Abstract
Background
Legionella pneumophila (L. pneumophila) is a causative agent of severe pneumonia. It is highly adapted to intracellular replication and manipulates host cell functions like vesicle trafficking and mRNA translation to its own advantage. However, it is still unknown to what extent microRNAs (miRNAs) are involved in the Legionella-host cell interaction.
Methods
WT and MyD88-/- murine bone marrow-derived macrophages (BMM) were infected with L. pneumophila, the transcriptome was analyzed by high throughput qPCR array (microRNAs) and conventional qPCR (mRNAs), and mRNA-miRNA interaction was validated by luciferase assays with 3´-UTR mutations and western blot.
Results
L. pneumophila infection caused a pro-inflammatory reaction and significant miRNA changes in murine macrophages. In MyD88-/- cells, induction of inflammatory markers, such as Ccxl1/Kc, Il6 and miR-146a-5p was reduced. Induction of miR-125a-3p was completely abrogated in MyD88-/- cells. Target prediction analyses revealed N-terminal asparagine amidase 1 (NTAN1), a factor from the n-end rule pathway, to be a putative target of miR-125a-3p. This interaction could be confirmed by luciferase assay and western blot.
Conclusion
Taken together, we characterized the miRNA regulation in L. pneumophila infection with regard to MyD88 signaling and identified NTAN1 as a target of miR-125a-3p. This finding unravels a yet unknown feature of Legionella-host cell interaction, potentially relevant for new treatment options.
Introduction
Legionella pneumophila (L. pneumophila), a gram-negative bacterium, is described as a causative pathogen of lung inflammation and life-threatening pneumonia [1]. Outbreaks still occur [2], and cases with as many as 450 patients have been reported [3]. Alveolar macrophages, which protect the lung from inhaled microorganisms and other insults, are their predominant host in the human lung. Upon infection, L. pneumophila form their replication niche inside macrophages, the Legionella-containing vacuole (LCV), where they replicate until host cell lysis. Infection of macrophages with L. pneumophila leads to a broad activation of signaling pathways, triggered by both extracellular and intracellular receptor molecules, such as Toll-like receptors (TLRs) that recognize pathogen associated molecular patterns (PAMPs). Upon formation of the LCV, Legionella shuttles an array of effector molecules into the host cytosol via type IV (T4S) and type II (T2S) secretion systems. The T2S –translocated effectors seem to attenuate MyD88 signaling and to enhance bacterial growth in mammalian host cells [4]. MyD88 is a central adaptor molecule that links all TLRs except TLR3 to their downstream signaling cascades. The consequence of signal cascade activation is typically the activation of gene transcription events that in sum constitute an anti-microbial defense. Coding and non-coding RNAs are both part of this response. microRNAs (miRNAs) are an example of short non-coding RNAs which are involved in the defense mechanisms of the host. Exemplarily, miR-146a-5p is broadly described as a negative regulator of IRAK1 and TRAF6, thereby limiting the immune response in a negative feedback loop [5]. By subduing the MyD88 response, Legionella is actively interfering with these processes. Therefore, it is still unknown to what extent miRNAs are involved in L. pneumophila infection, either as part of the host response, or as part of host cell rewiring by the pathogen. We characterized miRNA expression in murine macrophages upon L. pneumophila infection in the context of a MyD88 knockout to shed more light on this particular aspect of host-pathogen interaction. We found significant changes of miRNA expression, which partly depended on the MyD88-pathway. Specifically, miR-125a-3p was found to be regulated in a MyD88-dependent manner. We could furthermore show that it targets NTAN1, an amidase which converts residual asparagine to aspartic acid, the first step in a series of protein modifications leading to eventual proteolysis. Regulation of NTAN1 by miR-125a-3p might be an important feature of protein stability control during L. pneumophila infection.
Materials & methods
Bacterial strains and infection
L. pneumophila Corby wild type was kindly provided by the Robert Koch Institute Berlin, Germany (A. Flieger, K. Heuner), and routinely grown as described previously [6]. This strain (Corby) has been used to establish the GenBank entry under accession number CP000675. Cells were infected with L. pneumophila at indicated multiplicity of infection (MOI).
