Abstract
Background:
Randomized clinical trials showed improved overall survival (OS) of advanced gastroesophageal adenocarcinoma (GEA) patients treated with second-line taxane or irinotecan. However, most data on irinotecan efficacy in this setting come from large Asian trials. We retrospectively analyzed clinical effectiveness and toxicity of irinotecan in a cohort of patients with advanced GEA treated in our department.
Methods:
Advanced GEA patients who received at least one cycle of second-line irinotecan were eligible for inclusion. Irinotecan was administered every 3 weeks at an initial dose of 250 mg/m2 of body surface area with subsequent gradual (every 50 mg/m2) dose escalation up to 350 mg/m2, in the case of good treatment tolerance. OS was estimated using the Kaplan–Meier method. A multivariate Cox regression analysis was used to examine the association between clinical and laboratory parameters and survival.
Results:
A total of 48 patients were identified. Median OS was 6.2 months [95% confidence interval (CI): 3.9–7.6]. In multivariate analysis, age < 65 years, baseline total lymphocyte count (TLC) < 1500/µl and presence of peritoneal metastases were associated with shorter OS. Most adverse events were grade 1–2 and included: anemia (52.3%), leukocytopenia (40.9%), neutropenia (59.1%), nausea (25.0%), vomiting (31.8%), diarrhea (31.8%), anorexia (29.5%) and fatigue (43.2%). Febrile neutropenia occurred in three patients (6.8%). Nine patients (20.5%) experienced a toxicity grade 3–4 of any kind.
Conclusions:
This retrospective analysis confirms clinical effectiveness and manageable toxicity of second-line irinotecan in an unselected cohort of advanced GEA patients. Age < 65 years, baseline TLC < 1500/µl and presence of peritoneal metastases were independent prognostic factors associated with shorter OS.
Keywords: gastric cancer, gastroesophageal cancer, irinotecan, second-line chemotherapy
Introduction
Gastric cancer is the fifth most common malignancy and the third most common leading cause of cancer death worldwide [Torre et al. 2015]. In 2012, there were almost one million new cases of stomach cancer [Torre et al. 2015]. Around two- thirds of them are diagnosed in locally advanced or metastatic stages, in which palliative chemotherapy is the main treatment option. Prognosis of patients with advanced gastric cancer is poor, with 5-year survival at 5–20%, and median overall survival (OS) below 12 months [Power et al. 2010; Shah, 2015]. The efficacy of the second-line chemotherapy in advanced gastric adenocarcinoma was unclear until a few years ago when the results of phase III randomized clinical trials (RCTs) clearly demonstrated improvement in survival of patients treated with a single-agent taxane, irinotecan or ramucirumab, or a combination of ramucirumab and paclitaxel [Ford et al. 2014; Fuchs et al. 2014; Hironaka et al. 2013; Kang et al. 2012; Thuss-Patience et al. 2011; Wilke et al. 2014].
However, results from RCTs, due to stringent inclusion and exclusion criteria, may not always be replicable in a ‘real-world’ practice as characteristics of patients treated in RCTs may not always mirror the characteristics of a broader range of cancer patients in everyday clinical practice [Dreyer et al. 2010; Spigel, 2010]. Moreover, of all phase III studies, only the German AIO trial evaluated the role of irinotecan in the Western population and, due to poor accrual, it enrolled only 21 patients into the chemotherapy arm [Thuss-Patience et al. 2011]. Two other studies with a greater number of patients come from the Asian population [Hironaka et al. 2013; Kang et al. 2012]. As differences in both tumor biology and pharmacogenetics in Asian patients may play a role in chemotherapy efficacy and tolerability [Kim et al. 2010], we decided to launch this retrospective study to investigate the reproducibility of the data from RCTs with regard to irinotecan efficacy and safety in an unselected population of patients treated in our department (the ‘real-world’ setting).
Methods
Study design and patients
Eligible for registration were patients >18 years of age with metastatic or recurrent gastric or gastro-esophageal junction adenocarcinoma who progressed during or after completion of the first-line palliative chemotherapy and received at least one cycle of the second-line irinotecan at the Department of Oncology, University Hospital, Krakow, until November 2015. Exclusion criteria included prior second malignancy within 5 years before the diagnosis of gastric cancer or evidence of brain metastases at study entry. The following data were collected: patient demographics, Eastern Cooperative Oncology Group Performance Status (ECOG PS), hemoglobin level, total lymphocyte count (TLC), platelets level, body mass index (BMI), metastatic sites, primary tumor resection status, duration of response to prior chemotherapy, interval time between the end of first-line chemotherapy and the start of irinotecan, date of disease progression, death or last follow-up.
Irinotecan chemotherapy cycles were administered by intravenous infusion over 90 minutes every 3 weeks. In the first cycle, patients were administered a dose of 250 mg/m2 of body-surface area with a subsequent gradual (every 50 mg/m2) dose escalation up to 350 mg/m2, in the case of good treatment tolerance. Depending on the response to treatment and toxicity, chemotherapy was continued up to six or seven cycles, or was ceased earlier, if unacceptable toxicity or progression occurred. If a response to the treatment continued for at least 3 months after the completion of the last (sixth or seventh) chemotherapy cycle, a rechallenge with irinotecan at the last-administered dose level was possible, depending on patient performance status, laboratory tests and previous toxicity. The standard emetogenic prophylaxis consisted of 8 mg of ondansetron and 8 mg of dexamethasone, both given intravenously before irinotecan infusion. Dose and schedule modifications for any toxicity were at the discretion of the treating physician; this included maintaining the dose of irinotecan at the initial level of 250 mg/m2, dose reductions and/or administration of chemotherapy every 4 weeks. Supportive treatment with granulocyte colony stimulating factors (G-CSFs), erythropoiesis-stimulating agents (ESA), blood transfusions or megestrol acetate were also at the discretion of the treating physician and corresponded with national and international guidelines.
