Abstract
Background:
Mast cells (MCs) can stimulate angiogenesis, releasing several proangiogenic cytokines stored in their cytoplasm. In particular, MCs can release tryptase, a potent in vivo and in vitro proangiogenic factor via protease-activated receptor-2 (PAR-2) activation and mitogen-activated protein kinase (MAPK) phosphorylation. Nevertheless, no data are available concerning the relationship among tryptase MC density (TMCD), endothelial cells (ECs) positive to PAR-2 microvascular density (PAR-2-MVD) and classical MVD (C-MVD) in gastric cancer (GC) angiogenesis.
Methods:
In this study, we analyzed the correlation of TMCD, PAR-2-MVD, C-MVD with each other and with the main clinicopathological features in GC patients who underwent surgery. A series of 77 GC patients with stage T2-3N2-3M0 (classified by the American Joint Committee on Cancer for Gastric Cancer, 7th edition) were selected and then underwent surgery.
Results:
Tumour tissue samples were evaluated by mean of immunohistochemistry and image analysis methods in terms of numbers of TMCD, PAR-2-MVD and C-MVD. A significant correlation between the TMCD, PAR-2-MVD and C-MVD groups with each other was found by Pearson t-test analysis (r ranged from 0.64 to 0.76; p value ranged from 0.02 to 0.03). There was no other significant correlation between the above parameters and clinicopathological features.
Conclusions:
Our in vivo preliminary data suggest that TMCD and PAR-2-MVD may play a role in GC angiogenesis and they could be further evaluated as a target of antiangiogenic therapy.
Keywords: angiogenesis, gastric cancer, mast cells, PAR-2, tryptase
Introduction
Mast cells (MCs) play a role in tumour angiogenesis and their involvement has been demonstrated in several animal and human malignancies [Gulubova and Vlaykova, 2009; Marech et al. 2014a]. MCs can secrete classical proangiogenic factors, including vascular endothelial growth factor (VEGF), fibroblast growth factor-2, thymidine phosphorylase and interestingly, a nonclassical proangiogenetic factor, namely tryptase, stored in their secretory granules [Marech et al. 2014b; Ammendola et al. 2013a, 2014]. With special reference to tryptase, it induces in vitro microvascular endothelial cells’ (ECs) proliferation in the matrigel assay and displayed in vivo the capillary growth on the chick embryo chorioallantoic membrane, conversely, suppressed by tryptase inhibitors [Ammendola et al. 2013b; Marech et al. 2016b; Ribatti et al. 2011]. This proangiogenic stimulus induced by tryptase is mainly mediated via protease-activated receptor-2 (PAR-2) that belongs to the G protein-coupled receptor family [Blair et al. 1997; Stack and Johnson, 1994; Fajardo and Pejler, 2003; Itoh et al. 2005]. Four forms of PARs have been reported (PAR-1 through PAR-4). In particular, PAR-2 can be activated by proteases such as trypsin and tryptase. These proteases cleave the N terminus to generate a tethered ligand, which interacts and activates the receptor [Rickard et al. 2005; Matej et al. 2007; Morris et al. 2006; Ammendola et al. 2013c, 2014; Donato et al. 2014; Ranieri et al. 2009]. Signaling via PAR-2, expressed on ECs, elicits activation of the major members of the mitogen-activated protein kinase (MAPK) phosphorylation family and induces EC proliferation. PAR-2 activation also leads to the production of other proangiogenic factors, such as VEGF, interleukin-8 (IL-8), IL-6, granulocyte-macrophage colony-stimulating factor (GM-CSF) and macrophage colony-stimulating factor (M-CSF) [Ammendola et al. 2015; Ribatti and Ranieri, 2015; Malfettone et al. 2013; Soreide et al. 2006; Darmoul et al. 2001; Uusitalo-Jarvinen et al. 2007; Liu and Mueller, 2006].
In literature, no data have been published on the relationship among tryptase MC density (TMCD), ECs positive to PAR-2 forming microvascular density (PAR-2-MVD) and classical MVD (C-MVD) in gastric cancer (GC) angiogenesis [Yano et al. 1999; Sedda et al. 2014; Ribatti et al. 2010; Wang et al. 2013a; Zhang et al. 2012].
In this preliminary study, we analyzed the numbers of TMCD, PAR-2-MVD and C-MVD to analyse whether they correlated with each other in primary tumour tissue from GC patients undergoing surgery. The correlation among the above analysed parameters and the main clinicopathological features has been also performed.