Cell culture and genotyping
The Raw264.7 cell line was obtained from the America Type Culture Collection (ATCC). Mouse embryonic fibroblasts (MEF) were kindly supplied by Bastian Stielow, Marburg, Germany. Both cell lines were cultured in DMEM medium with 10% FCS (PAA Laboratories, Pasching, Austria) without antibiotics. Raw264.7 cells were used from passage 2–15. MEF cells, used as a vehicle for luciferase reporter assay, were cultured from passage 53 to 60. Bone marrow-derived macrophages (BMMs) were freshly prepared from femurs and tibiae of C57BL/6 wild type (Charles River Laboratories, Wilmington, USA) and MyD88-/- mice (kindly provided by C. Brunner, Ulm, Germany, [7]) and cultivated as described previously [8]. All cells were authenticated by microscopic morphology. Genotyping PCR for the MyD88-/- cells was performed using primers for the MyD88 gene (NC_000075.6) 5´-AGACAGGCTGAGTGCAAACTTGGTCTG-3´ (Primer A) and 5´-AGCCTCTACACCCTTCTCTTCTCCACA-3´ (Primer B) and with a primer for the neomycin resistance cassette, 5´-ATCGCCTTCTATCGCCTTCTTGACGAG-3´ (Primer C) (S1 Fig).
Quantitative RT-PCR
RNA was isolated using Isol-RNA Lysis Reagent (5 PRIME, Hamburg, Germany), quantified by Nanodrop and reverse transcribed with the High Capacity cDNA Reverse Transcription Kit or the microRNA reverse transcription kit (both Life Technologies) according to the manufacturer’s protocol. Quantitative RT-PCR was performed on a ViiA™ 7 Real-Time PCR System using Fast SYBR Green or Taqman Fast Advanced Master Mix (both Life Technologies). Primer sequences were obtained from the PrimerBank database (https://pga.mgh.harvard.edu/primerbank/) or from the referenced publications. The following primers were used: Ntan1 (NM_010946, sense: 5´-GGCATCGCTGTCAACATTAAAAC-3´, antisense: 5´-AATGCTAATCATTGGGCCTCC-3´, PrimerBank ID 87299633c2), Cxcl1/Kc (NM_008176, sense: 5´-ACTGCACCCAAACCGAAGTC-3´, antisense: 5´- TGGGGACACCTTTTAGCATCTT-3´, PrimerBank ID 229577225c1), Il6 (NM_031168, sense: 5´- CTGCAAGAGACTTCCATCCAG-3´, antisense: 5´-AGTGGTATAGACAGGTCTGTTGG-3´, PrimerBank ID 13624310c1), Sesn1 (NM_001162908, sense: 5´-ACACGGGATGCATGTCCCAAC-3´, antisense: 5´- TCCCACATCTGGATAGAGACGATTCA-3´, [9]), Gapdh (NM_001289726.1, sense: 5´-TGATGGGTGTGAACCACGAG-3´; antisense: 5´-TCAGTGTAGCCCAAGATGCC-3´, [10]). Commercial microRNA primers were purchased from Life Technologies. snoRNA202 served as endogenous control for microRNA analyses, while Gapdh was used for mRNA analyses. Samples were run in technical triplicates. Data were processed with the ViiA7 software (V. 1.2.4., Life Technologies) and analyzed with the 2-ddCt method [11].
High troughput qPCR and data analysis
Taqman Low Density Arrays (TLDAs) were performed with RNA samples from infected and uninfected WT and MyD88-/- BMMs according to the manufacturer´s instructions. For data interpretation, the Bioconductor R package HTqPCR (version 3.2) [12] with the limma package (version 3.24.15) [13] was used. The U6 snRNA was used for normalization.
Cloning
The 3´UTR of Ntan1 was amplified from murine macrophage cDNA with the following custom made primers: sense: 5´-GTTTTCTCGAGggagctaacatcagctcagag-3´; antisense 5´- GTTTTGCGGCCGCgaaagacaggaaggacggaag-3´. The 5´overhang (GTTTT) and restriction sites for XhoI (CTCGAG) and NotI (GCGGCCGC) are shown in capital letters. The amplified fragment was inserted into the psiCheck2 vector (Promega). The mutated 3´UTR of Ntan1 was purchased as a custom made gBlocks gene fragment from Integrated DNA Technologies.