The routine evaluation of treatment efficacy consisted of computed tomography (CT) scans of the abdomen and pelvis. The presence of lung metastases or pleural fluid was usually initially evaluated in plain chest X-rays (postero-anterior plus latero-lateral views). If based on a chest radiograph or an abdominal CT, there was suspicion of abnormality in thoracic organs, the CT of the chest was performed and repeated along with a CT evaluation of other body regions. Radiological evaluations were usually repeated every 10–12 weeks. Irinotecan was stopped if radiological or evident clinical progression occurred. The subsequent third-line treatment depended on a patient’s performance status, laboratory test results, duration of response and tolerance of previous chemotherapy and was at the discretion of the treating physician.
The primary objective of the study was OS calculated from the date of the first cycle of irinotecan until the date of death from any cause. The secondary objective was treatment toxicity assessed using the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.0). We also decided to carry out univariate and multivariate analyses of the recorded clinical prognostic factors for survival.
The protocol of the study was approved by The Bioethics Committee of the Jagiellonian University in Krakow (approval no. KBET/226/B/2014, date of issue 23 October 2014). All procedures followed were in accordance with the ethical standards of the responsible institutional committee on human experimentation and with the Declaration of Helsinki 1975, revised Hong Kong, 1989. A written informed consent was obtained from each patient before starting the treatment. Due to the retrospective nature of the study, formal consent for inclusion was waived by the ethics committee. All the data were collected and managed by the physicians of the Department of Oncology at the University Hospital in Krakow. This was an academic study with no external sponsors.
Statistical analysis
Continuous variables were presented as mean and standard deviation or as median and quartiles, as appropriate. Categorical variables were presented as counts and percentages.
The Kaplan–Meier method was used to estimate survival functions. The association between predictors and survival was investigated using the univariate Cox proportional hazard (CPH) model. Variables that showed an effect (p value < 0.1) on survival in univariate analyses were entered in a multivariate CPH model. The results of Cox models were presented as hazard ratios, along with tests of significance and 95% confidence intervals (CIs). There were no statistically significant violations of the proportionality assumption that underlies the CPH method. Categorization of continuous variables was prespecified.
A statistical analysis was performed in ‘R: A language and environment for statistical computing’. p values < 0.05 were considered statistically significant.
Results
Patient characteristics
Between March 2011 and November 2015, 49 eligible patients who received at least one cycle of the second-line palliative chemotherapy with irinotecan were registered. One patient with brain metastases was excluded from further analysis (Figure 1). The baseline characteristic of the 48 patients who completed all inclusion and exclusion criteria is shown in Table 1. The vast majority of patients had ECOG PS 0 or 1 (85.4%) and all patients had metastatic disease with “one third” (33.3%) having at least three metastatic sites. Except for one patient who received solely leucovorin and fluorouracil, all other patients received first-line platinum and fluoropirimide-based palliative chemotherapy, with the most common regimens being EOX (epirubicin/oxaliplatin/capecitabine; 43.8%) and mDCF (docetaxel/cisplatin/leucovorin/fluorouracil; 43.8%). Two patients with HER2 receptor overexpression received cisplatin/capecitabine chemotherapy plus trastuzumab. Mean interval time from the end of the first-line chemotherapy to begin irinotecan was 2.63 months, with 60.4% of patients progressing during first-line chemotherapy and 70.8% of patients starting irinotecan within 3 months from the last dose of the first-line treatment.
Figure 1.
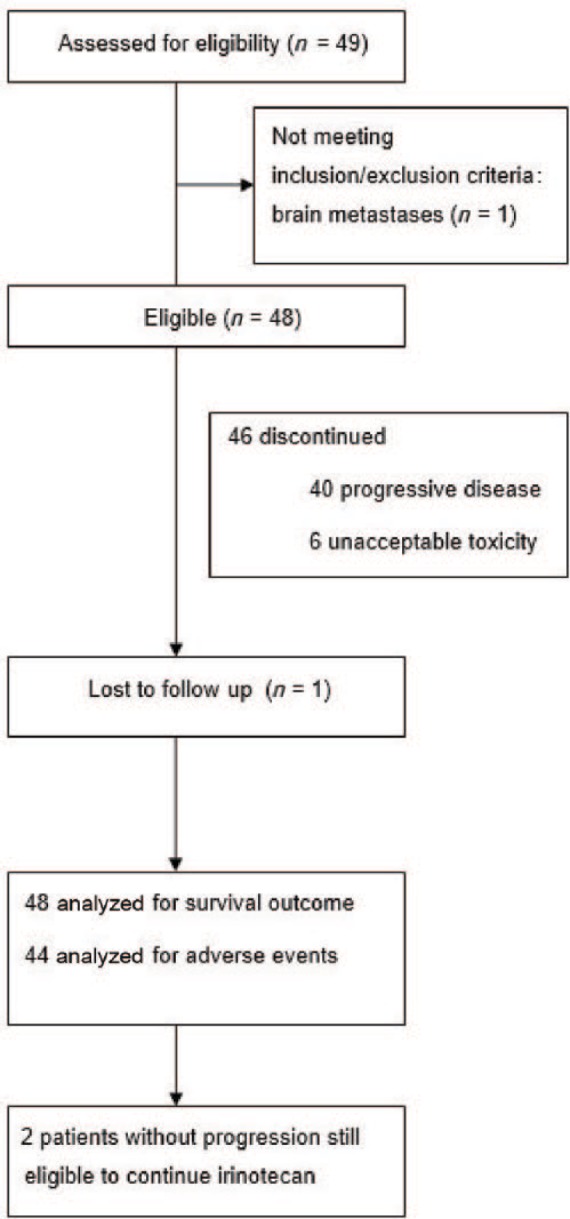
Trial profile.
