Abstract
Rhythmic activation and repression of the frequency (frq) gene are essential for normal function of the Neurospora circadian clock. WHITE COLLAR (WC) complex, the positive element of the Neurospora circadian system, is responsible for stimulation of frq transcription. We report that a C2H2 finger domain-containing protein IEC-1 and its associated chromatin remodeling complex INO80 play important roles in normal Neurospora circadian clock function. In iec-1KO strains, circadian rhythms are abolished, and the frq transcript levels are increased compared to that of the wild-type strain. Similar results are observed in mutant strains of the INO80 subunits. Furthermore, ChIP data show that recruitment of the INO80 complex to the frq promoter is IEC-1-dependent. WC-mediated transcription of frq contributes to the rhythmic binding of the INO80 complex at the frq promoter. As demonstrated by ChIP analysis, the INO80 complex is required for the re-establishment of the dense chromatin environment at the frq promoter. In addition, WC-independent frq transcription is present in ino80 mutants. Altogether, our data indicate that the INO80 complex suppresses frq transcription by re-assembling the suppressive mechanisms at the frq promoter after transcription of frq.
Author summary
Circadian clocks organize inner physiology to anticipate changes in the external environment. These clocks are controlled by the oscillation of central clock proteins which form the central oscillator. Transcriptional regulation is a critical step in the regulation of the oscillation of these core proteins. In eukaryotes, chromatin remodeling is a common mechanism to regulate gene transcription by conquering or establishing nucleosomal barriers for the transcription machinery. Here, we showed that a C2H2 finger domain-containing protein IEC-1 and its associated chromatin remodeling complex INO80 are required for transcriptional repression of the core clock gene frq in the Neurospora circadian system. Moreover, the activator WHITE COLLAR (WC) complex is responsible for the transcriptional activation of frq; thus, considering the different timing of the transcriptional activation and suppression of frq, there must be a mechanism that coordinates the two opposite processes. We also demonstrated that the WC-mediated open state of the frq promoter facilitates the binding of INO80 to this region, which prepares for subsequent transcriptional suppression. Collectively, our data provide novel insights into the regulation of the frq gene and the circadian clock.
Introduction
From the filamentous fungus Neurospora crassa to animals, circadian oscillation is a conserved mechanism based on an auto-regulatory feedback loop composed of negative and positive elements [1–6]. In Neurospora, the heterodimeric WHITE COLLAR (WC) complex, consisting of WC-1 and WC-2, acts as the positive element that binds to the frq promoter and activates frq transcription [7–13]. The negative elements FRQ and FRQ-interacting RNA helicase (FRH) form the FRQ/FRH complex and mediate the phosphorylation of WCs, which inhibits their WC complex activity and promotes the cytoplasmic localization of the WC complex [12,14–16]. Immediately following synthesis, FRQ is progressively phosphorylated by casein kinases (CKI and CKII) and other kinases throughout the subjective day and evening [12,17–20]. CKI participates in the regulation of both FRQ and the WCC [12]. The phosphorylation of FRQ by CKI increases the degradation rate of FRQ. FRQ protein also acts as a scaffold by bringing CKI to phosphorylate the WCC which leads to its inactivation and repression. Similar to CKI, many of the sites on FRQ protein can also be phosphorylated by CKII, which promotes its degradation [12,21]. While casein kinases regulate the stability of FRQ, the casein kinases are countered by multiple protein phosphatases, including PP1, PP2A and PP4 [22,23], The PP1 dephosphorylates and stabilizes FRQ protein while PP2a and PP4 activities influence frq transcription by dephosphorylating WC-2. Hyperphosphorylated FRQ is degraded through the ubiquitin-proteasome pathway [24]. However, a recent study showed that the cycle ends when FRQ is sufficiently hyperphosphorylated and becomes invisible to the circadian machinery [25]. When the activity of FRQ is not sufficient to suppress the activity of the WCs, frq transcription is reactivated by the WC complex. Recently, epigenetic modifications were reported to regulate frq transcription in Neurospora. SET-1 is required for normal expression of frq [26], and the SET-2 pathway is involved in the suppression of WC-independent frq transcription [27]. Moreover, antisense transcription was shown to inhibit sense expression by mediating chromatin modifications and premature termination of transcription in the frq locus [28].
Recent studies have shown that CLOCK:BMAL1 promote the removal of nucleosomes at its binding sites in mammalian clock genes during transcription activation [29]. Based on these results, nucleosomal barriers at the activator-binding sites should be established during transcriptional repression of the circadian cycle. A still unknown factor(s) may be responsible for the rhythmic incorporation of nucleosomes into chromatin at the activator-binding sites of the clock genes to establish rhythmic transcriptional repression.
Chromatin remodelers are capable of removing, destabilizing, ejecting, and restructuring nucleosomes by using the energy provided by ATP hydrolysis. In Neurospora, two ATP-dependent chromatin-remodeling factors, CLOCKSWITCH (CSW-1) and chromodomain helicase DNA-binding-1 (CHD1), have been reported to regulate frq transcription by modulating nucleosome density at the promoter region [7,30]. Recent studies have shown that SWI/SNF is recruited to the frq promoter by WC-1 to initiate frq transcription [31]. The INO80 complex is a highly conserved chromatin remodeler from yeast to humans [32]. INO80 can mobilize a mononucleosome to the center of a DNA fragment in vitro and remove histone variant H2A.Z from nucleosomes [33–36], which is involved in gene activation in yeast [37,38]. In yeast or mammalian systems, the recruitment of the INO80 complex by yeast Iec1 or mammalian Yin Yang 1 is a key event in gene regulation [39,40]. Moreover, the INO80 complex contributes to chromatin silencing of the boundaries of genes and heterochromatins [41]. However, little is known about the role of the INO80 complex in the suppression of protein-coding genes. Here, we demonstrate that the IEC-1-recruited INO80 complex is required for the Neurospora circadian clock system. WC-mediated transcriptional activation accounts for the rhythmic recruitment of the INO80 complex to the frq promoter; binding of the INO80 complex to the promoter region creates a dense chromatin environment and suppresses frq transcription. Loss of the INO80 complex leads to WC-independent frq transcription due to the accessible chromatin environment at the frq promoter.
Results
IEC-1 is required for normal circadian conidiation rhythm
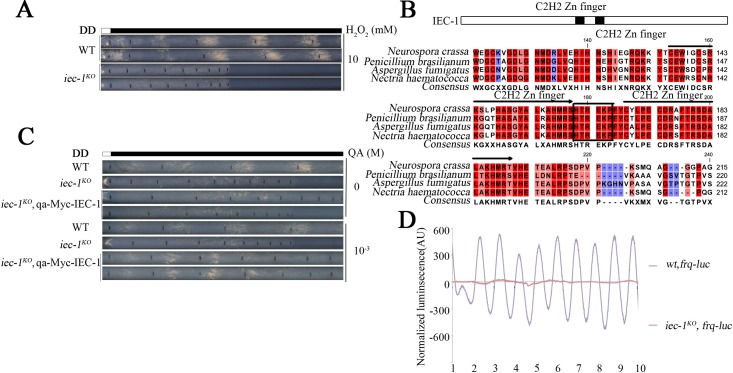
To characterize the transcription factors that are involved in the regulation of frq expression, we examined the conidiation rhythms of available knockout transcription factor mutants with race tube assays. As shown in Fig 1A, the strain with deletion of the iec-1 (ras-1+) (NCU03206) gene showed arrhythmic conidiation in a race tube assay compared to the wild-type strain, suggesting that IEC-1 plays a critical role in Neurospora circadian conidiation rhythm. The iec-1 gene codes a C2H2 finger domain-containing protein IEC-1, a homolog of mammalian Yin Yang 1 (YY1), Drosophila PcG PHO, and yeast Iec1 [39]. When the IEC-1 protein sequence was used in a BLAST search against protein databases, its homologs were found to be highly conserved in ascomycetes (Fig 1B). To confirm the phenotype of the iec-1 mutant, the iec-1 gene, which is driven by the quinic acid (QA)-inducible qa-2 promoter, was reintroduced to the iec-1KO strain. In addition, five copies of the c-Myc epitope with six histidine residues [42] were inserted at the N-terminus of the IEC-1 ORF to facilitate the detection of the expression of Myc-IEC-1 using a c-Myc monoclonal antibody (9E10). In QA-containing race tubes, the circadian conidiation rhythms of the iec-1KO, qa-Myc-IEC-1 strains were similar to those of the wild-type strains (Fig 1C). These data indicate that Myc-IEC-1 can partially complement the function of the endogenous IEC-1 protein in the iec-1KO strain. To test whether light exposure could entrain the conidiation rhythms, the wild-type strain and iec-1KO strains were assayed under light/dark cycles (LD) at 25°C. The race tube assays showed that the conidiation process of the iec-1KO strains was still entrained by LD cycles (S1A Fig). Meanwhile, a reduced light induction of FRQ expression by light was observed in the iec-1 mutant (S1B Fig). Notably, the basal level of FRQ is higher in the mutant. Nonetheless, this result suggests that IEC-1 also affect the light induction of FRQ expression. These data suggested that the clock phenotype of the mutant strain is not caused by a defect in the input pathway of clock. To further confirm the arrhythmic phenotype of the iec-1KO strain, we introduced bioluminescence reporter constructs (frq-luc) into the his-3 locus of the iec-1KO strains and the wild-type strains, in which the luciferase expression of both strains are driven by the frq promoter [43]. The sequence analysis of genomic DNA showed that the frq promoters in the luciferase-expressing strains were intact (S2 Fig). Consistent with the phenotype in the race tube assay, the robust bioluminescence rhythm of the wt, frq-luc strain was abolished in the iec-1KO, frq-luc strains (Fig 1D and S3A Fig), indicating that IEC-1 is critical for circadian clock function at the molecular level.
