Abstract
Discrimination has been associated with elevated cortisol as measured in saliva, blood, and urine. This study investigated the association between lifetime discrimination and hair cortisol concentrations, considered a measure of chronic stress. We recruited 180 young adults from diverse backgrounds. Participant responses to lifetime discrimination, home stress, and subjective status measures were recorded. Lifetime discrimination significantly predicted hair cortisol concentrations, supporting past research that discrimination experiences impact neuroendocrine systems. To our knowledge, these are the first findings associating hair cortisol concentrations with discrimination and supports prior evidence positing discrimination as a chronic stressor that serves as a risk factor for chronic disease.
Keywords: chronic stress, hair cortisol, perceived discrimination, subjective social status
The relationship between discrimination and stress has been well established (e.g. Clark et al., 1999; Pascoe and Smart Richmond, 2009). Experiences of social discrimination have been associated with numerous deleterious health outcomes, including heightened activity of the hypothalamus-pituitary-adrenal axis (HPAA), poorer cardiovascular functioning (Pascoe and Smart Richman, 2009), and poorer physical health outcomes (e.g. Clark et al., 1999; Coogan et al., 2014). Moreover, the physical symptoms linked to discrimination have been characterized as chronic stress (Clark et al., 1999). These findings have led to the hypothesis that chronic discrimination experiences can produce a sufficient level of chronic physiological stress in individuals to produce changes in the HPAA and health outcomes (Williams and Mohammed, 2009).
Discrimination has been established as a chronic stressor and influential on alterations in HPAA functioning, although not all findings are consistent; inconsistencies can be found depending on the cortisol measurement and the severity of the stressor. For example, in a meta-analysis by Miller et al. (2007), great variability was found in cortisol reactivity, depending on the timing of the cortisol assessment or the individual’s appraisal. Broadly, it was found that acute stressors may be associated with HPAA hyper-reactivity, whereas chronic stressors may be associated with HPAA hypo-reactivity, particularly when responsiveness is assessed at later time points. Yet inconsistencies remain in the literature, given the variability discussed above and the matrix for cortisol, that is, whether sampling saliva, urine, or blood (e.g. Yehuda et al., 2003).
When assessing the influence of discrimination or racism on HPAA functioning, several studies which examined diurnal profiles with salivary cortisol have found that African-Americans revealed flatter diurnal cortisol as compared to White participants (e.g. Hajat et al., 2010; Martin et al., 2012; Skinner et al., 2011). Flatter diurnal profiles are suggestive of hypothalamus-pituitary-adrenal (HPA) dysregulation, which has been associated with poor health outcomes (e.g. Skinner et al., 2011). In addition, Kaholokula et al. (2012) found for Native Hawaiians that morning and evening salivary cortisol samples were lower for those reporting higher versus lower attributed racism. While the association between hypocortisolism and chronic stress is well established (for review, see Heim et al., 2000), not all studies have found this hypo-responsivity when measuring HPAA activity.
For example, Zeiders et al. (2012) investigated discrimination-related stress in a sample of Mexican-American adolescents. Using salivary cortisol assessments to measure diurnal and awakening responses, they found that perceived discrimination was associated with greater overall cortisol output (area under the curve (AUC) and a nearly significant increase in the cortisol-awakening response (CAR) (p < .08). In another study of adolescents, Huynh et al. (2016) found that greater everyday discrimination was associated with greater daily cortisol (AUC). These associations were not significantly different by race or sex of participants.
Hair cortisol
Despite the vast amount of work on discrimination and HPAA reactivity and alterations, little work has yet investigated the potential association of hair cortisol concentrations (HCC) with this well-known chronic stressor: social discrimination. We used HCC as a biomarker of the cumulative secretion of cortisol into circulation over a 3-month period, which is now a well-established measure of HPAA activity. This methodological approach offers many advantages over measurement of cortisol in other sample matrices (for reviews, see Meyer and Novak, 2012; Russell et al., 2012; Wosu et al., 2013). Hair collection is simple and noninvasive, and samples can be collected in field or lab settings and stored in envelopes or vials until assayed (e.g. see O’Brien et al., 2013; Steudte et al., 2011). This avoids the necessity of storing samples refrigerated or frozen, which is particularly relevant given that the temporal distance from the stressor can be an important factor in quantifying the effects of chronic stress on HPAA activity (Miller et al., 2007). Moreover, cortisol assessments using blood, salivary, or urinary samples have shown a high degree of intra-individual variability. In contrast, recent studies found HCC to be relatively stable with high test–retest associations when samples were obtained two times over 1 year and three times over 2 months; test–retest associations resulted in rs between .68 and .79 (Stalder et al., 2012). HCC measurement additionally holds promise as a noninvasive measure that is feasible to use with large sample populations. Indeed, current application of the HCC methodology is a response to the call in the literature that multiple approaches are needed to get a full picture on the relationship between discrimination and its influence on stress systems (Juster et al., 2010). Furthermore, there is a rapidly progressing literature to explicate the neuroendocrine mechanisms underlying social stress and disease connections (for review, see Wosu et al., 2013).
