Abstract
Apart from the risks of obstetric complications like haemorrhage and eclampsia, a large number of medical conditions affect pregnancy and result in adverse outcomes for both the mother and offspring. Non-communicable diseases in pregnancy are becoming increasingly important in contributing to death and poor health. Changes in the patterns and distribution of these conditions mean that we need new perspectives and ways of dealing with these challenges for the future. This article reviews the burden of ill-health due to non-communicable diseases during pregnancy in low and middle income countries and presents some paradigms relevant to public health and health system needs of the future.
Keywords: Maternal mortality, complications, diabetes, general medicine, obesity
Maternal mortality in low and middle income countries
Maternal mortality in many countries is unacceptably high. In 2015, it was estimated that 303,000 maternal deaths occurred across the world, with 99% in low and middle income countries (LMIC). The maternal mortality ratio globally is 216 deaths per 100,000 live births. The world’s highest maternal mortality ratio is seen in sub-Saharan Africa at 546 deaths per 100,000 live births, where two-thirds of the maternal deaths in the world occur. This is in contrast to industrialised countries, where the maternal mortality ratio is 12 per 100,000 live births. Other developing regions of the world, e.g. Southern Asia, have a ratio of 176 deaths per 100,000 live births. Worldwide, maternal mortality has decreased by 44% since the 1990s. Between 1990 and 2015, rates of decrease were over 4% annually in South and East Asia but only 2.4% in sub-Saharan Africa.1
The determinants of maternal mortality were elegantly laid out many years ago.2 Distal and intermediate determinants are described. The distal determinants comprise factors like women’s education and income, as well as wider cultural and economic factors. Intermediate determinants comprise biomedical factors, including women’s general and reproductive health, and health care system factors, like access to care and use of services. The commonest causes of maternal death are the so-called direct causes, due to obstetric complications like haemorrhage during childbirth, eclampsia, abortion, obstructed labour and sepsis.3 For these reasons, efforts to reduce global maternal mortality have focused on obstetric interventions and maternity services. Less attention has been paid to the general health of women and medical conditions during pregnancy that result in maternal mortality and morbidity. This article focuses attention on the relatively neglected problem of non-communicable diseases (NCDs) during pregnancy in LMIC, and investigates the public health implications of changing patterns of health in pregnancy.
The burden of NCDs in pregnancy
It is estimated that in 2008, 29 million people died from NCDs in LMIC, making up 80% of NCD-related deaths worldwide.4 By 2030, a 50% increase is expected. NCDs are by no means confined to the older age groups. Those in reproductive age groups are increasingly affected, including women, during pregnancy.5
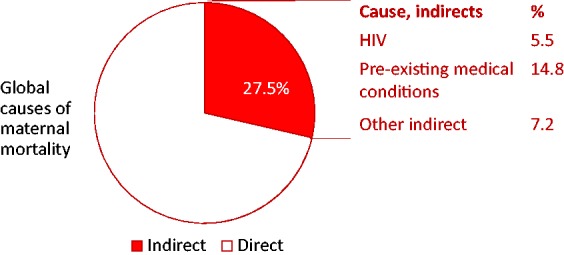
Indirect maternal deaths are a consequence of ‘previous existing disease or disease that developed during pregnancy and not due to direct obstetric causes, but aggravated by physiologic effects of pregnancy.’6 Indirect deaths make up almost one-third of all maternal deaths and can be due to NCDs, or communicable diseases, like HIV or malaria (Figure 1). Almost 15% of maternal deaths are due to pre-existing medical conditions.3 Over the last two decades, there have been decreases in the absolute number of maternal deaths from obstetric causes, possibly as a result of effective interventions to provide emergency obstetric care. On the other hand, only borderline declines have been observed for the indirect causes of maternal deaths. In terms of the proportion of deaths, an increase in indirect maternal deaths has occurred globally, from less than 10% to nearly 11%.7
Figure 1.
The burden of non-communicable diseases in pregnancy.