Fig 1. IEC-1 is required for normal circadian clock function.
(A) Race tube assays of the wild-type and iec-1KO strains. (B) Amino acid sequence alignment of the zf-C2H2 domains of IEC-1 from Neurospora crassa, Penicillium brasilianum, Aspergillus fumigates and Nectria haematococca. (C) Race tube assays of the wild-type strain, iec-1KO strain, and iec-1KO, qa-Myc-IEC-1 transformants in a race tube with or without QA. Growth media on the race tubes did not consist of glucose. (D) Luciferase reporter assay showing the frq promoter activity in the wt, frq-luc and iec-1KO, frq-luc strains grown in DD for several days. Raw data were normalized to subtract the baseline calculated by the LumiCycle analysis software.
IEC-1 suppresses frq transcription and rhythmically associates with the frq promoter
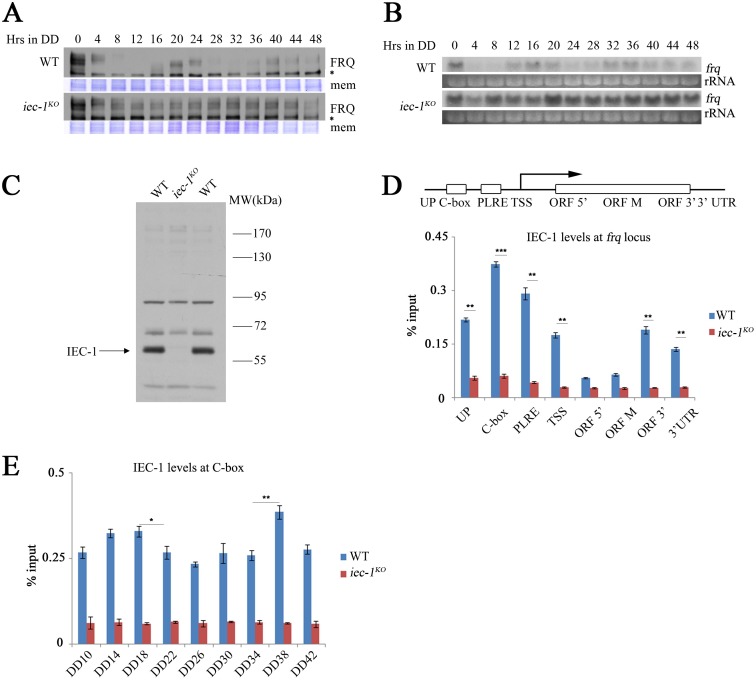
To further determine the role of IEC-1 in the circadian clock, we examined the FRQ expression profile at different time points in constant darkness (DD). Both FRQ rhythms and FRQ phosphorylation profiles were disrupted in the iec-1KO strain (Fig 2A). Northern blot analyses showed that the abolished FRQ rhythms were due to constant transcription of the frq gene in the iec-1KO strain (Fig 2B). To test whether IEC-1 directly regulates frq transcription, a chromatin immunoprecipitation (ChIP) assay was performed by using an IEC-1-specific polyclonal antibody (Fig 2C). Because the IEC-1 antibody recognizes nonspecific bands, the iec-1KO strain was used as the negative control in the ChIP assays. The ChIP assay revealed enrichment of IEC-1 at the frq promoter, ORF 3’, and the 3’UTR but not at ORF 5’ and the middle regions at DD18 (Fig 2D). Furthermore, the association of IEC-1 with C-box fluctuated from DD10 to DD42 (Fig 2E). Altogether, these results indicate that IEC-1 functions at the frq locus to regulate frq transcription.
Fig 2. IEC-1 suppresses frq transcription and rhythmically binds to the frq promoter.
(A) Western blot analysis showing the circadian oscillation of FRQ proteins in the wild-type and iec-1KO strains. The strains were grown in 2% glucose liquid media. The asterisk indicates a nonspecific cross-reacted protein band recognized by our FRQ antiserum. The Coomassie Brilliant Blue-stained membranes (mem) represent the total protein in each sample and were used as a loading control. (B) Northern blot analysis of frq transcription in the wild-type and iec-1KO strains. rRNA was used as a loading control. The strains were grown in 2% glucose liquid media. (C) Immunodetection of IEC-1 protein in the wild-type strain and the iec-1KO mutant using antiserum that specifically recognizes the IEC-1 protein in the wild-type strain. The arrow notes the specific IEC-1 protein band detected by our IEC-1 antibody. The strains were grown in 2% glucose liquid media. (D) ChIP analysis showing the recruitment of IEC-1 at different regions of the frq locus in the wild-type and iec-1KO strains at DD18. The strains were grown in 2% glucose liquid media. C-box, clock box; PLRE, proximal light-regulated element; TSS, transcription start site; ORF, open reading frame; UTR, untranslated region. (E) ChIP analysis showing the enrichment of IEC-1 at the C-box of the frq promoter in the wild-type and iec-1KO strains at the indicated time points. The strains were grown in 2% glucose liquid media. Significance was assessed by two-tailed t-test. *P<0.05, **P<0.01. Error bars show the mean ±S.D. (n = 3).
The INO80 complex is required for normal circadian conidiation rhythm and rhythmic frq transcription
Previous studies showed that yeast Iec1or mammalian Yin Yang 1 (YY1) co-purified with the INO80 complex in yeast or mammalian cells [39,44]. These transcription factors are required for the recruitment of the INO80 complex to target genes. Since the INO80 complex acts as a conserved co-factor of Iec1/YY1 in different organisms, we performed an IP assay to test the association of the INO80 complex with IEC-1 in Neurospora. The results showed that FLAG-IEC-1 was immunoprecipitated (IP) with INO80 (NCU08919) by the INO80 antiserum but not immunoprecipitated with the preimmune (PI) serum (S4A Fig). In the INO80 complex, INO80 is the catalytic subunit and IES-1 is a structural subunit [33,37,45]. To further identify whether the INO80 complex functions in the same pathway as IEC-1 in the regulation of the Neurospora circadian clock, we generated ino80 (NCU08919) and ies-1 (NCU01362) deletion mutants. As expected, both ino80KO and ies-1KO strains exhibited arrhythmic conidiation phenotypes in the race tube assays (S4B Fig). We also tried to generate mutants with a band background (ras-1bd), but we only obtained heterokaryotic strains. These strains also showed arrhythmic conidiation phenotypes in the race tube assays (S4 Fig). To further confirm that deletion of ino80 or ies-1 is the only cause of the clock defect in these mutants, rescue strains of the mutants were generated. The conidiation rhythms of the ino80KO, qa-Myc-INO80 and ies-1KO, qa-Myc-IES-1 transformants were not restored in the race tubes in the absence of QA (S4C and S4D Fig). Meanwhile, obvious conidiation rhythms were observed in the rescue strains but not in the knock-out strains in the race tube assays with 10−3 M QA (S4C and S4D Fig). However, we also observed a phase difference between the wild-type and the rescue strains. The results suggest that qa-2 promoter-driven expression of Myc-INO80 could not fully complement the function of endogenous INO80 due to an abnormal expression level or the presence of the Myc tag. The race tube assays showed that the conidiation processes of the ino80KO and ies-1KO strains are entrained by LD cycles, excluding the defect in the input pathway of clock in these mutants (S4E Fig). Notably, the growth rates of ino80KO strains were strongly dependent on glucose, indicating that ino80KO strain might be sensitive to glucose or carbon starvation. Bioluminescence rhythms in ino80KO, frq-luc and ies-1KO, frq-luc strains were also abolished (S4F, S3B and S3C Figs). Western blots showed disruption of both the FRQ rhythms and FRQ phosphorylation profiles in the ino80KO and ies-1KO strains (S4G Fig). Northern blot analyses showed that the abolished FRQ rhythms were due to constant transcription of the frq gene in the ino80KO and ies-1KO strains (S4H Fig). Altogether, these results indicate that the INO80 complex is critical for frq transcription repression.
