Abstract
Background
Neurite orientation dispersion and density imaging (NODDI) is a diffusion magnetic resonance imaging (MRI) technique with the potential to visualize the microstructure of the brain. Revolutionary histological methods to render the mouse brain transparent have recently been developed, but verification of NODDI by these methods has not been reported.
Purpose
To confirm the concordance of NODDI with histology in terms of density and orientation dispersion of neurites of the brain.
Material and Methods
Whole brain diffusion MRI of a thy-1 yellow fluorescent protein mouse was acquired with a 7-T MRI scanner, after which transparent brain sections were created from the same mouse. NODDI parameters calculated from the MR images, including the intracellular volume fraction (Vic) and the orientation dispersion index (ODI), were compared with histological findings. Neurite density, Vic, and ODI were compared between areas of the anterior commissure and the hippocampus containing crossing fibers (crossing areas) and parallel fibers (parallel areas), and the correlation between fiber density and Vic was assessed.
Results
The ODI was significantly higher in the crossing area compared to the parallel area in both the anterior commissure and the hippocampus (P = 0.0247, P = 0.00022, respectively). Neurite density showed a similar tendency, but was significantly different only in the hippocampus (P = 7.91E−07). There was no significant correlation between neurite density and Vic.
Conclusion
NODDI was verified by histology for quantification of the orientation dispersion of neurites. These results indicate that the ODI is a suitable index for understanding the microstructure of the brain in vivo.
Keywords: Magnetic resonance imaging (MRI), MR diffusion/perfusion, brain/brain stem, pathology
Introduction
There is growing interest in observing microstructure by diffusion magnetic resonance imaging (MRI). Neurite orientation dispersion and density imaging (NODDI) has detected microstructural alterations in the human brain in aging (1), Alzheimer’s disease (2), and epilepsy (3), but histological validation has not been reported.
NODDI is a newly proposed diffusion analysis technique that assumes three components of the tissue (stick for fiber, ball for free water, and others for tensor) and minimizes the partial volume effect of cerebrospinal fluid (CSF) (4). The parameters of NODDI, intra-cellular volume fraction (Vic), isotropic volume fraction (Viso), and the orientation dispersion index (ODI), represent each histological condition.
Comparison between diffusion MRI and histology is a robust method for understanding the underpinnings of the microstructure of the brain. Histological methods that render mouse brain transparent have recently been developed. These techniques allow three-dimensional (3D) whole brain microscopy with single-cell resolution (5), providing accurate imaging without the tissue damage that is inherent to two-dimensional (2D) examination of tissue slices. Kamagata et al. have used 3D imaging of the transparent mouse brain to examine the correlation between fractional anisotropy (FA) and neurite density (6), but to date, no study has validated NODDI metrics with 3D histology in a transparent mouse brain.
To confirm the concordance of NODDI with histology in terms of density and orientation dispersion of neurites, we compared Vic and ODI with histological findings in the anterior commissure and the hippocampus in a transparent mouse brain.
Material and Methods
Image acquisition
MR images of the brain of a 26-week-old male Thy-1-yellow fluorescent protein (YFP) mouse (B6. Cg-Tg [Thy1-cre/ERT2, -EYFP] AGfng/J; Jackson Laboratory, Bar Harbor, ME, USA) were acquired with a 7-T MRI scanner (Magnet: Kobelco and JASTEC, Japan; Console: Bruker Biospin, Ettlingen, Germany) with a 2-ch phased array cooled radiofrequency mouse brain coil (Brucker Biospin) for transmission and reception within two weeks from dissection and perfusion of the brain, after which 2-mm brain sections were prepared and cleared according to the clear, unobstructed brain imaging cocktails and computational analysis (CUBIC) (5) method, as previously described (6). All animal protocols were approved by the Animal Care and Use Committee of Juntendo University.
For the diffusion MRI sequence, a 2D diffusion-weighted spin echo-planar imaging (EPI) sequence was used with the following parameters: TR, 25 s; TE, 23.2 ms; field of view, 19.2 × 19.2 mm; matrix size, 128 × 128; number of slices, 100; slice thickness, 0.15 mm; slice gap, 0; number of acquisitions, 1; and number of segments for EPI, 8. Initial b = 0 s/mm2 images and four different b-values (b = 711, 1261, 2000, 2855 s/mm2) in 30 different directions (diffusion gradient duration = 6 ms, diffusion gradient separation = 12 ms) were acquired corresponding to the Jones30 scheme. Histological images (16-bit tiff; voxel dimensions in µm: x = 5; y = 5; z = 0.432 for images acquired with a ×40 objective) were acquired with a Carl Zeiss LSM 780 microscope equipped with a ×10 Plan Apochromat objective (numerical aperture, 0.45) and a ×40 c-Apochromat objective (numerical aperture, 1.2). Vic maps and ODI maps were created from the diffusion MR images.
