Abstract
Background:
The effects of metformin (MET) and curcumin (CUR) single treatments have been tested against breast cancer; however, their combination has not been explored. Here, we evaluated the antitumor activity of MET and CUR combination against breast cancer in mice.
Materials and methods:
The antiproliferative activity of single and combined treatments against breast cancer cell lines was determined. Vascular endothelial growth factor (VEGF) and Trp53 expression was examined in EMT6/P cells. In vivo studies were carried out by inoculating BALB/c mice with EMT6/P cells and examining tumor growth and apoptosis induction in tumor sections. Furthermore, serum levels of different cytokines and transaminases and creatinine were measured to detect the immune response and toxicity, respectively.
Results:
The combination treatment exhibited the highest effects against tumor proliferation and growth. It significantly reduced VEGF expression, induced Trp53 independent apoptosis, triggered Th2 immune response and showed no toxicity.
Conclusion:
The combination can be a potential therapeutic option to treat breast cancer. However, further testing is needed to measure the exact serum levels of MET and CUR and to further explain the obtained results.
Keywords: breast cancer, combination therapy, curcumin, metformin
Introduction
Breast cancer is one of the most common malignant tumors worldwide.1 It is the second most common cause of cancer-related death among women.2 Although early diagnosis of the disease has become possible due to advanced detection techniques, the mortality among women suffering from breast cancer increased.3 Traditional disease treatments which include surgery, chemotherapy, radiotherapy and hormone therapies are not fully effective in treating breast cancer, especially in patients with an advanced stage of the disease.3,4 Therefore, recent studies have focused on identifying alternative agents that reduce the incidence of the disease and cure it. Synthetic drugs or natural products that are highly selective and less toxic and show anticancer activity can be potential agents in breast cancer treatment.3,5 Another possibility that can be more effective is the combination therapy using synthetic, natural or both types of compounds.
Metformin (N’,N’-dimethylbiguanide hydrochloride; MET) is an oral antidiabetic drug, commonly used to treat type 2 diabetes mellitus.6 It lowers the high insulin level in hyperinsulinemia associated with type 2 diabetes by inhibiting hepatic gluconeogenesis via AMP-activated protein kinase (AMPK) activation. Also, it increases the insulin sensitivity and plasma glucose utilization by skeletal muscles and adipose tissues resulting in decreased glucose and insulin blood levels.7,8 Recently, MET has emerged as a potential anticancer drug. It shows an indirect effect as the increase in the insulin sensitivity and decrease in hyperinsulinemia reduces tumor proliferation.9 The drug also shows direct effects against a wide range of cancers, especially breast cancer which has high incidence of occurrence in diabetic women.6 In vitro and in vivo studies found that MET exhibits antiproliferative activity against different breast cancer subtypes by primarily inhibiting target of rapamycin and as a consequence reducing mRNA translation, ribosome biogenesis and cell growth.10,11 MET also shows other antitumor activities against breast cancer such as the induction of apoptosis,12–14 enhancement of cellular senescence14 and inhibition of the inflammatory response necessary for cell transformation and cancer stem cell formation.15 Finally, MET appears to have a paradox effect on angiogenesis as it promotes or inhibits expression of the pro-angiogenic vascular endothelial growth factor (VEGF) depending on breast cancer subtype.16–18
Curcumin (1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione; CUR) is a natural product derived from the rhizome of the plant turmeric (Curcuma longa Linn).3 It is a common Indian spice used for centuries in food recipes and folk remedies. CUR possesses a wide range of biological activities such as antimicrobial, anti-inflammatory, antioxidant, antiviral, anti-angiogenic and antibiotic activities, in addition to its role in the treatment of neurodegenerative diseases such as Alzheimer’s.19 It also shows chemopreventive and chemotherapeutic effects against different types of cancer.20 CUR’s effects against breast cancer have been largely explored. It has been found that CUR inhibits cell proliferation in different breast cancer cell lines by inducing cell arrest and inactivating growth signaling pathways. Also, it induces apoptosis by repressing anti-apoptotic genes and promoting pro-apoptotic ones.2,19,21 Furthermore, CUR exhibits anti-invasive/antimetastatic effects by preventing reattachment of circulatory tumor cells, downregulating the expression of pro-inflammatory cytokines and matrix metalloproteases, suppressing mesenchymal–epithelial transition and inhibiting cancer-associated fibroblast activation.19,21 CUR prevents angiogenesis in certain breast cancer subtypes by abrogating VEGF expression.22,23
Although CUR and MET have been shown to exhibit antitumor activity, the combination of both may produce more effective treatment against breast cancer. To the best of the authors’ knowledge, this combination has not been tested. Therefore, in the present study, we took a step toward exploring the effect of MET and CUR combination in the treatment of breast cancer in mice.
Materials and methods
Ethical statement
Animal care and use were conducted according to standard ethical guidelines, and all of the experimental protocols were approved by the Research and Ethical Committee at the Faculty of Pharmacy—Applied Science University, Amman, Jordan.
Chemicals and culture media
The following culture media were purchased from Sigma-Aldrich (Missouri, USA): Dulbecco’s Modified Eagle’s Medium (DMEM), Minimum Essential Medium (MEM) and Roswell Park Memorial Institute (RPMI)-1640 Medium. CUR (99%) was purchased from ChromaDex (California, USA) and MET (99%) was bought from Wanbury Ltd (Navi Mumbai, India).
Selection of MET and CUR doses for in vitro and in vivo studies
In the in vitro part of our study MET concentrations of 10–180 mm were selected based on previous results that reported antiproliferative activity of MET at various concentrations from 8 mm24 to 100 mm.25 We took four additional concentrations of MET above 100 mm to cover the different sensitivities of cell lines toward MET. Previous studies showed antiproliferative activity of CUR at concentrations below 100 µm26 and up to 160 µm.27 However, these concentrations were tested in our laboratory in a pilot study and showed limited inhibitory effect. Accordingly, we designed our own concentrations range (150–400 µm) to cover the various sensitivities of our cell lines toward CUR. For in vivo study, the dose of 50 mg/kg/day of CUR was selected based on a previous study that proved the activity of this dose against multidrug-resistant cancer implanted in mice.28 A dose of 80 mg/kg/day of MET was selected based on previous literatures and findings. This concentration was proved to be effective against implanted tumors with appreciated safety profile.29
In vitro cell proliferation (MTT) assay
The antiproliferative activity of CUR, MET and their combinations were tested using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. EMT6/p (mouse breast) and MCF-7 and T47D (human breast) carcinoma cell lines, as well as Vero (monkey kidney) normal cell line were cultured in 96-well microplates (100 µl; 1.5 × 104 cells per well) in a medium containing 10% fetal bovine serum (FBS), 1% L-glutamine, 1% penicillin–streptomycin and 0.1% gentamycin. Cells were incubated for 24 h at 37°C in a 5% CO2 enriched atmosphere. After that, the cells were treated with different concentrations of MET (10–180 mm), CUR (150–400 µm) and combination of MET and CUR (2–150 mm, 50–120 µm) for 48 h. Then, MTT was added to the wells according to the manufacturer’s instructions (Sigma-Aldrich, Missouri, USA). The calculated IC50 represents the treatment concentration that showed a lethal effect on 50% of cells. Cells treated with vincristine sulfate (an alkaloid from Madagascar periwinkle) were used as a positive control and those incubated with culture medium alone were used as a negative control. Vincristine sulfate was used as a positive control because it is a plant derived natural product and both agents (MET and CUR) used in this study were originated from plants.
Calculation of CI
The combined activity of MET and CUR was determined by calculating the combination index (CI) for both compounds in the four used cell lines (EMT6/P, MCF-7, T47D and Vero) using the following equation30: CI = (D1/DX1) + (D2/DX2) + α [(D1*D2)/(DX1*DX2)], where Dx1 = dose of drug 1 to produce 50% cell kill alone, D1 = dose of drug 1 to produce 50% cell kill in combination with D2, Dx2 = dose of drug 2 to produce 50% cell kill alone, D2 = dose of drug 2 to produce 50% cell kill in combination with D1, α = 0 for mutually exclusive or 1 for mutually nonexclusive modes of drug action. The results are interpreted as: CI > 1.3 antagonism; CI = 1.1–1.3 moderate antagonism; CI = 0.9–1.1 additive effect; CI = 0.8–0.9 slight synergism; CI = 0.6–0.8 moderate synergism; CI = 0.4–0.6 synergism; CI = 0.2–0.4 strong synergism.
