Abstract
Chronic obstructive pulmonary disease (COPD) is a leading cause of morbidity, mortality and health care expenditure throughout the world. COPD guidelines recommend the use of long-acting muscarinic antagonist (LAMA) either alone or in combination with a long-acting β2 agonist (LABA). For over 10 years, tiotropium was the only LAMA that was used in the management of COPD. Over the past few years, various new drugs have been identified that act on the muscarinic receptors and β2 receptors. Umeclidinium (Umec) is a new LAMA currently approved for use in patients with COPD either as monotherapy or in combination with vilanterol (Vil). Both Umec alone and in combination with Vil delivered through a multi-dose dry powder Ellipta™ device have shown improvement in lung function, health-related quality of life and exacerbation frequency in patients with COPD. This review provides an overview of the pharmacology, pharmacodynamics and pharmacokinetics of Umec, and evaluates the clinical efficacy and safety studies in patients with COPD.
Keywords: chronic obstructive pulmonary disease, glycopyrronium, long-acting muscarinic antagonist, tiotropium, umeclidinium, vilanterol trifenatate
Introduction
Chronic obstructive pulmonary disease (COPD) is a chronic lung condition characterized by progressive and airflow limitation secondary to chronic airway inflammation and injury to the lung parenchyma as a result of sustained cigarette smoke inhalation.1 COPD is increasingly being recognized as having a great impact on public health, and is estimated to become the third leading cause of mortality worldwide within the next two decades.1
Given the lack of any curative strategies, the main therapeutic goals in COPD are to prevent and control symptoms, attenuate the incidence and severity of exacerbations, improve health status and exercise capacity. Therapeutic options are hence based on disease severity and risk of exacerbations. These are usually topically administered and consist of bronchodilators and anti-inflammatory drugs.2 The two main classes of the former manifest their action via different mechanisms including beta-(β)2 receptor agonism and muscarinic receptor antagonism; both of these have been reported to result in improvements in subjective, objective, exacerbation and exercise tolerance parameters in patients with COPD.3
The Global Initiative on Obstructive Lung Disease (GOLD) guidelines classified patients with COPD into four categories, based on airflow limitation, subjective symptoms and the risk of exacerbations.1 GOLD recommends the use of bronchodilator monotherapy for patients with milder COPD, who have less severe symptoms and/or exacerbations; for patients with more severe and/or frequent symptoms, combination treatment with fixed doses of either bronchodilators, namely long-acting β2 agonists (LABAs) in combination with long-acting muscarinic antagonists (LAMAs) (LAMAs/LABAs) or inhaled corticosteroids (ICSs) combined with LABAs (ICS/LABAs) have been proposed. Increasing evidence suggests that the combination of LABAs and LAMAs are more effective than the individual monotherapies.4
In COPD, enhanced parasympathetic activity leads to increased airflow limitation, airway hyperresponsiveness and mucus secretion.5 Hence, the use of anti-cholinergic therapies may be advantageous. Inhaled anti-cholinergics have been used as bronchodilators for decades; however, their use initially was hampered by systemic adverse events and the short duration of action,6–8 until the development of tiotropium bromide (Tio) which had a high affinity for muscarinic receptors (M3) as well as 24-h bronchodilator activity. Despite the UPLIFT study showing significant improvement in lung function, health-related quality of life assessments, attenuated COPD exacerbations, respiratory failure episodes and COPD-related hospitalizations,9 Tio had some limitations, including delayed onset of action, localized adverse events and an expired drug patent. Additionally, other clinical unmet needs remain, which may have an impact on COPD-related parameters, including psychosocial issues and possibly mortality, as well as inadequate concordance related to complex procedures in inhaler use, alongside dozing frequency.10–12
In recent years, a number of new LAMAs have been developed to try to overcome some of the shortfalls associated with Tio, as well as the use of alternative devices in providing the patients with a choice of device, in anticipation that this may improve compliance and eventually clinical outcomes.13 These include glycopyrronium bromide and aclidinium bromide delivered through the Breezehaler™ (Novartis Pharmaceuticals, Camberley, UK) and Genuair™ (AstraZeneca, Luton, UK) devices, respectively. In this review, we focus on a new anti-cholinergic, umeclidinium (Umec) bromide (previously known as GSK573719; Glaxosmithkline®, Uxbridge, UK), once-daily inhalation therapy for use in COPD. We discuss its use as monotherapy and in combination with LABA, vilanterol (Vil) trifenatate. We focus on Umec pharmacokinetics and pharmacodynamics, efficacy (subjective, objective, exacerbation and exercise tolerance parameters) and safety.
Cholinergic receptors and their mechanism of action
In most mammals, including humans, the predominant innervation is via the cholinergic parasympathetic nerves.6 Although there are five subtypes of muscarinic acetylcholine (Ach) receptors (mAchRs), that is M1–5, only M1–3 are expressed in humans.14,15 These receptors are located in the vagal nerve, submucosal glands, pulmonary vasculature and bronchial smooth muscles in the airway. The mAchRs are 7-transmembrane domains, single-glycoprotein receptors united by intra- and extracellular loops that may be linked to ion channels (K+ or Ca2+). The binding of Ach ligand typically results in activation of adenylyl cyclase, activation of phospholipase C and opening of potassium channels. The release of Ach regulates airway tone, airway smooth muscle contraction, mucus secretion and vasodilatation through the interaction with mAchRs located on various structures of the airways.16–19 Detailed explanations of the binding and subsequent manifestations of mAchRs have been discussed elsewhere.6,13,20
The M3 Ach receptor is the primary mAchR, implicated in the regulation of various airway outcomes despite its lower expression than M2 receptors in the airways.6,20 COPD is characterized by increased parasympathetic activity21,22 which may be partially reversed with anti-cholinergic therapy and subsequently improve airflow limitation. The ideal anti-cholinergic agent for use in COPD should antagonize the M1 and M3 Ach receptors with little affinity for the M2 Ach receptors that counteract β2 adrenoceptor-mediated relaxant pathways.23 Recently, a number of new anti-cholinergic therapies have been licensed for use in COPD, designed to not only provide greater efficacy by attempting to inhibit more specifically the mAchRs subtypes, but also to have an enhanced safety profile.13
Umeclidinium bromide pharmacodynamics
Figure 1 shows the molecular structure of Umec. Preclinical studies of Umec using radioligand binding studies in recombinant assays as well as human and guinea pig tissue systems have been used to characterize its Ach binding and functional properties.24
Figure 1.