NCDs important in pregnancy include a large number of different medical conditions, which can affect all organ systems in the body. NCDs important in pregnancy include neoplasms; mental conditions; endocrine or metabolic conditions like diabetes mellitus, hypo and hyperthyroidism; conditions affecting the cardiovascular system, such as hypertension and chronic rheumatic heart disease; and the haematological conditions of anaemia, sickle cell disease or thalassemia. They cover the spectrum of acute, transient, chronic and permanent conditions. Medical and obstetric complications can often be closely interrelated. For example, in diabetes mellitus, immediate medical concerns are focused on glycemic control with related obstetric complications such as macrosomia, obstructed labour and increased risk of infection. Longer term medical complications such as chronic hypertension and obesity are pertinent to obstetric sequelae, which include the future need for Caesarean section with concurrent risks of surgical intervention and requirements for anaesthesia, in line with the individual’s overall health. Medical complications can thus affect not only the current pregnancy, but also future pregnancies and long-term health. There is also increasing evidence of the intergenerational effects of medical complications in pregnancy, which lead to compromised development and health of the offspring and their children.8
Particularly for the non-communicable fraction of mortality and morbidity in pregnancy, our knowledge of the prevalence, nature and consequences of the various conditions in pregnancy, pertinent to LMIC is limited. A systematic review of studies in LMIC found a number of population-based studies reporting on specific causes of pregnancy-related deaths due to NCDs.9 The review found that cardiovascular conditions, anaemia, neoplasms and diabetes featured amongst the commonest reported causes of death. Maternal mortality, however, gives a picture only of the ‘tip of the iceberg,’ as NCDs will rarely lead to death during pregnancy. However, some collated data are available. A systematic review found 12 population-based studies from LMIC, with prevalence of gestational diabetes mellitus from 0.4% to 17.2% in pregnant women.10 One population-based study reported the prevalence of type 1 and type 2 diabetes mellitus in pregnancy at 0.7%.11 The commonest complications of gestational diabetes mellitus reported in LMIC are not different from those in industrialised settings, and include maternal complications such as polyhydramnious, preterm labour, pregnancy-induced hypertension and neonatal conditions like macrosomia, jaundice, hypoglycaemia ad low birth weight. However, higher rates of operative deliveries and stillbirths are documented in LMIC, compared to industrialised settings. For example, a 40% Caesarean section rate was observed in LMIC compared to between 21% and 30% in Sweden, Australia and the United Kingdom. Stillbirth rates were 2.6% in LMIC in women with gestational diabetes mellitus, but are less than 0.5% in the United States, Sweden and Italy.12 These are somewhat crude comparisons and the observed differences may have several possible explanations, including poor quality epidemiological data in LMIC, and lack of reliable data. However, it is also plausible that diabetes mellitus in LMIC may be poorly managed and under-diagnosed in pregnancy, and that women lack access to medical care. The special needs of pregnant women with non-communicable conditions are unlikely to be met by poorly organised, under-resourced health services.