IEC-1- and WCC-driven frq transcription are responsible for the recruitment of INO80 at the frq C-box
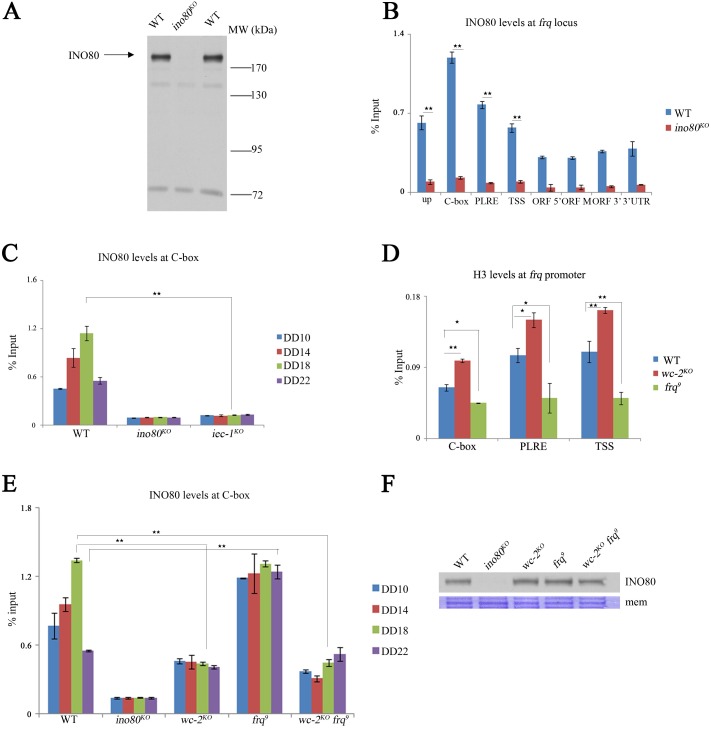
Previous studies have shown that the INO80 complex can be recruited to target genes by Iec1 or YY1 in yeast or mammalian cells [39,40]. To investigate the occupancy of the INO80 complex at the C-box of the frq gene, we performed a ChIP assay using INO80-specific antiserum at DD18 (Fig 3A). The ChIP results revealed that binding of INO80 to the frq gene peaks at the C-box and 3’-UTR (Fig 3B), similar to the binding pattern of IEC-1 (Fig 2D). Next, we assessed whether IEC-1 is required for recruitment of the INO80 complex at the C-box of the frq promoter. The ChIP assays conducted with an INO80 antibody showed a dramatic decrease in enrichment of INO80 at the C-box in iec-1KO strains (Fig 3C), indicating that efficient binding of the INO80 complex at the frq C-box is dependent on the expression of IEC-1.
Fig 3. INO80 is rhythmically recruited at the C-box by IEC-1 and WCC-driven frq expression.
(A) Immunodetection of INO80 in the wild-type strain and the ino80KO mutant using antiserum that specifically recognizes INO80 protein in the wild-type strain. The strains were grown in 2% glucose liquid media. The arrow indicates the INO80 specific band in the wild-type strains. (B) ChIP analysis showing the recruitment of INO80 at different regions of the frq locus in the wild-type and ino80KO strains at DD18. The ino80KO strain was used as a negative control in the ChIP assay. The strains were grown in 2% glucose liquid media. Significance was assessed by two-tailed t-test. *P<0.05, **P<0.01. Error bars show the mean ±S.D. (n = 3). (C) ChIP analysis showing the recruitment of INO80 at the C-box in the wild-type, ino80KO and iec-1KO strains at the indicated time points. The strains were grown in 2% glucose liquid media. Significance was assessed by two-tailed t-test. *P<0.05, **P<0.01. Error bars show the mean ±S.D. (n = 3). (D) ChIP analysis showing the enrichment of histone H3 at the C-box, PLRE or TSS of the frq promoter region in the wild-type, wc-2KO (bd) and frq9 (bd) mutant strains. The strains were grown in 2% glucose liquid media. Significance was assessed by two-tailed t-test. *P<0.05, **P<0.01. Error bars show the mean ±S.D. (n = 3). (E) ChIP analysis showing the recruitment of INO80 at the C-box of the frq promoter in the wild-type, ino80KO, wc-2KO(bd), frq9(bd), and wc-2KO frq9 (bd) strains at the indicated time points. The strains were grown in 2% glucose liquid media. Significance was assessed by two-tailed t-test. *P<0.05, **P<0.01. Error bars show the mean ±S.D. (n = 3). (F) Western blot analysis showing the INO80 protein levels in the wild-type, ino80KO, wc-2KO (bd), frq9 (bd) and wc-2KO frq9 (bd) strains. The strains were grown in 2% glucose liquid media.
To determine whether the association of INO80 with the C-box is rhythmic during circadian cycles, the recruitment of INO80 at four different time points in constant darkness were examined using a ChIP assay. The results demonstrated that the association of INO80 with the C-box of the frq promoter was rhythmic, peaking at DD18 (Fig 3C). In yeast or mammalian systems, recruitment of the INO80 complex by Iec1 or YY1 is a key event in gene expression [39,40]. Thus, these results suggest that IEC-1-dependent INO80 recruitment at the frq promoter is required for suppression of frq transcription after WCC-inactivation by FRQ. In the Neurospora circadian system, the transcriptional activators WC-1 and WC-2 are responsible for the transcriptional activation of the frq gene during circadian cycles [7,9–11,13,46,47]. In the frq9 mutant, constant frq transcription [48] causes a low H3 density at the frq promoter, whereas, in the wc-2KO strain, a lack of frq transcription [10] is associated with a high H3 density at the frq promoter (Fig 3D). These findings indicate that WC-mediated transcriptional activation leads to open chromatin states by nucleosomal removal, which increases the accessibility of the frq promoter. It is also possible that WC-mediated transcriptional activation of frq is required for rhythmic recruitment of the INO80 complex during the period of high expression of frq to prepare for repression of frq transcription. To assess this possibility, we examined enrichment of the INO80 complex at the frq C-box in the wc-2KO and frq9 strains. The ChIP results showed that the levels of INO80 enrichment in the wc-2 mutant were lower than that in the wild-type strain at the DD18 time point (Fig 3E). These data suggest that the rhythmic recruitment of the INO80 complex is dependent on WC-mediated transcriptional activation of frq. In contrast, in frq9 strains, the constant elevation of the levels of INO80 enrichment are due to the constant high elevation of frq expression, which is caused by malfunction of the negative feedback loop (Fig 3E). To verify that the high levels of recruitment of the INO80 complex in the frq9 mutant are due to the activity of the WC complex, we generated the wc-2KO frq9 double-mutant strain, and examined the recruitment of INO80 at the C-box. Loss of WC-2 restored the low levels of recruitment of INO80 at the C-box in the frq9 mutant (Fig 3E). Meanwhile, the INO80 expression of these mutants was similar to that of the wild-type strain (Fig 3F). These data indicate that the highly activated transcription of frq by the WC complex also contributes to the rhythmic recruitment of the INO80 complex to the C-box of frq for subsequent suppression of frq transcription during circadian cycles.