Several studies have examined the relationship between HCC and other long-term measures of cortisol secretion (for review see Russell et al., 2012). Specifically, positive correlations have been found between HCC and measures of diurnal HPA activity. For example, D’Anna-Hernandez et al. (2011) found HCC and diurnal salivary cortisol area under the curve with respect to ground (AUCg) were correlated throughout pregnancy. In earlier work, Suave et al. (2007) found positive correlations between HCC, serum, and 24-hour urine cortisol measurements. These findings have helped validate HCC as a useful biomarker of chronic cortisol secretion.
Perceived discrimination and subjective stress
This study used an established scale of lifetime discrimination to assess the relationship to HCC. The lifetime discrimination measure asks questions on structural perceived discrimination, perhaps where one’s greater socioeconomic status (SES) upward mobility may have been curtailed (McNeilly et al., 1996; Williams, 1999). Additionally, established perceived stress scales were used to examine relationships with both discrimination and HCC, including personal perceived stress and home stress. Associations between perceived stress measures and HCC have been inconsistent in the HCC-related literature (see Wosu et al., 2013 for review).
Subjective social status
The subjective social status (SSS) ladder (Operario et al., 2004) has been used extensively to assess perceived social standing or status, as compared to objective measures (e.g. income). Evidence has shown that SSS is associated with psychosocial factors and devaluation experiences more than objective SES measures (e.g. Subramanyam et al., 2013). SSS has also been found to be inversely related to physical health outcomes such as resting heart rate, obesity, and hypertension (Singh-Manoux et al., 2005), after statistically controlling for objective SES measures. Moreover, Adler et al. (2008), comparing the Whitehall II and Coronary Artery Risk Development in Young Adults (CARDIA) data, found that higher objective SES may not confer the same benefits to African-Americans as compared to other racial/ethnic groups, suggesting that implicit negative biases may still be operating in higher SES environments and that racism may influence the self-assessment of status.
Given that discrimination engages social devaluation and subjective status, we hypothesized that SSS would also moderate the relationships between discrimination and the stress indicators such that higher status may not confer the same benefit to high SES minorities reporting high discrimination.
While the health-gradient literature shows stepwise health improvements with objective measures of SES, life course or generational improvements in social status are not always accompanied by a parallel change in health outcomes or decreased stress (e.g. Colen et al., 2006; O’Brien et al., 2013), which raises questions about interactive effects arising from perceived status. In prior work with a diverse urban population, we found that higher SES conferred protection from both perceived stress and increased HCC among non-minorities but not among minorities (O’Brien et al., 2013). We conjectured that differences in discrimination could account for the minority group difference at the higher SES level.
This study was guided by the primary hypothesis that lifetime discrimination stress would be associated with chronically elevated HCC in a diverse sample. Given that temporal differences between HCC and shorter-term measures of cortisol can differ by days and months, lifelong experiences with adversity may be captured better with the longer-term measure of HCC. We predicted that a unique pattern would emerge in which reported lifetime discrimination would be a better predictor of elevated HCC than perceived stress. Finally, we investigated a potential moderator of the predicted discrimination-HCC association: subjective social status (i.e. the SSS ladder; Operario et al., 2004).