Transfection
For the Luciferase Reporter Assay, mouse embryonic fibroblasts (MEF) were co-transfected with (1) synthetic miR-125a-3p or a chemistry-matched scramble sequence at a final concentration of 50 nM and (2) 500 ng of psiCheck2 vector (Promega) containing either the wild type or mutated 3´UTR of Ntan1. For western blot analysis of NTAN1 protein abundance, Raw264.7 cells were transfected with synthetic miR-125a-3p or a chemistry-matched scramble sequence at a final concentration of 5 nM. Transfections were carried out with siPort NeoFX (Ambion) for Raw264.7 cells or Lipofectamine 2000 (Thermo Fisher) for MEF cells, according to the manufacturers´ recommendations.
Western blot
Western blot was performed as described previously [14]. Immunodetection was carried out with anti-NTAN1 (antikoerper-online.de, ABIN967387) and anti-tubulin (Santa Cruz, sc-5286) and visualized on a chemoluminescence imager (INTAS Science Imaging Instruments, Göttingen, Germany).
ELISA
ELISA against KC was performed with the Mouse CXCL1/KC DuoSet ELISA (R&D Systems) according to the manufacturer´s recommendations.
Luciferase reporter assay
At 72 h after transfection, MEF cells were lysed and substrates for Renilla and Firefly Luciferase were added, using the Dual Glo Assay System according to the manufacturer´s recommendations (Promega). Luminescence emission was detected on a Tecan Infinite M200 Pro.
Ethical statement
Animals were handled according to the EU council directive 86/609/EEC for the protection of animals and sacrificed by intraperitoneal administration of a fatal dose of ketamine and xylazine. The performed protocols were approved by the responsible animal ethics committee (Philipps-University Marburg; permit number: EX-22-2013), and all efforts were made to minimize suffering.
Statistical analyses
Data are presented as mean & SD (standard deviation) of at least three independent experiments. Effects were statistically evaluated employing the indicated appropriate tests. p-values < 0.05 were considered significant and adjusted for multiple testing where indicated.
Results
L. pneumophila characteristically changes expression of key miRNAs and mRNAs in a MyD88 dependent manner
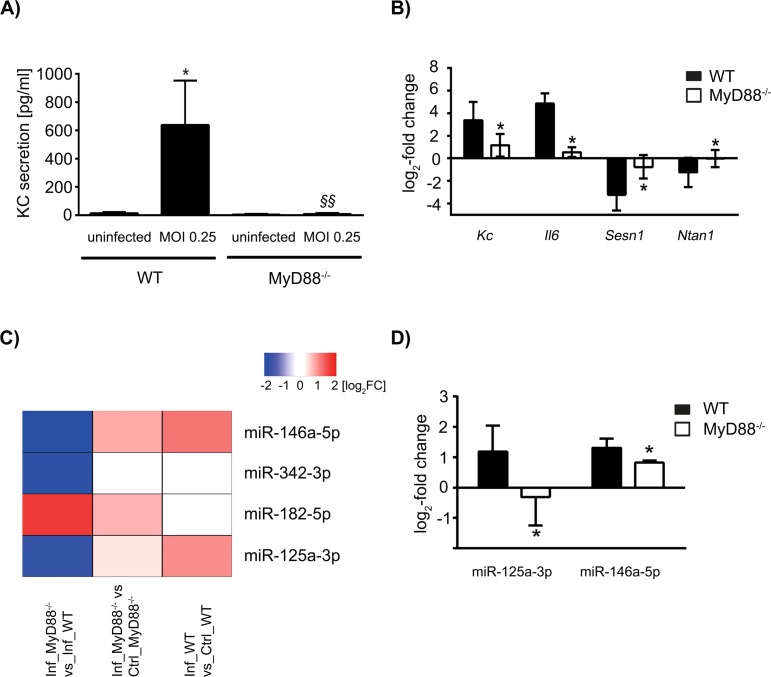
Firstly, we aimed to establish a solid set of markers to ensure efficient infection of BMMs with L. pneumophila and to show the perturbations inflicted by the MyD88 knockout. We detected increased expression of pro-inflammatory KC (protein and mRNA) and Il6 (mRNA) in WT BMMs and to a significantly lesser extent also in MyD88-/- cells at 24 h post infection (p.i., Fig 1A and 1B). Additionally, we confirmed a reduction in Sesn1 mRNA levels upon infection as previously described [9], which was significantly stronger in WT BMMs than in MyD88-/- BMMs at 24 h p.i. These established markers substantiated the pro-inflammatory macrophage phenotype that was expected to manifest after infection. In addition, we show Ntan1, which was down-regulated in WT cells, but was not regulated in MyD88-/- BMMs upon infection (Fig 1B).