WC-independent frq transcription occurs in the ies-1KO strain
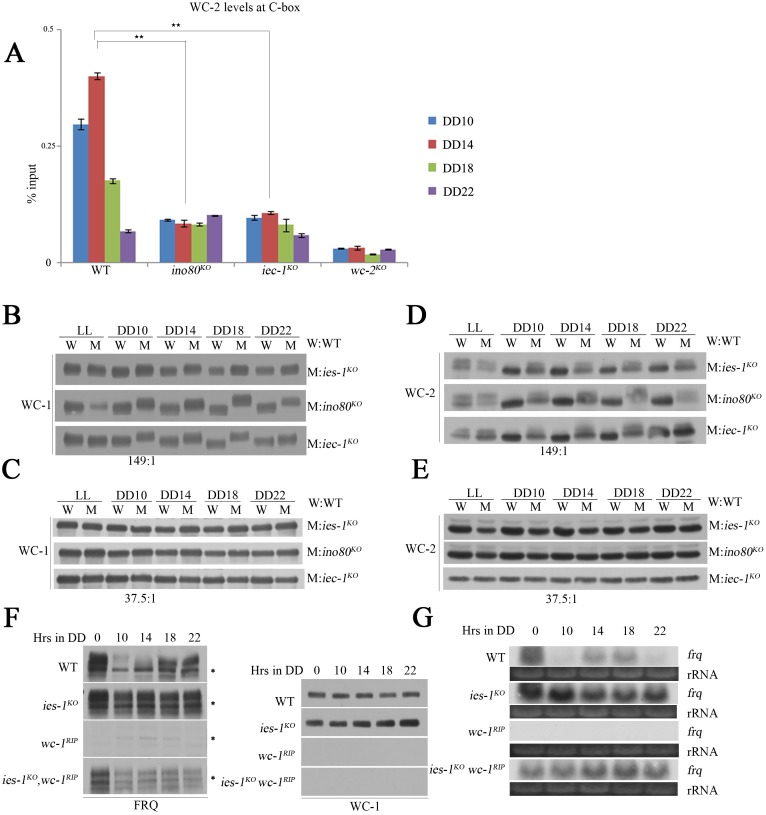
In the Neurospora circadian system, the WC complex, the positive transcription factor that binds to the C-box of frq, is responsible for rhythmic frq expression [7–9,13]. To test whether frq transcription is driven by the WC complex in ino80 and iec-1 mutants, we first examined the transcriptional activity of WC-2 in the ino80KO and iec-1KO strains. The ChIP results revealed that the rhythmic enrichment of WC-2 at the frq C-box corresponded to the rhythmic expression of frq in the wild type strain (Fig 4A). In contrast, the recruitment of WC-2 was dramatically decreased in the ino80KO and iec-1KO strains (Fig 4A), which was inconsistent with the constant expression of frq in these mutants. This finding suggests that the transcriptional activities of WC-1 and WC-2 are decreased in the ino80 and iec-1 mutants. Previous studies showed that hypophosphorylated WC-1 and WC-2 efficiently bind to the C-box for frq transcriptional activation [12,21] while hyperphosphorylated WC-1 and WC-2 exhibit lower binding activity at the C-box of frq [14,49]. Western blot analysis showed that the phosphorylation levels of WC-1 and WC-2 were increased in the ino80KO, ies-1KO and iec-1KO strains compared to those in the wild-type strains at different time points in DD (Fig 4B and 4D). In addition, we found no significant changes in the protein levels of WC-1 and a slight decrease in the levels of WC-2 in the ino80KO, ies-1KO and iec-1KO strains compared to those in the wild-type strains (Fig 4C and 4E). The levels of WC-1 protein did not exhibit a robust circadian rhythm in our hands, which is consistent with previous studies showing that the amplitudes of WC rhythms are variable. Since WC activity is mostly known to be regulated by phosphorylation, the variability of the WC-1 rhythms might be due to the use of different WC-1 antibodies in different laboratories which may have different sensitivity to different isoforms of WC-1. These results indicate the increased frq transcripts in these mutants are not caused by the increase of transcriptional activity of WCC. These data also suggest that the high levels of frq transcription could be partially independent of WC expression in these mutants. To further confirm this possibility, we generated an ies-1KO wc-1RIP double mutant and compared its FRQ levels to that of a wc-1 single mutant. Consistent with previous results, the FRQ levels were extremely low in the wc-1 single mutant (Fig 4F) [10]. However, the levels of FRQ protein and frq mRNA in the ies-1KO wc-1RIP double mutant were well detected with Western blot or Northern blot analyses (Fig 4F and 4G), indicating that WC-independent frq transcription exists in the ies-1 mutants. Altogether, these results demonstrate that recruitment of the INO80 complex at the C-box through high transcriptional activation prepares for suppression of frq transcription after WCC inactivation.
Fig 4. The INO80 complex is required for the suppression of WC-independent frq transcription.
(A) ChIP analysis showing WC-2 enrichment at the C-box in the wild-type, ino80KO, iec-1KO, and wc-2KO (bd) strains. The strains were grown in 2% glucose liquid media. Significance was assessed by two-tailed t-test. *P<0.05, **P<0.01. Error bars show the mean ±S.D. (n = 3). (B) Western blot analysis showing the phosphorylation of WC-1 in the wild-type, ies-1KO, ino80KO and iec-1KO strains. The numbers indicate the ratio of acrylamide/bisacrylamide used in the SDS-PAGE gel. The strains were grown in 2% glucose liquid media. (C) Western blot analysis showing the levels of WC-1 in the wild-type, ies-1KO, ino80KO and ies-1KO strains. The strains were grown in 2% glucose liquid media. (D) Western blot analysis showing the phosphorylation of WC-2 in the wild-type, ies-1KO, ino80KO and ies-1KO strains. The strains were grown in 2% glucose liquid media. (E) Western blot analysis showing the levels of WC-2 in the wild-type, ies-1KO, ino80KO and ies-1KO strains. The strains were grown in 2% glucose liquid media. (F) Western blot analysis of FRQ or WC-1 in the wild-type, ies-1KO, wc-1RIP (bd), and ies-1KO wc-1RIP strains. The strains were grown in 2% glucose liquid media. (G) Northern blot analysis showing the levels of frq mRNA in the wild-type, ies-1KO, wc-1RIP (bd), and ies-1KO wc-1RIP strains. The strains were grown in 2% glucose liquid media.
The INO80 complex contributed to the establishment of the dense chromatin environment at the frq promoter
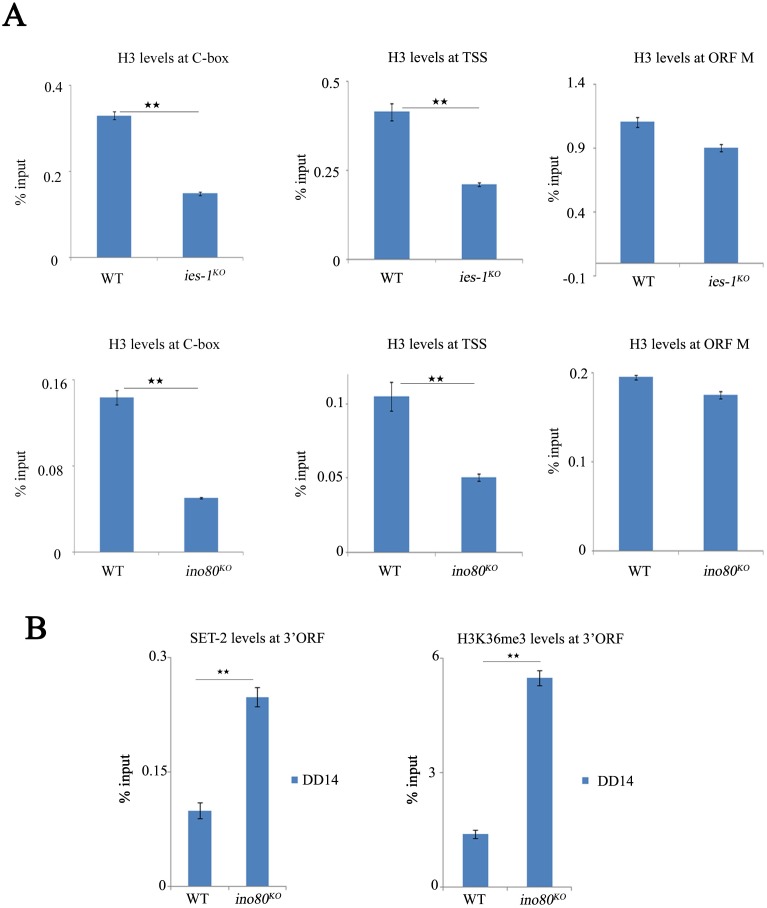
In S. cerevisiae, the first four nucleosomes at the transcription start site of genes have critical roles in the process of transcription initiation [50]. In Drosophila, the +1 nucleosome strongly inhibits the normal function of RNA polymerase II [51]. Although the INO80 complex occupied and peaked at the boundary of the genes, genome-wide ultra-high-resolution ChIP-exo data showed that the Arp5 subunit of the INO80 complex was particularly enriched at the +1 position in yeast [36,52]. Antisense transcripts qrf in the Neurospora circadian system revealed that induction of qrf promotes frq gene expression by creating a more accessible local chromatin environment, even in the absence of the WC complex [28]. Given that qrf-induced frq transcripts share the same properties with the WC-independent frq transcripts, the chromatin states in these two pathways should be similar. The ChIP assay showed that the H3 levels were dramatically reduced at both the C-box and TSS in the ino80KO and ies-1KO strains compared to that of the wild-type strains (Fig 5A). However, the H3 levels at the frq ORF in mutants were similar to that of the wild-type strains (Fig 5A). These results indicate that nucleosome density is decreased at the C-box and TSS of the frq gene in mutants.