Table 1.
Patients characteristics.
| Characteristic | n (%) |
|---|---|
| Sex | |
| Male | 27 (56.2%) |
| Female | 21 (43.8%) |
| Mean age, years (SD) | 59.5 (11.3) |
| Age | |
| <65 years | 33 (68.8%) |
| ⩾65 years | 15 (31.2%) |
| ECOG PS | |
| 0 | 14 (29.2%) |
| 1 | 27 (56.2%) |
| 2 | 7 (14.6%) |
| Mean baseline hemoglobin, g/dl (SD) |
11.4 (1.29) |
| Anemia | 23 (47.9%) |
| Platelets | |
| Normal | 39 (81.3%) |
| > Upper limit of normal | 9 (18.7%) |
| Mean baseline BMI (SD) |
22.6 (4.17) |
| BMI groups at baseline | |
| <17 (moderate and severe malnutrition) | 7 (14.6%) |
| 17–18.99 (mild malnutrition) | 3 (6.25%) |
| 19–24.99 (normal) | 24 (50.0%) |
| ⩾25.0 (overweight) | 14 (29.2%) |
| Malnutrition (baseline TLC < 1500/µl)* | 18 (38.3%) |
| Disease status | |
| Locally advanced | 0 (0%) |
| Metastatic disease | 48 (100%) |
| Location of metastases | |
| Liver | 26 (54.2%) |
| Peritoneum/ascites | 32 (66.7%) |
| Distant lymph nodes | 25 (52.1%) |
| Lungs | 7 (14.6%) |
| Ovaries** | 5 (23.8%) |
| Bones | 4 (8.33%) |
| Suprarenal glands | 4 (8.33%) |
| Other | 2 (4.17%) |
| Number of sites involved | |
| 1 | 13 (27.1%) |
| 2 | 19 (39.6%) |
| ⩾3 | 16 (33.3%) |
| First line chemotherapy regimen | |
| EOX | 21 (43.8%) |
| mDCF | 21 (43.8%) |
| Other | 6 (12.4%) |
| PFS on the first line chemotherapy | |
| <6 months | 22 (45.8%) |
| ⩾6 months | 26 (54.2%) |
| Interval time between the end of first-line chemotherapy and the start of irinotecan (months): mean (SD), median (quartiles) | 2.63 (3.37), 1.33 (0.59–3.77) |
| Interval from the end of first-line chemotherapy | |
| Progression during first-line chemotherapy | 29 (60.4%) |
| <3 months | 34 (70.8%) |
| ⩾3 months | 14 (29.2%) |
| Location of primary tumor | |
| Gastric | 35 (72.9%) |
| Gastroesophageal junction | 10 (20.8%) |
| Not specified | 3 (6.25%) |
| Histological subtype | |
| Intestinal | 13 (27.1%) |
| Diffuse | 9 (18.8%) |
| Mixed | 10 (20.8%) |
| Unknown | 16 (33.3%) |
| HER2 receptor over expression | |
| Positive | 5 (10.4%) |
| Negative | 36 (75.0%) |
| Unknown | 7 (14.6%) |
| Prior gastrectomy | |
| Yes | 25 (52.1%) |
| No | 23 (47.9%) |
Out of 47 patients for whom data were available.
Female population only.
SD, standard deviation; ECOG, Eastern Cooperative Oncology Group; PS, performance status; BMI, body mass index; PFS, progression-free survival; TLC, total lymphocyte count; EOX, epirubicin/oxaliplatin/capecitabine; mDCF, docetaxel/cisplatin/leucovorin/fluorouracil.
Treatment
The cut-off date for analysis was 10 May 2016, 6 months after the last patient started treatment with irinotecan and was enrolled into the study. At the time of the analysis, a total number of 198 cycles were administered with a median number of three [2.0–6.3] treatment cycles per patient. A total of 14 (29.2%) patients completed at least six cycles of chemotherapy. The maximum dose of irinotecan with good tolerance was 250 mg/m2 in 23 (47.9%) patients, 300 mg/m2 in 16 (33.3%) patients and 350 mg/m2 in 9 (18.8%) patients. Due to toxicity, delays in administration of subsequent cycles of chemotherapy or change to permanent administration of irinotecan every 4 weeks were necessary in eight (16.7%) patients. For the same reason, reductions in the irinotecan dose were necessary in 9 (18.8%) patients. Among patients who experienced disease progression at the time of the analysis or discontinued treatment due to toxicity (n =46), the rechallenge with irinotecan was possible in 6 (13.0%) patients. Only 5 (10.4%) patients received third-line chemotherapy. In eight (16.7%) patients, irinotecan was not continued beyond the first cycle due to fast cancer progression with significant deterioration of the ECOG PS in five (10.4%) patients or due to unacceptable toxicity in three (6.3%) patients.
Survival
At the time of the final analysis, 41 (85.4%) patients had died. One patient was lost to follow up after 3.5 months and although his status in the National Medical Security Database is ‘deceased’, the exact date of his death is unknown. Median OS was 6.2 months (95% CI: 3.9–7.6) and median progression-free survival was 2.2 months (95% CI: 1.8–3.9) (Figures 2 and 3). OS rate at 3 months was 75% and at 6 months was 50%. In 35 patients, evaluable for response, a disease-control rate was 51.4%.
Figure 2.
