Abstract
Purpose of review
Although elusive for many decades, transplantation tolerance can now be achieved in the clinic. This has prompted follow up investigations into its stability and longevity, as well as into barriers to its induction, which include memory T and B cells.
Recent findings
Clinical observations reveal that transplantation tolerance can be induced in adult recipients and that even episodes of acute rejection do not preclude successful weaning from immunosuppression to reveal tolerance. These observations appear to conflict with the currently accepted notion that adult transplant recipients harbor high frequencies of memory HLA-specific T cells that are a barrier to transplantation tolerance. We discuss how these observations may be rationalized, by proposing the generation of helpless effector CD8+ T cells that cannot develop into memory, and by highlighting recent findings on the ability of transplantation tolerance to be spontaneously restored after rejection. We speculate that in individuals who develop tolerance while on immunosuppression and then experience rejection, it is this restored tolerance that is revealed upon successful weaning of immunosuppression.
Summary
We have reviewed clinical and experimental data to explain how transplantation tolerance may be achieved in individuals who have experienced allograft rejection.
Keywords: Transplantation, Tolerance, Rejection, Alloreactivity, memory T cells
Introduction
It is widely accepted that a high frequency of alloreactive memory T cells is the main reason for why tolerance-inducing therapies that succeed in mice, fail to replicate in non-human primates and humans. Yet, seemingly paradoxical to this paradigm, is the clinical evidence that transplantation tolerance can emerge in individuals who have experienced allo-sensitization or allograft rejection. Here we review briefly the evidence that memory T cells and alloantibodies are important barriers to transplantation tolerance, and discuss potential explanations for how tolerance can nevertheless emerge after rejection, based on recent observations made in the clinic and in models of tolerance in mice, pigs and non-human primates.
Properties of Memory T cells
Memory T cells differ from naïve T cells in a cell-intrinsic manner and display a more rapid response upon antigen reencounter (Fig 1). As a result of increased T cell receptor nanoclustering, memory T cells have a lower threshold for activation and respond to antigen more rapidly [1,2], divide after a shorter lag time, exhibit increased numbers of cell divisions coupled with lower loss rates, and more quickly elaborate effector functions [3,4]. In addition to increased signaling through the TCR, memory T cells express a higher level and broader array of co-stimulatory molecules including ICOS, CD30, TNFR4 (OX40) and TNFR9 (4-1BB) [5,6] that enhance TCR signaling, and that regulate effector function and survival. Sensitized recipients harbor higher frequencies of antigen-specific T cells and potentially, circulating antibodies, which also contributes to more vigorous T cell responses in vivo, and altered sensitivity to immunosuppression (Fig 1) [5,7,8].
Figure 1. Mechanisms by which memory T and B cells resist tolerance.
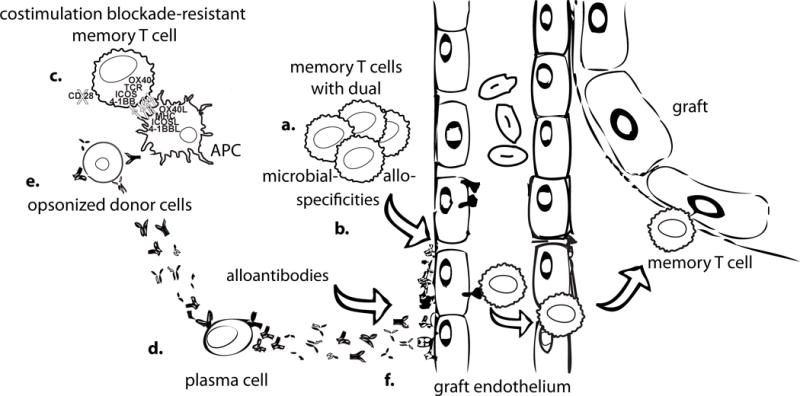
Memory T cells exist at higher frequencies (a) than naïve T cells and are able to infiltrate the allograft in sufficient numbers without having to proliferate first. Higher numbers of donor-reactive memory T cells can arise from prior exposure to donor antigens in the form of pregnancy, blood transfusions or prior transplantation, or through heterologous immunity (b) to antigens in the environment, including prior infections. Memory T cells can themselves be resistant to tolerance induction on a per cell basis through having relaxed costimulation requirements through the expression of multiple costimulatory molecules and/or the loss of CD28 (c). Plasma cells, can produce donor-specific alloantibodies continuously without the need to be restimulated (d). Alloantibodies in turn can act to opsonize donor cells, making them more immunogenic to antigen-presenting cells (APCs), and enhancing T cell responses (e). Alloantibodies can also exert direct damage to graft endothelial cells (f).