Region of interest (ROI) analysis
Portions of the left anterior commissure and the left hippocampus containing both crossing fibers and parallel fibers were selected for analysis.
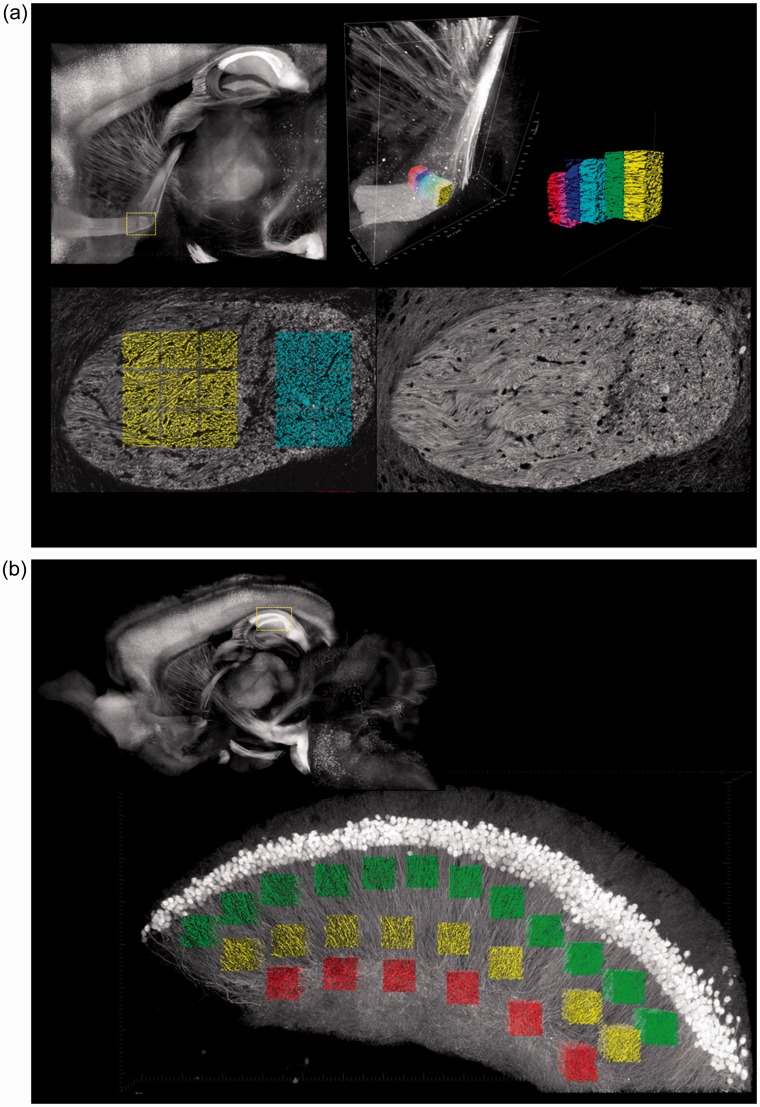
Seven sagittal slices from the histological specimen were selected from the transition zone between the anterior and posterior portions of the anterior commissure (Fig. 1a). The anterior portions of these slices contained crossing fibers and the posterior portions contained parallel fibers. Accordingly, the anterior portion was identified as the crossing area and the posterior portion was identified as the parallel area.
Fig. 1.
Regions of interest in the histological specimen. (a) Anterior commissure. ROIs of 75 µm/side were drawn at the transition zone between the anterior and posterior areas on seven sagittal slices. The anterior portions included mainly crossing fibers and the posterior portions included mainly parallel fibers. (b) Hippocampus. ROIs of 75 µm/side were drawn in field CA1 on a single sagittal slice. The lower portion included mainly crossing fibers and the upper and middle portions included mainly parallel fibers.
One sagittal slice from the histological specimen was selected from field CA1 in the hippocampus (Fig. 1b). Crossing fibers were visible in the lower portion and parallel fibers were visible in the upper and middle portions of this slice. Accordingly, the lower part was identified as the crossing area and the upper and middle parts were identified as the parallel area. ROIs of 75 µm/side were drawn in both the crossing areas and the parallel areas of the anterior commissure and the hippocampus.