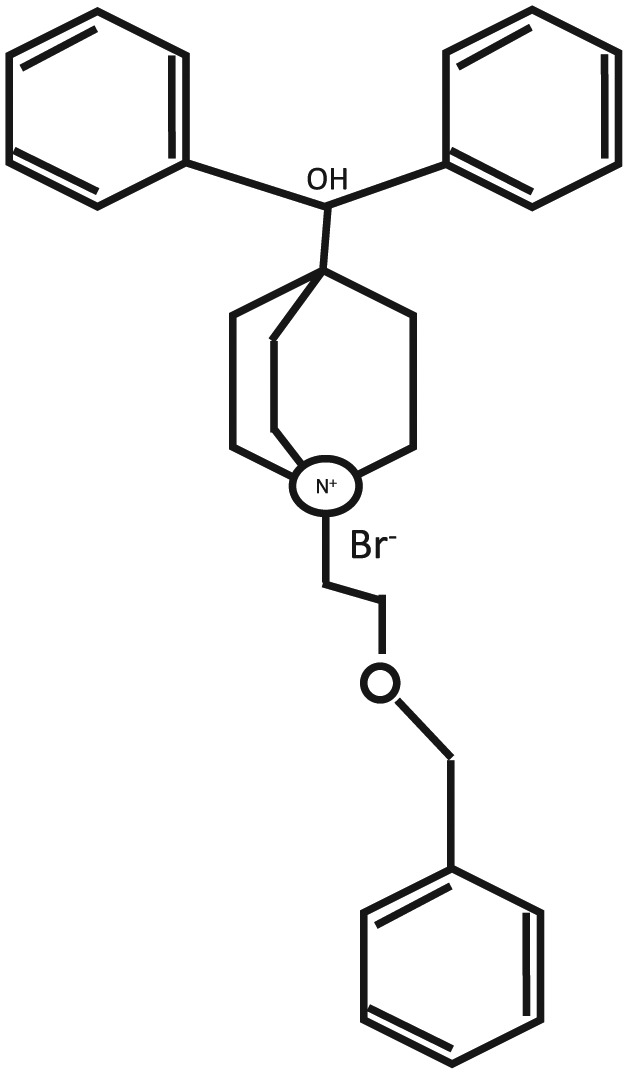
The molecular structure of umeclidinium.
The association and dissociation binding to the M2 and M3 receptors for Umec and Tio was rapid for each of the receptor types, and comparable, although the dissociation from the M3 mAchRs was far slower than from M2 mAchRs for Umec (82 versus 9 min, respectively) and Tio (273 versus 39 min, respectively).24 In Chinese hamster ovary cells transfected with recombinant human M3 mAchRs, Umec demonstrated low potency in Ach-mediated Ca2+ mobilization assay. Experiments with isolated bronchial strips showed that Umec was potent and showed competitive antagonism versus carbachol, and was slowly reversible in a concentration-dependent manner, similar to Tio. The time to 50% restoration of contraction at 10 nm was, however, shorter for Umec (381 min) than for Tio (413 min). Similar experiments at similar concentrations of carbachol (10 nm) in guinea pig trachea and strips showed little difference between Umec and Tio.
In in vivo mouse models of COPD, intranasal administration of Umec resulted in a blocking of nebulized methacholine in a dose-dependent manner.24 The inhibitory effect following a single-dose Umec administration was sustained at 50% or greater for up to 72 h. Similar findings were reported in the same study for Tio. Intranasal administration of once-daily Umec in mice on five consecutive days showed only a slight increase in inhibition with methacholine on the fifth day compared to the first; and after a 5-day gap similar inhibition was noted as at the first dose administered. Oral administration of Umec provided no observable protection against methacholine. Intratracheal instillation of Umec in conscious guinea pigs produced a dose-dependent Ach-induced bronchoconstriction for a prolonged period of time, but was not dose proportional. In anaesthetized guinea pigs, Umec caused an inhibition Ach-induced airway tone and a decrease in heart rate in a dose-dependent fashion. Similar outcomes were noted when Tio was administered to conscious and anaesthetized guinea pigs.
Based on the pharmacodynamics, Umec is a potent anti-cholinergic agent with slow functional reversibility at the human M3 mAchR with a prolonged duration of action in vitro and animal in vivo models. This would suggest that Umec is a good once-daily therapeutic option.