Determination of VEGF expression in EMT6/P cells
EMT6/P cells were suspended at a concentration of 1.5 × 106 cell/10 ml MEM and incubated for 24 h in four different tissue culture flasks. Tissue culture media were removed and cultured cells were subjected to the following treatments: 8 mm MET, 110 μm CUR, combination of 8 mm MET and 110 μm CUR and a blank MEM medium as a negative control. These concentrations represent two folds of IC50 values for MET and CUR in the combination treatment. The treated cultured cells were incubated for 48 h. After that, the medium in each flask was removed, and the cells were harvested using trypsin-EDTA solution, washed with phosphate buffered saline (PBS), and then centrifuged at 1500 RPM for 10 min. VEGF expression in cancer cells was measured using mouse VEGF enzyme-linked immunosorbent assay (ELISA) kit (catalogue # RAB0510; Sigma-Aldrich, Missouri, USA) according to the manufacturer’s instructions.
Experimental animals
A total of 40 female BALB/c mice were used in this study (4–6 weeks old, 23–25 g body weight). Separate cages with wooden shaving were used to keep mice. The environmental parameters in the animal room were: 50–60% humidity, 25°C temperature and continuous ventilation.
Tumor inoculation
EMT6/P mouse breast carcinoma cells were purchased from Public Health England (catalogue # 96042344; Salisbury, UK). Cells were maintained using MEM and left to grow for 48 h. After that, cells were harvested using trypsin-EDTA solution, centrifuged, washed and tested for viability using trypan blue exclusion method. Viable cells were re-suspended in PBS at density of 1 × 106 cells/ml. Mice were injected subcutaneously in the abdominal area with 1 × 105 cells suspended in 100 µl PBS.
Antitumor activity
After 14 days of inoculation, mice were distributed into four groups (n = 10 for each group). Group 1 received intraperitoneally 100 µl of MET (80 mg/kg) once a day.31 Group 2 received orally by gavage 100 µl of CUR (50 mg/kg) dissolved in olive oil once a day.32 Group 3 received a combination of MET and CUR with doses and administration methods similar to those introduced in single treatments. Group 4 served as a negative control and received a vehicle once a day by intraperitoneal injection of 100 µl PBS and oral gavage of 100 µl olive oil. All groups received their treatment for 14 days. The tumor size was measured according to the following equation: length × width2 × 0.5.33 At the end of the treatment period (day 14), mice were sacrificed (by cervical dislocation) and their tumors were isolated and kept in 10% buffered formalin. The percentage of tumor size was measured according to the following equation: [(final tumor size – initial tumor size)/(initial tumor size)]*100%.34
Histological examination of tumor sections
Formalin-fixed sample were gradually dehydrated in a series of ethanol concentrations, and then cleared in xylene. Following that, samples were infiltrated in wax and cut into 5 μm paraffin sections using a rotary microtome (Reichert, Nussloch, Germany). Standard hematoxylin and eosin (H&E) procedure was used to stain different sections, and images of the slides were visualized using light microscope equipped with computer-controlled digital camera.
Apoptosis detection in tumor sections and cultured EMT6/P cells
Apoptosis was detected in tumor sections using a terminal deoxynucleotidyl transferase (TdT)-mediated 16-deoxyuridine triphosphate (dUTP) Nick-End Labeling (TUNEL) kit (Promega, Wisconsin, USA). Xylene was used to de-paraffin tumor sections. Sections were rehydrated with gradual series of ethanol concentrations, and then washed several times with PBS. Following that, they were fixed with (4%) paraformaldehyde, washed with PBS and permeabilized with 20 μg/ml proteinase K solution. Fragmented DNA was labeled by incubating the sections with biotinylated dUTP in rTdT reaction mixture at 37°C for 1 h. Endogenous peroxidases were blocked using 0.3% hydrogen peroxide. After that, sections were incubated with streptavidin conjugated horse radish peroxide (HRP) at room temperature for 30 min, and fragmented DNA was visualized using hydrogen peroxide followed by the chromagen diaminobenzidine. The same procedure was followed to detect apoptosis in cultured EMT6/P cells after treatment with MET, CUR, and combination.
RNA extraction, cDNA synthesis and gene expression analysis
EMT6/P cells at a concentration of 1.5 × 106 cell/10 ml MEM were cultured in four different tissue culture flasks for 24 h. After that, the media were removed and cultured cells were subjected to the following treatment for 48 h at 37°C: 8 mm MET, 110 μm CUR, combination of 8 mm MET and 110 μm CUR and a blank medium as a control. The media were removed and cells were harvested using trypsin-EDTA solution. The harvested cells were washed with PBS and centrifuged at 1500 RPM for 10 min at 4°C. Following that, total RNA was extracted from treated and untreated control cells using 0.75 ml of TRIzol reagent (Invitrogen, California, USA) per 0.25 ml (5–10 × 106) of EMT6/P cells according to the manufacturer’s protocol. The RNA extracts were reverse-transcribed to cDNA using power cDNA synthesis kit (iNtRON Biotechnology, Inc., Gyeonggi-do, South Korea). Briefly, in each treatment, 1 µg of total RNA extract was used and the sample volume was completed to 9.5 µl using RNase free water. Then, 1 µl of Oligo (dT) was added to each RNA sample and the samples were heated at 75°C for 5 min. After that, they were placed on ice for at least 1 min. While on ice, 1 µl of RNase inhibitor, 4 µl of 5x RT buffer, 2 µl of dNTP, 2 µl of DTT and 0.5 µl of AMV RT enzyme were added to each tube and mixed gently. The samples were incubated at 42°C for 60 min, and then heated at 70ºC for 5 min to terminate the reaction. The synthesized cDNA was used to determine the expression level of Trp53 gene in treated and control cells using semiquantitative reverse transcriptase polymerase chain reaction (RT-PCR). The expression of β-actin gene was used as an internal control to normalize for initial variations in sample concentration. The PCR was carried out using KapaTaq Ready Mix DNA polymerase (Kapabiosystems, Massachusetts, USA) and gene specific primers. The following primers were designed according to a previous work35 and were used at a final concentration of 0.4 µm: p53 forward, 5′- CTGAGGTTGGCTCTGACTGTACCACCATCC-3′; p53 reverse, 5′-CTCATT CAGCTCTCGGAACATCTCGAAGCG-3′; β-actin forward, 5′-ACGGGGTCACCCA CACTGTGC-3′; and β-actin reverse, 5′-CTAGAA GCATTTGCGGTGGACGATG-3′. The PCR conditions used were: 94°C for 3 min, 35 cycles of 94°C for 45 s, 55°C (Trp53) or 57°C (β-actin) for 45 s and 72°C for 1 min, and a final extension step at 72°C for 5 min. The PCR products were visualized using 1.5% agarose gel stained with ethidium bromide. Gene expression analysis was performed using AlphaView Software in AlphaImager Mini Gel Documentation System (Proteinsimple, California, USA). In the negative control, the template cDNA was replaced by RNase-free water.
Detection of IFN-γ, IL-2, IL-4 and IL-10 serum levels
Serum samples were collected from fresh blood. Levels of interferon-γ (IFN-γ), interleukin-2 (IL-2), IL-4 and IL-10 were measured using Th1/Th2 ELISA kit (catalogue # 88-7711-44; affymetrix eBioscience, California, USA) following the procedure in the catalogue.
Assessment of liver functions in mice
Serum levels of both alanine transaminase (ALT) and aspartate transaminase (AST) were quantitatively measured according to a previously described method,36 using commercially available kits (BioSystems, Barcelona, Spain).
Assessment of kidney functions in mice
Serum level of creatinine was measured to assess any relative nephrotoxicity using creatinine detection kit (catalogue # C130613; Acromex, Amman, Jordan) following the manufacturer’s procedure.
Statistical analysis
Data were analyzed using IBM SPSS statistics version 21 (SPSS Inc., Chicago, Illinois, USA). They were presented as mean ± SEM (standard error of mean). One-way analysis of variance (ANOVA) followed by post hoc test was used to measure variations between different groups in VEGF and Trp53 expression analysis as well as cytokines, AST, ALT and creatinine determination. Paired-samples t test was used to examine the effects of different treatments on the tumor size and boy weight. Nonlinear regression analysis was applied to calculate the IC50 values of different treatments in different cell lines. The level of significance was set at p < 0.05.
