-12-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murray RZ, Wylie FG, Khromykh T, Hume DA, Stow JL. Syntaxin 6 and Vti1b form a novel SNARE complex, which is up-regulated in activated macrophages to facilitate exocytosis of tumor necrosis Factor-alpha. J Biol Chem. 2005;280:10478–10483. doi: 10.1074/jbc.M414420200. [DOI] [PubMed] [Google Scholar]
- 23.Pagan JK, Wylie FG, Joseph S, Widberg C, Bryant NJ, James DE, Stow JL. The t-SNARE syntaxin 4 is regulated during macrophage activation to function in membrane traffic and cytokine secretion. Curr Biol. 2003;13:156–160. doi: 10.1016/s0960-9822(03)00006-x. [DOI] [PubMed] [Google Scholar]
- 24.Cai DT, Ho YH, Chiow KH, Wee SH, Han Y, Peh MT, Wong SH. Aspirin regulates SNARE protein expression and phagocytosis in dendritic cells. Mol Membr Biol. 2011;28:90–102. doi: 10.3109/09687688.2010.525756. [DOI] [PubMed] [Google Scholar]
- 25.Ho YH, Cai DT, Wang CC, Huang D, Wong SH. Vesicle-associated membrane protein-8/endobrevin negatively regulates phagocytosis of bacteria in dendritic cells. J Immunol. 2008;180:3148–3157. doi: 10.4049/jimmunol.180.5.3148. [DOI] [PubMed] [Google Scholar]
- 26.Paumet F, Le Mao J, Martin S, Galli T, David B, Blank U, Roa M. Soluble NSF attachment protein receptors (SNAREs) in RBL-2H3 mast cells: functional role of syntaxin 4 in exocytosis and identification of a vesicle-associated membrane protein 8-containing secretory compartment. J Immunol. 2000;164:5850–5857. doi: 10.4049/jimmunol.164.11.5850. [DOI] [PubMed] [Google Scholar]
- 27.Vaidyanathan VV, Puri N, Roche PA. The last exon of SNAP-23 regulates granule exocytosis from mast cells. J Biol Chem. 2001;276:25101–25106. doi: 10.1074/jbc.M103536200. [DOI] [PubMed] [Google Scholar]
- 28.Lippert U, Ferrari DM, Jahn R. Endobrevin/VAMP8 mediates exocytotic release of hexosaminidase from rat basophilic leukaemia cells. FEBS Lett. 2007;581:3479–3484. doi: 10.1016/j.febslet.2007.06.057. [DOI] [PubMed] [Google Scholar]
- 29.Tiwari N, Wang CC, Brochetta C, Ke G, Vita F, Qi Z, Rivera J, et al. VAMP-8 segregates mast cell-preformed mediator exocytosis from cytokine trafficking pathways. Blood. 2008;111:3665–3674. doi: 10.1182/blood-2007-07-103309. [DOI] [PubMed] [Google Scholar]
- 30.Puri N, Roche PA. Mast cells possess distinct secretory granule subsets whose exocytosis is regulated by different SNARE isoforms. Proc Natl Acad Sci U S A. 2008;105:2580–2585. doi: 10.1073/pnas.0707854105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huse M, Lillemeier BF, Kuhns MS, Chen DS, Davis MM. T cells use two directionally distinct pathways for cytokine secretion. Nat Immunol. 2006;7:247–255. doi: 10.1038/ni1304. [DOI] [PubMed] [Google Scholar]
- 32.Murray RZ, Kay JG, Sangermani DG, Stow JL. A role for the phagosome in cytokine secretion. Science. 2005;310:1492–1495. doi: 10.1126/science.1120225. [DOI] [PubMed] [Google Scholar]
- 33.Lutz MB, Kukutsch N, Ogilvie AL, Rossner S, Koch F, Romani N, Schuler G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- 34.Orinska Z, Maurer M, Mirghomizadeh F, Bulanova E, Metz M, Nashkevich N, Schiemann F, et al. IL-15 constrains mast cell-dependent antibacterial defenses by suppressing chymase activities. Nat Med. 2007;13:927–934. doi: 10.1038/nm1615. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.