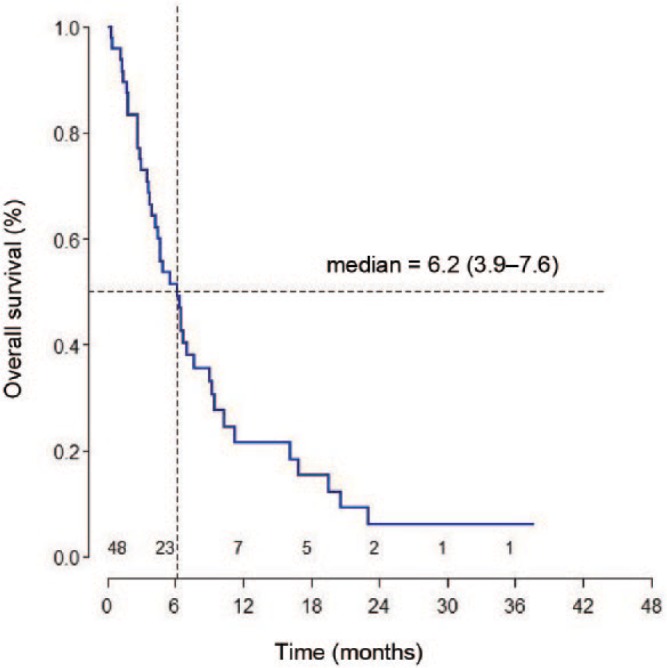
Overall survival from initiation of irinotecan.
Figure 3.
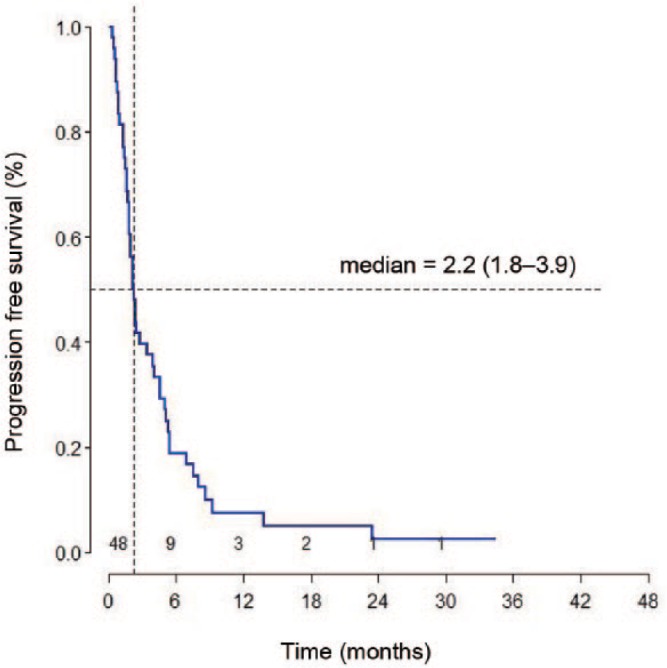
Progression-free survival from initiation of irinotecan.
Univariate and multivariate analysis of OS for independent prognostic factors
In the univariate analysis, age < 65 years, ECOG PS 2 and the presence of peritoneal metastases/ascites were associated with a significantly higher hazard of death. There was a trend for a higher hazard of death for patients with baseline TLC < 1500/µl and the absence of liver metastases (Table 2). In the multivariate analysis, three factors were negatively associated with the OS. These were: age < 65 years (p = 0.016), baseline TLC < 1500/µl (p = 0.005) and the presence of peritoneal metastases with or without ascites (p = 0.014) (Table 3).
Table 2.
Univariate analysis of factors associated with overall survival.
| Number of subjects |
Number of events |
Median OS, (95% CI) | HR (95% CI) |
p value (two sided) |
|
|---|---|---|---|---|---|
| Age | |||||
| <65 | 33 | 29 | 4.7 (3.0–6.7) | Ref. | |
| ⩾65 | 15 | 11 | 16.1 (3.6–20.6) | 0.407 (0.195–0.851) |
0.017 |
| Sex | |||||
| Female | 21 | 18 | 5.6 (3.5–6.7) | Ref. | |
| Male | 27 | 22 | 7.0 (3.0–11.2) | 0.728 (0.385–1.376) |
0.329 |
| ECOG PS | |||||
| 0–1 | 41 | 33 | 6.5 (4.4–9.4) | Ref. | |
| 2 | 7 | 7 | 3.6 (0.3–6.2) | 2.850 (1.208–6.724) |
0.017 |
| BMI baseline | |||||
| ⩽18.99 | 10 | 8 | 6.5 (1.9–11.2) | Ref. | |
| 19–24.99 | 24 | 20 | 6.5 (3.9–10.3) | 1.071 (0.470–2.436) |
0.871 |
| ⩾25 | 14 | 12 | 4.0 (1.4–9.0) | 1.388 (0.565–3.406) |
0.474 |
| Lymphocyte baseline | |||||
| <1500 | 18 | 17 | 3.8 (1.9–6.5) | Ref. | |
| ⩾1500 | 29 | 22 | 6.7 (4.7–10.3) | 0.544 (0.287–1.029) |
0.061 |
| Platelet baseline | |||||
| Normal | 39 | 32 | 6.2 (3.5–7.0) | Ref. | |
| >Upper limit of normal | 9 | 8 | 7.6 (1.4–20.6) | 0.646 (0.293–1.424) |
0.279 |
| Anemia | |||||
| No | 25 | 21 | 6.2 (3.6–9.0) | Ref. | |
| Yes | 23 | 19 | 6.4 (3.5–16.1) | 0.981 (0.522–1.846) |
0.953 |
| Lymph node metastases | |||||
| No | 23 | 20 | 4.7 (2.7–9.0) | Ref. | |
| Yes | 25 | 20 | 6.4 (3.9–10.3) | 1.083 (0.568–2.066) |
0.808 |
| Liver metastases | |||||
| No | 22 | 21 | 4.7 (2.7–7.6) | Ref. | |
| Yes | 26 | 19 | 6.5 (3.7–16.1) | 0.552 (0.289–1.052) |
0.071 |
| Lung metastases | |||||
| No | 41 | 34 | 6.5 (3.5–9.3) | Ref. | |
| Yes | 7 | 6 | 4.9 (4.4–6.5) | 0.947 (0.393–2.283) |
0.903 |
| Peritoneal metastases/ascites | |||||
| No | 16 | 12 | 9.0 (4.7–19.5) | Ref. | |
| Yes | 32 | 28 | 4.4 (2.9–6.7) | 2.483 (1.192–5.174) |
0.015 |
| Number of metastases | |||||
| 1–2 | 32 | 28 | 6.6 (2.7–9.4) | Ref. | |
| ⩾3 | 16 | 12 | 5.6 (3.7–6.5) | 1.360 (0.654–2.827) |
0.410 |
| Primary tumor resected | |||||
| Yes | 25 | 21 | 6.5 (4.3–9.3) | Ref. | |
| No | 23 | 19 | 4.9 (2.7–9.4) | 1.476 (0.775–2.810) |
0.236 |
| PFS on the first-line chemotherapy | |||||
| <6 months | 22 | 19 | 6.1 (3.7–9.4) | Ref. | |