Pharmacokinetics of umeclidinium
In healthy volunteers, one-off and multiple-dose inhalations of Umec have shown a Tmax of 5–15 min.25,26 At 14 days, with repeated inhalations of Umec a t1/2 of 26–28 h was achieved in the steady-state at 6–8 days of dose initiation.26 Similar Umec Tmax times have been reported in different ethnic populations as monotherapy or in combination with Vil.27,28 In a Chinese cohort of healthy participants, Umec accumulation following repeated dosing was 11–34% based on Cmax and 19–59% based on the area under the concentration–time curve from time 0 to 2 h (AUC0–2). Following single and repeat administration, the inter-subject coefficient of variation for all Umec pharmacokinetic parameter estimates ranged from 12% to 165% (including when in combination with Vil), indicating a wide range of variability in inhaled pharmacokinetic parameters.28 Urinary excretion of unchanged Umec was >1.5% and >4.5% of the total dose at day 1 and steady-state respectively.26 Single doses of oral and intravenous Umec have indicated negligible oral Umec bioavailability, low gut absorption, tissue distribution, biliary elimination of parent drug and its metabolites, and minimal urinary excretion.29 In a small study of patients with severe renal impairment and matched healthy volunteers, single doses of Umec monotherapy and in combination with Vil reported no clinically relevant increases in systemic exposure compared to healthy controls, hence implying no dose adjustment is essential.30
In order to assess changes in drug levels when used in combination with enzyme inhibitors, a number of studies have been conducted. Co-administration of Umec with verapamil (an inhibitor of P-glycoprotein and CYP3A4) or cytochrome p450 isoenzyme 2D6 (CYP2D6), an enhancer of Umec metabolism, did not alter levels of Umec or result in relevant drug interactions in healthy participants.31 Inhaled Umec monotherapy or in combination with Vil showed no clinically significant changes in QTcF when administered with placebo or moxifloxacin.32
As a secondary objective of a phase IIb, randomized, double-blind, double-dummy, multicentre, placebo-controlled, three-way crossover, incomplete block, 14-day study of various Umec doses (62.5–1000 µg) the pharmacokinetics at days 1, 7 and 14 were assessed in 176 patients with moderate-to-severe COPD. The AUC and geometric mean peak plasma concentrations showed a steady-state achievement by day 7 for most dose regimens, with little increase in the systemic Umec exposure from day 7 to day 14.33 Also, as a secondary objective in another 12-week, phase IIIa, randomized, double-blind, placebo-controlled, multicentre, parallel-group study, the pharmacokinetics of inhaled Umec (62.5 µg and 125 µg) were compared with placebo in moderate-to-severe COPD patients. At days 1 (single), 28 and 84 (multiple), the pharmacokinetics of both Umec doses were similar to those reported in the earlier study by Donohue and colleagues (Tmax of 5–15 min post-dose).34
To evaluate the impact of patient demographics and baseline characteristics on inhaled Umec and Vil exposure, Goyal and colleagues studied the population pharmacokinetics of these two drugs as monotherapies or in combination in 1635 COPD patients from two phase III, randomized, double-blind, parallel-group, placebo-controlled trials.35 Using the NONMEM® software (Leopardstown, Ireland), the researchers established that there were no apparent pharmacokinetic interactions when Umec and Vil were co-administered. Furthermore, no dose adjustments were essential on systemic exposure of Umec and Vil due to patient demographic, including age, body weight and creatinine clearance.
Clinical efficacy of umeclidinium in COPD
The efficacy and safety of Umec were examined in patients with moderate-to-severe COPD with two different doses (62.5 µg and 125 µg) over 12 weeks.34 Both doses improved trough forced expiratory volume in 1 s (FEV1); 62.5 µg by 127 ml and 125 µg by 152 ml. There was also improvement in the mean transitional dyspnoea index (TDI) and St. George’s Respiratory Questionnaire (SGRQ) scores compared to placebo.
A recent review and meta-analysis of Umec in patients with moderate-to-severe COPD showed significant improvements in lung function, including trough FEV1 as well as in exacerbation rates for COPD, breathlessness and quality of life. Anti-cholinergic side effects were uncommon with Umec, with the efficacy being similar to Tio.36
In a 52-week, double-blind, randomized controlled phase IIIa trial in 562 patients with COPD the safety of combination treatment with Umec and Vil (Umec/Vil), Umec was compared with placebo in an intention-to-treat analysis.37 This was essentially a safety study for Umec and Umec/Vil combination. There were fewer patients reporting COPD exacerbations with Umec/Vil 125/25 µg and Umec 125 µg (13% and 15% respectively) compared to placebo (24%). Overall, Umec/Vil 125/25 µg and Umec 125 µg were well tolerated over the 12 months of treatment in patients with COPD, providing greater improvements in lung function and rescue medication use compared to placebo. The incidence of adverse events, serious adverse events and drug-related adverse events were similar across active treatment groups and placebo. Headache was the most common adverse event across all treatments, followed by nasopharyngitis and ventricular extra-systoles. Adverse events leading to permanent discontinuation or withdrawal were reported in 8% of patients in the Umec/Vil 125/25 µg group, 9% of patients in the Umec 125 µg group, and 12% of patients in the placebo group. There was a lower overall incidence of cardiovascular adverse events reported in association with Umec/Vil 125/25 µg than Umec 125 µg or placebo. Umec 125 µg may be associated with an increase in atrial arrhythmias; however, the observations of supraventricular tachycardia and supraventricular extra-systoles were not associated with reports of clinically relevant symptoms like hypotension or syncope. Ocular effects and anti-cholinergic syndrome were reported in <1–2% of patients in each treatment group. There were no clinically significant changes from baseline in any clinical chemistry or haematological parameters in any treatment group, including glucose levels.
The clinical development programme of Umec regarding safety included eight completed monotherapy studies with Umec of a duration of >4 weeks. The most commonly reported adverse events with >3% incidence included headache, nasopharyngitis, cough and upper respiratory tract infections. The most common serious adverse event was exacerbation of COPD, which is not unexpected in this population. Cardiac-related side effects were noted in both Umec 62.5 µg and 125 µg groups compared to placebo. The most important cardiovascular findings were atrial arrhythmias (supraventricular tachycardia, atrial fibrillation and atrial ectopics). Given that patients with COPD are at risk for major adverse cardiovascular events (MACEs), additional analyses on MACEs was conducted; there was no evidence of increased risk of MACE with either dose of Umec compared with placebo. Pneumonia is a common background event in patients with COPD and Umec was not associated with an increased risk of pneumonia compared to placebo. The overall safety profile of Umec was similar to placebo and no difference in safety profile was observed in the two dosing (62.5 µg and 125 µg) groups.