Donor-specific memory is a barrier to tolerance
Sensitization in the clinic is diagnosed by the presence of donor-specific antibodies that may result from prior organ transplantation, pregnancy and/or blood transfusion [9▪]. These events likely also generate memory T cells. In addition, memory T cells developing from prior exposure to environmental antigens, microbiota and infections may cross-react with donor antigens, a phenomenon termed heterologous immunity [10] [11]. This cross-reactivity likely explains why high frequencies of memory T cells accumulate with age in healthy individuals [12], although currently there is no reliable way to quantify the frequency of donor-specific memory T cells in the clinic, nor to determine their provenance.
Seminal studies by Valujskikh et al. [13], reported on the ability of memory allospecific CD4+ or CD8+ T cells primed with allogeneic skin, to prevent anti-CD154/DST-mediated tolerance to allogeneic hearts. Subsequently, Pattenburg et al.[14], reported that Leishmania infection could induce memory CD4+ that cross-reacted with alloantigens, setting the stage for Adams et al. [10] to report that the generation of memory T cells through heterologous immunity following viral infections correlated with an acquired resistance to tolerance induction. This resistance correlated with the number of memory T cells (>105/recipient), and was most effectively mediated by central memory CD8+ T cells. Extending these observations, Brehm MA et al [15] showed that purified virus-specific CD8+ T cells adoptively transferred into SCID mice were sufficient for mediating the rejection of skin allografts. These collectively findings supported the notion that all adults, including those that had not been overtly exposed to alloantigens, are likely to harbor high frequencies of HLA-reactive memory T cells, and provided a unifying explanation for why tolerance-inducing therapies in laboratory mice do not translate into humans. Recent studies by Beura et al. [16▪▪] reported that feral and pet store mice have profound differences in both innate and adaptive immune systems that more closely reflect the immune systems of adult humans. Experimental data demonstrating these mice exhibit a resistance to transplantation tolerance that is correlated with the frequency of memory donor-reactive T cells would directly support this hypothesis and provide a model for studying the effects of heterologous immunity on transplantation tolerance induction and how they may be overcome.
Because of the preponderance of evidence supporting the barrier posed by memory T cells, considerable effort has been spent on developing strategies that can induce tolerance in recipients with high frequencies of memory T cells [17]. Many of these strategies are based on the notion that co-stimulation requirements of memory T cells are relaxed or redundant due to expression of post-activation co-stimulatory molecules or to the loss of CD28 in CD8+ T cells [18]. Indeed, antagonists of T cell adhesion in sensitized recipients improved graft survival when used in conjunction with costimulation blockade although at the considerable cost of reduced protective immunity [19]. Moreover, stable long-term tolerance to fully-mismatched allografts, even with these interventions, remained elusive [19,20]. Taking a different approach, Yamada et al. [21] reported that memory T cell responses in nonhuman primates can be mitigated by a sustained effort to delete CD8+ memory T cells and delaying donor bone marrow transplantation to induce tolerance. The rationale for delaying bone marrow transplantation is that a lower inflammatory environment promotes quiescence of the residual host-derived memory CD8+ T cells, which in turn, favors the survival of the transferred bone marrow cells and their ability to induce tolerance to the kidney allograft.
While less extensively investigated, humoral sensitization and resulting donor-specific antibodies can serve as a barrier to tolerance induction or persistence. Indeed, the inability to induce stable tolerance and the loss of established tolerance is most likely to occur in patients with donor-specific antibodies [22] [23,24▪]. In an experimental mouse model of cardiac allotransplantation, Burns et al. [8] reported that memory B cell responses and DSA are potent barriers to transplant tolerance induction. DSA generated immune complexes that efficiently promoted alloantigen-presentation and T cell activation even in the presence of co-stimulation blockade [7▪]. Furthermore, because of the direct effects of DSA on the graft endothelium, and the ability of long-lived plasma cells to spontaneously produce antibodies without requiring additional stimulation, the barrier that pre-transplant and even de novo DSA presents to tolerance is likely to be as formidable as, if not more than, memory T cells. There are scant insights into how this barrier to tolerance may be overcome.
Exceptions to the memory rule: tolerance in humans
Despite experimental evidence that memory T cells effectively prevent tolerance induction, recent clinical data paradoxically demonstrate that adult recipients harboring memory T cells can successfully develop tolerance induced with stem cell plus kidney transplantation, and also following the successful weaning of conventional immunosuppression. Since the impact of memory T cells is enhanced by the conditioning or induction protocols that result in the depletion of naïve and the relative sparing of memory T cells [25,26], successful induction of tolerance in these adults is even more remarkable. One potential explanation may be that, while all adults have comparably high frequencies of memory CD4+ and CD8+ T cells [27▪], the actual frequency of donor-reactive memory T cells may be variable. So it is possible that the fortunate but rare individual with minimal frequencies of memory donor-reactive T cells is more likely to develop tolerance (Fig 2A). Alternatively, the alloreactive memory T cells may have attained phenotypes that are more susceptible to tolerance induction, depending on the amount and duration of antigen exposure during the priming [28,29▪]. Prospective clinical trials on tolerance together with a reliable way to quantify the memory donor-reactive T cell repertoire will allow this possibility to be tested (Fig 2B).