This study
This study was guided by the primary hypothesis that lifetime discrimination stress would be associated with chronically elevated HCC in a diverse sample. Given that hair cortisol is arguably a better index of chronic HPAA activation than shorter-term measures such as salivary cortisol, lifelong experiences with adversity may be captured better using HCC. Finally, we investigated a potential moderator of the predicted discrimination-HCC association: subjective social status (i.e. the SSS ladder; Operario et al., 2004). To summarize the two major study goals: (1) Do lifetime discrimination experiences predict (a) elevated HPAA activity as measured by HCC and (b) perceived stress indicators and (2) Are the relationships between lifetime discrimination and HCC moderated by the SSS ladder.
Method
Participants and recruitment
The protocol was approved by the University of Massachusetts Boston (UMB) Institutional Review Board prior to recruitment. We recruited healthy adults from the UMB, an urban commuter campus, and through online community bulletin boards. The final sample included 180 adults, ages 18–30; M = 22.20, SD = 3.44, 52 percent female, race/ethnicity included 19.1 percent African-American/Black, 29.5 percent Asian, 18.6 percent Latin/Hispanic, and 32.8 percent European/White. All participants provided written informed consent and were paid an honorarium of US$25.00 for their participation.
Measures
Hair cortisol
Noting the scalp end, hair samples were trimmed to 3 cm, weighed, and stored in standard polypropylene test tubes at room temperature until assayed. Since hair grows approximately 1 cm per month, 3 cm is posited to capture retrospectively circulating cortisol for the past 3 months. Hair samples weighing 21–195 mg were processed and analyzed for cortisol (as described in Meyer et al., 2012). Intra- and inter-assay coefficients of variation were < 10 percent. Values were converted into pg/mg hair for data analysis and presentation.
Lifetime discrimination
This 12-item scale assesses the lifetime frequency of discrimination experiences across multiple domains including work, school, receiving services, and public life (adapted by adding one question referring to higher education advising, McNeilly et al., 1996; Williams et al., 2009). For example, “How many times in your life were you discouraged by a teacher or advisor from seeking higher education?” Respondents answer on a Likert scale from 0 to 4, where each anchor represents 1–2 instances. Respondents answered using similar anchors as above and the same open item question. The possible range is 0–48 (α = .93). For the following measures, items were reverse scored such that higher total values reflect higher endorsement of the construct assessed. One additional item was open and asked “Why” the participant felt that they were discriminated (e.g. race/ethnicity, sex, weight, nativity, sexual orientation, or other as an open-ended response).
Personal perceived stress
The personal perceived stress (PSS) is a standard validated subjective stress measure, which includes 10 items (Cohen and Williamson, 1988). The wording was modified to assess perceived stress over the past 3 months. Respondents answer on a Likert scale from 1 to 5, where 1 = never and 5 = very often. The possible range is 10–50 (α = .88).
Confusion, Hubbub, and Order
This reliable and validated 15-item measure assesses chaos/order of the home environment (Confusion, Hubbub, and Order Scale (CHAOS), Matheny et al., 1995). For example, “You can’t hear yourself think in our home.” Respondents answer on a Likert scale from 1 to 4, where 1 = strongly agree and 4 = strongly disagree. The possible range is 15–60 (α = .89).
Status measures
SSS
The SSS ladder was administered (Operario et al., 2004). Specifically, participants were presented an image of a ladder of 10 rungs and were asked to rate themselves in terms of their social standing in the community, where 1 is the lowest and 10 is the highest rank.
Socio-demographic
Age and sex
Participants listed their age in years and their sex.
Personal income
Personal income was obtained with the following scales: income: 1 = <US$5000.00, 2 = US$5000.00–9000.00, 3 = US$10,000.00–19,000.00, 4 = US$20,000.00–49,000.00, 5 = US$50,000.00–99,000.00, and 6 = US$100,000.00 and above. None of the respondents reported income greater than US$49,000.00, thus personal income was recoded into four categories where 1 = <US$5000.00, 2 = US$5000.00–9000.00, 3 = US$10,000.00–19,000.00, and 4 = US$20,000.00–49,000.00 (M = 1.9,SD = 1.09).
Race/ethnicity
Participants were selected from 13 race/ethnic categories, which were recoded into four major ethno/heritage groups: African (African-American/Black, Dominican, Haitian), Asian (Asian, Indian, Hawaiian, Pacific Islander), European (European/White, Arab/Middle Eastern), and Hispanic (Latino-Hispanic, Caribbean, Brazilian/Portuguese, Native-American), and other as an open response. Four race/ethnicity categories were constructed based on respondent’s selection of personal heritage group: African, Asian, Latin-Hispanic, and European-American.