| ⩾6 months | 26 | 21 | 6.2 (2.9–9.0) | 1.046 (0.561–1.950) |
0.887 |
| Interval from the end of first-line CTH | |||||
| <3 months | 34 | 28 | 6.0 (3.7–9.3) | Ref. | |
| ⩾3 months | 14 | 12 | 6.2 (1.3–9.0) | 1.124 (0.570–2.217) |
0.736 |
PFS, progression-free survival; CTH, chemotherapy; ECOG, Eastern Cooperative Oncology Group; PS, performance status; BMI, body mass index; OS, overall survival; CI, confidence interval.
Table 3.
Multivariate analysis of factors associated with overall survival.
| HR (95% CI) |
p value (two sided) |
|
|---|---|---|
| Age | ||
| <65 | Ref. | |
| ⩾65 | 0.402 (0.191–0.845) |
0.016 |
| ECOG PS | ||
| 0–1 | Ref. | |
| 2 | 1.957 (0.775–4.939) |
0.155 |
| Lymphocytes at baseline | ||
| <1500 | Ref. | |
| ⩾1500 | 0.331 (0.154–0.711) |
0.005 |
| Liver metastases | ||
| No | Ref. | |
| Yes | 0.768 (0.376–1.567) |
0.469 |
| Peritoneal metastases/ascites | ||
| No | Ref. | |
| Yes | 3.035 (1.256–7.336) |
0.014 |
HR, hazard ratio; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; PS, performance status.
Toxicity and supportive care
In four patients who received only one cycle of irinotecan, complete information on subsequent adverse events and supportive treatment were not available. Therefore, both the analysis of toxicity and supportive care during chemotherapy include 44 patients for whom these data were completely known. Most common adverse events were: anemia (52.3%), leukocytopenia (40.9%), neutropenia (59.1%), nausea (25.0%), vomiting (31.8%), diarrhea (31.8%), anorexia (29.5%) and fatigue (43.2%) (Table 4). However, these adverse events were predominantly of grade 1 and 2 with grades 3 and 4 having occurred in <10% of patients (except for neutropenia grade 3 and 4). Febrile neutropenia was observed in three patients (6.8%). Altogether, toxicity grade 3 or 4 of any kind occurred in nine (20.5%) patients.
Table 4.
Toxicity during irinotecan treatment.
| Toxicity* | All grades n (%) |
Grade 3 or 4 n (%) |
|---|---|---|
| Anemia | 23 (52.3%) | 2 (4.6%) |
| Leukocytopenia | 18 (40.9%) | 2 (4.6%) |
| Neutropenia | 26 (59.1%) | 5 (11.4%) |
| Febrile neutropenia | 3 (6.8%) | 3 (6.8%) |
| Thrombocytopenia | 1 (2.3%) | 0 (0%) |
| Nausea | 11 (25.0%) | 0 (0%) |
| Vomiting | 14 (31.8%) | 1 (2.3%) |
| Diarrhea | 14 (31.8%) | 1 (2.3%) |
| Anorexia | 13 (29.5%) | 0 (0%) |
| Fatigue | 19 (43.2%) | 1 (2.3% |
For 44 patients for whom complete data on the toxicity were available.
Due to toxicity, nine patients (20.5%) required at least one G-CSF administration, four patients (9.1%) were administered ESA and two (4.5%) required blood transfusions. Eight patients (18.2%) were given megestrol acetate for anorexia.
Discussion
Our retrospective analysis of an unselected patients’ cohort with advanced gastroesophageal adenocarcinoma (GEA) who progressed on or after first-line palliative chemotherapy confirms clinical effectiveness and manageable toxicity of second-line irinotecan with the median OS of 6.2 months.