Fig 1. KC, IL-6 and microRNAs are differentially regulated in wild type and MyD88-/- BMMs upon infection with Legionella pneumophila.
WT and MyD88-/- macrophages were infected with L. pneumophila for 24 h at a MOI of 0.25, and KC release was determined by ELISA (A). The expression patterns of selected mRNAs were analysed by qPCR (B). microRNAs were investigated by Taqman Low Density Array. The top 4 differentially regulated microRNAs as ranked by p-value are shown. The relative log2 fold induction in infected MyD88-/- vs. infected WT cells (left column), in infected MyD88-/- vs. uninfected MyD88-/- cells (middle column) or in infected WT vs. uninfected WT cells (right column) is depicted (C). Selected microRNAs were validated by qPCR (D). mRNA samples were normalized against GAPDH, while microRNA samples were normalized against snRNA U6 (C) or snoRNA202 (D). Data are shown as mean & SD of at least three independent experiments. Statistical tests were one-way ANOVA with post-hoc intergroup comparison (A) and Student´s T-Test with Bonferroni-Holm adjustment for multiple testing (B) and (D). *p<0.05 WT uninfected vs. WT MOI 0.25, §§p<0.05 MyD88-/- MOI 0.25 vs. WT MOI 0.25 (A), *padj<0.05 MyD88-/- vs. WT (B) and (D).
We furthermore characterized the response of WT and MyD88-/- BMMs on the miRNA level by Taqman Low Density Array. We found the miRNAs miR-146a-5p, miR-342-3p, miR-182-5p and miR-125a-3p to be subject to significant (p<0.05) differential expression between WT and MyD88-/- BMMs (Fig 1C). Within the scope of this work, we chose two miRNAs for a more detailed analysis on the basis of their immunomodulatory potential. In three additional independent confirmatory experiments, the well-characterized miR-146a-5p was up-regulated 24 h p.i. in WT cells to a significant extent when compared to MyD88-/- BMMs. Furthermore, miR-125a-3p was induced 24 h post L. pneumophila infection in WT BMMs but conversely showed a down-regulation in MyD88-/- BMMs (Fig 1D). This microRNA has been described to play a role in autophagy [15], obesity [16] and cancer [17, 18]. By in silico analysis (TargetScan Mouse Release 7.1), we found that binding site topology in the 3’ UTR of Ntan1 strongly suggests an interaction with miR-125a-3p. We subsequently investigated the relationship between miR-125a-3p and Ntan1, which both seem to be subject to downstream MyD88 signaling.