Materials and methods
Study population
A series of 77 GC patients with stage T2-3N2-3M0 (classified by the American Joint Committee on Cancer for Gastric Cancer, 7th edition) diagnosed with preoperative gastric endoscopy were selected and underwent to curative resection. Surgical approaches used were open total and subtotal gastrectomy, with D2 lymph node dissection. Patients were staged according to the American Joint Committee on Cancer 7th edition (AJCC-TNM) classification [Washington, 2010; Tamura et al. 2011; Verlato et al. 2014; Mrena et al. 2015]. All patients did not have distant metastases on computed tomography of the thorax, abdomen and pelvis. All samples evaluated in this study were of adenocarcinomas’ histological type. The clinicopathological features of the patients are summarized in Table 1. Full ethical approval and signed consent from individual patients were obtained. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of ‘Mater Domini’ Hospital, ‘Magna Graecia’ University, Catanzaro.
Table 1.
Clinicopathological features of patients (n = 77).
| Age | |
| ⩽65 | 31 (40%) |
| ⩾65 | 46 (60%) |
| Gender | |
| Male | 43 (56%) |
| Female | 34 (44%) |
| Tumour site | |
| Cardia | 11 (14%) |
| Lesser curvature | 7 (9%) |
| Greater curvature | 9 (12%) |
| Body and fundus | 24 (31%) |
| Pyloric area | 26 (34%) |
| TNM by AJCC stage and type by Lauren classification | |
| T2-3N2M0 | 47 (61%) |
| T2-3N3M0 | 30 (39%) |
| Intestinal type | 45 (58%) |
| Diffuse type | 32 (42%) |
| Histologic grade | |
| G1-G2 | 56 (73%) |
| G3 | 21 (27%) |
TNM, classification of cancer staging; AJCC, American Joint Committee on Cancer.
Immunohistochemistry
For the evaluation of TMCD, PAR-2-MVD and C-MVD, a three-layer biotin–avidin–peroxidase system was utilized [Ranieri et al. 2007]. Briefly, 6 μm-thick serial sections of formalin-fixed and paraffin-embedded tumour sample were cut. Sections were then microwaved at 500W for 10 minutes, after which, endogenous peroxidise activity was blocked with 3% hydrogen peroxide solution. Tumour sections were incubated with the following primary antibodies: antitryptase (clone AA1; Dako, Glostrup, Denmark) diluted 1:100 for 1 hour at room temperature specific for MC identification; anti-PAR-2 (C-17; sc-8205, Santa Cruz Biotechnology, Texas, USA), diluted 1:50 for 1 hour at room temperature and anti-CD34 antibody (QB-END 10; Bio-Optica Milan, Italy) diluted 1:50 for 1 hour at room temperature as a pan-endothelial marker, respectively. The bound antibody was visualized using a biotinylated secondary antibody, avidin–biotin–peroxidase complex and liquid permanent red (LPS, K0640, Dako, Glostrup, Denmark). Nuclear counterstaining was performed with Gill’s haematoxylin no.2 (Polysciences, Warrington, PA, USA). The primary antibody was omitted in negative controls.
Morphometrical assay
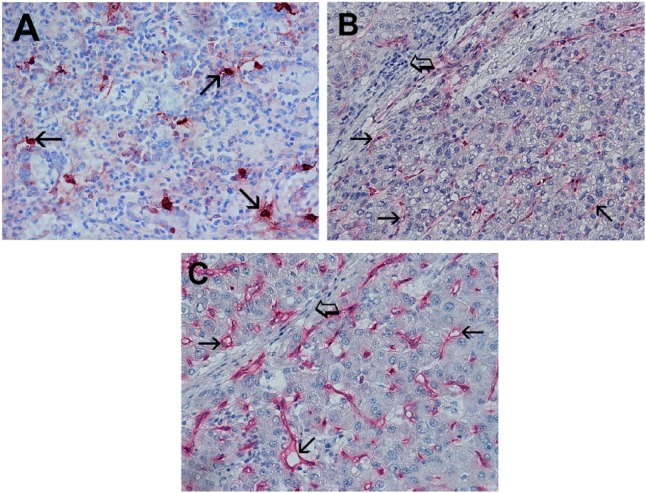
Light microscopy integrated with an image analysis system (Quantimet-500 Leica, Wetzlar, Germany) was utilized [Ranieri et al. 2007]. In tumour sections, immunostained areas (hot spots) were selected at ×100 magnification and TMCD (Figure 1A), PAR-2-MVD (Figure 1B) and C-MVD (Figure 1C) were assessed at ×400 magnification (0.19 mm2 area).