Results
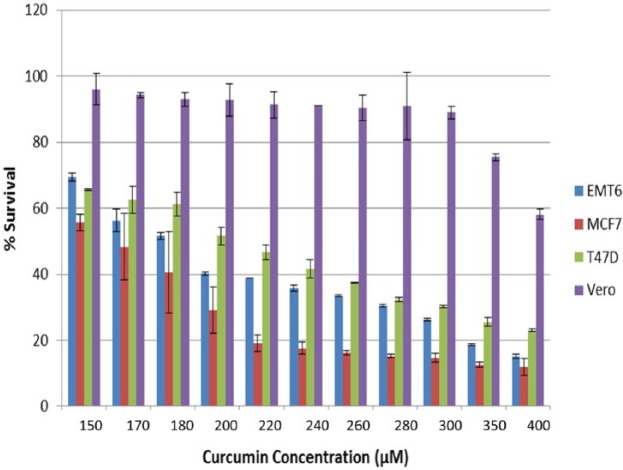
The antiproliferative activity of MET, CUR and their combination against EMT6/P, MCF7, T47D and Vero cell lines were tested in vitro using the MTT assay. A dose-dependent inhibition was observed in all cell lines after treatment with MET or CUR (Figures 1 and 2). The mouse cell line (EMT6) showed the highest sensitivity to MET treatment with >80% inhibition at MET concentration of 80 mm. Other cell lines exhibited various degrees of inhibition with increasing MET concentration with the highest degree of resistance observed in Vero cell lines followed by MCF7 and T47D, respectively (Figure 1). Different results were obtained after treatment of cell lines with increasing concentrations of CUR. The highest sensitivity was observed for MCF7 followed by EMT6, T47D and Vero cell lines, respectively (Figure 2). The IC50 for each treatment was calculated and compared with vincristine sulfate treatment. MET showed the highest antiproliferative effect against EMT6/P cells with IC50 value of 9 mm, while CUR was more effective against MCF7 with IC50 value of 155 µm. Moreover, the combination of MET and CUR caused a drastic reduction in IC50 values of MET and CUR in all cell types compared with the single treatments. The highest reduction was observed against EMT6/P cells with IC50 values of 4 mm and 55 µm for MET and CUR, respectively (Table 1). The calculated CI of different treatments revealed slight and moderate synergistic effect against EMT6/P and T47D cell lines, respectively.
Figure 1.
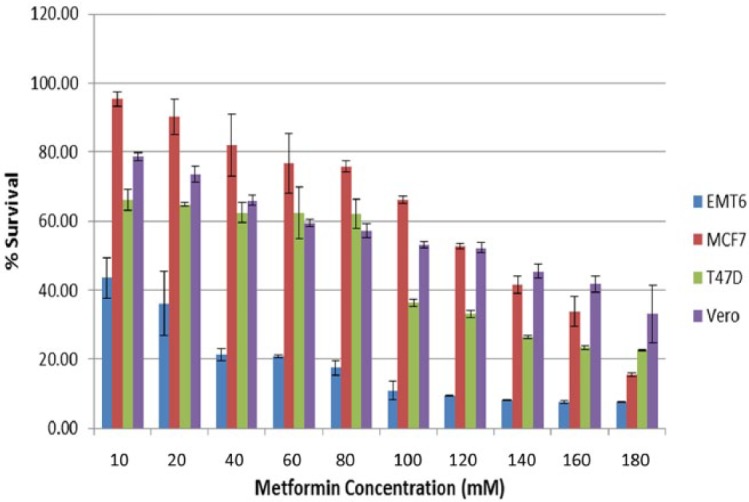
Effect of increasing concentrations of metformin (MET) on the viability of different cells lines (EMT6, MCF7, T47D and Vero).
Figure 2.
Effect of increasing concentrations of curcumin (CUR) on the viability of different cells lines (EMT6/P, MCF7, T47D and Vero).
Table 1.
The calculated IC50 and CI of MET, CUR, a combination of both and vincristine sulfate in EMT6/P, MCF7, T47D and Vero cell lines.
| Cell line | MET (mm) | CUR (µm) |
MET in combination (mm) | CUR in combination (µm) | Vincristine sulfate (µm) | CI | Interpretation |
|---|---|---|---|---|---|---|---|
| EMT6/P | 9 ± 5.533 | 180 ± 0.568 | 4 ± 1.198 | 55 ± 1.198 | 56 | 0.884 | Slight synergism |
| MCF7 | 130 ± 0.736 | 155 ± 10.061 | 22.5 ± 1.595 | 108 ± 1.595 | 15 | 1.069 | Additive effect |
| T47D | 90 ± 1.010 | 210 ± 2.132 | 13 ± 2.967 | 115 ± 2.967 | 14.11 | 0.7661 | Moderate synergism |
| Vero | 124 ± 1.448 | 416 ± 1.629 | 117.5 ± 0.543 | 66 ± 0.543 | 45.08 | 1.255 | Moderate antagonism |
Data are expressed as the mean ± SEM of three independent experiments, each performed in duplicate.
CI, combination index; CUR, curcumin; MET, metformin; SEM, standard error of the mean.
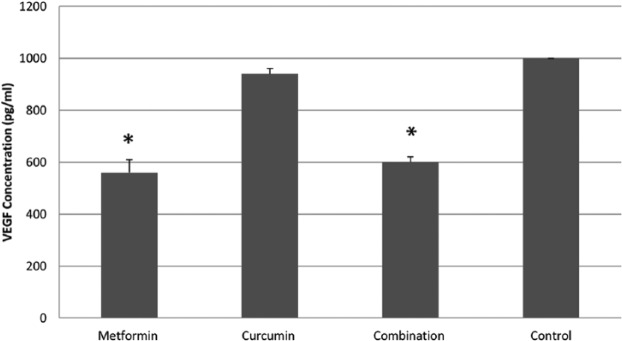
To test whether or not the inhibition of angiogenesis has a role in the observed antitumor activity, the expression of VEGF was measured in vitro for each treatment (Figure 3). Significant decrease of VEGF expression was observed in the cells treated with 8 mm MET (560 pg/ml) and those treated with a combination of 110 μm CUR and 8 mm MET (600 pg/ml) in comparison with untreated control cells (1000 pg/ml). Although the level of VEGF expression was reduced in cells treated with 110 μm CUR (960 pg/ml), this reduction was insignificant.
Figure 3.
The effect of different treatments on the expression of vascular endothelial growth factor (VEGF). Concentration of VEGF (pg/ml) in cells treated with 8 mm metformin (MET), 110 µm curcumin (CUR) and a combination of both, as well as in untreated control cells. Each treatment was performed in duplicate. Results are expressed as means (bars) ± SEM (lines). The asterisks represent significant values.
To examine the effect of each treatment in vivo, EMT6/P cells were inoculated in mice to induce tumor formation. Then, mice were treated with 80 mg/kg MET, 50 mg/kg CUR, combination of 80 mg/kg MET and 50 mg/kg CUR or treated with a vehicle as a control (Table 2). Although treatment with MET caused 15.83% increase in the tumor size, this percentage was statistically insignificant and far below that of the control group (201.82%). Treatment with CUR caused a −69.36% reduction in tumor size and it was statistically significant. However, treatment with MET and CUR combination showed the highest significant reduction in the tumor size (−98.59%). Moreover, the percentage change in body weight of mice at different treatments was measured (Table 2). The body weight reduced significantly in CUR (−6.49%) or combination of MET and CUR (−6.02%) treated groups. However, it was insignificantly reduced in MET treated group (−3.12%) and in the control (−3.77%).
Table 2.
Effect of 80 mg/kg MET, 50 mg/kg CUR and a combination of both on tumor size and body weight.
| Treatment | Initial tumor size (mm3) | Final tumor size (mm3) | % change in tumor size | p value | % change in body weight | p value | % of cured mice |
|---|---|---|---|---|---|---|---|
| MET | 364.97 ± 123.63 | 422.74 ± 250.11 | 15.83 | 0.728 | −3.119 | 0.121 | 60 |
| CUR | 227.67 ± 48.38 | 69.76 ± 49.81 | −69.36 | 0.037 | −6.493 | 0.001 | 60 |
| MET + CUR | 168.29 ± 55.52 | 2.37 ± 2.38 | −98.59 | 0.023 | −6.020 | 0.002 | 80 |
| Control | 460.693 ± 83.65 | 1390.466 ± 1103.74 | 201.821 | − | −3.772 | − | 0 |
Ten mice were used in each group (n = 10).
CUR, curcumin; MET, metformin.
For better understanding of the effects of different treatments on tumor histology, tumors of similar sizes from all different animal groups were subjected to hematoxylin and eosin staining (Figure 4). Necrotic areas were observed in 80 mg/kg MET (Figure 4A) and 50 mg/kg CUR (Figure 4B) treated groups, while no necrosis was observed in the vehicle treated group (Figure 4D). However, combination of MET and CUR resulted in larger necrotic areas (Figure 4C) compared with those observed in tumor sections from single treatments (Figure 4A and B).