Fig 5. The establishment of nucleosomal barriers at the frq promoter by the INO80 complex prevents RNA pol II initiation.
(A) ChIP analysis showing H3 density at the C-box, TSS or ORF middle regions of the frq locus in the wild-type, ies-1KO, and ino80KO strains. The strains were grown in 2% glucose liquid media. Significance was assessed by two-tailed t-test. *P<0.05, **P<0.01. Error bars show the mean ±S.D. (n = 3). (B) ChIP analysis showing the recruitment of SET-2 and enrichment of H3K36me3 at the ORF 3’ of frq in the wild-type and ino80KO strains. The strains were grown in 2% glucose liquid media. Significance was assessed by two-tailed t-test. *P<0.05, **P<0.01. Error bars show the mean ±S.D. (n = 3).
SET-2-mediated H3K36me3 is an important landmark on chromatin during transcription elongation [53]. To confirm the enhanced transcription of frq induced by the decreased nucleosomal barrier in the ino80KO strains, a ChIP assay was performed to examine the recruitment of SET-2 and enrichment of H3K36me3 at the 3’ORF of the frq gene. As expected, the recruitment of SET-2 and the enrichment of H3K36me3 were significantly increased at the 3’ ORF of the frq gene in the ino80KO strains compared to those of the wild-type strains (Fig 5B). Together, these results demonstrate that the INO80 complex contributes to establishment of the dense chromatin environment at the frq promoter, which is essential for suppressing WC-independent frq transcription.
Discussion
In this study, we identified a C2H2 finger domain-containing protein IEC-1 and its co-factor, the INO80 complex, which as part of their normal cellular roles in chromatin assembly, facilitate generalized repression of the clock gene frq in Neurospora. To investigate the role of IEC-1 and the INO80 complex, we generated the iec-1KO, ino80KO and ies-1KO strains and discovered that IEC-1 and the INO80 complex are required for normal circadian clock function. Based on the data presented in this manuscript, the INO80 complex is rhythmically recruited by IEC-1 to the frq promoter to suppress frq expression in the Neurospora clock. The recruitment of the INO80 complex to the frq locus by the transcription factor, IEC-1, is important for frq transcriptional repression, since the disruption of INO80 binding to the frq promoter in the iec-1KO, ino80KO and ies-1KO strains leads to high FRQ levels and loss of frq rhythmicity (Fig 2 and S4 Fig). A similar situation was observed in deletion of the transcriptional co-repressor RCO-1-RCM-1 [54,55]. Rhythmic activation and repression of frq transcription are required for the function of the Neurospora circadian clock. Therefore, normal suppression of frq expression is essential for the circadian auto-regulatory feedback loop function in the Neurospora clock. Nucleosomes at the TSS were identified as the strongest barriers for RNA Polymerase II in S. cerevisiae and Drosophila [50,51]. The FRQ expression levels in the iec-1KO, ino80KO and ies-1KO strains were high and arrhythmic (Fig 2 and S4 Fig), suggesting that the recruitment of the INO80 complex is a key step for establishing repressed state of chromatin at the frq promoter. Our ChIP data revealed that deletion of INO80 or IES-1 results in decreased nucleosome density at the frq promoter. These results suggest that the INO80 complex is required for establishing compact chromatin environments at the frq promoter.
We tried several times but failed to detect tight interaction between the WCC and the IEC-1-INO80 complex. However, weak or transient interaction between them might still occur. Our results showed that the rhythmic binding of the INO80 complex is also dependent on frq transcription activation by the WC complex in the wild-type strain (Fig 3E). Due to the constant transcriptional activation of frq, a significant decrease in nucleosome density along with increased recruitment of the INO80 complex at the frq promoter was observed in the frq9 mutant strains (Fig 3D and 3E). In contrast, the nucleosome density at the frq promoter was increased dramatically in the wc-2KO strains because of the inactivation of frq transcription and reduced INO80 binding (Fig 3D and 3E). These results indicate that binding of the WC complex to the frq promoter results in an open chromatin state in this region and recruitment of the INO80 complex by IEC-1. The progressive inactivation of the WC complex by accumulated FRQ stimulates the remodeling activity of the INO80 complex associated with the frq promoter, which increases the nucleosome density at the frq promoter (Fig 5A). In contrast, although high levels of INO80 recruitment at the frq promoter were observed in the frq9 mutant, the INO80 complex could not re-assemble the dense chromatin state at the frq promoter in this mutant due to the absence of the FRQ protein, which is needed to shut down the high levels of frq transcription driven by the WC complex. In the wild type strain, the lowest level of INO80 recruitment was found at DD22. At this time point the frq transcripts decline and the frq promoter is condensed which is in a line with the data above. These data suggest that at DD22, the chromatin structure mediated by IEC-1 and INO80 complex to suppress frq transcription has already been established which is unsuitable for the binding of INO80 complex. Current results indicate that the FRQ-FRH complex functions as the negative element in the Neurospora circadian auto-regulatory feedback loop to inactivate the WC complex [12,14–16] and activate INO80 to achieve complete repression of frq expression. Thus, the open chromatin state of WCC-driven frq transcription promotes the recruitment of the INO80 complex, which closes the negative feedback loop upon WCC inactivation by FRQ. Similar to the Neurospora clock, the rhythmic activation of the clock genes in Drosophila and mammals by a heterodimeric PAS domain containing the transcription factors, CLK:CYC or the CLOCK:BMAL1 complex is essential for circadian clock function. In the mammalian clock, the binding of CLOCK:BMAL1 to the E-box of clock genes promotes nucleosome eviction and incorporation of the histone variant H2A.Z [29], which suggests that activation of the clock genes by CLOCK:BMAL1 leads to changes in the chromatin structures. Whether the open chromatin states of animal clock genes activated by CLK:CYC or CLOCK:BMAL1 can promote the binding of a remodeler to promoters of clock genes, similar to our results in Neurospora, is not clear. Considering the conserved roles of IEC1/PHO/YY1 and the INO80 complex in different organisms, it is worth determining whether there are similar mechanisms in Drosophila and mammals. Altogether, these results suggest that a conserved repression mechanism involving chromatin regulation exists in eukaryotic circadian systems.