Figure 2. Possible ways tolerance can re-emerge after rejection.
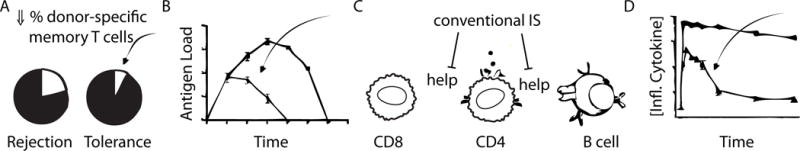
A. By chance, a recipient may have low percentages of memory cells reactive to the donor, which may facilitate tolerance to that donor.
B. Memory cells may have an altered phenotype based on the amount/duration of antigen exposure, which could allow them to be susceptible to tolerance induction.
C. Absnce of help from CD4+ cells to CD8+ cells, resulting in “helpless” CD8s that are impaired in their ability to act as memory cells. Absence of CD4+ help also prevents humoral sensitization. Fewer functional memory cells favor tolerance.
D. If tolerant T cells are exposed only to transient inflammation, they may temporarily gain effector functions sufficient to cause rejection, but after the inflammation subsides, they may become quiescient again, allowing tolerance to re-emerge.
Studies on spontaneously achieved operational tolerance revealed that a quarter of those recipients have a history of allo-immunization prior to transplantation, as detected by the presence of donor-specific antibodies and even in patients that had experienced an episode of biopsy-proven rejection, before immunosuppression withdrawal [30▪▪]. In a prospective immunosuppression weaning trial of 98 liver transplant recipients, equal percentages of recipients experienced an episode of rejection prior to weaning in the group that went on to develop tolerance (29%, N=41) and in the group that did not (26%, N=57) [31]. These observations are clearly at odds with the paradigm that donor-reactive memory T cells, when present in sufficient frequencies, are potent barriers to tolerance induction. We propose these observations may be explained by a hypothesis, that while under conventional immunosuppression, rejection is mediated by donor-specific CD8+ T effector cells that fail to differentiate into memory T cells (Fig 2C). Indeed, it has been established that “help” provided by CD4+ T cells is essential for generating functional CD8+ T cell memory but not their differentiation into effectors [32–35]. In subsequent mechanistic studies, Janssen et al. [36] reported that the absence of CD4 help functionally imprints in CD8+ T cells at an early time point the acquisition of a distinct genetic program that results in their activation-induced cell death during the secondary response. We speculate that infections that stimulate cross-reactive CD8+ T cells to differentiate into effector cells capable of mediating rejection, may not have stimulated the appropriate cross-reactive CD4+ T cell response. Under these circumstances, rejection can occur without the generation of functional donor-specific CD8+ memory T cells, and memory B cell responses are also prevented.
The hypothesis that helpless CD8+ T cells are unable to differentiate into memory cells cannot be extended to memory CD4+ T cells, which when generated, may infiltrate the graft to mediate CD4+ T cell-mediated rejection [37] and are proficient at providing help to B cells, thereby promoting humoral responses [38▪]. Thus, other explanations are required to reconcile the clinical observations of tolerance after rejection with the experimental data that memory T cells prevent tolerance induction.
Spontaneous restoration of tolerance after rejection in animal studies
Studies from experimental murine, non-human primates and swine models of transplantation tolerance have collectively demonstrated that established tolerance can be transiently abrogated by inflammatory insults to induce graft rejection [39–42▪▪]. But when the inflammation subsided, tolerance was spontaneously restored such that the memory of established tolerance dominated over the memory of rejection (Fig 2D). We therefore speculate that clinical tolerance revealed after the weaning of immunosuppression in recipients that had undergone rejection may reflect a similar scenario, whereupon these recipients had already acquired features of tolerance while under immunosuppression and the tolerance that was revealed after weaning of immunosuppression represented tolerance that was spontaneously restored after the rejection event was controlled. Indeed, Rebollo-Mesa et al. [43] recently reported that their validated signature of tolerance can identify patients on immunosuppression that may already be tolerant and suitable for weaning off immunosuppression.