miR-125a-3p functionally binds the Ntan1 3´-UTR and regulates NTAN1 protein abundance
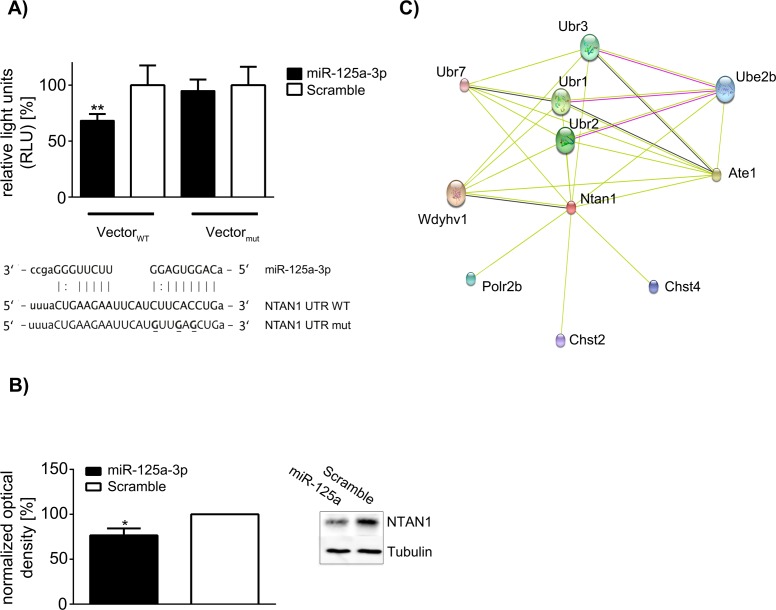
The hypothetical binding site for miR-125a-3p is partly conserved among several species, which indicates selective pressure on this site and therefore suggests a conserved function. We constructed a luciferase reporter vector including the 3’ UTR of Ntan1 to validate this putative molecular interaction. miR-125a-3p was capable of significantly decreasing the luminescence signal by approx. 30% when compared to a scramble control RNA. The mutation of 3 bases within the 3´UTR abrogated this effect (Fig 2A). Having found that this miRNA had no effect on Ntan1 transcript levels, we performed western blot and observed that overexpression of miR-125a-3p reduced NTAN1 protein by approx. 23% as opposed to scramble transfected control cells (Fig 2B). Furthermore, a network analysis based on the STRING database [19] highlights the involvement of NTAN1 in the n-end rule pathway by association of NTAN1 with the ubiquitin protein ligase E3 component n-recognin (Ubr) family and arginyltransferase 1 (Ate1, Fig 2C). In conclusion, we herewith reveal an interaction of the L. pneumophila-induced miR-125a-3p with NTAN1.
Fig 2. microRNA-125a-3p targets NTAN1 and regulates it on protein level.
microRNA-125a-3p shows partial homology to the 3´UTR of Ntan1 mRNA. To destroy the sequence compatibility in the seed region, 3 bases were exchanged (bold, underlined). MEF cells were co-transfected with the microRNA-125a-3p or a scramble sequence and the psiCheck2 vector carrying either the wild type Ntan1 3´UTR or the mutated sequence. Relative luminescence units (RLU) were determined after 72 h. Renilla luciferase signal was normalized against firefly luciferase. Relative luminescence units were calculated as a percentage of signal in scramble transfected cells (A). Raw264.7 cells were transfected with microRNA-125a-3p or a scramble sequence (5 nM), and NTAN1 protein levels were determined by western blot after 72 h. Densitometric analysis was performed with normalization against Tubulin. One representative blot is shown (B). In an interaction network, which was built from the STRING database, NTAN1 is shown to be associated with the N-end rule pathway. The observed interactions are based on experimental data (purple edges), co-expression (black edges) and textmining (green edges). Only direct interactions with an interaction score of minimum 0.4 (i.e. medium confidence) are shown. Interaction partners include arginyltransferase 1 (Ate1) and members of the ubiquitin protein ligase E3 component n-recognin (Ubr) family (C). Data are shown as mean & SD of at least four independent experiments. Statistical tests were one-way ANOVA with post-hoc intergroup comparison (A) and Mann Whitney U test (B). **p<0.01 Vector WT + miR-125a-3p vs. Vector Mutant + miR-125a-3p (A), *p<0.05 miR-125a-3p vs. Scramble (B).
Discussion
The aim of this study was to find miRNA-regulated events during L. pneumophila infection in dependency of the central signal transduction molecule MyD88. miRNAs constitute a cellular transcript control mechanism which is instrumental to the immune response but also constitutes a potential target for host cell subversion by a pathogen [20]. We infected murine WT and MyD88-/- BMMs to help unravel this particular aspect of host-pathogen interaction.