Figure 4.
Hematoxylin and eosin staining of tumors from different treatments. Tumors from animals treated once a day with 80 mg/kg metformin (MET) (A), 50 mg/kg curcumin (CUR) (B), combination of both (C) and a vehicle (D). N, necrotic area. Extensive necrosis was evident in tumors treated with a combination of MET and CUR (C). Four mice were used in each treatment.
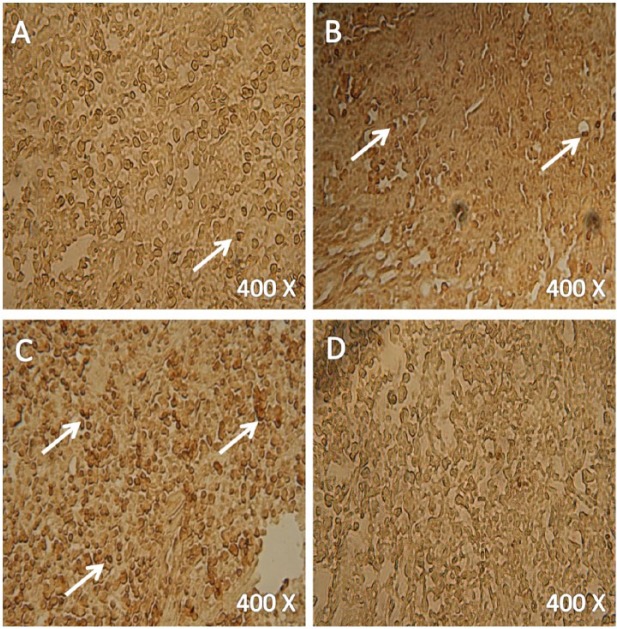
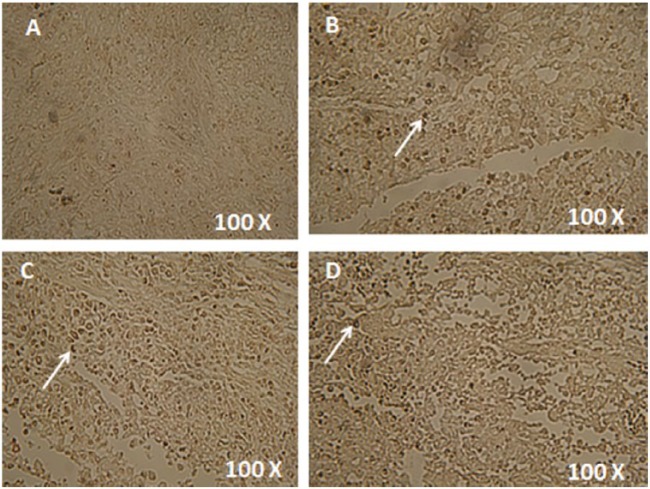
In order to evaluate the ability of different treatments to induce apoptosis, TUNEL colorimetric assay was used to stain tumor sections and cultured cells from different treatments (Figures 5 and 6). This assay detects fragmented DNA in cells undergoing apoptosis. The level of apoptosis increased in the cells from 80 mg/kg MET treated animals (Figure 5A) compared with those in vehicle treated group (Figure 5D). The level became even higher in the cells from 50 mg/kg CUR treated animals (Figure 5B). The highest level of apoptosis was shown in the cells from animals treated with a combination of MET and CUR (Figure 5C). Similar results were observed in vitro where the highest apoptosis rate were detected in cells treated with the combination therapy (Figure 6).
Figure 5.
Colorimetric TUNEL assay to detect the induction of apoptosis in tumor sections from different treatments. Tumors from animals treated once a day with 80 mg/kg metformin (MET) (A), 50 mg/kg curcumin (CUR) (B), combination of both (C) and a vehicle (D). Apoptotic nuclei are stained dark brown. Four mice were examined in each treatment.
Figure 6.
Colorimetric TUNEL assay to detect the induction of apoptosis in cultured EMT6/P cells after different treatments. Cancer cells treated for 48 h with 10 mm metformin (MET) (A), 180 µm curcumin (CUR) (B), combination of both (C) and a vehicle (D). Apoptotic nuclei are stained dark brown. Four mice were examined in each treatment.
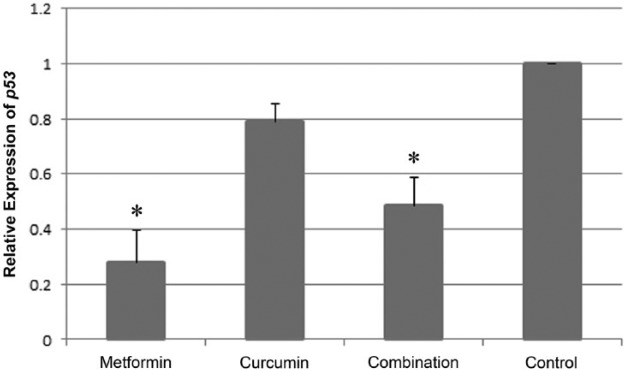
To uncover whether or not the apoptosis observed using TUNEL assay was due to upregulation in expression of the tumor suppressor gene Trp53 RT-PCR was carried out. Figure 7 shows the expression patterns of Trp53 and the internal control β-actin in EMT6/P cells treated with 8 mm MET, 110 µm CUR, combination of both and in the untreated control cells. In each treatment, the expression of Trp53 relative to that in control cells was normalized to corresponding expression of β-actin relative to that in the control (Figure 7). The expression of Trp53 in MET and combination of MET and CUR treated cells was significantly reduced compared to that in the control (Figure 8). Trp53 expression was also reduced in CUR treated cells, however, this reduction was statistically insignificant (Figure 8).
Figure 7.
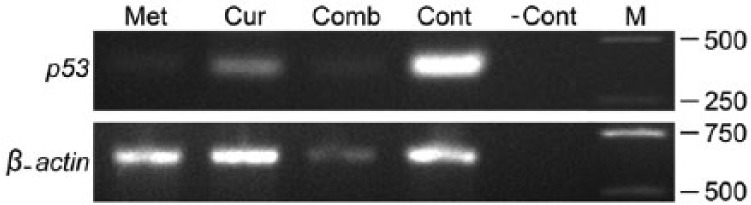
The expression of Trp53 and β-actin genes from cells under different treatments. Electrophoretic bands represent the expression of Trp53 (371 bp; upper row) and β-actin (659 bp; lower row) in cells treated with 8 mm metformin (MET), 110 µm curcumin (CUR), a combination of both (Comb), untreated control cells (Cont) and RT-PCR negative control (-Cont) which includes RNase free water instead of cDNA. M represents DNA markers with molecular weights in base pairs (bp) shown at the right.
Figure 8.
Relative expression of Trp53 from cells under different treatments. The mean of Trp53 relative expression normalized to the corresponding β-actin relative expression in cells treated with 8 mm metformin (MET), 110 µm curcumin (CUR) and a combination of both, as well as in untreated control cells. In each treatment, five independent experiments were performed and each experiment was done in duplicate. Results are expressed as means (bars) ± SEM (lines). The asterisks represent significant values.
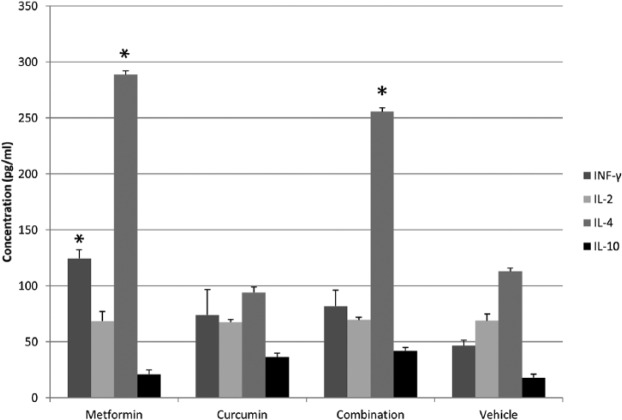
To explore the effect of different treatments on the immune response of animals, the serum levels of IFN-γ, IL-2, IL-4 and IL-10 were detected (Figure 9). Although the level of IFN-γ increased in 80 mg/kg MET (124.50 pg/ml), 50 mg/kg CUR (74.0 pg/ml) and combination of MET and CUR (82.0 pg/ml) treated animals compared to that in the control (46.50 pg/ml), this increase was only significant in MET treated group. The level of IL-2 was almost stable and did not change significantly across the different treatments compared with the control. In contrast, the level of IL-4 increased significantly in the MET (288.50 pg/ml) and combination of MET and CUR (255.5 pg/ml) treated animals compared to the control (113 pg/ml), and it was insignificantly reduced in the CUR treated group (94 pg/ml). IL-10 level did not significantly change among different treatments relative to the control.