Until a few years ago, the role of the second-line palliative chemotherapy in advanced gastroesophageal cancer had remained unknown. Nowadays, however, we have a tremendous body of evidence from RCTs unquestionably proving the efficacy of a few agents in this setting. In the first published trial of the German Arbeitsgemeinschaft Internistische Onkologie (AIO) group (n = 40), irinotecan monotherapy was compared with best supportive care (BSC) and showed improvement in OS in favor of chemotherapy [4.0 versus 2.4 months, hazard ratio (HR) = 0.48, 95% CI: 0.25–0.92; p = 0.012] [Thuss-Patience et al. 2011]. Subsequently, the efficacy of a second-line chemotherapy in advanced gastroesophageal cancer was confirmed in the Korean phase III study (n = 202), where both docetaxel and irinotecan improved median OS compared with BSC (5.2 and 6.5 months, respectively versus 3.8 months in BSC arm) [Kang et al. 2012] and in the phase III British trial COUGAR-2 (n = 168) where second-line docetaxel significantly improved median OS compared with active symptom control (ASC) (5.2 versus 3.6 months for ASC, HR = 0.67; p = 0.01) [Ford et al. 2014]. A Japanese WJOG 4007 phase III study (n = 219) showed equal efficacy of paclitaxel and irinotecan in the second-line treatment of advanced gastric adenocarcinoma refractory to the treatment with fluoropyrimidine and platinum (median OS 9.5 months for paclitaxel and 8.4 months for irinotecan, HR = 1.13; p = 0.38) [Hironaka et al. 2013]. Furthermore, ramucirumab, a fully human IgG1 monoclonal antibody that is a VEGFR-2 antagonist, in the second-line setting improved median OS in monotherapy compared with BSC in the REGARD study (n = 355) (5.2 versus 3.8 months, HR = 0.776; p = 0.047) [Fuchs et al. 2014], as well as in combination with paclitaxel versus paclitaxel alone in the RAINBOW trial (n = 665) (9.6 versus 7.4 months, HR = 0.807; p = 0.017) [Wilke et al. 2014]. Finally, recently published meta-analyses clearly confirmed improved OS of patients with advanced gastroesophageal cancer treated with second-line chemotherapy compared with BSC alone [Iacovelli et al. 2014; Janowitz et al. 2016; Kim et al. 2013].
We decided to launch this study to analyze effectiveness and safety of the second-line irinotecan in a ‘real-world’ practice in patients with advanced GEA treated at our department. The OS of patients in our analysis is consistent with the results of the abovementioned trials examining a taxane or irinotecan monotherapy in the second-line treatment, with the exception of the study by Hironaka and colleagues, who reported longer median OS for both paclitaxel- and irinotecan-treated groups. A few factors may be responsible for that difference. Firstly, in the WJOG 4007 study, 89.9% of paclitaxel and 72.1% of irinotecan-treated patients received third-line chemotherapy. In the Korean study, 40% of patients in the chemotherapy arm received further treatment, and median OS was longer for those patients who received subsequent therapy compared with those who did not, regardless of the treatment arm (8.0 versus 3.7 months; p < 0.001). In our study, only five patients (10.4%) received third-line chemotherapy, which is similar to the 14.3% in the AIO trial (three patients from the irinotecan arm). Secondly, in both Asian trials, there were few patients with ECOG PS 2 (none in the study by Kang and colleagues and only 3.6% in the irinotecan arm in the Hironaka and colleagues’ trial) compared with 19% in the German study and 14.6% in our report. Thirdly, Asian patients seem to represent a different tumor biology with more limited metastatic spread; only one metastatic site was observed in 57.7% of patients receiving irinotecan in the Japanese trial and in 32% of patients receiving chemotherapy in the Korean study. Moreover, the WJOG 4007 study included only 25.2% of patients with peritoneal metastases, excluding those with severe peritoneal metastases. In our study, at baseline, only 27.1% of patients had one metastatic site and 66.7% were known to have peritoneal metastases. In this regard, the population in our analysis is more similar to the German AIO study where 29% of patients had one metastatic site and 43% had peritoneal metastases. All of these factors are proved to negatively impact the outcome of patients with advanced gastroesophageal cancer and may explain the differences in reported median OS between the studies [Chau et al. 2004, 2009].
However, despite the median OS of 6.2 months in the whole group of patients included in our study, it seems that not all patients benefit equally from the second-line irinotecan. We performed a multivariate regression analysis of OS and found that patients with peritoneal metastases with or without ascites had a significantly shorter median OS compared with patients who did not have them. This is not surprising as the presence of peritoneal metastases has been proven to be a negative prognostic factor for the patients’ outcome [Chau et al. 2004, 2009]. Similarly, patients with the baseline TLC < 1500/µl had a shorter median OS compared with patients having a baseline TLC ⩾ 1500/µl. TLC is an indirect indicator of the nutritional status and low lymphocyte count may reflect patient malnutrition. It has been shown that baseline cachexia in cancer patients undergoing treatment, including patients with gastrointestinal malignancies, is associated with more severe dose-limiting toxicity, less chemotherapy administration, reduced response to chemotherapy and shorter OS [Andreyev et al. 1998; Ross et al. 2004; Van Bokhorst-de van der Schuer et al. 1999; Van Cutsem and Arends, 2005]. Thirdly, patients under 65 years of age seem to have shorter median OS in this analysis. This finding is consistent with that in the meta-analysis by Janowitz and colleagues, where older age was found to be a positive predictor of improved OS with second-line chemotherapy [Janowitz et al. 2016]. In addition, some studies have shown that younger patients with gastric cancer, especially those under 35 years of age, tend to have more aggressive tumors associated with a shorter survival [Kim et al. 2015; Matley et al. 1988; Smith and Stabile, 2009].