Hair treatment
Six questions assessed the frequencies of hair washing and styling treatments that may influence cortisol capture in the hair matrix. These include the frequency of washing, dyeing, and chemical treatments.
Medications
Six questions were asked about medications that potentially alter HPAA activity (e.g. corticosteroids, beta-blockers), as well as depression, anxiety, and hormones (e.g. birth control).
Procedure
After obtaining written informed consent, the gloved experimenter clipped a 3-cm sample of hair from the posterior vertex for the HCC assay. Human hair grows approximately 1 cm per month; therefore, the collection of 3 cm is posited to capture cortisol secretion retrospectively for the past 3 months. Participants then completed the socio-demographic and perceived stress and discrimination surveys online via PsychData (State College, PA). Upon completion of surveys, participants were debriefed, paid, and thanked.
Results
Data analyses
Initial examination of the data revealed that HCC values (pg/mg) fell within a range that is consistent with hair cortisol findings from both clinical and non-clinical populations (i.e. range: 3.10–99.00 pg/mg, M = 16.83, SD = 19.16). However, assessment of normality by means of the Shapiro–Wilk and Kolmogorov–Smirnov tests showed that the distribution was positively skewed; consequently, the data were subjected to log-transformation for further analysis (transformed range: .49–2.02, M = 1.07, SD = 0.33). The frequencies of hair washing, dyeing, use of weave, or chemical treatments were singularly entered as covariates in the first step of HCC analyses. There were no significant differences in hair treatment or medication use on HCC levels (all ps n.s.) and, therefore, these were not included as covariates in the remaining statistical models. Table 1 shows the zero-order correlations, means, and standard deviations of major variables.
Table 1.
Means, standard deviations, and zero-order correlations for major variables.
| Variable | M | SD | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|---|---|
| 1. Life discrimination | 29.03 | 7.12 | – | ||||||
| 2. HCC | 1.07 | 0.32 | .17* | – | |||||
| 3. Chaos | 29.02 | 7.11 | .23** | .02 | – | ||||
| 4. Personal stress | 27.74 | 6.50 | .17 | −.05 | .49** | – | |||
| 5. SES | 0.00 | 0.68 | .02 | .05 | −.14 | −.10 | – | ||
| 6. Subjective status | 5.76 | 1.87 | −.24** | −.03 | −.182* | −.10 | .09 | – | |
| 7. Race/ethnicity | 2.65 | 1.13 | −.03 | .02 | −.06 | −.02 | −.19** | .03 | – |
HCC: hair cortisol concentration; SD: standard deviation; SES: socioeconomic status.
N = 180. Race/ethnicity is coded 1–4, where 1 is African, 2 is Asian, 3 is Latin, and 4 is European-American.
p < .05; **p< .01.
Analytic plan
Using IBM’s SPSS version 19 statistical software, hierarchical linear regression modeling was used to assess whether the dependent variables were predicted by perceived discrimination experiences. Dependent measures included HCC and perceived stress. Independent variables included lifetime discrimination and the SSS ladder.
Simple and moderation effects of subjective status were assessed by the following. In step 1, primary predictors were entered, in step 2, the moderator variable was entered, and in step 3, interaction terms were entered. Independent variables were centered prior to creating the interaction term to control for multicollinearity (Aiken and West, 1991; Baron and Kenney, 1986; Cohen et al., 2013). Models assessed covariates by entering them simultaneously in step 1. Moderation effects were examined using standard procedures outlined in Aiken and West (1991) and Baron and Kenney (1986). Specifically, significant two-way interactions were probed via regression analyses that included a conditional moderator variable. Regression lines were then plotted based on the regression equations. Analysis of covariance (ANCOVA) was used to examine simple and interaction effects of race/ethnicity by discrimination on the dependent measures.
Lifetime discrimination and social status
The first model produced a significant main effect of lifetime discrimination on HCC, after controlling for age and sex (B = .26, t(1, 171) = 2.21, p < .01, ΔR2 = .03*; Figure 1). There was no main effect of SSS and the interaction was n.s. In the next model, perceived stress was not predicted by lifetime discrimination, status, or the interaction term.
Figure 1.

Mean of lifetime discrimination (+/- the mean) on hair cortisol concentrations as measured in pg/mg). Those highest in reported discrimination showed the highest cortisol, a measure of cortisol secretion retrospectively for approximately 3 months.