Materials and methods
Strains and culture conditions
The wild-type strain (4200) was used as a control. The iec-1 or ies-1 genes were deleted by the replacement of their ORFs with a hygromycin resistance gene (hph) on the ku70RIP (bd, a) background strain. The ku70RIP iec-1KO and ku70RIP ies-1KO strains were crossed with the 774–10 (A, his-3-) strain to obtain homokaryotic iec-1KO and ies-1KO strains, respectively. The ku70::bar ino80KO strain was generated by the replacement of its ORF with the hygromycin resistance gene (hph) on the ku70::bar background strain and microconidia purification. The ku70::bar ras-1bd ino80KO and ras-1bd ino80KO strains were generated in the same manner as the ku70::bar ino80KO strain. For rescue strains, the plasmid qa-5Myc-6his-IEC-1 was transformed into the iec-1KO strain. The ino80KO, qa-5Myc-6his-INO80 and ies-1KO, qa-5Myc-6his-IES-1 transformants were obtained in the same manner as the iec-1KO, qa-5Myc-6his-IEC-1 transformants. A plasmid containing the full-length frq promoter fused to luciferase was transformed into the iec-1KO, ino80KO and ies-1KO strains to generate the iec-1KO, frq-luc, ino80KO, frq-luc and ies-1KO, frq-luc strains. Liquid culture conditions were the same as a previously published method [56]. The wc-2KO, frq9, wc-2KO frq9, wc-1RIP and ies-1KO wc-1RIP all contain the band mutation.
Race tube assay
The race tube medium contained 1× Vogel’s salts, 0.1% glucose, 0.17% arginine, 50 ng/mL biotin and 1.5% agar supplemented with or without 10 mM H2O2. Conidia of different strains were inoculated at one end of each race tube and were grown under constant light (LL) for 1 day to synchronize the clock. The race tubes were then transferred to constant darkness (DD), and the position of the advancing mycelia front was marked at 24 h intervals on the tube. When growth was completed, tubes were scanned, and the growth period of each strain was calculated.
Generation of antiserum against INO80 and IEC-1
GST-INO80 (containing INO80 amino acids 1–341) and GST-IEC-1 (containing IEC-1 amino acids 9–175) fusion proteins were expressed in BL21 cells by induction of IPTG. After purification, the recombinant proteins were used as antigens to immunize rabbits, which yielded rabbit polyclonal antiserums [57].
ChIP analysis
ChIP assay was performed as previously described [55]. Neurospora tissues were fixed by shaking in 1% formaldehyde for 15 min at 25°C, and cross-linking reactions were stopped by adding glycine at a final concentration of 125 mM. The cross-linked chromatin was sheared by sonication to approximately 200–500 bp fragments. A 1 mL aliquot of protein (2 mg/mL) was used per immunoprecipitation, and 10 μL was maintained as the input DNA. The chromatin immunoprecipitation reaction was carried out with 2 μL antibody to WC-2, 2.5 μL antibody to H3 (2650; Cell Signaling Technology), 2.5 μL antibody to IEC-1, 5 μL antibody to INO80, 5 μL antibody to SET-2, and 2 μL antibody to H3K36me3 (4909; Cell Signaling Technology). Immunoprecipitated DNA was quantified using real-time PCR. The primers for real-time PCR were designed according to a previously published protocol [27]. The ChIP-qPCR data were normalized by the input DNA, and the results were presented as the percentage of input DNA. Each experiment was independently performed at least three times.
Protein analyses
Protein extraction, quantification and western blot analysis were performed as previously described [58,59]. Western blot analyses were performed by using antibodies against the proteins of interest. Equal amounts of total protein (40 μg) were loaded in each lane. After electrophoresis, proteins were transferred onto PVDF membranes, and western blot analysis was performed.
RNA analyses
RNA was extracted by TRIzol [60] and analyzed by northern blotting as previously reported [56]. Shortly, equal amounts of total RNA (20 μg) were loaded onto agarose gels for electrophoresis, and the gels were blotted and probed with an RNA probe specific for frq mRNA.
Luciferase reporter assay
The luciferase reporter assay was performed as previously reported [54]. The bioluminescence reporter construct (frq-luc), in which luciferase expression is driven by the frq promoter, was introduced into the his-3 locus of the iec-1KO, iec-1KO, ino80KO and wild-type strains. One drop of conidia suspensions in water was placed on AFV medium and grown in constant light (LL) overnight at 25°C. The cultures were then transferred to constant darkness, and luminescence was recorded in real time using LumiCycle after one day in DD at 25°C. The data were then normalized with LumiCycle analysis software by subtracting the baseline luciferase signal, which increases as the cells grow. Under our experimental condition, luciferase signals are highly variable during the first day in the LumiCycle but become stabilized afterwards, which is likely due to the light-dark transfer of the cultures. Thus, the results were recorded after one day in DD at 25°C.
Supporting information
(A) Race tube assays of the wild-type and iec-1KO strains under light-dark cycles at 25°C. (B) Western blot analysis showing FRQ protein levels in the wild-type and iec-1KO strains in the indicated time points after exposure to light from the samples in DD24.
(TIF)
(TIF)
(A) Luciferase reporter assay showing the frq promoter activity in the wt, frq-luc and iec-1KO, frq-luc strains grown in DD for several days. (B) Luciferase reporter assay showing the frq promoter activity in the wt, frq-luc and ies-1KO, frq-luc strains grown in DD for several days. (C) Luciferase reporter assay showing the frq promoter activity in the wt, frq-luc and ino80KO, frq-luc strains grown in DD for several days.
(TIF)
(A) IP analysis showing the interaction between INO80 and FLAG-IEC-1. The extracts of the wt, FLAG-IEC-1 strain were immunoprecipitated by the preimmune serum (PI) or the INO80 antiserum (IP), followed by western blot analysis using the FLAG or INO80 antibodies. The strains were grown in 2% glucose liquid media. (B) Race tube assays of the wild-type, ino80KO and ies-1KO strains. (C) Race tube assays of the wild-type, ino80KO, and ino80KO, qa-Myc-INO80 strains with or without QA. Growth media on the race tubes did not consist of glucose. (D) Race tube assays of the wild-type, ies-1KO, and ies-1KO, qa-Myc-IES-1 strains with or without QA. Growth media on the race tubes did not consist of glucose. (E) Race tube assays of the wild-type, ino80KO and iec-1KO strains under light-dark cycles at 25°C. (F) Luciferase reporter assays showing the frq promoter activity in the wt, frq-luc, ino80KO, frq-luc and ies-1KO, frq-luc strains grown in DD for several days. Raw data were normalized to subtract the baseline calculated by the LumiCycle analysis software. (G) Western blot analysis showing the circadian oscillation of FRQ in the wild-type, ies-1KO and ino80KO strains. The strains were grown in 2% glucose liquid media. The asterisk indicates a nonspecific cross-reacted protein band recognized by our FRQ antiserum. “mem” indicates the membrane stained by Coomassie Brilliant Blue used as a loading control. (H) Northern blot analysis showing the levels of frq mRNA in the wild-type, ino80KO and ies-1KO strains. rRNA was used as a loading control. The strains were grown in 2% glucose liquid media.
(TIF)
The ino80KO strain was used as a positive control.