Figure 9.
The effect of different treatments on the immune response of animals. The measured serum levels (pg/ml) of interferon-γ (IFN-γ), interleukin-2 (IL-2), IL-4 and IL-10 from animals treated once a day with 80 mg/kg metformin (MET), 50 mg/kg curcumin (CUR), combination of both and a vehicle (control). Each treatment was performed in duplicate. Results are expressed as means (bars) ± SEM (lines). The asterisks represent significant values.
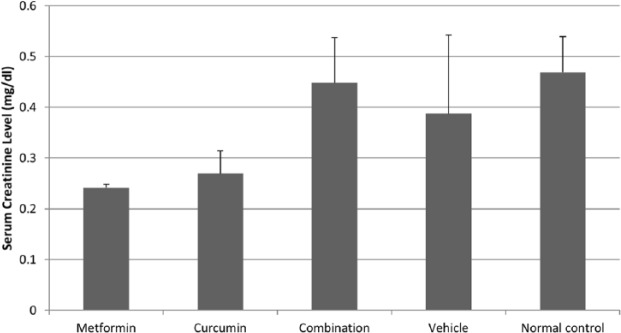
The potential of developing toxicity associated with different treatments was investigated by measuring the serum levels of AST and ALT liver enzymes as well as the creatinine kidney enzyme. No significant change in the AST level was observed across vehicle treated animals (58.33 IU/l), healthy normal animals (62.77 IU/l) and animals treated with a combination of 80 mg/kg MET and 50 mg/kg CUR (65.55 IU/l) (Figure 10). Although the AST level in MET (41.66 IU/l) and CUR (42.22 IU/l) treated groups was lower than that in the normal group, the reduction in the reading was statistically insignificant (Figure 10). The ALT level in the combination treated group (30 IU/l) was higher than in the normal group (17.36 IU/l), however, this level rise was not statistically significant (Figure 10). Moreover, in all other treated groups no significant changes in ALT levels were detected (Figure 10). Finally, in all different treatments, the creatinine levels did not significantly change compared with those in the normal group (Figure 11). Therefore, AST, ALT and creatinine levels were considered to be normal compared to their levels in the normal group.
Figure 10.
The effect of different treatments on serum levels of aspartate transaminase (AST) and alanine transaminase (ALT). The measured levels (IU/l) of AST and ALT in animals treated once a day with 80 mg/kg metformin (MET), 50 mg/kg curcumin (CUR), combination of both and a vehicle, as well as in normal control animals. Each experiment was performed in duplicate. Results are expressed as means (bars) ± SEM (lines).
Figure 11.
The effect of different treatments on serum levels of creatinine. The measured level (mg/dl) of creatinine in animals treated once a day with 80 mg/kg metformin (MET), 50 mg/kg curcumin (CUR), combination of both and a vehicle, as well as in normal control animals. Each experiment was performed duplicate. Results were expressed as means (bars) ± SEM (lines).
Discussion
In the present study, the antitumor activity of MET and CUR combination was evaluated against mice breast cancer cells. In vitro and in vivo studies showed high ability of the combination to reduce tumor proliferation and growth, inhibit angiogenesis, induce apoptosis and trigger Th2 immune response.
We found that CUR and MET single treatments exhibited antiproliferative effect against cultured mouse (EMT6/P) and human (MCF-7 and T47D) breast cancer cell lines in a dose-dependent manner. These results are consistent with those from previous studies which demonstrated the inhibitory effect of MET9,37–39 and CUR40–42 on the proliferation of several breast cancer cell lines. MET exhibited its highest antiproliferative activity against EMT6/P cells while CUR was more active against MCF7 cells. Such difference may indicate that these two agents have different targets that are expressed at different levels on various cell lines. The combination of MET and CUR reduced dramatically cell proliferation in all cell types compared with the single treatments. The highest antiproliferative activity was against EMT6/P cells. However, we noticed that synergism was observed in only two cell lines: this difference could be due to tissue-specific response where different cell lines exhibit different sensitivity toward MET and CUR combination.
Inhibition of angiogenesis prevents tumor proliferation and expansion. In our study we used VEGF expression as a marker to test whether or not the different treatments exerted anti-angiogenic activity against EMT6/P cells. Although MET and CUR single treatments caused reduction in the VEGF expression compared with the control, the reduction was only significant in MET treatment. However, the inhibitory effect of CUR was augmented when combined with MET. The combination significantly reduced the expression of VEGF. Previous study showed that CUR had different effects on two human breast cancer cell lines. It markedly reduced VEGF transcript level in triple-negative MDA-MB-231 cells but had little effect on MCF-7 cells.22 Another study demonstrated the inhibitory effect of CUR on VEGF expression in 4T1 mouse mammary tumor cell line.23 These results suggest that the effect of CUR can vary depending on breast cancer subtype. This may explain the slight effect of CUR single treatment on VEGF expression in EMT6/P cells used in our experiments. The reduction in VEGF expression under MET single treatment is in agreement with previous findings which elucidated its inhibitory action against VEGF expression in triple-negative MDA-MB-453 cells.18 In MCF-7 cells, one study reported an increase in VEGF expression after MET treatment.16 By contrast; another study showed a decrease in VEGF expression even though MET had a paradox effect on angiogenesis. It caused general decrease of both anti-angiogenic and pro-angiogenic proteins.17
We used mice bearing EMT6/P tumor cells to examine the effect of each treatment in vivo. Variable tumor sizes within each group were observed at the end of the study in all groups as indicated by high standard deviation values. Such difference is mainly due to the difference in response toward each treatment. Although all mice were inoculated using the same concentration of EMT6/P cells, not all inoculated cells were able to attach and start tumor colonies. A few millimeters increase in tumor size means millions of dividing cancer cells that makes therapy less effective. We found that MET treatment did not reduce tumor size; in contrast, the treatment caused a slight nonsignificant increase in the size. Several studies showed that the effect of MET against implanted breast tumors can vary according to the administrative route, duration of treatment or dosage concentration. For example, in a previous study, MET at a concentration of 2 mg/ml was provided in the animals’ drinking water either on day 8 following triple-negative MDA-MB-231 tumor inoculation or 1 week before inoculation.43 The mice in both groups were sacrificed when the tumor size reached 2 cm or at 90 days (first group) and 103 days (second group) post implantation. In both groups MET significantly reduced tumor growth. In another study, mice were inoculated with triple-negative MDA-MB-231 cells.44 Then, MET was orally administered by diluting it in drinking water at a concentration of 3 mg/ml. The drug remarkably reduced tumor size in the group under prolonged treatment (1 month); however, it showed no inhibitory effect against the group under pulse treatment (48 h). In a different study, MET (20 mg/kg) was injected locally for 15 consecutive days into mice bearing MCF-7 cells.45 No inhibition of tumor progression was observed as tumor volume did not change between the control and MET treated groups. Our results are consistent with the last study. We also administered the drug by injection and the treatment similarly lasted for 2 weeks. One difference, however, between our experiment and their experiment was the dose concentration which was higher (80 mg/kg) in our case; nonetheless, no inhibition of tumor growth was detected. In addition, the concentration of MET (80 mg/kg) used in our study is extremely lower than concentrations given orally to control blood glucose (350 mg/kg). This difference is due to the difference in administration route where intraperitoneal injection proved to be more effective compared with the oral route46 and a recent study showed that a concentration of 50 mg/kg was effective in lowering blood glucose if injected intraperitoneally.47 We also examined the effect of CUR against tumor growth in vivo, and we found that it significantly reduced the tumor size. Our findings are in agreement with previous studies which reported the ability of CUR to reduce tumor size in human41,48 or mice23,49 breast cancer cells. Although <1% of oral CUR actually enter the plasma,50 most studies depend on oral administration of CUR to treat different diseases.51 In our study we selected oral route to deliver CUR based on the promising results obtained in previous studies and the fact that CUR is a major component of widely consumed spices used to prepare different foods. Compared with single treatments, the combination treatment showed the strongest effect against tumor growth. We observed drastic decrease in tumors size which almost disappeared in the treated animals. The significant reduction in tumor size was correlated to significant reduction in body weight. This might be a consequence of losing large mass of tumor growth and was not due to side effects resulted from the administration of combination treatment. Indeed, 80% of the animals were cured at the end of the experiment.