We identified the sagittal sections on the NODDI Vic and ODI maps by using the coordinate data and manually selected 12 voxels in the anterior commissure and 20 voxels in the hippocampus corresponding to the ROIs in the histological specimens.
Statistical analysis
Independent t-test was performed to compare density, Vic, and ODI between the crossing area and the parallel area of the anterior commissure and between the crossing area and the parallel area the hippocampus. Correlation between fiber density and Vic was evaluated with Pearson’s correlation coefficient. Two extremely high values were excluded from the ODI of the hippocampus. False discovery rate (FDR) correction was applied to P values.
Results
Neurite density and NODDI metrics for crossing areas and parallel areas are presented in Table 1.
Table 1.
Neurite density and NODDI parameters in crossing areas and parallel areas, with t-test results.
| Crossing area | Parallel area | P value | |
|---|---|---|---|
| Anterior commissure | |||
| Density (%) | 44.2 ± 4.06 | 42.9 ± 3.58 | n.s. |
| Vic | 0.778 ± 0.0685 | 0.847 ± 0.173 | n.s. |
| ODI | 0.227 ± 0.169 | 0.0178 ± 0.0108 | 0.0247* |
| Hippocampus | |||
| Density (%) | 17.6 ± 1.67 | 12.06 ± 1.51 | 7.91E–07*** |
| Vic | 0.339 ± 0.0316 | 0.362 ± 0.0357 | n.s. |
| ODI | 0.589 ± 0.0856 | 0.373 ± 0.0997 | 0.000219** |
Each set of values represents the mean ± standard deviation.
P < 0.05.
P < 0.001.
P < 0.0001.
Vic, intracellular volume fraction; ODI, orientation dispersion index.
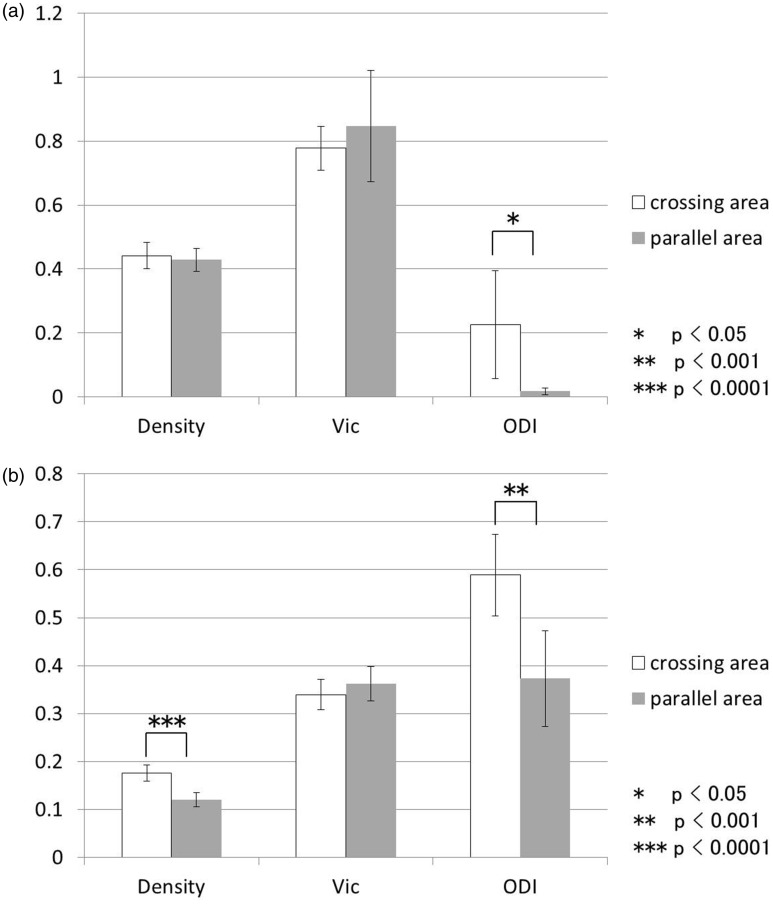
In the histological specimens, neurite density was significantly higher in the crossing area of the hippocampus in comparison with the parallel area (Fig. 2b, P = 7.91E–07), while there was no significant difference in neurite density between the crossing area and the parallel area of the anterior commissure.
Fig. 2.