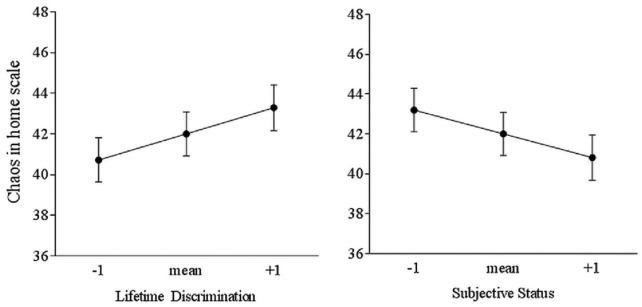
In the next model, lifetime discrimination predicted the home CHAOS measure (B = .458, t(1, 178) = 2.63, p < .01, ΔR2 = .12**). There was also a main effect of social status ladder (B = −.173, t(1, 158) = 2.20, p < .05, ΔR2 = .11*), but the interaction term was not significant (Figure 2).
Figure 2.
The left figure shows the mean of lifetime discrimination (+/- the mean) on Chaos in the home scale. Those highest in reported discrimination also reported the highest Chaos, compared to those in the lowest group. The right figure shows the mean of subjective status (+/- the mean) on Chaos. Those in the lowest report subjective status also reported the lowest, compared to those reporting high status.
An ANCOVA assessed the interaction between race/ethnicity (4) by lifetime discrimination on HCC and perceived stress measures. There were no main effects, and the interaction of race/ethnicity by lifetime discrimination was not significant for any other dependent measures, controlling for sex and age.
Finally, an omnibus analysis of variance (ANOVA) found a trend, but no significant between-group differences in lifetime discrimination by race/ethnicity (p = .80). The prevalence of lifetime discrimination experiences by race/ethnicity is presented in Table 2. Responses to the open-ended questions about the perceived reason “Why were you treated this way?” for discrimination varied tremendously and included race, sex, age, looks, and other attributions, but no clear pattern emerged.
Table 2.
Prevalence of lifetime discrimination reported by self-reported race/ethnic heritage group.
| Lifetime discrimination | African | Asian | Latino | European | Total |
|---|---|---|---|---|---|
| Mean | 4.78 | 2.77 | 3.63 | 3.73 | 3.62 |
| % of Total N | 18.1% | 29.9% | 18.1% | 33.9% | 100% |
| Minimum | 0 | 0 | 0 | 0 | 0 |
| Maximum | 13 | 10 | 14 | 14 | 14 |
Discussion
The results reported in this study support the primary hypothesis: discrimination experiences can lead to chronically elevated cortisol in healthy young adults. This relationship was found using HCC as a biomarker of chronic or repeated HPA activity and a self-reported measure of discrimination experience in a diverse sample in terms of race/ethnicity and social class. The findings provide the first evidence of a positive association between frequency of self-reported lifetime discrimination experience and HCC. It is notable that this association was not found for a daily discrimination scale (Kessler et al., 1999), that was also administered (data not shown), but only for the more cumulative and chronic experiences of discrimination. It would be of interest to replicate this finding as well as examine HCC and other acute or short-term assessments of social devaluation, such as microaggressions (e.g. Torres-Harding et al., 2012). In addition, it would be of interest to examine the relationship between cumulative concentrations of cortisol in hair with the disrupted diurnal dynamics of cortisol secretion reported in association with perceived discrimination in several recent studies (e.g. Thayer and Kuzawa, 2015).
Measurement of HCC is a compelling noninvasive approach for the physiological assessment of chronic stress, and the extant research using HCC is expanding rapidly (for reviews, see Meyer and Novak, 2012; Russell et al., 2012). The findings in this report indicate that it can also be a useful tool for teasing out the additive and interactive effects of the multiple social stressors found in health disparity populations and thought to be consequential for more frequent or earlier onset of various chronic diseases. The now considerable literature has established HCC as a stable, reliable, and efficiently obtained biomarker of average circulating cortisol over months. It can be obtained in a single sample collected noninvasively that takes no more than a few minutes to obtain, has few specific storage needs (e.g. can be stored in tin foil in a plastic bag), and can be repeated over time with methodological ease. These features suit it well to further research aimed to understand interrelations among chronic physiological stress, discrimination, and other pervasive social stressors, neurobehavioral pathways, and health outcomes.