In a mouse model of heart allograft tolerance, Wang et al. [39] and Miller et al. [40▪] reported that infection with Listeria monocytogenes resulted in the abrogation of established tolerance in approximately 40% of recipients. Upon clearance of the infection, tolerance spontaneously returned such that a donor-matched cardiac allograft, transplanted 7–42 days after a Listeria-mediated rejection of the first allograft, was accepted without additional tolerance-inducing therapy. The frequency of donor-reactive IFNγ producing T cells showed a transient increase post-Listeria infection, coincident with Listeria infection-induced serum IL-6 and IFNβ concentrations, that subsided when the inflammation receded. Indeed, IL-6 and IFNβ were shown to be necessary and sufficient to abrogate tolerance [39], by promoting the ability of Tconv cells to escape regulation and to produce IFNγ. These studies provide a proof-of-principle that infections can transiently overcome established tolerance through bystander mechanisms, and that tolerance can spontaneously return when the infection is cleared.
In non-human primates, where tolerance was induced with transient mixed bone marrow chimerism, multiple injections of high-dose IL-2 restored donor-specific T cell IFNγ responses and precipitated kidney allograft rejection in 4 of 4 recipients. Furthermore, cessation of IL-2 administration promptly aborted the rejection process, and kidney graft function was spontaneously restored within 7 days of IL-2 cessation [41▪▪]. These observations confirm that established tolerance is difficult to overcome, but when it is overcome, the reawakening of alloreactivity is unexpectedly transient and tolerance can be spontaneously restored. These findings, when taken together, underscore an unexpected feature of robust tolerance and raise an intriguing possible explanation for the ability to reveal tolerance upon immunosuppression withdrawal even in individuals that had experienced rejection. This possibility can be tested in the clinic when unambiguous biomarkers for tolerance, that can be detectable despite pharmacological immunosuppression, become available.
Restored tolerance after rejection is eroded
An important feature of restored tolerance after rejection is that it is inferior to the original state of tolerance. In the model of established murine tolerance, wherein an acute infection with Listeria caused the full rejection of the allograft, defined as a complete loss of heartbeat, but where tolerance spontaneously returned to mediate the acceptance a second donor-matched heart transplanted 7 days later [40▪], we showed that the quality of restored tolerance was reduced. Treatment with anti-CD25 antibodies to deplete regulatory T cells was sufficient to prevent the return of tolerance and result in the rejection of the second donor-matched heart, whereas the same treatment had no significant effect on the acceptance of a second heart allograft in recipients with established tolerance but no infection [40▪]. We therefore reasoned that the quality of the restored tolerance was less robust compared to the tolerance without infection.
Furthermore, because clinical rejection is defined by pathology long before full cessation of heart function, we also investigated the impact of Listeria infection that resulted in a rejection crisis without heartbeat cessation [44▪]. In the surviving allografts on day 8 post-infection, graft-infiltrating cell numbers increased and there was a dramatic loss in the tolerance gene signature exhibited by the CD45+ cells infiltrating the allograft. The tolerance signature was broadly restored by day 30 post-infection but with a discernable reduction in the expression of a subset of genes that marked tolerance. The tolerant state after Listeria infection was further shown to be functionally eroded, as rejection of the long-term surviving graft could be induced with anti-PD-L1 blocking antibody treatment alone, a treatment that did not precipitate graft survival in non-infected tolerant mice. Collectively, those observations demonstrated that tolerance was eroded following exposure to the bystander immune responses elicited by infection that caused a transient reawakening of alloreactivity without causing the full rejection of the allograft.
Similar observations were made in a porcine model of tolerance to MHC Class-I mismatched kidney allografts induced with a 12-day treatment course with cyclosporine A. Scalea et al. [42▪▪] reported that donor-specific transfusion to re-stimulate alloreactivity, together with leukapheresis to induce homeostatic proliferation of remaining T cells, and the removal of the tolerant kidney graft to eliminate regulatory T cells, triggered rejection of second donor-matched kidney grafts in 7 of 10 recipients. While 3 fully rejected the freshly transplanted second kidney allograft, the remaining four underwent a transient rejection crisis but survived for >100 days without additional intervention. A donor-matched skin graft was placed in 2 of 4 recipients at ≥100 days after the rejection crisis had been resolved, and in both of these recipients, the rejection of the skin graft precipitated the rejection of the accepted kidney allograft. In contrast, the other 2 recipients without the skin transplant maintained their kidney allograft without rejection. Finally, in a single control recipient that received the same tolerance-breaking regimen but did not undergo a rejection crisis, rejection of the skin graft did not precipitate rejection of the accepted kidney graft. The authors concluded from these observations that the quality of tolerance had been weakened by the first rejection crisis.