Comparison of neurite density, Vic, and ODI in crossing areas and parallel areas. (a) Anterior commissure. The ODI in the crossing area was significantly higher than that of parallel area. (b) Hippocampus. Neurite density and ODI in the crossing area were significantly higher than neurite density and ODI in the parallel area. Vic, intracellular volume fraction; ODI, orientation dispersion index.
On NODDI, the ODI was significantly higher in the crossing area compared to the parallel area in both the anterior commissure (Fig. 2a, P = 0.0247) and the hippocampus (Fig. 2b, P = 0.00022).
No consistent tendency for Vic was observed in comparisons between the crossing areas and the parallel areas, and the t-test did not show significant difference in the anterior commissure or in the hippocampus. There was no significant correlation between neurite density and Vic.
Discussion
We found that the ODI was significantly higher in the crossing area compared to the parallel area in both the anterior commissure (Table 1, Fig. 2a) and the hippocampus (Table 1, Fig. 2b) of the transparent mouse brain. The utility of ODI was histologically confirmed.
NODDI is one of the novel diffusion MRI techniques that have the potential to reveal the microstructure of the brain by distinguishing diffusion patterns according to neurite orientation dispersion, neurite density, and isotropic features like edema. Traditional diffusion tensor imaging (DTI) detects microstructural changes mainly as decreases of fractional anisotropy (FA). However, FA relies on biological factors, such as decrease of neurite density, demyelination, and edema, and therefore does not specifically represent histological features. Complexities of the tissue environment including crossing fibers or contamination of CSF or gray matter can cause errant metrics in DTI (7,8). In particular, it is well-known that the presence of crossing fibers is a major cause of inaccuracies in FA estimation in complex architectures (9,10). Increases in FA without definite cause have also been reported (11–13). Kamagata et al. have found that the correlation between FA and neurite density is weak in the caudate nucleus and putamen, which contain crossing fibers, in the transparent mouse brain (6).
An important feature of NODDI is that the degree of orientation dispersion of axons is quantitatively represented by the ODI. While microstructural features, including axonal density and diameter, can be estimated by other multi-shell compartment model-based methods, such as CHARMED or AxCaliber, these methods do not cover crossing, fanning, or bending of axons (4). Several authors have reported precise visualization of crossing fibers by high resolution or advanced DTI with histologic validation (14,15). However, histologic validation of the ODI for quantification of the degree of orientation dispersion of axons has not been previously reported. In this study, we have compared NODDI metrics and histological findings in brain regions containing crossing fibers in 3D. The ODI was in good agreement with the histological findings.
Our finding that neither neurite density nor Vic was significantly different between crossing areas and parallel areas in the anterior commissure implies that there is not much variation in density or Vic. Therefore, it would be expected that Vic would not show significant correlation with density.
In the hippocampus, although neurite density was significantly different between the crossing area and the parallel area, there was no significant difference in Vic, and Vic did not show significant correlation with neurite density. Discrepancy in t-test results between neurite density and Vic can be well explained in terms of the microstructure of the hippocampus. Only YFP-labeled neurons in the histological section are countable for neurite density calculation. YFP expression is essentially restricted to projection neurons in the hippocampus, i.e. pyramidal neurons in CA1 (16). As opposed to the anterior commissure, the hippocampus contains various types of interneurons that do not express YFP (17). It is noted that transparent brain histological analysis does not completely represent the whole brain.
Depending on the clearing method, the size and microstructure of the brain can be partially altered during the brain clearing process (5,6). Thus, there may be small registration errors between NODDI and histological imaging. However, we think that the tendency of the fiber orientation was maintained because no significant morphological alterations were observed in our targeted brain regions. Another methodological issue between NODDI and histology is the difference of the spatial resolution and the partial volume effect. One pixel of NODDI corresponds to several pixels of the histologic images. Thus, congruent registration for the two methods was difficult.
In spite of these limitations, non-invasive diffusion MRI is highly expected to have a key role as a method for in vivo evaluation of the microstructure of the brain. Histological validation of the tissues and comparison with MRI will be the basis of imaging assessment of the microstructure. In the future, these tools may lead to clinical use of MRI of the whole human brain microstructure for evaluation and treatment at the molecular level.
In conclusion, our results showed significant increases of ODI in crossing areas, which was consistent with histology in terms of the orientation dispersion of neurites. There was no significant correlation between Vic and neurite density because of the low degree of variation in neurite density in the anterior commissure and the YFP expression mismatch in the hippocampus. Our results suggest that the ODI is a suitable index for understanding the microstructure of the brain in vivo.