The linkage between HCC and discrimination stress found in this study provides further evidence to the broad literature that has evinced physiological mechanisms underlying the discrimination and poor health link. Perceived discrimination has been associated in prior research with poorer cardiovascular, mental, and health outcomes (e.g. Clark et al., 1999; Coogan et al., 2014; Williams et al., 2008), and this has not always been race-based discrimination (Smart Richman et al., 2009).
In this study, race/ethnicity did not interact with perceived discrimination or subjective status to predict HCC or subjective stress and perceived lifetime discrimination was reported by participants in all four of the major racial/ethnic groups (Table 2). The lack of interaction effects may be due to the diversity of the sample in both race/ethnicity and SES. For example, the present sample includes a relatively low SES sample, with the lowest reported income was <US$5000.00, the highest reported income was approximately US$49,000.00, which is lower than both US and Massachusetts median incomes. The sample was also 50 percent students who also work part-time or full time. However, there was evidence of differences in the pervasiveness of discrimination across these race/ethnic groups; African-Americans reported the highest levels of lifetime discrimination experiences (Table 2). Given the diversity of this sample, in this first iteration investigating discrimination and HCC, we did not primarily predict that discrimination would always be race based. The open-ended questions revealed enormous variability in “Why” participants felt discrimination; race was often reported with gender, sexual orientation, or age. Other responses were ambiguous and included “class,” “not enough,” and “religion.” In future studies, the open-ended response could be constrained to disambiguate the reasons for perceived discrimination.
The complex pattern of social stress and status relationships reported in the literature led us to examine protective factors for the impact of lifetime discrimination stress on outcome measures. We hypothesized that social status would have moderating effects, and this was confirmed with the chaos variable (i.e. higher status was associated with lower chaos in the home). It is important for understanding the complex interplay of these social variables and for guiding the selection of interventions that the moderator hypotheses were supported. In addition, higher discrimination was predictive of higher chaos, perhaps highlighting pathways of stress experiences that can infuse other domains.
Questions for future research include other protective factors that can be assessed experimentally and titrated to specific discrimination stress, in order to specify the moderator’s strengths and contextual constraints. The real world significance of this work supports the categorization of discrimination as chronic stress.
Limitations
This study has several limitations that warrant discussion. First, our sample includes a relatively young sample of diverse adults, ages between 18 and 30 years, and does not include long-term health outcomes or assessments of acute or chronic diseases. While we can merely assess discrimination–stress relationship as a potential risk for deleterious health later in life, this study focused primarily on one factor of risk: elevated cortisol secretions in hair, which may be prospective. Future studies could add measures specifically associated with allostatic load and actual health outcomes (e.g. chronic diseases) for a more complete picture. Furthermore, we did not examine objective SES (income) as a predictor or moderator, so these findings are independent of income in this sample. Future samples could recruit greater variability in SES.
The coding of race/ethnicity was based on participant’s selection of 13 groups as suggested by National Institutes of Health (NIH). Based on these reports, the race category was constructed into four groups based on respondent’s answer to one question (primary heritage group; NIH, 2017). Given a larger sample size, with a more focused recruitment on specific race/ethnicity and variability in SES, one could investigate differences between related heritage groups that also have vast cultural differences, as well as class, status, and discrimination experiences in the United States. For example, Vietnamese and Japanese individuals could be examined as separate groups, rather than together as Asian.
Finally, there are limits related to HCC that need to be considered (Meyer and Novak, 2012). Most importantly, measurement of hair cortisol does not capture details about the dynamics of physiological stress response systems, such as extent and timing of the rise and recovery of cortisol secretion during a stress response or diurnal patterning of HPA activity, which feed into the cumulated cortisol sum. These stress-related dynamics have downstream effects on cardiovascular and other homeostatic systems, which can result in “wear and tear” on the systems over time (Wosu et al., 2013). Because of the limitations of the hair cortisol approach, we do not yet know whether the elevated HCC levels found in this study reflect higher baseline cortisol levels or increased responsiveness to daily stressful experiences (including those that are discrimination related). For this reason, further research is needed to define more completely the nature of HPAA dysregulation in people who have suffered high levels of lifetime social discrimination.