Collectively these observations suggest that peripheral transplantation tolerance remains responsive to being reshaped by ongoing immunological cues, and that while a resilient tolerance can spontaneously return after rejection, it is eroded in quality. These observations have profound implications for the ability of tolerance to maintain the function of an allograft for the life of the recipient. They also underscore the need for tolerance to be robust enough to withstand erosion and to be resilient so that it can accommodate the pro-inflammatory effects of protective immunity to infections and then still be able to return to a state of tolerance. This robust tolerance can be characterized by the perfect induction of single tolerance mechanisms, such as complete clonal deletion of donor-reactive T cells, or by the cooperative induction of multiple non-redundant mechanisms. Consistent with this latter notion are the recent observations with a mouse model of robust tolerance induced with anti-CD154 plus donor-splenocyte transfer, that established tolerance could be overcome by a combination of anti-PD-L1, anti-CD25 and the adoptive transfer of moderate numbers of alloreactive TCR-transgenic T cells but not through individual perturbations [45▪].
Conclusion
The barrier that memory T cells pose to the induction of transplantation tolerance has been clearly established. These memory alloreactive T cells are generated as a result of exposure to alloantigen, or as a result of cross-reactivity to environment antigens, microbiota or infections. Hence, all adults, even those who have not been overtly exposed to alloantigens, will likely harbor memory HLA-reactive T cells and be resistant to tolerance induction. Yet, somewhat paradoxically, clinical tolerance can be induced intentionally with hematopoietic stem cell transplantation in adult recipients and can be revealed after conventional immunosuppression is stopped, even in recipients who have undergone biopsy-proven rejection. Here we speculate on two possible mechanisms that may reconcile these observations – the induction of “helpless” effector CD8+ T cells that cannot differentiate into memory cells, and the notion that tolerance may be resilient and come back even if transiently broken. It is this restored, albeit eroded, tolerance that may be revealed when immunosuppression is stopped in the subset of patients that had undergone prior rejection events.
Key points.
Memory alloreactive T cells, generated as a result of exposure to alloantigen, or as a result of cross-reactivity to environment antigens, microbiota or infections, are detected in most adult humans and act as a barrier to successful induction of transplantation tolerance.
Paradoxically, clinical tolerance can be induced intentionally with hematopoietic stem cell transplantation in adult recipients and can be revealed after conventional immunosuppression is stopped even in recipients that have undergone biopsy-proven rejection.
Tolerance after rejection can be explained by the induction of “helpless” effector CD8+ T cells that induce rejection but cannot differentiate into memory cells.
Established robust tolerance may be overcome by some infections, resulting in graft rejection, but may be spontaneously restored after the infection is cleared.
Tolerance that returns after rejection is eroded and thus more susceptible to reversal.
Acknowledgments
We would like to thank current and former members for their contribution to the studies discussed in this review.
Financial support and sponsorship This work was supported in part by grants (P01AI097113) from the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health to A.S.C and M.L.A. M.L.M. was funded by the Cardiovascular Pathophysiology and Biochemistry Training Grant (T32 HL07237), HHMI Med-into-Grad Program training grant (56006772) and two American Heart Association Midwest pre-doctoral fellowships (13PRE14550022 and 15PRE22180007).
Footnotes
Conflicts of Interest The authors have no conflicts of interest to declare.
References
- 1.Saito T. Nanocluster formation: more with memory. Immunity. 2011;35:318–320. doi: 10.1016/j.immuni.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 2.Kumar R, Ferez M, Swamy M, Arechaga I, Rejas MT, Valpuesta JM, Schamel WW, Alarcon B, van Santen HM. Increased sensitivity of antigen-experienced T cells through the enrichment of oligomeric T cell receptor complexes. Immunity. 2011;35:375–387. doi: 10.1016/j.immuni.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 3.Veiga-Fernandes H, Walter U, Bourgeois C, McLean A, Rocha B. Response of naive and memory CD8+ T cells to antigen stimulation in vivo. Nat Immunol. 2000;1:47–53. doi: 10.1038/76907. [DOI] [PubMed] [Google Scholar]