Figure 1.
(A) Primary gastric cancer (GC) tissue section. Many scattered red immunostained mast cells positive to the antitryptase antibody. Each arrow indicates single mast cells, ×400 magnification (0.19 mm2). (B) Primary GC tissue section. Each small arrow indicates single red immunostained microvessel positive to the antiprotease-activated receptor-2 antibody. Big arrows indicate stromal collagen and lymphocytes infiltrate, ×400 magnification (0.19 mm2). (C) Primary GC tissue section. Each small arrow indicate single red immunostained microvessel positive to the anti-CD34 antibody. Big arrow indicates stromal collagen with scattered lymphocytes, ×400 magnification (0.19 mm2).
Statistical analysis
Mean values ± 1 standard deviation (SD) of all the tissue-evaluated parameters are reported in Table 2. Correlations between TMCD, PAR-2-MVD, and C-MVD were calculated using Pearson’s (r) analysis. Correlations among the all analyzed parameters and the main clinicopathological features listed in Table 1 were performed by Chi-square test (χ2). A p < 0.05 was considered significant. All statistical analyses were performed with the SPSS statistical software package (SPSS, Inc., Chicago, IL).
Table 2.
Tryptase mast cell density, protease-activated receptor-2 microvascular density and classical microvascular density means ± standard deviation as a function of gastric cancer tumour tissue, respectively.
| Tissue | TMCD 400× (0.19 mm2) |
PAR-2-MVD 400× (0.19 mm2) |
C-MVD 400× (0.19 mm2) |
|---|---|---|---|
| Primary tumour | 10.87 ± 4.21 | 24.32 ± 8.67 | 27.22 ± 9.12 |
TMCD, tryptase mast cell density; PAR-2-MVD, protease-activated receptor-2 microvascular density; C-MVD, classical microvascular density.
Results
Immunohistochemical staining by using the antibodies antitryptase, anti-PAR-2 and anti-CD34, demonstrates that tryptase-positive MCs are well recognizable as red-stained ovoid cells with thin prolongations and generally, they are located in perivascular position (Figure 1A). A close topographic association between TMCD and PAR-2-MVD and between TMCD and C-MVD was often observed. The mean value ± SD of TMCD, PAR-2-MVD and C-MVD was 10.87 ± 4.21, 24.32 ± 8.67 and 27.22 ± 9.12 respectively, and these results are summarized in Table 2.
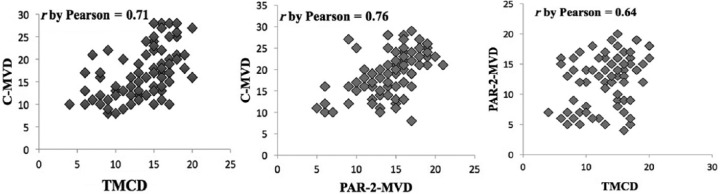
There was a significant correlation between TMCD and C-MVD (r = 0.71, p = 0.03), between PAR-2-MVD and C-MVD (r = 0.76, p = 0.02), and between TMCD and PAR-2-MVD (r = 0.64 p = 0.02) (Figure 2). There was no correlation between TMCD, PAR-2-MVD and C-MVD and the main clinicopathological features found.
Figure 2.
Correlation between tryptase mast cells’ density (TMCD) and classical microvascular density (C-MVD) (r = 0.71, p = 0.03), protease-activated receptor-2 microvascular density (PAR-2-MVD) and C-MVD (r = 0.76, p = 0.02), TMCD and PAR-2-MVD (r = 0.64, p = 0.02).
TMCD, tryptase mast cell density; PAR-2-MVD, protease-activated receptor-2 microvascular density; C-MVD, classical microvascular density.
Discussion
Currently, a lot of data supported the central role of angiogenesis in GC development and progression but few data regarding the role of MCs in GC angiogenesis have been published [Geng et al. 2014; Wang et al. 2013a; Zhao et al. 2012]. In particular, in a study performed by Mukherjee and colleagues, the authors studied MC density in tissue from patients with gastric ulcers and in tissue from GC patients [Mukherjee et al. 2009]. In the above study, the histochemical method of toluidine blue was employed to identify and count MC density. Data from this study indicated that MC density in benign gastric ulcers and in cancers was much higher than control and correlated with angiogenesis.