Pharmacology of vilanterol
Vil trifenatate is a novel selective LABA with bronchodilatory effects lasting for 24 h. Vil is an ante-drug analogue of salmeterol, with a higher intrinsic activity at the β2 adrenoceptor.38 The development of Vil was based on the modification of the salmeterol molecule to create homochiral compounds with the (R)-configuration, as the (R)-enantiomer of salmeterol is more potent. The pharmacological effects of Vil are essentially due to the β2 agonistic effect. Saturation binding studies with Vil report that the drug is bound to one or two β2 receptors with an enhanced affinity interaction with the β2 receptor. Vil is highly selective, with over 1000-fold selectivity to the β2 receptor compared to β1 or β3 adrenoceptor.39 Vil’s β2 selectivity is similar to salmeterol but markedly higher than formoterol or indacaterol. The drug has extensive first-pass metabolism and is primarily excreted in the urine. Vil per se does not require any caution in patients with hepatic and renal impairment. Unlike other bronchodilators, Vil is not available for use as a single agent and is approved for use in COPD only in fixed-dose combination with Umec or with fluticasone furoate (FF). Umec/Vil is an orally inhaled dry powder inhaler through an Ellipta™ (GlaxosmithKline, Uxbridge, UK) device. The dosage of one Umec/Vil inhalation is 62.5/25 µg once daily, equivalent to the delivered dose of 55/22 µg.40
Efficacy in patients with chronic obstructive pulmonary disease
The combination of Umec/Vil has been evaluated in several clinical trials and included >9000 patients with COPD to evaluate the efficacy and safety profile of the treatment.37,41–44 The duration of the studies ranged from 12 weeks to 52 weeks. Phase II and III trials in patients with COPD examined Umec/Vil and compared it to the mono-components – that is Vil 25 µg, Umec 125 µg or 62.5 µg, Tio 18 µg or fluticasone propionate combined with salmeterol. The studies and their outcomes are shown in Table 1.
Table 1.
A summary of the studies involving umeclidinium.
| Study | Trial design | Subjects | Study duration | Study drug | Outcomes |
|---|---|---|---|---|---|
| Trivedi et al.34 | Randomized placebo-controlled | 168 | 12 weeks | Umec 62.5 µg and 125 µg | Both doses improved trough FEV1 by 127 ml and 152 ml respectively, significant SGRQ improvement compared to placebo for both doses, significant improvement in TDI for Umec 125 µg |
| Donohue et al.37 | Randomized double-blind placebo-controlled | 562 | 52 weeks | Umec/Vil 125/25 µg, Umec 125 µg, or placebo |
Incidents of on-treatment AEs, serious AEs and drug-related AEs was similar between groups, incidents of atrial arrhythmias were similar between groups, both active treatment improved lung functions compared to placebo |
| Celli et al.41 | Double-blind placebo-controlled parallel-group | 1493 | 24 weeks | Umec/Vil 125/25 µg, Umec 125 µg, Vil 25 µg, or placebo | All active treatments improved trough FEV1 compared to placebo, improvements for Umec/Vil 125/25 µg versus placebo were observed for the TDI, rescue albuterol use at weeks 1–24 and SGRQ |
| Donohue et al.43 | Double-blind placebo-controlled parallel-group | 532 | 4 weeks | Umec/Vil 62.5/25 µg, Umec 62.5 µg, Vil 25 µg or placebo | All active treatments improved FEV1 compared to placebo, increases with Umec/Vil 62.5/25 µg were significantly greater than monotherapies |
| Decramer et al.37 | Randomized blinded double-dummy parallel-group | 1141 | 24 weeks | Umec/Vil 125/5 µg, Umec/Vil 62.5/25 µg, Tio 18 µg, and either Vil 25 µg (study 1) or Umec 125 µg (study 2) | FEV1 improved in both studies on day 169 compared to Tio monotherapy, both doses of Umec/Vil improved FEV1 compared to Vil alone, all treatments produced improvement in dyspnoea and health-related quality of life |
| Maleki- Yazdi et al.44 | Randomized blinded double-dummy parallel-group | 1191 | 24 weeks | Umec/Vil 62.5/25 µg versus Tio 18 µg | A significant improvement in FEV1 in favour of Umec/Vil compared to Tio, Umec/Vil improved SGRQ and reduced the need for rescue medication compared to Tio |
AE, adverse events; FEV1, forced expiratory volume in 1 s; ml, millilitres; SGRQ, St. George’s respiratory questionnaire; TDI, transitional dyspnoea index; Tio, tiotropium; Umec, umeclidinium; Vil, vilanterol.
In patients with COPD, the combination of Umec/Vil improved trough FEV1 by at least 100 ml (clinically significant) above baseline compared to patients treated with Umec alone. Furthermore, Umec/Vil significantly increased the percentage of patients with minimal clinically important difference (MCID) in TDI and use of rescue medication compared to monotherapy. There was no significant difference in mortality or serious adverse events between the treatment arms. No significant differences were observed between the Umec doses (62.5 µg or 125 µg) either in combination with Vil or as monotherapy.45
Results from five studies41–43,46 that compared Umec/Vil (125/25 µg or 62.5 µg) with Vil 25 µg showed that the trough FEV1 improved by 110 ml at the end of treatment, favouring the combination. Overall 53.6% of patients achieved an increase of >100 ml in trough FEV1 in the combination compared to 37.9% in the monotherapy. Furthermore, Umec/Vil reduced COPD exacerbations significantly (6.1% versus 8.5%). Safety outcomes revealed significant reduction in serious adverse events, favouring the Umec/Vil combination.