- 4.Kersh EN, Fitzpatrick DR, Murali-Krishna K, Shires J, Speck SH, Boss JM, Ahmed R. Rapid demethylation of the IFN-gamma gene occurs in memory but not naive CD8 T cells. J Immunol. 2006;176:4083–4093. doi: 10.4049/jimmunol.176.7.4083. [DOI] [PubMed] [Google Scholar]
- 5.Ford ML. T Cell Cosignaling Molecules in Transplantation. Immunity. 2016;44:1020–1033. doi: 10.1016/j.immuni.2016.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol. 2013;13:227–242. doi: 10.1038/nri3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burns AM, Chong AS. Alloantibodies prevent the induction of transplantation tolerance by enhancing alloreactive T cell priming. J Immunol. 2011;186:214–221. doi: 10.4049/jimmunol.1001172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burns AM, Ma L, Li Y, Yin D, Shen J, Xu J, Chong AS. Memory alloreactive B cells and alloantibodies prevent anti-CD154-mediated allograft acceptance. J Immunol. 2009;182:1314–1324. doi: 10.4049/jimmunol.182.3.1314. [DOI] [PubMed] [Google Scholar]
- 9.Redfield RR, Scalea JR, Zens TJ, Mandelbrot DA, Leverson G, Kaufman DB, Djamali A. The mode of sensitization and its influence on allograft outcomes in highly sensitized kidney transplant recipients. Nephrol Dial Transplant. 2016 doi: 10.1093/ndt/gfw099. The results of this study suggest a greater barrier to long-term graft acceptance in patients who have undergone retransplantation than those with a history of pregnancy or blood transfusions, and therefore predict a more precise way to quantify memory cells in sensitized patients could help identify those at greatest risk of graft loss. [DOI] [PubMed] [Google Scholar]
- 10.Adams AB, Williams MA, Jones TR, Shirasugi N, Durham MM, Kaech SM, Wherry EJ, Onami T, Lanier JG, Kokko KE, et al. Heterologous immunity provides a potent barrier to transplantation tolerance. J Clin Invest. 2003;111:1887–1895. doi: 10.1172/JCI17477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Su LF, Kidd BA, Han A, Kotzin JJ, Davis MM. Virus-specific CD4(+) memory-phenotype T cells are abundant in unexposed adults. Immunity. 2013;38:373–383. doi: 10.1016/j.immuni.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farber DL, Yudanin NA, Restifo NP. Human memory T cells: generation, compartmentalization and homeostasis. Nat Rev Immunol. 2014;14:24–35. doi: 10.1038/nri3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Valujskikh A, Pantenburg B, Heeger PS. Primed allospecific T cells prevent the effects of costimulatory blockade on prolonged cardiac allograft survival in mice. Am J Transplant. 2002;2:501–509. doi: 10.1034/j.1600-6143.2002.20603.x. [DOI] [PubMed] [Google Scholar]
- 14.Pantenburg B, Heinzel F, Das L, Heeger PS, Valujskikh A. T cells primed by Leishmania major infection cross-react with alloantigens and alter the course of allograft rejection. J Immunol. 2002;169:3686–3693. doi: 10.4049/jimmunol.169.7.3686. [DOI] [PubMed] [Google Scholar]
- 15.Brehm MA, Daniels KA, Priyadharshini B, Thornley TB, Greiner DL, Rossini AA, Welsh RM. Allografts stimulate cross-reactive virus-specific memory CD8 T cells with private specificity. Am J Transplant. 2010;10:1738–1748. doi: 10.1111/j.1600-6143.2010.03161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beura LK, Hamilton SE, Bi K, Schenkel JM, Odumade OA, Casey KA, Thompson EA, Fraser KA, Rosato PC, Filali-Mouhim A, et al. Normalizing the environment recapitulates adult human immune traits in laboratory mice. Nature. 2016;532:512–516. doi: 10.1038/nature17655. Provides an alternative pre-clinical model to specific-pathogen free mice and a potential explanation for why many agents manipulating the immune system have not translated well to humans in the clinic. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krummey SM, Ford ML. New insights into T-cell cosignaling in allograft rejection and survival. Curr Opin Organ Transplant. 2015;20:43–48. doi: 10.1097/MOT.0000000000000151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leitner J, Herndler-Brandstetter D, Zlabinger GJ, Grubeck-Loebenstein B, Steinberger P. CD58/CD2 Is the Primary Costimulatory Pathway in Human CD28−CD8+ T Cells. J Immunol. 2015;195:477–487. doi: 10.4049/jimmunol.1401917. [DOI] [PubMed] [Google Scholar]