(TIF)
Acknowledgments
We would like to thank Yubo He and Zhijun Wang for critically evaluating the manuscript. We would also like to thank Zhihong Xue for providing the RNA extraction protocol.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This project is supported by grants from a project supported by the State Key Program of National Natural Science of China (31330004) (http://www.nsfc.gov.cn/)and National Basic Research Program of China (973 Program) grant (2012CB947600)(http://www.most.gov.cn/) to QH. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Dunlap JC (2006) Proteins in the Neurospora circadian clockworks. J Biol Chem 281: 28489–28493. 10.1074/jbc.R600018200 [DOI] [PubMed] [Google Scholar]
- 2.Liu Y, Bell-Pedersen D (2006) Circadian rhythms in Neurospora crassa and other filamentous fungi. Eukaryot Cell 5: 1184–1193. 10.1128/EC.00133-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dunlap JC (1999) Molecular Bases for Circadian Clocks. Cell 96: 271–290. [DOI] [PubMed] [Google Scholar]
- 4.Young MW, Kay SA (2001) Time zones: a comparative genetics of circadian clocks. Nat Rev Genet 2: 702–715. 10.1038/35088576 [DOI] [PubMed] [Google Scholar]
- 5.Bell-Pedersen D, Cassone VM, Earnest DJ, Golden SS, Hardin PE, et al. (2005) Circadian rhythms from multiple oscillators: lessons from diverse organisms. Nat Rev Genet 6: 544–556. 10.1038/nrg1633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heintzen C, Liu Y (2007) The Neurospora crassa Circadian Clock In: Jeffery CH, editor. Advances in Genetics: Academic Press; pp. 25–66. 10.1016/S0065-2660(06)58002-2 [DOI] [PubMed] [Google Scholar]
- 7.Belden WJ, Loros JJ, Dunlap JC (2007) Execution of the circadian negative feedback loop in Neurospora requires the ATP-dependent chromatin-remodeling enzyme CLOCKSWITCH. Mol Cell 25: 587–600. 10.1016/j.molcel.2007.01.010 [DOI] [PubMed] [Google Scholar]
- 8.Cheng P, Yang Y, Gardner KH, Liu Y (2002) PAS Domain-Mediated WC-1/WC-2 Interaction Is Essential for Maintaining the Steady-State Level of WC-1 and the Function of Both Proteins in Circadian Clock and Light Responses of Neurospora. Mol Cell Biol 22: 517–524. 10.1128/MCB.22.2.517-524.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng P, Yang Y, Liu Y (2001) Interlocked feedback loops contribute to the robustness of the Neurospora circadian clock. Proc Natl Acad Sci U S A 98: 7408–7413. 10.1073/pnas.121170298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crosthwaite SK, Dunlap JC, Loros JJ (1997) Neurospora wc-1 and wc-2: Transcription, Photoresponses, and the Origins of Circadian Rhythmicity. Science 276: 763–769. [DOI] [PubMed] [Google Scholar]
- 11.Froehlich AC, Loros JJ, Dunlap JC (2003) Rhythmic binding of a WHITE COLLAR-containing complex to the frequency promoter is inhibited by FREQUENCY. Proc Natl Acad Sci U S A 100: 5914–5919. 10.1073/pnas.1030057100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He Q, Cha J, He Q, Lee HC, Yang Y, et al. (2006) CKI and CKII mediate the FREQUENCY-dependent phosphorylation of the WHITE COLLAR complex to close the Neurospora circadian negative feedback loop. Genes Dev 20: 2552–2565. 10.1101/gad.1463506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He Q, Liu Y (2005) Molecular mechanism of light responses in Neurospora: from light-induced transcription to photoadaptation. Genes Dev 19: 2888–2899. 10.1101/gad.1369605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schafmeier T, Haase A, Kaldi K, Scholz J, Fuchs M, et al. (2005) Transcriptional feedback of Neurospora circadian clock gene by phosphorylation-dependent inactivation of its transcription factor. Cell 122: 235–246. 10.1016/j.cell.2005.05.032 [DOI] [PubMed] [Google Scholar]
- 15.Cha J, Chang SS, Huang G, Cheng P, Liu Y (2008) Control of WHITE COLLAR localization by phosphorylation is a critical step in the circadian negative feedback process. EMBO J 27: 3246–3255. 10.1038/emboj.2008.245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hong CI, Ruoff P, Loros JJ, Dunlap JC (2008) Closing the circadian negative feedback loop: FRQ-dependent clearance of WC-1 from the nucleus. Genes Dev 22: 3196–3204. 10.1101/gad.1706908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Y, Loros J, Dunlap JC (2000) Phosphorylation of the Neurospora clock protein FREQUENCY determines its degradation rate and strongly influences the period length of the circadian clock. Proc Natl Acad Sci U S A 97: 234–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang Y, Cheng P, He Q, Wang L, Liu Y (2003) Phosphorylation of FREQUENCY protein by casein kinase II is necessary for the function of the Neurospora circadian clock. Mol Cell Biol 23: 6221–6228. 10.1128/MCB.23.17.6221-6228.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang Y, Cheng P, Zhi G, Liu Y (2001) Identification of a calcium/calmodulin-dependent protein kinase that phosphorylates the Neurospora circadian clock protein FREQUENCY. J Biol Chem 276: 41064–41072. 10.1074/jbc.M106905200 [DOI] [PubMed] [Google Scholar]
- 20.Pregueiro AM, Liu Q, Baker CL, Dunlap JC, Loros JJ (2006) The Neurospora checkpoint kinase 2: a regulatory link between the circadian and cell cycles. Science 313: 644–649. 10.1126/science.1121716 [DOI] [PubMed] [Google Scholar]
- 21.Huang G, Chen S, Li S, Cha J, Long C, et al. (2007) Protein kinase A and casein kinases mediate sequential phosphorylation events in the circadian negative feedback loop. Genes Dev 21: 3283–3295. 10.1101/gad.1610207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang Y, He Q, Cheng P, Wrage P, Yarden O, et al. (2004) Distinct roles for PP1 and PP2A in the Neurospora circadian clock. Genes Dev 18: 255–260. 10.1101/gad.1152604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cha J, Chang SS, Huang G, Cheng P, Liu Y (2008) Control of WHITE COLLAR localization by phosphorylation is a critical step in the circadian negative feedback process. Embo j 27: 3246–3255. 10.1038/emboj.2008.245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He Q, Cheng P, Yang Y, He Q, Yu H, et al. (2003) FWD1—mediated degradation of FREQUENCY in Neurospora establishes a conserved mechanism for circadian clock regulation. EMBO J 22: 4421–4430. 10.1093/emboj/cdg425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Larrondo LF, Olivares-Yanez C, Baker CL, Loros JJ, Dunlap JC (2015) Circadian rhythms. Decoupling circadian clock protein turnover from circadian period determination. Science 347: 1257277 10.1126/science.1257277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raduwan H, Isola AL, Belden WJ (2013) Methylation of histone H3 on lysine 4 by the lysine methyltransferase SET1 protein is needed for normal clock gene expression. J Biol Chem 288: 8380–8390. 10.1074/jbc.M112.359935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun G, Zhou Z, Liu X, Gai K, Liu Q, et al. (2016) Suppression of WHITE COLLAR-independent frequency Transcription by Histone H3 Lysine 36 Methyltransferase SET-2 Is Necessary for Clock Function in Neurospora. J Biol Chem 291: 11055–11063. 10.1074/jbc.M115.711333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li N, Joska TM, Ruesch CE, Coster SJ, Belden WJ (2015) The frequency natural antisense transcript first promotes, then represses, frequency gene expression via facultative heterochromatin. Proc Natl Acad Sci U S A 112: 4357–4362. 10.1073/pnas.1406130112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Menet JS, Pescatore S, Rosbash M (2014) CLOCK:BMAL1 is a pioneer-like transcription factor. Genes Dev 28: 8–13. 10.1101/gad.228536.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Belden WJ, Lewis ZA, Selker EU, Loros JJ, Dunlap JC (2011) CHD1 remodels chromatin and influences transient DNA methylation at the clock gene frequency. PLoS Genet 7: e1002166 10.1371/journal.pgen.1002166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang B, Kettenbach AN, Gerber SA, Loros JJ, Dunlap JC (2014) Neurospora WC-1 recruits SWI/SNF to remodel frequency and initiate a circadian cycle. PLoS Genet 10: e1004599 10.1371/journal.pgen.1004599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Conaway RC, Conaway JW (2009) The INO80 chromatin remodeling complex in transcription, replication and repair. Trends Biochem Sci 34: 71–77. 10.1016/j.tibs.2008.10.010 [DOI] [PubMed] [Google Scholar]
- 33.Shen X, Ranallo R, Choi E, Wu C (2003) Involvement of Actin-Related Proteins in ATP-Dependent Chromatin Remodeling. Mol Cell 12: 147–155. [DOI] [PubMed] [Google Scholar]