L. pneumophila is an intracellular pathogen that causes shifts in the gene expression of the host cell upon infection. While the cellular immune response aims to remove the pathogen, L. pneumophila manipulates host gene expression to its own advantage [21]. We have described previously that TNFAIP2 is up-regulated upon L. pneumophila infection, a factor which is beneficial for bacterial replication inside the cell [22]. Aside from coding RNAs, non-coding RNAs such as miRNAs are also regulated upon infection with L. pneumophila. In a high throughput screening by Taqman Low Density Array, we identified, among others, miR-146a-5p and miR-125a-3p as significantly regulated between WT and MyD88-/- BMMs. Subsequent further testing confirmed regulation of miR-146a-5p, which has been extensively characterized with regard to its anti-inflammatory properties [23], in WT and MyD88-/- BMMs. While markedly weaker in MyD88-/- BMMs, this miRNA was induced in both cases. This suggests that MyD88 is not strictly required but merely an accessory to the induction of miR-146a-5p. In accordance with this finding, pro-inflammatory cytokines showed a lower induction on mRNA level in MyD88-/- cells when compared to WT cells, and KC secretion was close to the detection limit (Fig 1A). The induction of miR-125a-3p, however, revealed a strong dependence on MyD88, as it was down-regulated in MyD88-/- cells upon infection, while robustly induced in WT cells. The induction of this miRNA under infectious conditions and its dependency on TLR2 and MyD88 has been shown previously [24]. We found a functional interaction between miR-125a-3p and the Ntan1 3´UTR by Luciferase Reporter Assay, which was abolished by the mutation of three bases in the miRNA-binding region of the Ntan1 3´UTR. Whilst no detectable down-regulation of Ntan1 transcript was achieved upon artificial overexpression of miR-125a-3p, we confirmed down-regulation on the protein level by western blot.
NTAN1 is an aminohydrolase which converts N-terminal asparagine to aspartic acid within the N-end rule pathway of protein degradation [25]. While its involvement in Legionella infection remains to be elucidated, the N-end rule pathway is known to play a role in antigen degradation and the generation of epitopes during infection with Listeria monocytogenes [26, 27], a pathogen also known to induce miR-125a-3p [24].
Our study reveals the NTAN1/miR-125a-3p interaction as a new feature of the host´s response against L. pneumophila. Intriguingly, Legionella have developed sophisticated mechanisms of host cell exploitation during their co-evolution with eukaryotic host cells. They secrete more than a hundred effector proteins into the host cytosol [28], and have been described to hijack the host proteasome by specifically labelling factors with ubiquitin for degradation [29]. NTAN1 as part of the protein degradation machinery is associated with several E3 ubiquitin protein ligases (Fig 2C). MyD88-dependent regulation of miR-125a-3p may prove to be a mechanism to control antigen stability and epitope generation via NTAN1 and the N-end rule pathway upon bacterial infection.
The integration of these events into one regulatory unit is supported by the fact that another member of the miR-125 family, miR-125b-5p, has been shown to target MyD88 [30]. In our high-throughput dataset, while not statistically significant, this microRNA shows an up-regulation in the wild type upon infection (1.26 log2 FC) and a down-regulation in MyD88-/- cells (-2.06 log2 FC), mirroring the expression pattern we show for miR-125a-3p (Fig 1D). We suggest the existence of a functional, self-regulating node in infection, which contains MyD88, miR-125a-3p, miR-125b-5p and NTAN1. It remains to be studied whether this mechanism works in favor of Legionella, or whether it antagonizes it. In addition, our observations are based on primary murine cells and will have to be validated in human cells.
In summary, this study characterizes for the first time changes in miRNA expression upon macrophage infection by L. pneumopila in the context of MyD88 deletion. We describe the regulation of the factor NTAN1 by miR-125a-3p, which is MyD88-dependent. This finding underlines the importance of miRNAs in innate immune reactions against bacterial pathogens and adds understanding to a node downstream of MyD88 in the complex interaction network between bacterium and host cell [28].
Supporting information
PCR was performed to validate the WT and MyD88-/- genotype of the used BMMs. The primer pair for the MyD88 gene (A+B) yielded a product only in the WT cells, while the primer pair designed to detect the inserted neomycin resistance cassette (B+C) only yielded a product in the MyD88-/- cells. A no-template control (NTC) was included to ensure method fidelity.