- 19.Anderson DJ, Lo DJ, Leopardi F, Song M, Turgeon NA, Strobert EA, Jenkins JB, Wang R, Reimann KA, Larsen CP, et al. Anti-Leukocyte Function-Associated Antigen 1 Therapy in a Nonhuman Primate Renal Transplant Model of Costimulation Blockade-Resistant Rejection. Am J Transplant. 2016;16:1456–1464. doi: 10.1111/ajt.13628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Espinosa JR, Samy KP, Kirk AD. Memory T cells in organ transplantation: progress and challenges. Nat Rev Nephrol. 2016;12:339–347. doi: 10.1038/nrneph.2016.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamada Y, Boskovic S, Aoyama A, Murakami T, Putheti P, Smith RN, Ochiai T, Nadazdin O, Koyama I, Boenisch O, et al. Overcoming memory T-cell responses for induction of delayed tolerance in nonhuman primates. Am J Transplant. 2012;12:330–340. doi: 10.1111/j.1600-6143.2011.03795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brouard S, Pallier A, Renaudin K, Foucher Y, Danger R, Devys A, Cesbron A, Guillot-Guegen C, Ashton-Chess J, Le Roux S, et al. The natural history of clinical operational tolerance after kidney transplantation through twenty-seven cases. Am J Transplant. 2012;12:3296–3307. doi: 10.1111/j.1600-6143.2012.04249.x. [DOI] [PubMed] [Google Scholar]
- 23.Leventhal J, Abecassis M, Miller J, Gallon L, Ravindra K, Tollerud DJ, King B, Elliott MJ, Herzig G, Herzig R, Ildstad ST. Chimerism and tolerance without GVHD or engraftment syndrome in HLA-mismatched combined kidney and hematopoietic stem cell transplantation. Sci Transl Med. 2012;4:124ra28. doi: 10.1126/scitranslmed.3003509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kawai T, Sachs DH, Sprangers B, Spitzer TR, Saidman SL, Zorn E, Tolkoff-Rubin N, Preffer F, Crisalli K, Gao B, et al. Long-term results in recipients of combined HLA-mismatched kidney and bone marrow transplantation without maintenance immunosuppression. Am J Transplant. 2014;14:1599–1611. doi: 10.1111/ajt.12731. Results from one of the first clinical trials that prospectively induced tolerance successfully across different HLAs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yao Z, Jones J, Kohrt H, Strober S. Selective resistance of CD44hi T cells to p53-dependent cell death results in persistence of immunologic memory after total body irradiation. J Immunol. 2011;187:4100–4108. doi: 10.4049/jimmunol.1101141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neujahr DC, Chen C, Huang X, Markmann JF, Cobbold S, Waldmann H, Sayegh MH, Hancock WW, Turka LA. Accelerated memory cell homeostasis during T cell depletion and approaches to overcome it. J Immunol. 2006;176:4632–4639. doi: 10.4049/jimmunol.176.8.4632. [DOI] [PubMed] [Google Scholar]
- ▪.Thome JJ, Yudanin N, Ohmura Y, Kubota M, Grinshpun B, Sathaliyawala T, Kato T, Lerner H, Shen Y, Farber DL. Spatial map of human T cell compartmentalization and maintenance over decades of life. Cell. 2014;159:814–828. doi: 10.1016/j.cell.2014.10.026. First study to take a comprehensive look at T cell subsets in humans across different ages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Floyd TL, Koehn BH, Kitchens WH, Robertson JM, Cheeseman JA, Stempora L, Larsen CP, Ford ML. Limiting the amount and duration of antigen exposure during priming increases memory T cell requirement for costimulation during recall. J Immunol. 2011;186:2033–2041. doi: 10.4049/jimmunol.1003015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Badell IR, Kitchens WH, Wagener ME, Lukacher AE, Larsen CP, Ford ML. Pathogen Stimulation History Impacts Donor-Specific CD8(+) T Cell Susceptibility to Costimulation/Integrin Blockade-Based Therapy. Am J Transplant. 2015;15:3081–3094. doi: 10.1111/ajt.13399. Authors detail how not all heterologous memory T cells behave the same, and thus a patient’s unique immune history may directly impact how large a barrier to tolerance induction they may have. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Massart A, Pallier A, Pascual J, Viklicky O, Budde K, Spasovski G, Klinger M, Sever MS, Sorensen SS, Hadaya K, et al. The DESCARTES-Nantes survey of kidney transplant recipients displaying clinical operational tolerance identifies 35 new tolerant patients and 34 almost tolerant patients. Nephrol Dial Transplant. 2016;31:1002–1013. doi: 10.1093/ndt/gfv437. Extremely large-scale study attempting to determine the frequency of operationally tolerant kidney transplant recipients, and it discovers three additional now-tolerant patients that had prior episodes of rejection. [DOI] [PubMed] [Google Scholar]
- 31.Benitez C, Londono MC, Miquel R, Manzia TM, Abraldes JG, Lozano JJ, Martinez-Llordella M, Lopez M, Angelico R, Bohne F, et al. Prospective multicenter clinical trial of immunosuppressive drug withdrawal in stable adult liver transplant recipients. Hepatology. 2013;58:1824–1835. doi: 10.1002/hep.26426. [DOI] [PubMed] [Google Scholar]
- 32.Bourgeois C, Rocha B, Tanchot C. A role for CD40 expression on CD8+ T cells in the generation of CD8+ T cell memory. Science. 2002;297:2060–2063. doi: 10.1126/science.1072615. [DOI] [PubMed] [Google Scholar]
- 33.Shedlock DJ, Shen H. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science. 2003;300:337–339. doi: 10.1126/science.1082305. [DOI] [PubMed] [Google Scholar]