Ribatti and colleagues studied tumour samples from GC patients by mean of immunohistochemistry employing antitryptase and antichymase antibodies to stain MCs. In this study, a correlation between MVD and tryptase and chymase-positive MCs with histopathological type was found [Ribatti et al. 2010].
Interestingly, MCs have been shown as important players in tumour angiogenesis because of the release of proangiogenic factors stored in their secretory granules [Bhattacharyya et al. 1998; Marech et al. 2016a].
In the tumour microenvironment, MCs can be activated in different ways such as: c-Kit receptor activation and phosphorylation by stem cell factor, IgE mechanism mediated by T-lymphocytes–MC interaction and other microenvironmental stimuli [Ammendola et al. 2016a; Patruno et al. 2014]. After intensive or piecemeal activation, degranulation of secretory granules occurs, depending on the MC-activation mechanism, and MC-derived proangiogenic factors are released in the tumour microenvironment, stimulating angiogenesis [Ribatti and Ranieri, 2015; Wasiuk et al. 2009]. Among them, tryptase has been characterized as a powerful nonclassical angiogenic factor [Marech et al. 2016b; Norrby, 2002; Visciano et al. 2015; Marone et al. 2015].
Preclinical data showed that tryptase is an agonist of PAR-2 in vascular ECs that stimulates their proliferation. Signaling via PAR-2 on ECs elicits activation of the major members of the MAPK phosphorylation family and contributes to proliferation of ECs and angiogenesis. Experimental data also suggest that PAR-2 activation leads to the production of other proangiogenic factors, such as VEGF, IL-8, IL-6, GM-CSF and M-CSF [Malfettone et al. 2013; Soreide et al. 2006; Darmoul et al. 2001; Uusitalo-Jarvinen et al. 2007; Liu and Mueller, 2006; Yano et al. 1999; Sedda et al. 2014; Ribatti et al. 2010; Wang et al. 2013b; Zhang et al. 2012; Washington, 2010; Tamura et al. 2011; Verlato et al. 2014; Mrena et al. 2015; Loffredo et al. 2014; Zhang et al. 2013; Rasmussen et al. 2012; Chang et al. 2013; Ammendola et al. 2016b]. With special regard to GC, it is important to underscore that the role of TMCD in angiogenesis has been little investigated and no data have been published regarding MVD in terms of PAR-2 endothelial expressing cells. Our results demonstrated an association between TMCD, PAR-2-MVD and C-MVD, supporting the central role of tryptase as a main proangiogenic factor in primary GC tissue. On the other hand, these first data need to be interpreted with caution, due to following limitations: the medium size of analyzed samples series; the lack of a previous standardized method to evaluate PAR-2-MVD and the possible inter-observer variability in the evaluation of PAR-2-MVD. Although our results are preliminary and they need to be confirmed to further awaited studies, it is intriguing to speculate that the inhibition of MC degranulation by means of c-Kit receptor tyrosine kinase inhibitors (e.g. masitinib) or the inhibition of tryptase by means of gabexate mesilate or nafamostat mesilate, which could be novel antiangiogenic strategies worthy of clinical investigation [Erba et al. 2001; Mori et al. 2003; Humbert et al. 2010; Marech et al. 2014c; Deplanque et al. 2015; Ammendola et al. 2016c].
Footnotes
Author contributions: Ammendola M and Ranieri G conceived the research. Vescio G, Kocak IF and Ozgurtas T performed the critical review of the literature. Ammendola M Sammarco G Sacco R performed surgical procedures. Zuccalà V, Patruno R, C Gadaleta, Zizzo N contributed to immunohistochemistry and tissue’s study. Marech I, Ruggieri R, Luposella M and Gadaleta CD elaborated data analysis. All authors wrote the manuscript. Ranieri G reviewed the manuscript.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Michele Ammendola, Department of Medical and Surgical Sciences, Clinical Surgery Unit, University of Catanzaro ‘Magna Graecia’ Medical School, Viale Europa – Germaneto, 88100, Catanzaro, Italy.
Rosario Sacco, Department of Medical and Surgical Sciences, Clinical Surgery Unit, University of Catanzaro ‘Magna Graecia’ Medical School, Catanzaro, Italy.
Giuseppina Vescio, Department of Medical and Surgical Sciences, Clinical Surgery Unit, University of Catanzaro ‘Magna Graecia’ Medical School, Catanzaro, Italy.