Acknowledgements
The authors thank Masaaki Hori and Sayaka Shibata for their contribution to the acquisition and analysis of the data.
Declaration of conflicting interests
The author(s) declare no potential conflicts of interest with respect to the research, authorship, and/or publication of the article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by JSPS KAKENHI Grant Number 16K19853 and JP16H06280. A part of this work was carried out under MEXT-Supported Program for the Private University Research Branding Project and the Brain Mapping by Integrated Neurotechnologies for Disease Studies (Brain/MINDS) from Japan Agency for Medical Research and development, AMED. Aurelien Kerever is an international research fellow of the Japan Society for the Promotion of Science.
References
- 1.Billiet T, Vandenbulcke M, Mädler B, et al. Age-related microstructural differences quantified using myelin water imaging and advanced diffusion MRI. Neurobiol Aging 2015; 36: 2107–2121. [DOI] [PubMed] [Google Scholar]
- 2.Colgan N, Siow B, O’Callaghan JM, et al. Application of neurite orientation dispersion and density imaging (NODDI) to a tau pathology model of Alzheimer's disease. Neuroimage 2016; 125: 739–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lemkaddem A, Daducci A, Kunz N, et al. Connectivity and tissue microstructural alterations in right and left temporal lobe epilepsy revealed by diffusion spectrum imaging. Neuroimage Clin 2014; 5: 349–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang H, Schneider T, Wheeler-Kingshott CA, et al. NODDI: practical in vivo neurite orientation dispersion and density imaging of the human brain. Neuroimage 2012; 61: 1000–1016. [DOI] [PubMed] [Google Scholar]
- 5.Susaki EA, Tainaka K, Perrin D, et al. Whole-brain imaging with single-cell resolution using chemical cocktails and computational analysis. Cell 2014; 157: 726–739. [DOI] [PubMed] [Google Scholar]
- 6.Kamagata K, Kerever A, Yokosawa S, et al. Quantitative histological validation of diffusion tensor MRI with two-photon microscopy of cleared mouse brain. Magn Reson Med Sci 2016; 15: 416–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Le Bihan D, van Zijl P. From the diffusion coefficient to the diffusion tensor. NMR Biomed 2002; 15: 431–434. [DOI] [PubMed] [Google Scholar]
- 8.Mori S, Zhang J. Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron 2006; 51: 527–539. [DOI] [PubMed] [Google Scholar]
- 9.Jbabdi S, Behrens TE, Smith SM. Crossing fibres in tract-based spatial statistics. Neuroimage 2010; 49: 249–256. [DOI] [PubMed] [Google Scholar]
- 10.Vos SB, Jones DK, Jeurissen B, et al. The influence of complex white matter architecture on the mean diffusivity in diffusion tensor MRI of the human brain. Neuroimage 2012; 59: 2208–2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sato K, Ishigame K, Ying SH, et al. Macro- and microstructural changes in patients with spinocerebellar ataxia type 6: assessment of phylogenetic subdivisions of the cerebellum and the brain stem. Am J Neuroradiol 2015; 36: 84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abe O, Yamasue H, Kasai K, et al. Voxel-based diffusion tensor analysis reveals aberrant anterior cingulum integrity in posttraumatic stress disorder due to terrorism. Psychiatry Res 2006; 146: 231–242. [DOI] [PubMed] [Google Scholar]
- 13.Abdallah CG, Tang CY, Mathew SJ, et al. Diffusion tensor imaging in studying white matter complexity: a gap junction hypothesis. Neurosci Lett 2010; 475: 161–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Budde MD, Annese J. Quantification of anisotropy and fiber orientation in human brain histological sections. Front Integr Neurosci 2013; 7: 3–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leergaard TB, White NS, de Crespigny A, et al. Quantitative histological validation of diffusion MRI fiber orientation distributions in the rat brain. PLoS One 2010; 5: e8595–e8595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Porrero C, Rubio-Garrido P, Avendaño C, et al. Mapping of fluorescent protein-expressing neurons and axon pathways in adult and developing Thy1-eYFP-H transgenic mice. Brain Res 2010; 1345: 59–72. [DOI] [PubMed] [Google Scholar]
- 17.Yi F, Catudio-Garrett E, Gábriel R, et al. Hippocampal “cholinergic interneurons” visualized with the choline acetyltransferase promoter: anatomical distribution, intrinsic membrane properties, neurochemical characteristics, and capacity for cholinergic modulation. Front Synaptic Neurosci 2015; 7: 4–4. [DOI] [PMC free article] [PubMed] [Google Scholar]