Three trials from two studies compared Umec/Vil versus Tio.42,43 At the end of treatment, the trough FEV1 improved by 90 ml in the Umec/Vil group compared to Tio. There was no difference in the risk of serious adverse events between the groups. No significant difference in MCID in TDI, percentage of patients with MCID in SGRQ, exacerbations of COPD or withdrawal from clinical study due to any reason were reported. The fixed-dose combination of Umec/Vil was compared in a 12-week randomized controlled trial with once-daily Tio 18 µg and indacaterol 150 µg by Kalberg and colleagues.47 There was non-inferiority between the two groups, with similar improvements in trough FEV1. The improvements in lung function measures were paralleled by comparable improvements in patient-reported measures of dyspnoea, health-related quality of life and rescue medication use. Furthermore, the incidence of adverse events and COPD exacerbation were similar between the groups with headache and nasopharyngitis reported most frequently.
Closed triple therapy
Triple combination therapy with ICS, LABA and LAMA agents is a recommended option for patients with COPD at high risk of exacerbations (GOLD stage D). Sousa and colleagues recently evaluated the effect of Umec added to ICS/LABA in patients with moderate-to-severe COPD.48 Umec combined with ICS/LABA resulted in significant improvements in trough FEV1 compared with placebo. The adverse events were similar between the active group and placebo. Pneumonia was reported in 3% of the study population in the Umec group compared to 2% in the placebo. The exacerbation frequency for COPD was similar in both groups (14%).
FF/Vil/Umec is a once-daily ICS/LABA/LAMA in phase III development in patients with COPD. Initial pharmacokinetic studies suggest no clinically relevant difference in systemic exposure of FF, Umec or Vil when administered as a triple therapy compared to the approved FF/Vil or Umec/Vil combinations.49 The study also showed that closed triple therapy allows the three drugs to be administered through a single inhaler device, offering the advantage of improved compliance and better outcomes in patients with severe COPD and frequent exacerbations.
The phase III study of once-daily closed triple combination with FF/Umec/Vil in patients with COPD compared with twice-daily budesonide/formoterol met its endpoints, including improvements in FEV1 and health-related quality of life as measured by SGRQ scores. There was a 171 ml improvement in the trough FEV1 and a 2.2 unit reduction in SGRQ score. These were statistically significant at 24 weeks. At 24 and 52 weeks the most common side effects noted across both treatment arms were nasopharyngitis, exacerbation of COPD and headache. At 52 weeks, the incidence of investigator-reported serious adverse events was 10.0% for FF/Umec/Vil and 12.7% for budesonide/formoterol, respectively, of which the incidence of worsening COPD was 2.4% and 9.1%; for pneumonia was 1.9% and 1.8%; and for cardiac disorders was 1.4% and 0.9%, respectively.50
The recently completed TRILOGY trial evaluated the combination of extra-fine beclomethasone, formoterol, and glycopyrronium (BDP/FM/GB) in patients with COPD.51 At 26 weeks the triple combination improved pre- and post-dose FEV1 and TDI significantly compared to BDP/FM. There was a 23% reduction in the annual adjusted moderate-to-severe exacerbation frequencies. Closed triple therapy has the potential for improving compliance and hence improved efficacy outcomes.
Comparison studies
To establish non-inferiority and/or superiority, head-to-head comparisons are essential. In the last year, two head-to-head studies comparing Umec and other licensed LAMAs have been conducted.52,53
In a 12-week, multicentre, randomized, blinded, double-dummy, parallel-group study, 1017 (976 in the per-protocol population) participants with moderate-to-severe COPD were randomly assigned to receive once-daily 62.5 µg Umec or 18 µg Tio (1:1).52 At day 85, the primary endpoint, change in trough FEV1 from baseline, in both the intention-to-treat (53 ml) and per-protocol (59 ml) populations were greater than the non-inferiority threshold, in favour of Umec. Additionally, Umec resulted in a statistically significant difference in the mean change from baseline in trough FEV1 and forced vital capacity (FVC) compared to Tio at days 28, 56 and 84. Similar-weighted mean FEV1 outcomes were reported at day 84 in the 0–12 h and 0–24 h post-dose times, although there was a significant improvement in favour of Umec in the 12–24 h post-dose outcomes. Importantly, subjective parameter outcomes including TDI focal score, SGRQ total score and CAT scores, as well as rescue medication use, were similar for both treatments at day 84. Likewise, similar overall incidences of adverse events were noted. A post-hoc analysis showed a greater change in trough FEV1 in the Umec- versus Tio-treated patients with GOLD stage 2 COPD at day 84; however, this was not the case for patients at GOLD grade 3 COPD.
Another 12-week, multicentre, randomized, open-label, two-arm, parallel-group non-inferiority study conducted by Rheault and colleagues compared once-daily 62.5 µg Umec with glycopyrronium 44 µg (1:1) in 1034 (986 in the per-protocol population) COPD subjects with moderate-to-severe airflow obstruction.53 There was no difference observed in the primary endpoint of trough FEV1 at day 85 between the two treatments in the intention-to-treat and per-protocol populations. Likewise, there were no significant differences between the two treatments in trough FVC, and 0–24, 0–12 and 12–24 h weighted mean FEV1 outcomes. Similar lack of difference between the two treatments was noted in subjective parameters of TDI, SGRQ and CAT scores, as well as rescue medication use. Adverse event profiles were comparable for the two treatment groups.
Conclusion
Inhaled Umec, available as monotherapy or in combination with Vil, is a potent antagonist at the M3 cholinergic receptor with a prolonged duration of action comparable to other anti-cholinergic agents. Furthermore, Umec is safe to use in patients taking drugs for other conditions as well as in patients with renal impairment.