Valeria Zuccalà, Health Science Department, Pathology Unit, University of Catanzaro ‘Magna Graecia’ Medical School, Catanzaro, Italy.
Maria Luposella, Cardiovascular Disease Unit, ‘San Giovanni di Dio’ Hospital, Crotone, Italy.
Rosa Patruno, Chair of Pathology, University ‘Aldo Moro’ Veterinary Medical School, Bari, Italy.
Nicola Zizzo, Chair of Pathology, University ‘Aldo Moro’ Veterinary Medical School, Bari, Italy.
Claudia Gadaleta, Chair of Pathology, University ‘Aldo Moro’ Veterinary Medical School, Bari, Italy.
Ilaria Marech, Diagnostic and Interventional Radiology Unit with Integrated Section of Translational Medical Oncology, National Cancer Research Centre, ‘Giovanni Paolo II’, Bari, Italy.
Roberta Ruggieri, Diagnostic and Interventional Radiology Unit with Integrated Section of Translational Medical Oncology, National Cancer Research Centre, ‘Giovanni Paolo II’, Bari, Italy.
Ibrahim Furkan Kocak, Department of Biochemistry, Gulhane Military Medical Academy Etlik, Ankara, Turkey.
Taner Ozgurtas, Department of Biochemistry, Gulhane Military Medical Academy Etlik, Ankara, Turkey.
Cosmo Damiano Gadaleta, Diagnostic and Interventional Radiology Unit with Integrated Section of Translational Medical Oncology, National Cancer Research Centre, ‘Giovanni Paolo II’, Bari, Italy.
Giuseppe Sammarco, Department of Medical and Surgical Sciences, Clinical Surgery Unit, University of Catanzaro ‘Magna Graecia’ Medical School, Catanzaro, Italy.
Girolamo Ranieri, Diagnostic and Interventional Radiology Unit with Integrated Section of Translational Medical Oncology, National Cancer Research Centre, ‘Giovanni Paolo II’, Bari, Italy.
References
- Ammendola M., Leporini C., Marech I., Gadaleta C., Scognamillo G., Sacco R., et al. (2014) Targeting mast cells tryptase in tumor microenvironment: a potential antiangiogenetic strategy. Biomed Int Res 2014: 154702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammendola M., Marech I., Sammarco G., Zuccalà V., Luposella M., Zizzo N., et al. (2015) Infiltrating mast cells correlate with angiogenesis in bone metastases from gastric cancer patients. Int J Mol Sci 16: 3237–3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammendola M., Patruno R., Sacco R., Marech I., Sammarco G., Zuccalà V., et al. (2016a) Mast cells positive to tryptase and tumour-associated macrophages correlate with angiogenesis in locally advanced colorectal cancer patients undergone to surgery. Expert Opin Ther Targets 20: 533–540. [DOI] [PubMed] [Google Scholar]
- Ammendola M., Sacco R., Donato G., Zuccalà V., Russo E., Luposella M., et al. (2013a) Mast cell positivity to tryptase correlates with metastatic lymph nodes in gastrointestinal cancer patients treated surgically. Oncology 85: 111–116. [DOI] [PubMed] [Google Scholar]
- Ammendola M., Sacco R., Sammarco G., Donato G., Montemurro S., Ruggieri E., et al. (2014) Correlation between serum tryptase, mast cells positive to tryptase and microvascular density in colo-rectal cancer patients: possible biological-clinical significance. PLoS One 9: e99512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammendola M., Sacco R., Sammarco G., Donato G., Zuccalà V., Luposella M., et al. (2014) Mast cells density positive to tryptase correlates with angiogenesis in pancreatic ductal adenocarcinoma patients having undergone surgery. Gastroenterol Res Pract 2014: 951957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammendola M., Sacco R., Sammarco G., Donato G., Zuccalà V., Romano R., et al. (2013b) Mast cells positive to tryptase and c-Kit receptor expressing cells correlates with angiogenesis in gastric cancer patients surgically treated. Gastroenterol Res Pract 2013: 703163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammendola M., Sacco R., Sammarco G., Luposella M., Patruno R., Gadaleta C., et al. (2016b) Mast cell-targeted strategies in cancer therapy. Transfus Med Hemother 43: 109–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammendola M., Sacco R., Sammarco G., Piardi T., Zuccalà V., Patruno R., et al. (2016c) Mast cells positive to tryptase, endothelial cells positive to protease-activated receptor-2, and microvascular density correlate among themselves in hepatocellular carcinoma patients who have undergone surgery. Onco Targets Ther 9: 4465–4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammendola M., Zuccalà V., Patruno R., Russo E., Luposella M., Amorosi A., et al. (2013c) Tryptase-positive mast cells and angiogenesis in keloids: a new possible post-surgical target for prevention. Updates Surg 65: 53–57. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S., Drucker I., Reshef T., Kirshenbaum A., Metcalfe D., Mekori Y. (1998) Activated T lymphocytes induce degranulation and cytokine production by human mast cells following cell-to-cell contact. J Leukoc Biol 63: 337–341. [DOI] [PubMed] [Google Scholar]
- Blair R., Meng H., Marchese M., Ren S., Schwartz L., Tonnesen M., et al. (1997) Human mast cells stimulate vascular tube formation. Tryptase is a novel, potent angiogenic factor. J Clin Invest 99: 2691–2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L., Pan S., Lai C., Tsai A., Teng C. (2013) Activated PAR-2 regulates pancreatic cancer progression through ILK/HIF-α-induced TGF-α expression and MEK/VEGF-A-mediated angiogenesis. Am J Pathol 183: 566–575. [DOI] [PubMed] [Google Scholar]
- Darmoul D., Marie J., Devaud H., Gratio V., Laburthe M. (2001) Initiation of human colon cancer cell proliferation by trypsin acting at protease-activated receptor-2. Br J Cancer 85: 772–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deplanque G., Demarchi M., Hebbar M., Flynn P., Melichar B., Atkins J., et al. (2015) A randomized, placebo-controlled phase III trial of masitinib plus gemcitabine in the treatment of advanced pancreatic cancer. Ann Oncol 26: 1194–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato G., Conforti F., Camastra C., Ammendola M., Donato A., Renzulli A., et al. (2014) The role of mast cell tryptases in cardiac myxoma: histogenesis and development of a challenging tumor. Oncol Lett 8: 379–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erba F., Fiorucci L., Pascarella S., Menegatti E., Ascenzi P., Ascoli F. (2001) Selective inhibition of human mast cell tryptase by gabexate mesylate, an antiproteinase drug. Biochem Pharmacol 61: 271–276. [DOI] [PubMed] [Google Scholar]
- Fajardo I., Pejler G. (2003) Human mast cell beta-tryptase is a gelatinase. J Immunol 171: 1493–1499. [DOI] [PubMed] [Google Scholar]
- Geng Y., Chen X., Qiu J., Zhou Y., Wang J., Liu L. (2014) Human epidermal growth factor receptor-2 expression in primary and metastatic gastric cancer. Int J Clin Oncol 19: 303–311. [DOI] [PubMed] [Google Scholar]
- Gulubova M., Vlaykova T. (2009) Prognostic significance of mast cell number and microvascular density for the survival of patients with primary colorectal cancer. J Gastroenterol Hepatol 24: 265–275. [DOI] [PubMed] [Google Scholar]
- Humbert M., Castéran N., Letard S., Hanssens K., Iovanna J., Finetti P., et al. (2010) Masitinib combined with standard gemcitabine chemotherapy: in vitro and in vivo studies in human pancreatic tumour cell lines and ectopic mouse model. PLoS One 5: e9430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh Y., Sendo T., Oishi R. (2005) Physiology and pathophysiology of proteinase-activated receptors (PARs): role of tryptase/PAR-2 in vascular endothelial barrier function. J Pharmacol Sci 97: 14–19. [DOI] [PubMed] [Google Scholar]
- Liu Y., Mueller B. (2006) Protease-activated receptor-2 regulates vascular endothelial growth factor expression in MDA-MB-231 cells via MAPK pathways. Biochem Biophys Res Commun 344: 1263–1270. [DOI] [PubMed] [Google Scholar]
- Loffredo S., Staiano R., Granata F., Genovese A., Marone G. (2014) Immune cells as a source and target of angiogenic and lymphangiogenic factors. Chem Immunol Allergy 99: 15–36. [DOI] [PubMed] [Google Scholar]