Data on the prevalence of other medical conditions in pregnancy are piecemeal. Chronic hypertension was reported at 4% in Brazil with associated complications of small for gestational age and a neonatal mortality of 6.2%.9,13 A hospital-based study in Eritrea recorded a prevalence of rheumatic heart defects of 6.6%,14 although other estimates from India and South Africa ranged from 0.5% to 2%.15–17 Mortality from chronic rheumatic heart disease was not insubstantial, with studies reporting a contribution of 0.5% of all maternal deaths in Egypt18 and 6.2% in Sri Lanka.19 Haemoglobin disorders are relatively common in many LMIC, with over 7% of pregnant women carrying a variant.20 Cervical cancer is particularly implicated in pregnancy and linked with early marriage, early age at first pregnancy and sexually transmitted genital infection with the human papillomavirus. Due to poor access to screening and treatment services, more than 90% of deaths occur in LMIC.21,22 Mental health disorders are also common in LMIC with reported prevalence rates varying from 10% to 41%.23
Obesity is a well-recognised risk factor for various chronic diseases in pregnancy. Although malnutrition in pregnant women is still common and has adverse effects in pregnancy, the overall body mass index is increasing in women.24 In sub-Saharan Africa for example, the rate of increase in body mass index is larger in women than men, with a change of 0.8 kg/m2 per decade between 1980 and 2008. In Southern African women, the baseline body mass index is 26 kg/m2, above the upper limit of 25 kg/m2 considered normal. High levels of obesity in women of reproductive age are seen across several African countries, e.g. at 23% in Malawi and 38% in Kenya, in urban settings, and also amongst educated and richer women.25 Although the prevalence of obesity was much lower in rural areas, for example at 18% in Kenya, there are indications that the rate of increase in urban overweight/obesity was substantially higher among non-educated women, in comparison with their educated counterparts. The prevalence of overweight/obesity appears to be increasing by about 5% per year on average in the countries in the study between 1992 and 2003.25
The reliability of the data obtained in many of the above studies can be questioned. Most data are from hospital-based studies and in LMIC, where women often cannot reach health facilities, and where health facility birth rates can be less than 50%, hospital studies are likely to underestimate the burden of disease. The large variations in reported prevalence levels may be related to geographical, lifestyle, cultural and ethnic predispositions, but may also be because data were limited and subject to inconsistent definitions and poor standardisation of diagnostic criteria. Nevertheless, it is becoming increasingly clear that we cannot ignore the consequences of these epidemiological changes and how they will affect women of childbearing age in the poorest countries.
The obstetric transition
We have observed how maternal mortality has fallen over the last 25 years especially in relation to obstetric complications. Interventions to reduce maternal mortality have, up to now, quite rightly focused on maternity services and care for emergency complications. The information gap on NCDs during pregnancy in LMIC points towards our neglect of this emerging global concern. There have been recent efforts to increase the attention paid to severe maternal morbidities,26 to which NCDs contribute. The increasing risks of maternal ill-health from NCDs in LMIC are part of what others have termed an ‘obstetric transition.’ This concept refers to changing patterns in maternal mortality and morbidity, encompassing the reduction of levels of maternal deaths from direct obstetric causes, alongside an increasing proportion of indirect causes stemming from NCDs, an ageing maternal population and increasing use of health facilities for maternity care.27
The obstetric transition phenomenon prompts us to consider a few questions. Are we, as development practitioners and maternal health advocates, failing to grasp an opportunity to deal with NCDs and chronic diseases in pregnancy? Are we adequately prepared to deal with these changes in health need? Have we assessed the added value of managing NCDs in pregnancy not only for the immediate well-being of the mother and baby, but also for their longer term health and that of future offspring? In contemplating these concerns, we consider the application of a life-course perspective in women’s health care.
A life-course perspective and primordial prevention
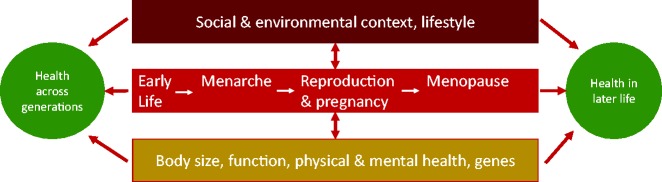
A life-course perspective allows an exploration of social, behavioural and biological factors during the various phases of early life, childhood, young adulthood, gestation and later life, also extending across generations28 (Figure 2). From an obstetric viewpoint, this approach broadens the focus of attention from maternal events and underscores the linkages and continuity across an individual’s lifespan, and to future generations of offspring. The life-course approach highlights firstly, the potential for early intervention to reduce disease risk or severity, and secondly, triggers health care needs in a more predictable fashion,29 so is a particularly useful paradigm for public health considerations.
Figure 2.
A life course perspective.