There is no one ‘gold standard’ regimen of irinotecan administration in the second-line treatment of advanced gastroesophageal cancer. In the original German AIO study, irinotecan was administered at a dose of 250 mg/m2 in the first cycle with the escalation to 350 mg/m2 in subsequent cycles [Thuss-Patience et al. 2011]. In both Asian trials [Hironaka et al. 2013; Kang et al. 2012], irinotecan was administered at the dose of 150 mg/m2 every 2 weeks. Out of these trials, the rate of grade 3 or 4 toxicity with regard to neutropenia and diarrhea was highest in the German study (16% and 26%, respectively). In our department, we adopted a similar, but not identical, second-line irinotecan regimen to that presented by Thuss-Patience and colleagues. It consists of irinotecan 250 mg/m2 of body-surface area in the first cycle with a subsequent but gradual (every 50 mg/m2) dose escalation up to 350 mg/m2, in the case of good treatment tolerance. With this regimen, as compared with that described in the German AIO study, our patients experienced less grade 3 or 4 toxicity with regard to anemia (4.6% versus 11%), leukocytopenia (4.6% versus 21%), neutropenic fever (6.8% versus 16%) and diarrhea (2.3% versus 26%). It is noteworthy that 37% of patients in the German trial were escalated to the dose 350 mg/m2 compared with only 18.8% of patients in our study. Therefore, it seems that the dose of 350 mg/m2 may be associated with increased toxicity in this patient population.
Since the efficacy of irinotecan in the second-line setting has been proven to be comparable with the efficacy of taxane (docetaxel or paclitaxel), we believe that irinotecan remains a valuable second-line option in patients who received taxane in the first line. Although the addition of docetaxel to the combination of platinum and fluoropirimidine (PF) in the first-line treatment is a matter of an ongoing debate, recently published studies and meta-analysis provide more data on a higher efficacy (with regard to response rate and survival) of a three-drug combination docetaxel/PF versus PF in this setting [Haj Mohammad et al. 2015; Shah et al. 2015; Wang et al. 2016]. These findings make docetaxel-based regimens especially attractive in the perioperative treatment of gastric cancer and in the palliative therapy, where rapid tumor shrinkage is the objective. Moreover, the utility of irinotecan as second-line therapy is further supported by the fact that in many countries (including Poland) ramucirumab is still unavailable for the treatment of advanced gastroesophageal cancer.
Our study has several limitations. First of all, it is a retrospective analysis with a higher probability of bias compared with randomized, prospective trials. For example, although toxicity observed in our study seems to be favorable compared with the German trial, some patient-reported complaints might not have been collected as precisely in medical charts as it tends to be done in randomized, prospective studies. Secondly, some subgroups of patients analyzed in the univariate and multivariate analysis were small, and it is possible that if a small but true difference existed between the analyzed subgroups, this study may have been underpowered to detect it. Finally, due to the retrospective character of the analysis and lack of access to the imaging studies of some patients while preparing the database, we were unable to provide an objective response rate.
In conclusion, in our retrospective study we have demonstrated that irinotecan in the second-line treatment of advanced gastric and gastro-esophageal adenocarcinoma is associated with a tolerable toxicity, and is an effective salvage regimen with a median OS comparable with that from randomized phase III trials. Although ramucirumab combined with paclitaxel is now considered a ‘gold standard’ second-line regimen in this setting in many countries, a considerable number of patients may not be appropriate candidates for paclitaxel due to taxane administration in the first-line chemotherapy or due to its specific toxicity. Therefore, it seems necessary to examine the role of the irinotecan and ramucirumab combination in this setting in randomized phase III trials.
Acknowledgments
We are grateful to Joanna Gołąb for language editing.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: Dr. Ochenduszko received speaker honoraria from Roche, Amgen and Lilly and received financial support for attending symposia from Roche, Janssen and Pfizer. All other authors declare that they have no conflicts of interest.
Contributor Information
Sebastian Ochenduszko, University Hospital in Krakow – Department of Oncology, ul.Sniadeckich 10, Krakow 31–531, Poland.
Mirosława Puskulluoglu, Jagiellonian University Medical College – Department of Oncology, Krakow, Poland.
Kamil Konopka, University Hospital in Krakow – Department of Oncology, Krakow, Poland.
Kamil Fijorek, Cracow University of Economics – Department of Statistics, Krakow, Poland.
Agnieszka Julia Slowik, Jagiellonian University Medical College – Department of Oncology, Krakow, Poland.
Michał Pędziwiatr, Jagiellonian University Medical College – 2nd Department of General Surgery, Krakow, Poland.
Andrzej Budzyński, Jagiellonian University Medical College – 2nd Department of General Surgery, Krakow, Poland.
