Abstract
Theiler murine encephalomyelitis viruses (TMEVs) are picornaviruses that cause enteric and neurological disease in mice. The GDVII strain and other members of the GDVII subgroup are highly virulent and cause an acute, fatal polioencephalomyelitis following intracerebral inoculation, whereas the DA stain and other members of the TO subgroup cause a persistent, demyelinating infection. We previously produced a full-length, infectious DA cDNA clone. We now describe the generation of a full-length, infectious GDVII cDNA clone and the subsequent production of intratypic chimeric cDNAs and intratypic recombinant viruses. Inoculation of the recombinant viruses into mice demonstrated that a major determinant of TMEV neurovirulence is within the GDVII 1B (capsid protein VP2)-2C coding region, most likely in the GDVII 1B (VP2)-2A coding region. Genomic sequences 5' to this region of GDVII RNA also contribute to expression of the full neurovirulence phenotype. These data demonstrate the multigenic nature of TMEV neurovirulence, as has been reported for other viruses.
Full text
PDF
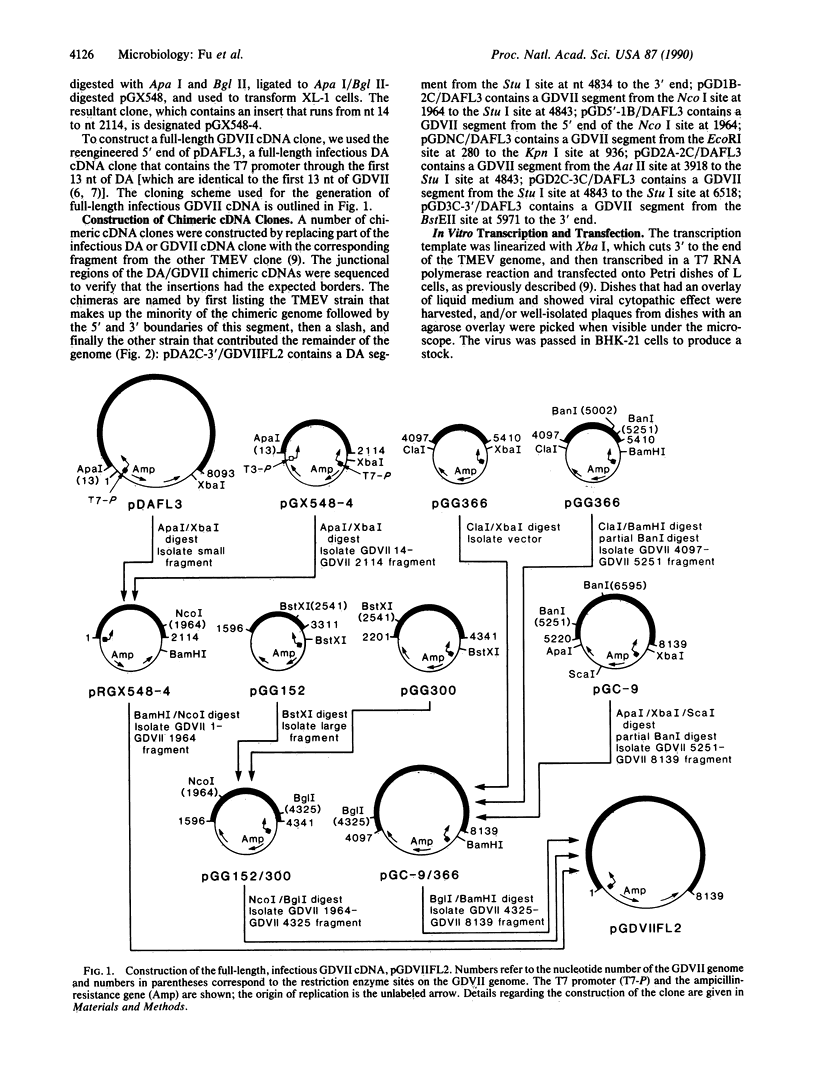


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cash E., Chamorro M., Brahic M. Theiler's virus RNA and protein synthesis in the central nervous system of demyelinating mice. Virology. 1985 Jul 15;144(1):290–294. doi: 10.1016/0042-6822(85)90327-7. [DOI] [PubMed] [Google Scholar]
- Dalziel R. G., Lampert P. W., Talbot P. J., Buchmeier M. J. Site-specific alteration of murine hepatitis virus type 4 peplomer glycoprotein E2 results in reduced neurovirulence. J Virol. 1986 Aug;59(2):463–471. doi: 10.1128/jvi.59.2.463-471.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field H. J., Coen D. M. Pathogenicity of herpes simplex virus mutants containing drug resistance mutations in the viral DNA polymerase gene. J Virol. 1986 Oct;60(1):286–289. doi: 10.1128/jvi.60.1.286-289.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field H. J., Wildy P. The pathogenicity of thymidine kinase-deficient mutants of herpes simplex virus in mice. J Hyg (Lond) 1978 Oct;81(2):267–277. doi: 10.1017/s0022172400025109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javier R. T., Izumi K. M., Stevens J. G. Localization of a herpes simplex virus neurovirulence gene dissociated from high-titer virus replication in the brain. J Virol. 1988 Apr;62(4):1381–1387. doi: 10.1128/jvi.62.4.1381-1387.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye K. M., Spriggs D. R., Bassel-Duby R., Fields B. N., Tyler K. L. Genetic basis for altered pathogenesis of an immune-selected antigenic variant of reovirus type 3 (Dearing). J Virol. 1986 Jul;59(1):90–97. doi: 10.1128/jvi.59.1.90-97.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton H. L. Persistent Theiler's murine encephalomyelitis virus infection in mice depends on plaque size. J Gen Virol. 1980 Jan;46(1):169–177. doi: 10.1099/0022-1317-46-1-169. [DOI] [PubMed] [Google Scholar]
- Lorch Y., Friedmann A., Lipton H. L., Kotler M. Theiler's murine encephalomyelitis virus group includes two distinct genetic subgroups that differ pathologically and biologically. J Virol. 1981 Nov;40(2):560–567. doi: 10.1128/jvi.40.2.560-567.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A., Wychowski C., Couderc T., Crainic R., Hogle J., Girard M. Engineering a poliovirus type 2 antigenic site on a type 1 capsid results in a chimaeric virus which is neurovirulent for mice. EMBO J. 1988 Sep;7(9):2839–2847. doi: 10.1002/j.1460-2075.1988.tb03140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray M. G., Bradley J., Yang X. F., Wimmer E., Moss E. G., Racaniello V. R. Poliovirus host range is determined by a short amino acid sequence in neutralization antigenic site I. Science. 1988 Jul 8;241(4862):213–215. doi: 10.1126/science.2838906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohara Y., Senkowski A., Fu J. L., Klaman L., Goodall J., Toth M., Roos R. P. Trypsin-sensitive neutralization site on VP1 of Theiler's murine encephalomyelitis viruses. J Virol. 1988 Sep;62(9):3527–3529. doi: 10.1128/jvi.62.9.3527-3529.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohara Y., Stein S., Fu J. L., Stillman L., Klaman L., Roos R. P. Molecular cloning and sequence determination of DA strain of Theiler's murine encephalomyelitis viruses. Virology. 1988 May;164(1):245–255. doi: 10.1016/0042-6822(88)90642-3. [DOI] [PubMed] [Google Scholar]