Conclusion
The present findings add to the body of evidence indicating that discrimination is a chronic stressor influential enough to alter the HPA axis and related stress systems, potentially increasing the growing gap in health disparities. Moreover, these findings inform clinical intervention and social policy in that both discrimination and moderators of these specific stress effects need to be taken into account in interventions designed to reduce health disparities in racial and ethnic minorities and other devalued groups who report discrimination.
Acknowledgments
The authors thank Kendra Rosenberg for performing the hair cortisol assays.
Footnotes
Funding: The project described was supported by Award No. P20MD002290-05 from the National Institute on Minority Health and Health Disparities (NIMHD) and by a Department of Psychology Research Trust Fund award to C.L.M. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIMHD or the National Institutes of Health.
References
- Adler N, Singh-Manoux A, Schwartz J, et al. (2008) Social status and health: A comparison of British civil servants in Whitehall-II with European- and African-Americans in CARDIA. Social Science & Medicine 66(5): 1034–1045. [DOI] [PubMed] [Google Scholar]
- Aiken LS, West SG. (1991) Multiple Regression: Testing and Interpreting Interactions. Newbury Park, CA: SAGE. [Google Scholar]
- Baron RM, Kenny DA. (1986) The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology 51(6): 1173–1182. [DOI] [PubMed] [Google Scholar]
- Clark R, Anderson NB, Clark VR, et al. (1999) Racism as a stressor for African Americans: A biopsychosocial model. American Psychologist 54(10): 805–816. [DOI] [PubMed] [Google Scholar]
- Cohen J, Cohen P, West SG, et al. (2013) Applied Multiple Regression/Correlation Analysis for the Behavioral Sciences. London: Routledge. [Google Scholar]
- Cohen S, Williamson G. (1988) Perceived stress in a probability sample of the US. In: Spacapam S, Oskamp S. (eds) The Social Psychology of Health: Claremont Symposium on Applied Social Psychology. Newbury Park, CA: SAGE, pp. 31–67. [Google Scholar]
- Colen CG, Geronimus AT, Bound J, et al. (2006) Maternal upward socioeconomic mobility and Black-White disparities in infant birthweight. American Journal of Public Health 96(11): 2032–2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coogan PF, Yu J, O’Connor GT, et al. (2014) Experiences of racism and the incidence of adult-onset asthma in the black women’s health study. CHEST Journal 145(3): 480–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Anna-Hernandez KL, Ross RG, Natvig CL, et al. (2011) Hair cortisol levels as a retrospective marker of hypothalamic-pituitary axis activity throughout pregnancy: Comparison to salivary cortisol. Physiology & Behavior 104(2): 348–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajat A, Diez-Roux A, Franklin TG, et al. (2010) Socioeconomic and race/ethnic differences in daily salivary cortisol profiles: The multi-ethnic study of atherosclerosis. Psychoneuroendocrinology 35(6): 932–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Ehlert U, Hellhammer DH. (2000) The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology 25(1): 1–35. [DOI] [PubMed] [Google Scholar]
- Huynh VW, Guan SS, Almeida DM, et al. (2016) Everyday discrimination and diurnal cortisol during adolescence. Hormones and Behavior 80: 76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juster RP, McEwen BS, Lupien SJ. (2010) Allostatic load biomarkers of chronic stress and impact on health and cognition. Neuroscience & Biobehavioral Reviews 35(1): 2–16. [DOI] [PubMed] [Google Scholar]
- Kaholokula JK, Grandinetti A, Keller S, et al. (2012) Association between perceived racism and physiological stress indices in native Hawaiians. Journal of Behavioral Medicine 35(1): 27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Mickelson KD, Williams DR. (1999) The prevalence, distribution, and mental health correlates of perceived discrimination in the United States. Journal of Health and Social Behavior 208–30. [PubMed] [Google Scholar]
- McNeilly MD, Anderson NB, Armstead CA, et al. (1996) The perceived racism scale: A multidimensional assessment of the experience of white racism among African Americans. Ethnicity & Disease 6(1–2): 154–166. [PubMed] [Google Scholar]