- 34.Papamichos-Chronakis M, Watanabe S, Rando OJ, Peterson CL (2011) Global regulation of H2A.Z localization by the INO80 chromatin-remodeling enzyme is essential for genome integrity. Cell 144: 200–213. 10.1016/j.cell.2010.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Udugama M, Sabri A, Bartholomew B (2011) The INO80 ATP-Dependent Chromatin Remodeling Complex Is a Nucleosome Spacing Factor. Mol Cell Biol 31: 662–673. 10.1128/MCB.01035-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yen K, Vinayachandran V, Pugh BF (2013) SWR-C and INO80 chromatin remodelers recognize nucleosome-free regions near +1 nucleosomes. Cell 154: 1246–1256. 10.1016/j.cell.2013.08.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jonsson ZO, Jha S, Wohlschlegel JA, Dutta A (2004) Rvb1p/Rvb2p recruit Arp5p and assemble a functional Ino80 chromatin remodeling complex. Mol Cell 16: 465–477. 10.1016/j.molcel.2004.09.033 [DOI] [PubMed] [Google Scholar]
- 38.Barbaric S, Luckenbach T, Schmid A, Blaschke D, Horz W, et al. (2007) Redundancy of chromatin remodeling pathways for the induction of the yeast PHO5 promoter in vivo. J Biol Chem 282: 27610–27621. 10.1074/jbc.M700623200 [DOI] [PubMed] [Google Scholar]
- 39.Hogan CJ, Aligianni S, Durand-Dubief M, Persson J, Will WR, et al. (2010) Fission yeast Iec1-ino80-mediated nucleosome eviction regulates nucleotide and phosphate metabolism. Mol Cell Biol 30: 657–674. 10.1128/MCB.01117-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cai Y, Jin J, Yao T, Gottschalk AJ, Swanson SK, et al. (2007) YY1 functions with INO80 to activate transcription. Nat Struct Mol Biol 14: 872–874. 10.1038/nsmb1276 [DOI] [PubMed] [Google Scholar]
- 41.Xue Y, Van C, Pradhan SK, Su T, Gehrke J, et al. (2015) The Ino80 complex prevents invasion of euchromatin into silent chromatin. Genes Dev 29: 350–355. 10.1101/gad.256255.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.He Q, Cheng P, He Q, Liu Y (2005) The COP9 signalosome regulates the Neurospora circadian clock by controlling the stability of the SCF(FWD-1) complex. Genes Dev 19: 1518–1531. 10.1101/gad.1322205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gooch VD, Mehra A, Larrondo LF, Fox J, Touroutoutoudis M, et al. (2008) Fully codon-optimized luciferase uncovers novel temperature characteristics of the Neurospora clock. Eukaryot Cell 7: 28–37. 10.1128/EC.00257-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu S, Shi Y, Mulligan P, Gay F, Landry J, et al. (2007) A YY1-INO80 complex regulates genomic stability through homologous recombination-based repair. Nat Struct Mol Biol 14: 1165–1172. 10.1038/nsmb1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shen X MG, Hamiche A, Wu C. (2000) A chromatin remodelling complex involved in transcription and DNA processing. Nature 406: 541–544. 10.1038/35020123 [DOI] [PubMed] [Google Scholar]
- 46.Cheng P, Yang Y, Gardner KH, Liu Y (2002) PAS Domain-Mediated WC-1/WC-2 Interaction Is Essential for Maintaining the Steady-State Level of WC-1 and the Function of Both Proteins in Circadian Clock and Light Responses of Neurospora. Molecular and Cellular Biology 22: 517–524. 10.1128/MCB.22.2.517-524.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.He Q, Cha J, He Q, Lee H-C, Yang Y, et al. (2006) CKI and CKII mediate the FREQUENCY-dependent phosphorylation of the WHITE COLLAR complex to close the Neurospora circadian negative feedback loop. Genes Dev 20: 2552–2565. 10.1101/gad.1463506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aronson BD, Johnson Ka, Loros Jj, Dunlap JC Negative feedback defining a circadian clock: autoregulation of the clock gene frequency. [DOI] [PubMed] [Google Scholar]
- 49.Cha Joonseok C SS, Huang Guocun, Cheng Ping, Liu Yi (2008) Control of WHITE COLLAR localization by phosphorylation is a critical step in the circadian negative feedback process. The EMBO Journal 2008: 3246–3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Churchman LS, Weissman JS (2011) Nascent transcript sequencing visualizes transcription at nucleotide resolution. Nature 469: 368–373. 10.1038/nature09652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weber CM, Ramachandran S, Henikoff S (2014) Nucleosomes are context-specific, H2A.Z-modulated barriers to RNA polymerase. Mol Cell 53: 819–830. 10.1016/j.molcel.2014.02.014 [DOI] [PubMed] [Google Scholar]
- 52.Yen K, Vinayachandran V, Batta K, Koerber RT, Pugh BF (2012) Genome-wide nucleosome specificity and directionality of chromatin remodelers. Cell 149: 1461–1473. 10.1016/j.cell.2012.04.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li B, Carey M, Workman JL (2007) The role of chromatin during transcription. Cell 128: 707–719. 10.1016/j.cell.2007.01.015 [DOI] [PubMed] [Google Scholar]
- 54.Zhou Z, Liu X, Hu Q, Zhang N, Sun G, et al. (2013) Suppression of WC-independent frequency transcription by RCO-1 is essential for Neurospora circadian clock. Proc Natl Acad Sci U S A 110: E4867–4874. 10.1073/pnas.1315133110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu X, Li H, Liu Q, Niu Y, Hu Q, et al. (2015) A role for protein kinase A in the Neurospora circadian clock by regulating WC-independent frequency transcription through phosphorylation of RCM-1. Mol Cell Biol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aronson BD, Johnson KA, Dunlap JC (1994) Circadian clock locus frequency: protein encoded by a single open reading frame defines period length and temperature compensation. Proc Natl Acad Sci U S A 91: 7683–7687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xu H, Wang J, Hu Q, Quan Y, Chen H, et al. (2010) DCAF26, an adaptor protein of Cul4-based E3, is essential for DNA methylation in Neurospora crassa. PLoS Genet 6: e1001132 10.1371/journal.pgen.1001132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang J, Hu Q, Chen H, Zhou Z, Li W, et al. (2010) Role of individual subunits of the Neurospora crassa CSN complex in regulation of deneddylation and stability of cullin proteins. PLoS Genet 6: e1001232 10.1371/journal.pgen.1001232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhou Z, Wang Y, Cai G, He Q (2012) Neurospora COP9 signalosome integrity plays major roles for hyphal growth, conidial development, and circadian function. PLoS Genet 8: e1002712 10.1371/journal.pgen.1002712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xue Z, Ye Q, Anson SR, Yang J, Xiao G, et al. (2014) Transcriptional interference by antisense RNA is required for circadian clock function. Nature 514: 650–653. 10.1038/nature13671 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Race tube assays of the wild-type and iec-1KO strains under light-dark cycles at 25°C. (B) Western blot analysis showing FRQ protein levels in the wild-type and iec-1KO strains in the indicated time points after exposure to light from the samples in DD24.
(TIF)
(TIF)
(A) Luciferase reporter assay showing the frq promoter activity in the wt, frq-luc and iec-1KO, frq-luc strains grown in DD for several days. (B) Luciferase reporter assay showing the frq promoter activity in the wt, frq-luc and ies-1KO, frq-luc strains grown in DD for several days. (C) Luciferase reporter assay showing the frq promoter activity in the wt, frq-luc and ino80KO, frq-luc strains grown in DD for several days.
(TIF)
(A) IP analysis showing the interaction between INO80 and FLAG-IEC-1. The extracts of the wt, FLAG-IEC-1 strain were immunoprecipitated by the preimmune serum (PI) or the INO80 antiserum (IP), followed by western blot analysis using the FLAG or INO80 antibodies. The strains were grown in 2% glucose liquid media. (B) Race tube assays of the wild-type, ino80KO and ies-1KO strains. (C) Race tube assays of the wild-type, ino80KO, and ino80KO, qa-Myc-INO80 strains with or without QA. Growth media on the race tubes did not consist of glucose. (D) Race tube assays of the wild-type, ies-1KO, and ies-1KO, qa-Myc-IES-1 strains with or without QA. Growth media on the race tubes did not consist of glucose. (E) Race tube assays of the wild-type, ino80KO and iec-1KO strains under light-dark cycles at 25°C. (F) Luciferase reporter assays showing the frq promoter activity in the wt, frq-luc, ino80KO, frq-luc and ies-1KO, frq-luc strains grown in DD for several days. Raw data were normalized to subtract the baseline calculated by the LumiCycle analysis software. (G) Western blot analysis showing the circadian oscillation of FRQ in the wild-type, ies-1KO and ino80KO strains. The strains were grown in 2% glucose liquid media. The asterisk indicates a nonspecific cross-reacted protein band recognized by our FRQ antiserum. “mem” indicates the membrane stained by Coomassie Brilliant Blue used as a loading control. (H) Northern blot analysis showing the levels of frq mRNA in the wild-type, ino80KO and ies-1KO strains. rRNA was used as a loading control. The strains were grown in 2% glucose liquid media.
(TIF)
The ino80KO strain was used as a positive control.
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.