References
- Andreyev H., Norman A., Oates J., Cunningham D. (1998) Why do patients with weight loss have a worse outcome when undergoing chemotherapy for gastrointestinal malignancies? Eur J Cancer 34: 503–509. [DOI] [PubMed] [Google Scholar]
- Chau I., Ashley S., Cunningham D. (2009) Validation of the Royal Marsden Hospital prognostic index in advanced esophagogastric cancer using individual patient data from the REAL 2 study. J Clin Oncol 27: 3–4. [DOI] [PubMed] [Google Scholar]
- Chau I., Norman A., Cunningham D., Waters J., Oates J., Ross P. (2004) Multivariate prognostic factor analysis in locally advanced and metastatic esophago-gastric cancer - pooled analysis from three multicenter, randomized, controlled trials using individual patient data. J Clin Oncol 22: 2395–2403. [DOI] [PubMed] [Google Scholar]
- Dreyer N., Tunis S., Berger M., Ollendorf D., Mattox P., Gliklich R. (2010) Why observational studies should be among the tools used in comparative effectiveness research. Health Aff 29: 1818–1825. [DOI] [PubMed] [Google Scholar]
- Ford H., Marshall A., Bridgewater J., Janowitz T., Coxon F., Wadsley J., et al. (2014) Docetaxel versus active symptom control for refractory oesophagogastric adenocarcinoma (COUGAR-02): an open-label, phase 3 randomised controlled trial. Lancet Oncol 15: 78–86. [DOI] [PubMed] [Google Scholar]
- Fuchs C., Tomasek J., Yong C., Dumitru F., Passalacqua R., Goswami C., et al. (2014) Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 383: 31–39. [DOI] [PubMed] [Google Scholar]
- Haj Mohammad N., ter Veer E., Ngai L., Mali R., van Oijen M., van Laarhoven H. (2015) Optimal first-line chemotherapeutic treatment in patients with locally advanced or metastatic esophagogastric carcinoma: triplet versus doublet chemotherapy: a systematic literature review and meta-analysis. Cancer Metastasis Rev 34: 429–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hironaka S., Ueda S., Yasui H., Nishina T., Tsuda M., Tsumura T., et al. (2013) Randomized, open-label, phase III study comparing irinotecan with paclitaxel in patients with advanced gastric cancer without severe peritoneal metastasis after failure of prior combination chemotherapy using fluoropyrimidine plus platinum: WJOG 4007 trial. J Clin Oncol 31: 4438–4444. [DOI] [PubMed] [Google Scholar]
- Iacovelli R., Pietrantonio F., Farcomeni A., Maggi C., Palazzo A., Ricchini F., et al. (2014) Chemotherapy or targeted therapy as second-line treatment of advanced gastric cancer: a systematic review and meta-analysis of published studies. PLoS One 9: e108940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janowitz T., Thuss-Patience P., Marshall A., Kang J., Connell C., Cook N., et al. (2016) Chemotherapy vs supportive care alone for relapsed gastric, gastroesophageal junction, and oesophageal adenocarcinoma: a meta-analysis of patient-level data. Br J Cancer 114: 381–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J., Lee S., Lim D., Park K., Oh S., Kwon H., et al. (2012) Salvage chemotherapy for pretreated gastric cancer: a randomized phase III trial comparing chemotherapy plus best supportive care with best supportive care alone. J Clin Oncol 30: 1513–1518. [DOI] [PubMed] [Google Scholar]
- Kim H., Kim H., Kim S., Kim T., Lee K., Baek S., et al. (2013) Second-line chemotherapy versus supportive cancer treatment in advanced gastric cancer: a meta-analysis. Ann Oncol 24: 2850–2854. [DOI] [PubMed] [Google Scholar]
- Kim H., Kim K., Kim S., Park S. (2015) Outcomes of metastatic gastric cancer in young adult patients treated with first-line combination chemotherapy. Int J Cancer Ther Oncol 3: 344. [Google Scholar]
- Kim J., Sun C., Mailey B., Prendergast C., Artinyan A., Bhatia S., et al. (2010) Race and ethnicity correlate with survival in patients with gastric adenocarcinoma. Ann Oncol 21: 152–160. [DOI] [PubMed] [Google Scholar]
- Matley P., Dent D., Madden M., Price S. (1988) Gastric carcinoma in young adults. Ann Surg 208: 593–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power D., Kelsen D., Shah M. (2010) Advanced gastric cancer – slow but steady progress. Cancer Treat Rev 36: 384–392. [DOI] [PubMed] [Google Scholar]
- Ross P., Ashley S., Norton A., Priest K., Waters J., Eisen T., et al. (2004) Do patients with weight loss have a worse outcome when undergoing chemotherapy for lung cancers? Br J Cancer 90: 1905–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah M. (2015) Update on metastatic gastric and esophageal cancers. J Clin Oncol 33: 1760–1769. [DOI] [PubMed] [Google Scholar]
- Shah M., Janjigian Y., Stoller R., Shibata S., Kemeny M., Krishnamurthi S., et al. (2015) Randomized multicenter phase II study of modified docetaxel, cisplatin, and fluorouracil (DCF) versus DCF plus growth factor support in patients with metastatic gastric adenocarcinoma: a study of the US Gastric Cancer Consortium. J Clin Oncol 33: 3874–3879. [DOI] [PubMed] [Google Scholar]
- Smith B., Stabile B. (2009) Extreme aggressiveness and lethality of gastric adenocarcinoma in the very young. Arch Surg 144: 506–510. [DOI] [PubMed] [Google Scholar]
- Spigel D. (2010) The value of observational cohort studies for cancer drugs. Biotechnol Healthc 7: 18–24. [PMC free article] [PubMed] [Google Scholar]
- Thuss-Patience P., Kretzschmar A., Bichev D., Deist T., Hinke A., Breithaupt K., et al. (2011) Survival advantage for irinotecan versus best supportive care as second-line chemotherapy in gastric cancer - a randomised phase III study of the Arbeitsgemeinschaft Internistische Onkologie (AIO). Eur J Cancer 47: 2306–2314. [DOI] [PubMed] [Google Scholar]
- Torre L., Bray F., Siegel R., Ferlay J., Lortet-Tieulent J., Jemal A. (2015) Global cancer statistics, 2012. CA Cancer J Clin. 65: 87–108. [DOI] [PubMed] [Google Scholar]
- Van Bokhorst-de van der Schuer M., van Leeuwen P., Kuik D., Klop W., Sauerwein H., Snow G., et al. (1999) The impact of nutritional status on the prognoses of patients with advanced head and neck cancer. Cancer 86: 519–527. [PubMed] [Google Scholar]
- Van Cutsem E., Arends J. (2005) The causes and consequences of cancer-associated malnutrition. Eur J Oncol Nurs 9: S51–S63. [DOI] [PubMed] [Google Scholar]
- Wang J., Xu R., Li J., Bai Y., Liu T., Jiao S., et al. (2016) Randomized multicenter phase III study of a modified docetaxel and cisplatin plus fluorouracil regimen compared with cisplatin and fluorouracil as first-line therapy for advanced or locally recurrent gastric cancer. Gastric Cancer 19: 234–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilke H., Muro K., Van Cutsem E., Oh S., Bodoky G., Shimada Y., et al. (2014) Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol 15: 1224–1235. [DOI] [PubMed] [Google Scholar]