Umec is delivered using the unique Ellipta delivery device. This enables drug delivery with a minimal number of manoeuvres for activation and hence reduced critical patient errors. Using the appropriate inhaler device is known to improve adherence to medication and also provide patient satisfaction, both of which have a direct impact on treatment outcomes.54 Additionally, the Ellipta device has been designed to deliver a standardized distribution of the active ingredients to the airways even in cases of poor inspiratory capacity. When compared to other inhaler delivery systems in patients with COPD, the Ellipta device was not only shown to be preferable, but associated with the least number of critical areas associated with inhaler use.55 This is also the case with just reading the patient information leaflet in the absence of any formal healthcare professional input.56 Overall, the Ellipta delivery device seems to be a robust and easy-to-use system with good drug delivery; the characteristics of the device are particularly appreciated by patients, especially those with COPD. The ability of Umec to be used as monotherapy, dual therapy with Vil and also as triple therapy with Vil and FF may allow an easy step-up regimen as needed, as the same delivery device in the form of the Ellipta is used. Likewise, the step down in therapy can also be easily achieved. This novelty may be advantageous for individuals initiated on therapies using the devices.
Data from clinical trials have shown that not only is Umec an effective treatment as monotherapy, but also dual bronchodilator therapy in combination with Vil and triple therapy (Umec, Vil and FF) in improving lung function and subjective parameters, and rescue medication use, besides being safe to use. Moreover, recent studies have shown that Umec is as effective, if not marginally superior, in terms of improvements in lung function parameters compared to currently licensed inhaled anti-cholinergic therapies.
The critical goals of COPD management are to reduce disease progression and mortality. The availability of newer molecules and improved combinations combined with a significant input into the inhaler devices by pharmaceutical companies has provided patients with COPD with options of inhaler device and LAMA, LAMA/LABA, LAMA/LABA/ICS to choose from to control the symptoms and improve their quality of life. Future studies aimed at enhancing compliance and halting the progression of the disease would go a long way in improving care for patients with COPD. Additionally, head-to-head studies of LAMAs/LABAs with some of the LAMA monotherapies and ICS/LABAs would pave the way for identifying the optimal combination for patients.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Conflict of interest statement: KSB has received honoraria for speaking and financial support to attend meetings from Wyeth, Chiesi, GSK, Teva, Novartis, Boehringer Ingelheim and AstraZeneca. JBM has received honoraria for speaking and financial support to attend meetings/advisory boards from Wyeth, Chiesi, Pfizer, MSD, Boehringer Ingelheim, Teva, GSK/Allen & Hanburys, Napp, Almirall, AstraZeneca and Novartis.
Contributor Information
Kesavan Suresh Babu, Department of Respiratory Medicine, Queen Alexandra Hospital, Cosham, Portsmouth, UK.
Jaymin Bhagwanji Morjaria, Department of Respiratory Medicine, Harefield Hospital, Hill End Road, Harefield UB9 6JH, UK.
References
- 1. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management and prevention of COPD, http://www.goldcopd.org (2014, accessed May 2016).
- 2. Donohue JF, Ohar JA. Bronchodilator therapy of airway disease. In: Chung KF, Barnes PJ. (eds) Pharmacology and therapeutics of airways disease. New York: Informa Healthcare USA, 2009, pp.198–225. [Google Scholar]
- 3. Ohar JA, Donohue JF. Mono- and combination therapy of long-acting bronchodilators and inhaled corticosteroids in advanced COPD. Semin Respir Crit Care Med 2010; 31: 321–333. [DOI] [PubMed] [Google Scholar]
- 4. Rodrigo GJ, Plaza V, Castro-Rodriguez JA. Comparison of three combined pharmacological approaches with tiotropium monotherapy in stable moderate to severe COPD: a systematic review. Pulm Pharmacol Ther 2012; 25: 40–47. [DOI] [PubMed] [Google Scholar]
- 5. Barnes PJ. The role of anticholinergics in chronic obstructive pulmonary disease. Am J Med 2004; 117(Suppl. 12A): 24S–32S. [DOI] [PubMed] [Google Scholar]
- 6. Belmonte KE. Cholinergic pathways in the lungs and anticholinergic therapy for chronic obstructive pulmonary disease. Proc Am Thorac Soc 2005; 2: 297–304; discussion 11–12. [DOI] [PubMed] [Google Scholar]
- 7. Gross NJ, Skorodin MS. Role of the parasympathetic system in airway obstruction due to emphysema. N Engl J Med 1984; 311: 421–425. [DOI] [PubMed] [Google Scholar]
- 8. Flohr E, Bischoff KO. Oxitropium bromide, a new anticholinergic drug, in a dose–response and placebo comparison in obstructive airway diseases. Respiration 1979; 38: 98–104. [DOI] [PubMed] [Google Scholar]
- 9. Tashkin DP, Celli B, Senn S, et al. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med 2008; 359: 1543–1554. [DOI] [PubMed] [Google Scholar]