We performed subsequent histological analysis to further explore the effect of each treatment against implanted tumors. We found that MET and CUR single treatments were able to promote the formation of large necrotic areas and to induce apoptosis. Our findings are consistent with previous findings that reported the effect of MET and CUR against breast cancer and other cancers. MET was shown to enhance necrosis in tumor sections from mice bearing breast tumors treated with the drug for long45 or short period of time.44 Also, it was able to induce apoptosis in pancreatic cancer cells52 and adrenocortical carcinoma cells implanted in mice.53 Similarly, CUR was reported to enhance the formation of large necrotic areas in nasopharyngeal carcinoma sections54 and to induce apoptosis in LNCaP prostate cancer cells55 and uterine leiomyosarcoma cells56 inoculated in mice. In our study, although MET and CUR single treatments showed obvious effect against breast tumors, the effect of their combination was even larger. The necrotic areas extended and more cells underwent apoptosis following the combination treatment.
There are two well-characterized pathways for apoptosis activation: the extrinsic pathway initiates by cell surface death receptors, and the intrinsic pathway operates through changes in mitochondrial integrity.57 The pro-apoptotic protein p53 acts as a transcription factor that enhances or represses transcription of several genes involved in the two apoptotic pathways.58 In our study, we examined the expression of Trp53 to determine whether apoptosis induced in the three different treatments is due to upregulation in Trp53 expression relative to that in the control. We found that none of the different treatments triggered Trp53 upregulation. Therefore, apoptosis appears to be induced by a Trp53-independent mechanism. Our results are inconsistent with those which showed the ability of MET to induce Trp53-dependent apoptosis in MDA-MB-231 and MCF-7 breast cancer cells39 and those reported the ability of CUR to activate Trp53-dependent apoptosis in MDA-MB-231 cells.59 One possible explanation is the use of different breast cancer cell line (EMT6/P) in our study. On the other hand, our results are in agreement with previous findings that showed the ability of CUR to induce apoptosis in the ovarian carcinoma,60 melanoma61 and colon cancer cells62 in a Trp53-independent manner. CUR was also reported to cause the degradation of p53 protein and to inhibit p53-induced apoptosis in normal thymocytes and myeloid leukemia cells.63 Moreover, MET was found to enhance efficacy of the anthracycline derivative WP 631 in promoting apoptosis in hepatocarcinoma cells without increasing Trp53 expression level.64
The immune system did not exhibit significant response in the CUR treated animals. In contrast, in MET-treated animals, the serum levels of both IFN-γ and IL-4 cytokines were significantly increased compared with those in vehicle-treated animals. Originally, Th1/Th2 model postulated that IFN-γ is selectively produced by T helper1 (Th1) cells which express the master regulator T-bet, whereas IL-4 is selectively made by T helper2 (Th2) cells which express Gata-3.65–67 T helper (Th1 or Th2) cells were also viewed as having the ability to reinforce the production of their own signature cytokines but repress the expression of other cell cytokines.68 However, several studies showed that Th1, Th2 and other cell lineages (T helper17, Th17; T regulatory, Treg; T follicular helper, Tfh) originate from CD4+ T cells are plastic and can change their phenotypes.69 One example is the reprogramming of Th2 cells that caused coproduction of IL-4 and IFN-γ upon viral infection.68 The phenotypic plasticity of T helper cells may explain the rise in both IFN-γ and IL-4 levels following MET administration in our study. IFN-γ was found to play an important regulatory role in the antitumor response against pancreatic cancer cells.70 It was also reported to interact synergistically with IL-4 to induce an immune action. For example, both IFN-γ and IL-4 cooperate to activate murine macrophages against Leishmania major amastigotes.71 They also synergize to enhance MHC class II expression on the surface of melanoma and breast cancer cells.72 Furthermore, IL-4 was shown not to inhibit IFN-γ-induced activation of human colostral macrophages.73 Similarly, IFN-γ alone or in combination with IL-4 might elicit an antitumor immune response against breast cancer cells after MET treatment in our experiments. Furthermore, previous study showed that treatment of mice bearing RLmale1 leukemia cells with MET increased the number of CD8+ tumor-infiltrating lymphocytes (TILs).74 These TILs were capable of producing IFN-γ, IFN-α and IL-2 which were responsible for tumor rejection. Although we found almost stable level of IL-2, the rise in IFN-γ level is in agreement with the previous study74 that also reported a simultaneous increase in the number of CD4+ cells. Likewise, CD8+ TILs might have been activated in our study. Finally, the combination of MET and CUR resulted in a significant increase in IL-4 level without any significant change in IFN-γ, IL-2 and IL-10 levels. This suggests the trigger of Th2 immune response under combination treatment. Previous work demonstrated the ability of IL-4 to inhibit growth72,75–77 and induce apoptosis77 in human breast cancer cells. IL-4 also showed antiproliferative effect against other tumor cells including renal carcinoma78 lung carcinoma79 and gastric carcinoma.80
To evaluate the safety of using MET, CUR and their combination, we measured the serum levels of AST, ALT and creatinine. We found that none of the different treatments significantly changed the levels of these enzymes compared with their levels in normal controls. Therefore, our results suggest that neither single treatments nor combined one had toxicity side effects on liver and kidney functions. Previous work also showed the safe use of MET and CUR in cancer treatment. In one study, MET was used to treat therapy-induced hyperglycemia in children with acute lymphoblastic leukemia, and it did not cause significant increase in AST and creatinine levels.81 In another work, CUR was administered to rats bearing diethylnitrosamine-induced liver tumor and it caused significant reduction in the high levels of ALT and AST enzymes82 or partially normalized them.83 Furthermore, CUR was used to treat glioblastoma and it did not significantly change the levels of transaminases and creatinine.84
In conclusion, compared with single treatments, the combination of MET and CUR showed the highest effect against cell proliferation in vitro and tumor growth in vivo. The action of CUR against angiogenesis was augmented by its combination with MET. Moreover, the combination showed the highest effect on necrosis enhancement and apoptosis induction. It is also safe and did not exhibit toxicity side effects against liver and kidney functions. However, further studies are required to explore the underlying mechanisms of apoptosis induction and immune response trigger. Also the accurate measurement of MET and CUR serum levels is essential to clearly understand the observed response. Taken together, our results suggest that the combination of MET and CUR can be a possible candidate for additional clinical investigations related to breast cancer therapeutics.
Footnotes
Funding: This research project was fully financially supported by Applied Science Private University, Amman, Jordan.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Rabah Rashad Falah, Department of Clinical Pharmacy and Therapeutics, Applied Science Private University, Amman, Jordan.
Wamidh H. Talib, Department of Clinical Pharmacy and Therapeutics, Applied Science Private University, Amman, 11931-166, Jordan.
Seba Jamal Shbailat, Department of Biology and Biotechnology, The Hashemite University, Zarqa, Jordan.