- Malfettone A., Silvestris N., Saponaro C., Ranieri G., Russo A., Caruso S., et al. (2013) High density of tryptase-positive mast cells in human colorectal cancer: a poor prognostic factor related to protease-activated receptor 2 expression. J Cell Mol Med 17: 1025–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marech I., Ammendola M., Gadaleta C., Zizzo N., Oakley C., Gadaleta C., et al. (2014a) Possible biological and translational significance of mast cells density in colorectal cancer. World J Gastroenterol 20: 8910–8920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marech I., Ammendola M., Sacco R., Capriuolo G., Patruno R., Rubini R., et al. (2014b) Serum tryptase, mast cells positive to tryptase and microvascular density evaluation in early breast cancer patients: possible translational significance. BMC Cancer 14: 534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marech I., Ammendola M., Sacco R., Sammarco G., Zuccalà V., Zizzo N., et al. (2016a) Tumour-associated macrophages correlate with microvascular bed extension in colorectal cancer patients. J Cell Mol Med 20: 1373–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marech I., Leporini C., Ammendola M., Porcelli M., Gadaleta C., Russo E., et al. (2016b) Classical and non-classical proangiogenic factors as a target of antiangiogenic therapy in tumor microenvironment. Cancer Lett 28; 380: 216–226. [DOI] [PubMed] [Google Scholar]
- Marech I., Patruno R., Zizzo N., Gadaleta C., Introna M., Zito A., et al. (2014c) Masitinib (AB1010), from canine tumour model to human clinical development: where we are? Crit Rev Oncol Hematol 91: 98–111. [DOI] [PubMed] [Google Scholar]
- Marone G., Varricchi G., Loffredo S., Granata F. (2015) Mast cells and basophils in inflammatory and tumor angiogenesis and lymphangiogenesis. Eur J Pharmacol 778: 146–151. [DOI] [PubMed] [Google Scholar]
- Matej R., Mandàkovà P., Netikovà I., Poucková P., Olejár T. (2007) Proteinase-activated receptor-2 expression in breast cancer and the role of trypsin on growth and metabolism of breast cancer cell line MDA MB-231. Physiol Res 56: 475–484. [DOI] [PubMed] [Google Scholar]
- Mori S., Itoh Y., Shinohata R., Sendo T., Oishi R., Nishibori M. (2003) Nafamostat mesilate is an extremely potent inhibitor of human tryptase. J Pharmacol Sci 92: 420–423. [DOI] [PubMed] [Google Scholar]
- Morris D., Ding Y., Ricks T., Gullapalli A., Wolfe B., Trejo J. (2006) Protease-activated receptor-2 is essential for factor VIIa and Xa-induced signaling, migration, and invasion of breast cancer cells. Cancer Res 66: 307–314. [DOI] [PubMed] [Google Scholar]
- Mrena J., Mattila A., Böhm J., Jantunen I., Kellokumpu I. (2015) Surgical care quality and oncologic outcome after D2 gastrectomy for gastric cancer. World J Gastroenterol 21: 13294–13301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee S., Bandyopadhyay G., Dutta C., Bhattacharya A., Karmakar R., Barui G., et al. (2009) Evaluation of endoscopic biopsy in gastric lesions with a special reference to the significance of mast cell density. Indian J Pathol Microbiol 52: 20–24. [DOI] [PubMed] [Google Scholar]
- Norrby K. (2002) Mast cells and angiogenesis. APMIS 110: 355–371. [DOI] [PubMed] [Google Scholar]
- Patruno R., Marech I., Zizzo N., Ammendola M., Nardulli P., Gadaleta C., et al. (2014) C-Kit expression, angiogenesis, and grading in canine mast cell tumour: a unique model to study c-Kit driven human malignancies. Biomed Res Int 2014: 730246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranieri G., Ammendola M., Patruno R., Celano G., Zito F., Montemurro S., et al. (2009) Tryptase-positive mast cells correlate with angiogenesis in early breast cancer patients. Int J Oncol 35: 115–120. [DOI] [PubMed] [Google Scholar]
- Ranieri G., Grammatica L., Patruno R., Zito A., Valerio P., Iacobellis S., et al. (2007) A possible role of thymidine phosphorylase expression and 5-fluorouracil increased sensitivity in oropharyngeal cancer patients. J Cell Mol Med 11: 362–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen J., Riis S., Frobert O., Yang S., Kastrup J., Zachar V., et al. (2012) Activation of protease-activated receptor 2 induces VEGF independently of HIF-1. PLoS One 7: e46087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribatti D., Guidolin D., Marzullo A., Nico B., Annese T., Benagiano V., et al. (2010) Mast cells and angiogenesis in gastric carcinoma. Int J Exp Pathol 91: 350–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribatti D., Ranieri G. (2015) Tryptase, a novel angiogenic factor stored in mast cell granules. Exp Cell Res 332: 157–162. [DOI] [PubMed] [Google Scholar]