- 34.Sun JC, Bevan MJ. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science. 2003;300:339–342. doi: 10.1126/science.1083317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Janssen EM, Lemmens EE, Wolfe T, Christen U, von Herrath MG, Schoenberger SP. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature. 2003;421:852–856. doi: 10.1038/nature01441. [DOI] [PubMed] [Google Scholar]
- 36.Janssen EM, Droin NM, Lemmens EE, Pinkoski MJ, Bensinger SJ, Ehst BD, Griffith TS, Green DR, Schoenberger SP. CD4+ T-cell help controls CD8+ T-cell memory via TRAIL-mediated activation-induced cell death. Nature. 2005;434:88–93. doi: 10.1038/nature03337. [DOI] [PubMed] [Google Scholar]
- 37.Pietra BA, Wiseman A, Bolwerk A, Rizeq M, Gill RG. CD4 T cell-mediated cardiac allograft rejection requires donor but not host MHC class II. J Clin Invest. 2000;106:1003–1010. doi: 10.1172/JCI10467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gorbacheva V, Fan R, Fairchild RL, Baldwin WM, 3rd, Valujskikh A. Memory CD4 T Cells Induce Antibody-Mediated Rejection of Renal Allografts. J Am Soc Nephrol. 2016 doi: 10.1681/ASN.2015080848. As the humoral arm of the immune system has been shown to augment cellular alloimmunity, this paper illustrates how the cellular arm can similarly boost humoral responses to induce rejection even if the T cells are not persistent. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang T, Ahmed EB, Chen L, Xu J, Tao J, Wang CR, Alegre ML, Chong AS. Infection with the intracellular bacterium, Listeria monocytogenes, overrides established tolerance in a mouse cardiac allograft model. Am J Transplant. 2010;10:1524–1533. doi: 10.1111/j.1600-6143.2010.03066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miller ML, Daniels MD, Wang T, Chen J, Young J, Xu J, Wang Y, Yin D, Vu V, Husain AN, et al. Spontaneous restoration of transplantation tolerance after acute rejection. Nat Commun. 2015;6:7566. doi: 10.1038/ncomms8566. The first study to experimentally show tolerance to cardiac allografts can spontaneously re-emerge after an infection-mediated rejection event and that the quality of tolerance that returns is not as robust as in uninfected recipients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamada Y, Nadazdin O, Boskovic S, Lee S, Zorn E, Smith RN, Colvin RB, Madsen JC, Cosimi AB, Kawai T, et al. Repeated Injections of IL-2 Break Renal Allograft Tolerance Induced via Mixed Hematopoietic Chimerism in Monkeys. Am J Transplant. 2015;15:3055–3066. doi: 10.1111/ajt.13382. First study to show tolerance can be reversibly lost and regained multiple times in the same recipient. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scalea JR, Okumi M, Villani V, Shimizu A, Nishimura H, Gillon BC, Torabi R, Cormack T, Moran S, LeGuern C, et al. Abrogation of renal allograft tolerance in MGH miniature swine: the role of intra-graft and peripheral factors in long-term tolerance. Am J Transplant. 2014;14:2001–2010. doi: 10.1111/ajt.12816. The authors identify multiple perturbations that can disrupt long-term tolerance, which hint at the underlying mechanisms that are important for the maintenance of robust tolerance. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rebollo-Mesa I, Nova-Lamperti E, Mobillo P, Runglall M, Christakoudi S, Norris S, Smallcombe N, Kamra Y, Hilton R, Indices of Tolerance EUC et al. Biomarkers of Tolerance in Kidney Transplantation: Are We Predicting Tolerance or Response to Immunosuppressive Treatment? Am J Transplant. 2016 doi: 10.1111/ajt.13932. The study authors provide new markers of tolerance that have predictive value for identifying which patients are tolerant and can be safely weaned from immunosuppression, which has the potential to increase the safety of weaning and management of patients post-transplant. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Young JS, Daniels MD, Miller ML, Wang T, Zhong R, Yin D, Alegre ML, Chong AS. Erosion of Transplantation Tolerance After Infection. Am J Transplant. 2016 Apr 11; doi: 10.1111/ajt.13821. [Epub ahead of print] A study in cardiac transplantation that showed that the restored state of tolerance after rejection is not as good as it was initally, suggesting patients that have undergone rejection crises prior to revealing their operational tolerance may be more susceptible to later graft loss than those with no prior rejection episodes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miller ML, Daniels MD, Wang T, Wang Y, Xu J, Yin D, Chong AS, Alegre ML. Tracking of TCR-Tg T cells reveals multiple mechanisms maintain cardiac transplant tolerance in mice. Am J Transplant. 2016 Apr 18; doi: 10.1111/ajt.13814. [Epub ahead of print] Study identifies several mechanisms underlying tolerance in a mouse model, a strategy that could impact the rational design of combinations of therapies to use in patients to induce and bolster tolerance. [DOI] [PMC free article] [PubMed] [Google Scholar]