- 10. Calverley PM. COPD: what is the unmet need? Br J Pharmacol 2008; 155: 487–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Partridge MR, Karlsson N, Small IR. Patient insight into the impact of chronic obstructive pulmonary disease in the morning: an internet survey. Curr Med Res Opin 2009; 25: 2043–2048. [DOI] [PubMed] [Google Scholar]
- 12. Agusti A, Hedner J, Marin JM, et al. Night-time symptoms: a forgotten dimension of COPD. Eur Respir Rev 2011; 20: 183–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Prakash A, Babu KS, Morjaria JB. Novel anti-cholinergics in COPD. Drug Discov Today 2013; 18: 1117–1126. [DOI] [PubMed] [Google Scholar]
- 14. Caulfield MP, Birdsall NJ. International Union of Pharmacology. XVII. Classification of muscarinic acetylcholine receptors. Pharmacol Rev 1998; 50: 279–290. [PubMed] [Google Scholar]
- 15. van Koppen CJ, Blankesteijn WM, Klaassen AB, et al. Autoradiographic visualization of muscarinic receptors in pulmonary nerves and ganglia. Neurosci Lett 1987; 83: 237–240. [DOI] [PubMed] [Google Scholar]
- 16. Gosens R, Zaagsma J, Meurs H, et al. Muscarinic receptor signaling in the pathophysiology of asthma and COPD. Respir Res 2006; 7: 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Eglen RM, Hegde SS, Watson N. Muscarinic receptor subtypes and smooth muscle function. Pharmacol Rev 1996; 48: 531–565. [PubMed] [Google Scholar]
- 18. Villetti G, Pastore F, Bergamaschi M, et al. Bronchodilator activity of (3R)-3-[[[(3-fluorophenyl)[(3,4,5-trifluorophenyl)methyl]amino] carbonyl]oxy]-1-[2-oxo-2-(2-thienyl)ethyl]-1-azoniabicyclo[2.2.2]octane bromide (CHF5407), a potent, long-acting, and selective muscarinic M3 receptor antagonist. J Pharmacol Exp Ther 2010; 335: 622–635. [DOI] [PubMed] [Google Scholar]
- 19. Paredes-Gamero EJ, Medeiros VP, Farias EH, et al. Heparin induces rat aorta relaxation via integrin-dependent activation of muscarinic M3 receptors. Hypertension 2010; 56: 713–721. [DOI] [PubMed] [Google Scholar]
- 20. Karakiulakis G, Roth M. Muscarinic receptors and their antagonists in COPD: anti-inflammatory and antiremodeling effects. Mediators Inflamm 2012; 2012: 409580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wessler IK, Kirkpatrick CJ. The non-neuronal cholinergic system: an emerging drug target in the airways. Pulm Pharmacol Ther 2001; 14: 423–434. [DOI] [PubMed] [Google Scholar]
- 22. Gosens R, Zaagsma J, Grootte Bromhaar M, et al. Acetylcholine: a novel regulator of airway smooth muscle remodelling? Eur J Pharmacol. 2004; 500: 193–201. [DOI] [PubMed] [Google Scholar]
- 23. Restrepo RD. Use of inhaled anticholinergic agents in obstructive airway disease. Respir Care 2007; 5: 833–851. [PubMed] [Google Scholar]
- 24. Salmon M, Luttmann MA, Foley JJ, et al. Pharmacological characterization of GSK573719 (umeclidinium): a novel, long-acting, inhaled antagonist of the muscarinic cholinergic receptors for treatment of pulmonary diseases. J Pharmacol Exp Ther 2013; 345: 260–270. [DOI] [PubMed] [Google Scholar]
- 25. Cahn A, Lovick R, Newlands A, et al. Safety, tolerability, pharmocodynamics (PD0 and pharmocokinetics (PK) of GSK573719 inhalation powder in healthy subjects. Eur Respir J 2011; 38(Suppl. 55): 723s; A3971. [Google Scholar]
- 26. Mehta R, Hardes K, Cahn A, et al. Safety, tolerability and pharcokinetics (PK) of repeated doses of GSK573719 inhalation powder, a new long-acting muscarinic antagonist, in healthy adults. Eur Respir J 2011; 38(Suppl. 55): 723s; A3972. [Google Scholar]
- 27. Kelleher DL, Mehta RS, Jean-Francois BM, et al. Safety, tolerability, pharmacodynamics and pharmacokinetics of umeclidinium and vilanterol alone and in combination: a randomized crossover trial. PLoS One 2012; 7: e50716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hu C, Jia J, Dong K, et al. Pharmacokinetics and tolerability of inhaled umeclidinium and vilanterol alone and in combination in healthy Chinese subjects: a randomized, open-label, crossover trial. PLoS One 2015; 10: e0121264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kelleher D, Hughes S, Mehta R, et al. Absorption, distribution, metabolism, and elimination (ADME) of umeclidinium (UMEC) in healthy adults. Eur Respir J 2012; 40(Suppl. 56): 384s; A2153. [Google Scholar]
- 30. Mehta R, Hardes K, Brealey N, et al. Effect of severe renal impairment on umeclidinium and umeclidinium/vilanterol pharmacokinetics and safety: a single-blind, nonrandomized study. Int J Chron Obstruct Pulmon Dis 2015; 10: 15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mehta R, Kelleher D, Preece A, et al. Effect of verapamil on systemic exposure and safety of umeclidinium and vilanterol: a randomized and open-label study. Int J Chron Obstruct Pulmon Dis 2013; 8: 159–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kelleher D, Tombs L, Preece A, et al. A randomized, placebo- and moxifloxacin-controlled thorough QT study of umeclidinium monotherapy and umeclidinium/vilanterol combination in healthy subjects. Pulm Pharmacol Ther 2014; 29: 49–57. [DOI] [PubMed] [Google Scholar]
- 33. Donohue JF, Anzueto A, Brooks J, et al. A randomized, double-blind dose-ranging study of the novel LAMA GSK573719 in patients with COPD. Respir Med 2012; 106: 970–979. [DOI] [PubMed] [Google Scholar]
- 34. Trivedi R, Richard N, Mehta R, et al. Umeclidinium in patients with COPD: a randomised, placebo-controlled study. Eur Respir J 2014; 43: 72–81. [DOI] [PubMed] [Google Scholar]