The uniqueness of pregnancy as an event in the life-course of a woman is that the physiological demands of pregnancy act as a ‘stress test.’29 The state of being pregnant can thus reveal underlying or undiagnosed disease which may have been dormant, as well as the risk of future chronic conditions.
In relation to NCDs and their consequences in pregnancy, a life-course perspective opens opportunities for prevention early in life. For example, women who have had a pregnancy complicated by gestational diabetes, or a hypertensive disorder, are at increased risk of cardiovascular disease later in life. Dietary, lifestyle and screening interventions could take place in younger women, instead of waiting until the disease presents itself later in life.29 Some have extended the concept of primary prevention to ‘primordial prevention,’ i.e. prevention of the risk factors themselves.30 Highly predictive pregnancy outcomes can open opportunities to identify women at risk later in their lives. This is important in cardiovascular disease, especially as risk factors can be more difficult to predict in women than in men. At least from studies in industrialised countries, there is evidence that women (even if they have no overt signs of disease themselves during pregnancy) who deliver preterm, or who have growth restricted babies, have an elevated risk of developing cardiovascular disease much later in their lives.31,32 Other adverse pregnancy outcomes, like stillbirth and placental abruption, have also been linked to poor health and mortality later in a woman’s life.30 Hence, pregnancy may serve as an optimal time for the early identification of women at risk for future cardiovascular disease. Our understanding of the complex mechanisms through which these associations arise, is still limited, so it may only be a proportion of adverse pregnancy outcomes which contribute to the associations observed. A combination of genetic predisposition, underlying physiology, and sharing of common risk factors may also be in play, so the future possibilities for life course prevention will rely on developing an improved understanding of the causal and disease pathways involved.
Health systems for NCDs in pregnancy
‘A health system designed to deliver longitudinal management of a chronic health condition is distinctly different from one designed for the management of serial acute episodes.’33
The urgent need for high-quality emergency obstetric care in LMIC have thus far favoured the development of acute care, and are designed to manage serial events occurring during pregnancy. The obstetric transition has profound implications for health systems; and services will need to be reorganised and made fit for the future. We cannot underestimate the complexity and resource requirements to meet the needs of women with NCDs, during pregnancy.
Individualised care that is linked across reproductive experiences and the lifespan is required. There needs to be sufficient priority given to preventive and early interventions, and to developing a seamless health system, with integrated services at community to primary and referral level, as well as across medical specialities, clinical and social care. Medical conditions affecting pregnant women will differ across settings, and between individuals, so a ‘one-size-fits-all’ solution will not necessarily be successful.29 In addition to core service provision, better understanding of social and cultural norms, life-style, diet and physical activity in LMIC will allow implementation of acceptable and effective interventions. Ascertaining the disease burden is essential, and in the case of maternal health, there will be a need to relook at how changes can be implemented, to document and track maternal morbidities and the proportion of indirect and late maternal deaths attributable to NCDs.26
Even industrialised countries have difficulties in providing services which are tailored to individuals, and not fragmented.29 In resource-poor settings, such requisites might seem arduous and problematic, as many health services have not even got on top of providing reliable, focused emergency obstetric care. The additional burden of NCDs must not be ignored however, and major changes will have to be thought through in the face of resource constraints. The health system ‘re-engineering,’7 required to prepare for the new challenges that lie ahead, will reap benefits not only for women and their offspring of today, but for the generations that will come.
Conclusion
What we know: Levels of maternal mortality have fallen globally over the last 25 years. Interventions for the reduction of maternal mortality have focused on use and access to maternity care – and this seems to have resulted in reducing deaths from direct obstetric causes. Less attention has been paid to the determinants of maternal mortality and morbidity related to NCDs in pregnancy that have close links to lifestyle, social, cultural and economic factors, and are proportionately increasingly represented in the maternal burden of disease.