References
- 1. Wang Y, Yu J, Cui R, et al. Curcumin in treating breast cancer: a review. J Lab Autom 2016; 21: 723–731. [DOI] [PubMed] [Google Scholar]
- 2. Liu D, Chen Z. The effect of curcumin on breast cancer cells. J Breast Cancer 2013; 16: 133–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ko EY, Moon A. Natural products for chemoprevention of breast cancer. J Cancer Prev 2015; 20: 223–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sahin K, Tuzcu M, Sahin N, et al. Inhibitory effects of combination of lycopene and genistein on 7,12- dimethyl benz(a)anthracene-induced breast cancer in rats. Nutr Cancer 2011; 63: 1279–1286. [DOI] [PubMed] [Google Scholar]
- 5. Grossmann ME, Yang D, Guo Z, et al. Metformin treatment for the prevention and/or treatment of breast/mammary tumorigenesis. Curr Pharmacol Rep 2015; 1: 312–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hatoum D, McGowan EM. Recent advances in the use of metformin: can treating diabetes prevent breast cancer? Biomed Res Int 2015; 2015: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Giannarelli R, Aragona M, Coppelli A, et al. Reducing insulin resistance with metformin: the evidence today. Diabetes Metab 2003; 29: 6S28–6S35. [DOI] [PubMed] [Google Scholar]
- 8. Rena G, Pearson ER, Sakamoto K. Molecular mechanism of action of metformin: old or new insights? Diabetologia 2013; 56: 1898–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Queiroz EA, Puukila S, Eichler R, et al. Metformin induces apoptosis and cell cycle arrest mediated by oxidative stress, AMPK and FOXO3a in MCF-7 breast cancer cells. PLoS One 2014; 9: e98207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cazzaniga M, Bonanni B. Breast cancer metabolism and mitochondrial activity: the possibility of chemoprevention with metformin. Biomed Res Int 2015; 2015: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chae YK, Arya A, Malecek MK, et al. Repurposing metformin for cancer treatment: current clinical studies. Oncotarget 2016; 7: 40767–40780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Del Barco S, Vazquez-Martin A, Cufí S, et al. Metformin: multi-faceted protection against cancer. Oncotarget 2011; 2: 896–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhuang Y, Miskimins WK. Metformin induces both caspase-dependent and poly(ADP-ribose) polymerase-dependent cell death in breast cancer cells. Mol Cancer Res 2011; 9: 603–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li P, Zhao M, Parris A, et al. p53 is required for metformin-induced growth inhibition, senescence and apoptosis in breast cancer cells. Biochem Biophys Res Commun 2015; 464: 1267–1274. [DOI] [PubMed] [Google Scholar]
- 15. Hirsch H, Iliopoulos D, Struhl K. Metformin inhibits the inflammatory response associated with cellular transformation and cancer stem cell growth. Proc Natl Acad Sci 2012; 110: 972–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Phoenix K, Vumbaca F, Claffey KP. Therapeutic metformin/AMPK activation promotes the angiogenic phenotype in the ERα negative MDA-MB-435 breast cancer model. Breast Cancer Res Treat 2008; 113: 101–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bruno A, Dallaglio K, Cantelmo AR, et al. Paradoxic effects of metformin on endothelial cells and angiogenesis. Carcinogenesis 2014; 35: 1055–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang J, Li G, Wang Y, et al. Suppression of tumor angiogenesis by metformin treatment via a mechanism linked to targeting of HER2/HIF-1α/VEGF secretion axis. Oncotarget 2014; 6: 44579–44592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bandyopadhyay D. Farmer to pharmacist: curcumin as an anti-invasive and antimetastatic agent for the treatment of cancer. Front Chem 2014; 2: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nagaraju GP, Aliya S, Zafar SF, et al. The impact of curcumin on breast cancer. Integr Biol 2012; 4: 996–1007. [DOI] [PubMed] [Google Scholar]
- 21. Mock CD, Jordan BC, Selvam C. Recent advances of curcumin and its analogues in breast cancer prevention and treatment. RSC Adv 2015; 5: 75575–75588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shao ZM, Shen ZZ, Liu CH, et al. Curcumin exerts multiple suppressive effects on human breast carcinoma cells. Int J Cancer 2002; 98: 234–240. [DOI] [PubMed] [Google Scholar]
- 23. Farhangi B, Alizadeh A, Khodayari H, et al. Protective effects of dendrosomal curcumin on an animal metastatic breast tumor. Eur J Pharmacol 2015; 758: 188–196. [DOI] [PubMed] [Google Scholar]
- 24. Grossmann ME, Yang DQ, Guo Z, et al. Metformin treatment for the prevention and/or treatment of breast/mammary tumorigenesis. Curr Pharmacol Rep 2015; 1: 312–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Barbieri F, Thellung S, Ratto A, et al. In vitro and in vivo antiproliferative activity of metformin on stem-like cells isolated from spontaneous canine mammary carcinomas: translational implications for human tumors. BMC Cancer 2015; 15: 228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Iqbal B. Antiproliferative and apoptotic effect of curcumin and TRAIL (TNF related apoptosis inducing ligand) in chronic myeloid leukaemic cells. J Clin Diagn Res 2016; 10: XC01–XC05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Patel P, Thakkar V, Patel J. Cellular effect of curcumin and citral combination on breast cancer cells: induction of apoptosis and cell cycle arrest. J Breast Cancer 2015; 18: 225–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lu W, Qin Y, Yang C, et al. Effect of curcumin on human colon cancer multidrug resistance in vitro and in vivo. Clinics 2013; 68: 694–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhuang Y, Chan D, Haugrud A, et al. Mechanisms by which low glucose enhances the cytotoxicity of metformin to cancer cells both in vitro and in vivo. PLoS One 2014; 9: e108444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ichite N, Chougule M, Jackson T, et al. Enhancement of docetaxel anticancer activity by a novel diindolylmethane compound in human non-small cell lung cancer. Clinical Cancer Res 2009; 15: 543–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhuang Y, Chan DK, Haugrud AB, et al. Mechanisms by which low glucose enhances the cytotoxicity of metformin to cancer cells both in vitro and in vivo. PLoS One 2014; 9: e108444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. He HJ, Wang GY, Gao Y, et al. Curcumin attenuates NRF2 signaling defect, oxidative stress in muscle and glucose intolerance in high fat diet-fed mice. World J Diabetes 2012; 3: 94–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Agrawal N, Bettegowda C, Cheong I, et al. Bacteriolytic therapy can generate a potent immune response against experimental tumors. Proc Natl Acad Sci 2004; 101: 15172–15177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Talib WH, AbuKhader MM. Combinatorial effects of thymoquinone on the anticancer activity and hepatotoxicity of the prodrug CB 1954. Sci Pharm 2013; 81: 519–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Azizi E, Abdolmohammadi MH, Fouladdel SH, et al. Evaluation of p53 and Bcl-2 genes and proteins expression in human breast cancer T47D cells treated with extracts of Astrodaucus persicus (Boiss.) Drude in comparison to Tamoxifen. DARU 2009; 17: 181–186. [Google Scholar]
- 36. Al-Ghamdi MS. Protective effect of Nigella sativa seeds against carbon tetrachloride-induced liver damage. Am J Chin Med 2003; 31: 721–728. [DOI] [PubMed] [Google Scholar]
- 37. Topcul M, Cetin I. Effects of metformin on cell kinetic parameters of MCF-7 breast cancer cells in vitro. Asian Pac J Cancer Prev 2015; 16: 2351–2354. [DOI] [PubMed] [Google Scholar]
- 38. Wang Y, Wei J, Li L, et al. Combined use of metformin and everolimus is synergistic in the treatment of breast cancer cells. Oncol Res 2015; 22: 193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Marinello PC, da Silva TN, Panis C, et al. Mechanism of metformin action in MCF-7 and MDA-MB-231 human breast cancer cells involves oxidative stress generation, DNA damage, and transforming growth factor β1 induction. Tumor Biol 2015; 37: 5337–5346. [DOI] [PubMed] [Google Scholar]
- 40. Nejati-Koshki K, Akbarzadeh A, Pourhassan-Moghaddam M. Curcumin inhibits leptin gene expression and secretion in breast cancer cells by estrogen receptors. Cancer Cell Int 2014; 14: 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ferreira LC, Arbab AS, Jardim-Perassi BV, et al. Effect of curcumin on pro-angiogenic factors in the xenograft model of breast cancer. Anticancer Agents Med Chem 2015; 15: 1285–1296. [DOI] [PubMed] [Google Scholar]
- 42. Khazaei Koohpar Z, Entezari M, Movafagh A, et al. Anticancer activity of curcumin on human breast adenocarcinoma: role of Mcl-1 gene. Iran J Cancer Preven 2015; 8: e2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Liu B, Fan Z, Edgerton S, et al. Metformin induces unique biological and molecular responses in triple negative breast cancer cells. Cell Cycle 2009; 8: 2031–2040. [DOI] [PubMed] [Google Scholar]