- 35. Goyal N, Beerahee M, Kalberg C, et al. Population pharmacokinetics of inhaled umeclidinium and vilanterol in patients with chronic obstructive pulmonary disease. Clin Pharmacokinet 2014; 53: 637–648. [DOI] [PubMed] [Google Scholar]
- 36. Pleasants RA, Wang T, Gao J, et al. Inhaled umeclidinium in COPD patients: a review and meta-analysis. Drugs 2016; 76: 343–361. [DOI] [PubMed] [Google Scholar]
- 37. Donohue JF, Niewoehner D, Brooks J, et al. Safety and tolerability of once-daily umeclidinium/vilanterol 125/25 mcg and umeclidinium 125 mcg in patients with chronic obstructive pulmonary disease: results from a 52-week, randomized, double-blind, placebo-controlled study. Respir Res 2014; 15: 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Procopiou PA, Barrett VJ, Bevan NJ, et al. Synthesis and structure–activity relationships of long-acting beta2 adrenergic receptor agonists incorporating metabolic inactivation: an antedrug approach. J Med Chem 2010; 53: 4522–4530. [DOI] [PubMed] [Google Scholar]
- 39. Slack RJ, Barrett VJ, Morrison VS, et al. In vitro pharmacological characterization of vilanterol, a novel long-acting beta2-adrenoceptor agonist with 24-hour duration of action. J Pharmacol Exp Ther 2013; 344: 218–230. [DOI] [PubMed] [Google Scholar]
- 40. Malerba M, Radaeli A, Montuschi P, et al. Vilanterol trifenatate for the treatment of COPD. Expert Rev Respir Med 2016; 10: 719–731. [DOI] [PubMed] [Google Scholar]
- 41. Celli B, Crater G, Kilbride S, et al. Once-daily umeclidinium/vilanterol 125/25 mcg in COPD: a randomized, controlled study. Chest 2014; 145: 981–991. [DOI] [PubMed] [Google Scholar]
- 42. Decramer M, Anzueto A, Kerwin E, et al. Efficacy and safety of umeclidinium plus vilanterol versus tiotropium, vilanterol, or umeclidinium monotherapies over 24 weeks in patients with chronic obstructive pulmonary disease: results from two multicentre, blinded, randomised controlled trials. Lancet Respir Med 2014; 2: 472–486. [DOI] [PubMed] [Google Scholar]
- 43. Donohue JF, Maleki-Yazdi MR, Kilbride S, et al. Efficacy and safety of once-daily umeclidinium/vilanterol 62.5/25 mcg in COPD. Respir Med 2013; 107: 1538–1546. [DOI] [PubMed] [Google Scholar]
- 44. Maleki-Yazdi MR, Kaelin T, Richard N, et al. Efficacy and safety of umeclidinium/vilanterol 62.5/25 mcg and tiotropium 18 mcg in chronic obstructive pulmonary disease: results of a 24-week, randomized, controlled trial. Respir Med 2014; 108: 1752–1760. [DOI] [PubMed] [Google Scholar]
- 45. Rodrigo GJ, Neffen H. A systematic review of the efficacy and safety of a fixed-dose combination of umeclidinium and vilanterol for the treatment of COPD. Chest 2015; 148: 397–407. [DOI] [PubMed] [Google Scholar]
- 46.[cited 2016. September 10]; Available from: http://www.gsk-clinicalstudyregister.com/study/114418?study_ids=114418#rs
- 47. Kalberg C, O’Dell D, Galkin D, et al. Dual bronchodilator therapy with umeclidinium/vilanterol versus tiotropium plus indacaterol in chronic obstructive pulmonary disease: a randomized controlled trial. Drugs R D 2016; 16: 217–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sousa AR, Riley JH, Church A, et al. The effect of umeclidinium added to inhaled corticosteroid/long-acting beta2-agonist in patients with symptomatic COPD: a randomised, double-blind, parallel-group study. NPJ Prim Care Respir Med 2016; 26: 16031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Brealey N, Gupta A, Renaux J, et al. Pharmacokinetics of fluticasone furoate, umeclidinium, and vilanterol as a triple therapy in healthy volunteers. Int J Clin Pharmacol Ther 2015; 53: 753–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lomas D, Lipson D, Barnacle H, et al. Single inhaler triple therapy (ICS/LAMA/LABA) in patients with advanced COPD: results of the FULFIL trial. Eur Respir J 2016; 48(Suppl. 60): A4629. [Google Scholar]
- 51. Singh D, Papi A, Corradi M, et al. Single inhaler triple therapy versus inhaled corticosteroid plus long-acting beta2-agonist therapy for chronic obstructive pulmonary disease (TRILOGY): a double-blind, parallel group, randomised controlled trial. Lancet 2016; 388: 963–973. [DOI] [PubMed] [Google Scholar]
- 52. Feldman G, Maltais F, Khindri S, et al. A randomized, blinded study to evaluate the efficacy and safety of umeclidinium 62.5 μg compared with tiotropium 18 μg in patients with COPD. Int J Chron Obstruct Pulmon Dis 2016; 11: 719–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Rheault T, Khindri S, Vahdati-Bolouri M, et al. A randomised, open-label study of umeclidinium versus glycopyrronium in patients with COPD. ERJ Open Res 2016; 2: 00101-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Anderson P. Patient preference for and satisfaction with inhaler devices. Eur Respir Rev 2005; 14: 109–116. [Google Scholar]
- 55. van der Palen J, Thomas M, Chrystyn H, et al. Inhaler preference in patients with COPD: a comparison of Ellipta® with five other inhaler devices. AJRCCM 2016; 193: A6813. [Google Scholar]
- 56. Thomas M, van der Palen J, Chrystyn H, et al. Inhaler errors after reading the patient information leaflet in patients with asthma: Ellipta® compared with three inhaler devices. AJRCCM 2016; 196: A1738. [Google Scholar]