What we need to do: The optimists amongst us will say that maternal mortality can be expected to continue decreasing with continued promotion of what we know works, i.e. by reducing communicable diseases, providing good quality maternity services, family planning and abortion care, all of which can improve maternal health and reduce both direct and indirect causes of maternal death. Yet, the changing trends and patterns in maternal ill-health and mortality mean that we have to prepare for an obstetric transition, where NCDs feature prominently. Too many health systems in LMIC are unprepared for the implications of the obstetric transition, and corrections need to be made to fragmented health services which focus on acute emergencies.
What we need to know: Improving how we monitor and assess the burden of NCDs in pregnancy will be a continuing task. Making a case for dealing with NCDs in pregnancy, and showing added value to mobilise interest and funds will require concepts and knowledge beyond a singular maternal focus toward a life-course perspective. Yet maternal health holds the key which could unlock the door, not only for the wellbeing of women today, but for future generations.
Acknowledgements
I acknowledge Professor Vincent De Brouwere who suggested I present on this topic at a meeting, which led to the conception of this article. Thanks also to Dr Lovney Kanguru for sharing her PhD thesis, from which some of the material for this article was drawn; and to Dr Tabassum Firoz who invited me to write this article.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Guarantor
JH confirms the accuracy of the manuscript in her role as sole author. She was responsible for conceiving the content of the article, gathering and analysing the data, and writing the manuscript.
References
- 1.World Health Organization. Trends in maternal mortality: 1990 to 2015. Estimates by WHO, UNICEF, UNFPA, the World Bank and the United Nations Population Division. World Health Organization Report, Geneva, Switzerland, 2015.
- 2.McCarthy J, Maine D. A framework for analyzing the determinants of maternal mortality. Stud Fam Plan 1992; 23: 23–33. [PubMed] [Google Scholar]
- 3.Say L, Chou D, Gemmil A, et al. Global causes of maternal death: A WHO systematic analysis. Lancet Global Health 2014; 2: e323–e333. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. Global status report on non-communicable diseases 2010. World Health Organization Report, Geneva, Switzerland, 2011.
- 5.World Bank. The growing danger of non-communicable diseases. Acting now to reverse the course. World Bank Report, Washington, USA, 2011.
- 6.World Health Organization. The WHO application of ICD-10 to deaths during pregnancy, childbirth and puerperium: ICD MM. World Health Organization Report, Geneva, Switzerland, 2012.
- 7.Kassebaum NJ, Bertozzi-Villa A, Coggeshall MS, et al. Global, regional, and national levels and causes of maternal mortality during 1990-2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014; 384: 980–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Battista MC, Hivert MF, Duval K, et al. Intergenerational cycle of obesity and diabetes: How can we reduce the burdens of these conditions on the health of future generations? Exp Diab Res 2011. Article ID 596060: 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kanguru L. Diabetes mellitus and hypertension in pregnancy in low and middle income countries, and a case study of the health system in Jamaica. PhD Thesis, University of Aberdeen, UK, 2015.