- Martin CG, Bruce J, Fisher PA. (2012) Racial and ethnic differences in diurnal cortisol rhythms in preadolescents: the role of parental psychosocial risk and monitoring. Hormones and Behavior 61(5): 661–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matheny AP, Wachs TD, Ludwig JL, et al. (1995) Bringing order out of chaos: Psychometric characteristics of the confusion, hubbub, and order scale. Journal of Applied Developmental Psychology 16(3): 429–444. [Google Scholar]
- Meyer JS, Novak MA. (2012) Mini review: Hair cortisol: A novel biomarker of hypothalamic-pituitary-adrenocortical activity. Endocrinology 153(9): 4120–4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, Zhou ES. (2007) If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychological Bulletin 133(1): 25–45. [DOI] [PubMed] [Google Scholar]
- National Institutes of Health (2017) Available from: https://grants.nih.gov/grants/guide/notice-files/NOT-OD-15-089.html
- O’Brien KM, Tronick EZ, Moore CL. (2013) Relationship between hair cortisol and perceived chronic stress in a diverse sample. Stress and Health 29(4): 337–344. [DOI] [PubMed] [Google Scholar]
- Operario D, Adler NE, Williams DR. (2004) Subjective social status: Reliability and predictive utility for global health. Psychology and Health 19(2): 237–246. [Google Scholar]
- Pascoe EA, Smart Richman L. (2009) Perceived discrimination and health: A meta-analytic review. Psychological Bulletin 135(4): 531–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell E, Koren G, Rieder M, et al. (2012) Hair cortisol as a biological marker of chronic stress: Current status, future directions and unanswered questions. Psychoneuroendocrinology 37(5): 589–601. [DOI] [PubMed] [Google Scholar]
- Singh-Manoux A, Marmot MG, Adler NE. (2005) Does subjective social status predict health and change in health status better than objective status? Psychosomatic Medicine 67(6): 855–61. [DOI] [PubMed] [Google Scholar]
- Skinner ML, Shirtcliff EA, Haggerty KP, et al. (2011) Allostasis model facilitates understanding race differences in the diurnal cortisol rhythm. Development and Psychopathology 23(04): 1167–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalder T, Steudte S, Miller R, et al. (2012) Intraindividual stability of hair cortisol concentrations. Psychoneuroendocrinology 37(5): 602–610. [DOI] [PubMed] [Google Scholar]
- Steudte S, Kolassa IT, Stalder T, et al. (2011) Increased cortisol concentrations in hair of severely traumatized Ugandan individuals with PTSD. Psychoneuroendocrinology 36(8): 1193–1200. [DOI] [PubMed] [Google Scholar]
- Subramanyam MA, James SA, Diez-Roux AV, et al. (2013) John Henryism and blood pressure among African-Americans in the Jackson Heart Study. Social Science & Medicine 93: 139–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer ZM, Kuzawa CW. (2015) Ethnic discrimination predicts poor self-rated health and cortisol in pregnancy: Insights from New Zealand. Social Science & Medicine 128: 36–42. [DOI] [PubMed] [Google Scholar]
- Torres-Harding SR, Andrade AL, Jr, Romero Diaz CE. (2012) The Racial Microaggressions Scale (RMAS): A new scale to measure experiences of racial microaggressions in people of color. Cultural Diversity and Ethnic Minority Psychology 18(2): 153–164. [DOI] [PubMed] [Google Scholar]
- Williams DR. (1999) Race, socioeconomic status, and health the added effects of racism and discrimination. Annals of the New York Academy of Sciences 896(1): 173–188. [DOI] [PubMed] [Google Scholar]
- Williams DR, Mohammed SA. (2009) Discrimination and racial disparities in health: Evidence and needed research. Journal of Behavioral Medicine 32(1): 20–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wosu AC, Valdimarsdóttir U, Shields AE, et al. (2013) Correlates of cortisol in human hair: Implications for epidemiologic studies on health effects of chronic stress. Annals of Epidemiology 23(12): 797–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R, Halligan SL, Yang RK, et al. (2003) Relationship between 24-hour urinary-free cortisol excretion and salivary cortisol levels sampled from awakening to bedtime in healthy subjects. Life Sciences 73(3): 349–358. [DOI] [PubMed] [Google Scholar]
- Zeiders KH, Doane LD, Roosa MW. (2012) Perceived discrimination and diurnal cortisol: Examining relations among Mexican American adolescents. Hormones and Behavior 61(4): 541–8. [DOI] [PMC free article] [PubMed] [Google Scholar]