- 44. Marini C, Salani B, Massollo M, et al. Direct inhibition of hexokinase activity by metformin at least partially impairs glucose metabolism and tumor growth in experimental breast cancer. Cell Cycle 2013; 12: 3490–3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gao S, Jiang J, Li P, et al. Attenuating tumour angiogenesis: a preventive role of metformin against breast cancer. Biomed Res Int 2015; 2015: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Abd AH, Hussein AG, Mahmood AS. Efficacy of intraperitoneal administration of metformin and 5-fluoruracil in prevention of induced colorectal aberrant crypt foci in mice. Int J Pharmacy Pharmaceut Sci 2014; 6: 305–308. [Google Scholar]
- 47. Madiraju A, Erion D, Rahimi Y, et al. Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase. Nature 2014; 510: 542–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lv ZD, Liu XP, Zhao WJ, et al. Curcumin induces apoptosis in breast cancer cells and inhibits tumor growth in vitro and in vivo. Int J Clin Exp Pathol 2014; 7: 2818–2824. [PMC free article] [PubMed] [Google Scholar]
- 49. Shiri S, Alizadeh AM, Baradaran B, et al. Dendrosomal curcumin suppresses metastatic breast cancer in mice by changing m1/m2 macrophage balance in the tumor microenvironment. Asian Pac J Cancer Prev 2015; 16: 3917–3922. [DOI] [PubMed] [Google Scholar]
- 50. Helson L. Curcumin (diferuloylmethane) delivery methods: a review. BioFactors 2013; 39: 21–26. [DOI] [PubMed] [Google Scholar]
- 51. Prasad S, Tyagi A, Aggarwal B. Recent developments in delivery, bioavailability, absorption and metabolism of curcumin: the golden pigment from golden spice. Cancer Res Treat 2014; 46: 2–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Shi Y, He Z, Jia Z, et al. Inhibitory effect of metformin combined with gemcitabine on pancreatic cancer cells in vitro and in vivo Mol Med Rep 2016; 14: 2921–2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Poli G, Cantini G, Armignacco R, et al. Metformin as a new anti-cancer drug in adrenocortical carcinoma. Oncotarget 2016; 7: 49636–49648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Xie YQ, Wu XB, Tang SQ. Curcumin treatment alters ERK-1/2 signaling in vitro and inhibits nasopharyngeal carcinoma proliferation in mouse xenografts. Int J Clin Exp Med 2014; 7: 108–114. [PMC free article] [PubMed] [Google Scholar]
- 55. Dorai T, Cao Y, Dorai B, et al. Therapeutic potential of curcumin in human prostate cancer. III. Curcumin inhibits proliferation, induces apoptosis, and inhibits angiogenesis of LNCaP prostate cancer cells in vivo. Prostate 2001; 47: 293–303. [DOI] [PubMed] [Google Scholar]
- 56. Wong TF, Takeda T, Li B, et al. Curcumin targets the AKT–mTOR pathway for uterine leiomyosarcoma tumor growth suppression. Int J Clin Oncol 2014; 19: 354–363. [DOI] [PubMed] [Google Scholar]
- 57. Blatt NB, Glick GD. Signaling pathways and effector mechanisms pre-programmed cell death. Bioorg Med Chem 2001; 9: 1371–1384. [DOI] [PubMed] [Google Scholar]
- 58. Fridman JS, Lowe SW. Control of apoptosis by p53. Oncogene 2003; 22: 9030–9040. [DOI] [PubMed] [Google Scholar]
- 59. Bimonte S, Barbieri A, Palma G, et al. Dissecting the role of curcumin in tumour growth and angiogenesis in mouse model of human breast cancer. Biomed Res Int 2015; 2015: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Watson JL, Greenshields A, Hill R, et al. Curcumin-induced apoptosis in ovarian carcinoma cells is p53-independent and involves p38 mitogen-activated protein kinase activation and downregulation of Bcl-2 and survivin expression and Akt signaling. Mol Carcinog 2010; 49: 13–24. [DOI] [PubMed] [Google Scholar]
- 61. Bush JA, Cheung KJ, Jr, Li G. Curcumin induces apoptosis in human melanoma cells through a fas receptor/caspase-8 pathway independent of p53. Exp Cell Res 2001; 271: 305–314. [DOI] [PubMed] [Google Scholar]
- 62. Jaiswal AS, Marlow BP, Gupta N, et al. β-Catenin-mediated transactivation and cell–cell adhesion pathways are important in curcumin (diferuylmethane)-induced growth arrest and apoptosis in colon cancer cells. Oncogene 2002; 21: 8414–8427. [DOI] [PubMed] [Google Scholar]
- 63. Tsvetkov P, Asher G, Reiss V, et al. Inhibition of NAD(P)H:quinone oxidoreductase 1 activity and induction of p53 degradation by the natural phenolic compound curcumin. Proc Natl Acad Sci 2005; 102: 5535–5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sliwinska A, Rogalska A, Marczak A, et al. Metformin, but not sitagliptin, enhances WP 631-induced apoptotic HepG2 cell death. Toxicol In Vitro 2015; 29: 1116–1123. [DOI] [PubMed] [Google Scholar]
- 65. Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol 1989; 7: 145–173. [DOI] [PubMed] [Google Scholar]
- 66. Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature 1996; 383: 787–793. [DOI] [PubMed] [Google Scholar]
- 67. Murphy KM, Reiner SL. The lineage decisions of helper T cells. Nat Rev Immunol 2002; 2: 933–944. [DOI] [PubMed] [Google Scholar]
- 68. Hegazy AN, Peine M, Helmstetter C, et al. Interferons direct Th2 cell reprogramming to generate a stable GATA-3+T-bet+ cell subset with combined Th2 and Th1 cell functions. Immunity 2010; 32: 116–128. [DOI] [PubMed] [Google Scholar]
- 69. Nakayamada S, Takahashi H, Kanno Y, et al. Helper T cell diversity and plasticity. Curr Opin Immunol 2012; 24: 297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Yamamoto M, Kamigaki T, Yamashita K, et al. Enhancement of anti-tumor immunity by high levels of Th1 and Th17 with a combination of dendritic cell fusion hybrids and regulatory T cell depletion in pancreatic cancer. Oncol Rep 2009; 22: 337–343. [PubMed] [Google Scholar]
- 71. Bogdan C, Stenger S, Röllinghoff M, et al. Cytokine interactions in experimental cutaneous leishmaniasis. Interleukin 4 synergizes with interferon-γ to activate murine macrophages for killing of Leishmania major amastigotes. Eur J Immunol 1991; 21: 327–333. [DOI] [PubMed] [Google Scholar]
- 72. Obiri NI, Siegel JP, Varrichhio F, et al. Expression of high-affinity IL-4 receptors on human melanoma, ovarian and breast carcinoma cells. Clin Exp Immunol 1994; 95: 148–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Lehn M, Kandil O, Arena C, et al. Interleukin-4 fails to inhibit interferon-γ-induced activation of human colostral macrophages. Cell Immunol 1992; 141: 233–242. [DOI] [PubMed] [Google Scholar]
- 74. Eikawa S, Nishida M, Mizukami S, et al. Immune-mediated antitumor effect by type 2 diabetes drug, metformin. Proc Natl Acad Sci 2015; 112: 1809–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Toi M, Bicknell R, Harris AL. Inhibition of colon and breast carcinoma cell growth by interleukin-4. Cancer Res 1992; 52: 275–279. [PubMed] [Google Scholar]
- 76. Blais Y, Gingras S, Haagensen DE, et al. Interleukin-4 and interleukin-13 inhibit estrogen-induced breast cancer cell proliferation and stimulate GCDFP-15 expression in human breast cancer cells. Mol Cell Endocrinol 1996; 121: 11–18. [DOI] [PubMed] [Google Scholar]
- 77. Gooch JL, Lee AV, Yee D. Interleukin 4 inhibits growth and induces apoptosis in human breast cancer cells. Cancer Res 1998; 58:4199–4205. [PubMed] [Google Scholar]
- 78. Obiri NI, Hillman GG, Haas GP, et al. Expression of high affinity interleukin-4 receptors on human renal cell carcinoma cells and inhibition of tumor cell growth in vitro by interleukin-4. J Clin Invest 1993; 91: 88–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Topp MS, Papadimitriou CA, Eitelbach F, et al. Recombinant human interleukin 4 has antiproliferative activity on human tumor cell lines derived from epithelial and nonepithelial histologies. Cancer Res 1995; 55: 2173–2176. [PubMed] [Google Scholar]
- 80. Morisaki T, Uchiyama A, Yuzuki D, et al. Interleukin 4 regulates G1 cell cycle progression in gastric carcinoma cells. Cancer Res 1994; 54: 1113–1118. [PubMed] [Google Scholar]
- 81. Bostrom B, Uppal P, Chu J, et al. Safety and efficacy of metformin for therapy-induced hyperglycemia in children with acute lymphoblastic leukemia. J Pediatr Hematol Oncol 2013; 35: 504–508. [DOI] [PubMed] [Google Scholar]
- 82. Huang CZ, Huang WZ, Zhang G, et al. In vivo study on the effects of curcumin on the expression profiles of anti-tumour genes (VEGF, CyclinD1 and CDK4) in liver of rats injected with DEN. Mol Biol Rep 2013; 40: 5825–5831. [DOI] [PubMed] [Google Scholar]
- 83. Abouzied MM, Eltahir HM, Abdel Aziz MA, et al. Curcumin ameliorate DENA-induced HCC via modulating TGF-β, AKT, and caspase-3 expression in experimental rat model. Tumor Biol 2014; 36: 1763–1771. [DOI] [PubMed] [Google Scholar]
- 84. Zanotto-Filho A, Braganhol E, Edelweiss MI, et al. The curry spice curcumin selectively inhibits cancer cells growth in vitro and in preclinical model of glioblastoma. J Nutr Biochem 2012; 23: 591–601. [DOI] [PubMed] [Google Scholar]