- 10.Kanguru L, Bezawada N, Hussein J, et al. The burden of diabetes mellitus during pregnancy in low- and middle-income countries: A systematic review. Global Health Action 2014; 7: 23987–23987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davilla H, Pena M, Matos Z. Clinical and epidemiological profile of diabetes mellitus in pregnancy, Isle of Youth, 2008. Medic Rev 2011; 13: 29–34. [DOI] [PubMed] [Google Scholar]
- 12.Wang Z, Kanguru L, Hussein J, et al. The incidence of adverse outcomes of gestational diabetes mellitus (GDM) in low and middle income countries: A systematic review. Int J Gynec Obstet 2013; 121: 14–29. [DOI] [PubMed] [Google Scholar]
- 13.Gaio P, Schmidt M, Duncan B, et al. Hypertensive disorders in pregnancy: Frequency and associated factors in a cohort of Brazilian women. Hypertens Preg 2001; 20: 269–281. [DOI] [PubMed] [Google Scholar]
- 14.Otto H, Saether SG, Banteyrga L, et al. High prevalence of subclinical rheumatic heart disease in pregnant women in a developing country: An echocardiographic study. Echocardiography 2011; 28: 1049–1053. [DOI] [PubMed] [Google Scholar]
- 15.Bhatla N, Lal S, Behera G, et al. Cardiac disease in pregnancy. Int J Gynec Obstet 2003; 82: 153–159. [DOI] [PubMed] [Google Scholar]
- 16.Sawhney H, Aggarwal N, Suri V, et al. Maternal and perinatal outcome in rheumatic heart disease. Int J Gynec Obstet 2003; 80: 9–14. [DOI] [PubMed] [Google Scholar]
- 17.Soma-Pillay P, MacDonald AP, Mathivha TM, et al. Cardiac disease in pregnancy-a four-year audit at Pretoria Academic Hospital (2002-2005). South Afr Med J 2008; 98: 553–556. [PubMed] [Google Scholar]
- 18.Campbell O, Gipsom R, Hakim AH, et al. National maternal mortality ratio in Egypt halved between 1992-93 and 2000. Bull World Health Organ 2005; 83: 462–471. [PMC free article] [PubMed] [Google Scholar]
- 19.Haththotuwa HR, Attygalle D, Jayatilleka AC, et al. Maternal mortality due to cardiac disease in Sri Lanka. Int J Gynec Obstet 2009; 104: 194–198. [DOI] [PubMed] [Google Scholar]
- 20.Modell B, Darlison M. Global epidemiology of haemoglobin disorders and derived service indicators. Bull World Health Organ 2008; 86: 480–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Louie KS, de Sanjose S, Diaz M, et al. Early age at first sexual intercourse and early pregnancy are risk factors for cervical cancer in developing countries. Br J Cancer 2009; 100: 1191–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.World Health Organization. Women’s health. Fact sheet No. 334, http://www.who.int/mediacentre/factsheets/fs334/en/ (2013, accessed 4 May 2016).
- 23.World Health Organization. Maternal mental health and child health and development in low and middle income countries. World Health Organization Report, Geneva, Switzerland, January–February 2008.
- 24.Finucane MM, Stevens GA, Cowan MJ, et al. National, regional, and global trends in body-mass index since 1980: Systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9·1 million participants. Lancet 2011; 377: 557–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ziraba AK, Fotso JC, Ochako R. Overweight and obesity in urban Africa: A problem of the rich or the poor? BMC Public Health 2009; 9: 465–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Firoz T, Chou D, von Dadelszen P. Measuring maternal health: Focus on maternal morbidity. Bull World Health Organ 2013; 91: 794–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Souza JP, Tuncalp O, Vogel JP, et al. Obstetric transition: The pathway towards ending preventable maternal deaths. BJOG 2014; 121: 1–4. [DOI] [PubMed] [Google Scholar]
- 28.Mishra G, Cooper R, Kuh D. A life course approach to reproductive health: Theory and methods. Maturitas 2010; 65: 92–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.RCOG. Why should we consider a life course approach to women’s health care? RCOG Scientific Impact paper no. 27, 2011.
- 30.Bohrer J, Ehrenthal D. Other adverse pregnancy outcomes and future chronic disease. Semin Perinat 2015; 39: 259–263. [DOI] [PubMed] [Google Scholar]
- 31.Hastie CE, Smith GC, Mackay DF, et al. Maternal risk of ischaemic heart disease following elective and spontaneous pre-term delivery: Retrospective cohort study of 750350 singleton pregnancies. Int J Epidemiol 2011; 40: 914–919. [DOI] [PubMed] [Google Scholar]
- 32.Mongraw-Chaffin ML, Cirillo PM, Cohn BA. Preeclampsia and cardiovascular disease death: Prospective evidence from the child health and development studies cohort. Hypertens 2010; 56: 166–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reidpath DD, Allotey P. The burden is great and the money little: Changing chronic disease management in low- and middle-income countries. J Global Health 2012; 2: 020301–020301. [DOI] [PMC free article] [PubMed] [Google Scholar]