- Ribatti D., Ranieri G., Nico B., Benagiano V., Crivellato E. (2011) Tryptase and chymase are angiogenic in vivo in the chorioallantoic membrane assay. Int J Dev Biol 55: 99–102. [DOI] [PubMed] [Google Scholar]
- Rickard A., Portell C., Kell P., Vinson S., McHowat J. (2005) Protease-activated receptor stimulation activates a Ca2+-independent phospholipase A2 in bladder microvascular endothelial cells. Am J Physiol Renal Physiol 288: F714–F721. [DOI] [PubMed] [Google Scholar]
- Sedda S., Marafini I., Caruso R., Pallone F., Monteleone G. (2014) Proteinase activated-receptors-associated signaling in the control of gastric cancer. World J Gastroenterol 20: 11977–11984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soreide K., Janssen E., Körner H., Baak J. (2006) Trypsin in colorectal cancer: molecular biological mechanisms of proliferation, invasion, and metastasis. J Pathol 209: 147–156. [DOI] [PubMed] [Google Scholar]
- Stack M., Johnson D. (1994) Human mast cell tryptase activates single-chain urinary-type plasminogen activator (pro-urokinase). J Biol Chem 269: 9416–9419. [PubMed] [Google Scholar]
- Tamura S., Takeno A., Miki H. (2011) Lymph node dissection in curative gastrectomy for advanced gastric cancer. Int J Surg Oncol 2011: 748745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uusitalo-Jarvinen H., Kurokawa T., Mueller B., Andrade-Gordon P., Friedlander M., Ruf W. (2007) Role of protease activated receptor 1 and 2 signaling in hypoxia-induced angiogenesis. Arterioscler Thromb Vasc Biol 27: 1456–1462. [DOI] [PubMed] [Google Scholar]
- Verlato G., Giacopuzzi S., Bencivenga M., Morgagni P., De Manzoni G. (2014) Problems faced by evidence-based medicine in evaluating lymphadenectomy for gastric cancer. World J Gastroenterol 20: 12883–12891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visciano C., Prevete N., Liotti F., Marone G. (2015) Tumor-associated mast cells in thyroid cancer. Int J Endocrinol 2015: 705169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G., Wang Y., Li D., Deng B. (2013a) Expression of protease-activated receptor-2 in human gastric stromal tumor and its clinicopathological significance. Hepatogastroenterology 60: 2125–2128. [DOI] [PubMed] [Google Scholar]
- Wang X., Chen X., Fang J., Yang C. (2013b) Overexpression of both VEGF-A and VEGF-C in gastric cancer correlates with prognosis, and silencing of both is effective to inhibit cancer growth. Int J Clin Exp Pathol 6: 586–597. [PMC free article] [PubMed] [Google Scholar]
- Washington K. (2010) 7th edition of the AJCC cancer staging manual: stomach. Ann Surg Oncol 17: 3077–3079. [DOI] [PubMed] [Google Scholar]
- Wasiuk A., de Vries V., Hartmann K., Roers A., Noelle R. (2009) Mast cells as regulators of adaptive immunity to tumours. Clin Exp Immunol 155: 140–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano H., Kinuta M., Tateishi H., Nakano Y., Matsui S., Monden T., et al. (1999) Mast cell infiltration around gastric cancer cells correlates with tumour angiogenesis and metastasis. Gastric Cancer 2: 26–32. [DOI] [PubMed] [Google Scholar]
- Zhang C., Gao G., Lv C., Zhang B., Zhang Z., Zhang X. (2012) Protease-activated receptor-2 induces expression of vascular endothelial growth factor and cyclooxygenase-2 via the mitogen-activated protein kinase pathway in gastric cancer cells. Oncol Rep 28: 1917–1923. [DOI] [PubMed] [Google Scholar]
- Zhang X., Wang W., Mize G., Takayama T., True L., Vessella R. (2013) Protease-activated receptor 2 signaling up regulates angiogenic growth factors in renal cell carcinoma. Exp Mol Pathol 94: 91–97. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Wu K., Cai K., Zhai R., Tao K., Wang G., et al. (2012) Increased numbers of gastric-infiltrating mast cells and regulatory T cells are associated with tumor stage in gastric adenocarcinoma patients. Oncol Lett 4: 755–758. [DOI] [PMC free article] [PubMed] [Google Scholar]