- Paquette Y., Hanna Z., Savard P., Brousseau R., Robitaille Y., Jolicoeur P. Retrovirus-induced murine motor neuron disease: mapping the determinant of spongiform degeneration within the envelope gene. Proc Natl Acad Sci U S A. 1989 May;86(10):3896–3900. doi: 10.1073/pnas.86.10.3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pevear D. C., Borkowski J., Calenoff M., Oh C. K., Ostrowski B., Lipton H. L. Insights into Theiler's virus neurovirulence based on a genomic comparison of the neurovirulent GDVII and less virulent BeAn strains. Virology. 1988 Jul;165(1):1–12. doi: 10.1016/0042-6822(88)90652-6. [DOI] [PubMed] [Google Scholar]
- Pevear D. C., Calenoff M., Rozhon E., Lipton H. L. Analysis of the complete nucleotide sequence of the picornavirus Theiler's murine encephalomyelitis virus indicates that it is closely related to cardioviruses. J Virol. 1987 May;61(5):1507–1516. doi: 10.1128/jvi.61.5.1507-1516.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos R. P., Kong W. P., Semler B. L. Polyprotein processing of Theiler's murine encephalomyelitis virus. J Virol. 1989 Dec;63(12):5344–5353. doi: 10.1128/jvi.63.12.5344-5353.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos R. P., Richards O. C., Green J., Ehrenfeld E. Characterization of a cell culture persistently infected with the DA strain of Theiler's murine encephalomyelitis virus. J Virol. 1982 Sep;43(3):1118–1122. doi: 10.1128/jvi.43.3.1118-1122.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos R. P., Stein S., Ohara Y., Fu J. L., Semler B. L. Infectious cDNA clones of the DA strain of Theiler's murine encephalomyelitis virus. J Virol. 1989 Dec;63(12):5492–5496. doi: 10.1128/jvi.63.12.5492-5496.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos R. P., Stein S., Routbort M., Senkowski A., Bodwell T., Wollmann R. Theiler's murine encephalomyelitis virus neutralization escape mutants have a change in disease phenotype. J Virol. 1989 Oct;63(10):4469–4473. doi: 10.1128/jvi.63.10.4469-4473.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szurek P. F., Yuen P. H., Jerzy R., Wong P. K. Identification of point mutations in the envelope gene of Moloney murine leukemia virus TB temperature-sensitive paralytogenic mutant ts1: molecular determinants for neurovirulence. J Virol. 1988 Jan;62(1):357–360. doi: 10.1128/jvi.62.1.357-360.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taha M. Y., Clements G. B., Brown S. M. A variant of herpes simplex virus type 2 strain HG52 with a 1.5 kb deletion in RL between 0 to 0.02 and 0.81 to 0.83 map units is non-neurovirulent for mice. J Gen Virol. 1989 Mar;70(Pt 3):705–716. doi: 10.1099/0022-1317-70-3-705. [DOI] [PubMed] [Google Scholar]
- Thompson R. L., Devi-Rao G. V., Stevens J. G., Wagner E. K. Rescue of a herpes simplex virus type 1 neurovirulence function with a cloned DNA fragment. J Virol. 1985 Aug;55(2):504–508. doi: 10.1128/jvi.55.2.504-508.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuffereau C., Leblois H., Bénéjean J., Coulon P., Lafay F., Flamand A. Arginine or lysine in position 333 of ERA and CVS glycoprotein is necessary for rabies virulence in adult mice. Virology. 1989 Sep;172(1):206–212. doi: 10.1016/0042-6822(89)90122-0. [DOI] [PubMed] [Google Scholar]
- Weiner H. L., Drayna D., Averill D. R., Jr, Fields B. N. Molecular basis of reovirus virulence: role of the S1 gene. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5744–5748. doi: 10.1073/pnas.74.12.5744. [DOI] [PMC free article] [PubMed] [Google Scholar]