(TIF)
Acknowledgments
We thank Kerstin Hoffmann and Nadine Siebert (Philipps University Marburg, Germany) for excellent technical assistance. We also thank Sebastian Müller for technical support during his internship. This work contributes in part to the master theses of E.J. and M. B.
Data Availability
All Taqman Low Density Array data are available from the ncbi database: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?token=ajqpcogehvapzwb&acc=GSE92600.
Funding Statement
The work was in part supported by grants from the Deutsche Forschungsgesellschaft, (TR84), Bundesministerium für Bildung und Forschung (e:bio 0316175A, e:Med 01X1304E, Progress FKZ 01KI1010K) and Hessisches Ministerium für Wissenschaft und Kunst (LOEWE UGMLC and LOEWE Medical RNomics) to BS. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Fraser DW, Tsai TR, Orenstein W, Parkin WE, Beecham HJ, Sharrar RG, et al. Legionnaires' disease: description of an epidemic of pneumonia. The New England journal of medicine. 1977;297(22):1189–97. doi: 10.1056/NEJM197712012972201 [DOI] [PubMed] [Google Scholar]
- 2.Kuroki T, Amemura-Maekawa J, Ohya H, Furukawa I, Suzuki M, Masaoka T, et al. Outbreak of Legionnaire's Disease Caused by Legionella pneumophila Serogroups 1 and 13. Emerging infectious diseases. 2017;23(2):349–51. PubMed Central PMCID: PMC5324795. doi: 10.3201/eid2302.161012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garcia-Fulgueiras A, Navarro C, Fenoll D, Garcia J, Gonzalez-Diego P, Jimenez-Bunuales T, et al. Legionnaires' disease outbreak in Murcia, Spain. Emerging infectious diseases. 2003;9(8):915–21. PubMed Central PMCID: PMC3020623. doi: 10.3201/eid0908.030337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mallama CA, McCoy-Simandle K, Cianciotto NP. The Type II Secretion System of Legionella pneumophila Dampens the MyD88 and Toll-Like Receptor 2 Signaling Pathway in Infected Human Macrophages. Infection and immunity. 2017;85(4). PubMed Central PMCID: PMC5364298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Griss K, Bertrams W, Sittka-Stark A, Seidel K, Stielow C, Hippenstiel S, et al. MicroRNAs Constitute a Negative Feedback Loop in Streptococcus pneumoniae-Induced Macrophage Activation. The Journal of infectious diseases. 2016;214(2):288–99. doi: 10.1093/infdis/jiw109 [DOI] [PubMed] [Google Scholar]
- 6.Schmeck B, N'Guessan PD, Ollomang M, Lorenz J, Zahlten J, Opitz B, et al. Legionella pneumophila-induced NF-kappaB- and MAPK-dependent cytokine release by lung epithelial cells. The European respiratory journal. 2007;29(1):25–33. doi: 10.1183/09031936.00141005 [DOI] [PubMed] [Google Scholar]
- 7.Adachi O, Kawai T, Takeda K, Matsumoto M, Tsutsui H, Sakagami M, et al. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 1998;9(1):143–50. [DOI] [PubMed] [Google Scholar]
- 8.Sester DP, Trieu A, Brion K, Schroder K, Ravasi T, Robinson JA, et al. LPS regulates a set of genes in primary murine macrophages by antagonising CSF-1 action. Immunobiology. 2005;210(2–4):97–107. doi: 10.1016/j.imbio.2005.05.004 [DOI] [PubMed] [Google Scholar]
- 9.Fortier A, Faucher SP, Diallo K, Gros P. Global cellular changes induced by Legionella pneumophila infection of bone marrow-derived macrophages. Immunobiology. 2011;216(12):1274–85. doi: 10.1016/j.imbio.2011.06.008 [DOI] [PubMed] [Google Scholar]
- 10.Opitz B, Puschel A, Schmeck B, Hocke AC, Rosseau S, Hammerschmidt S, et al. Nucleotide-binding oligomerization domain proteins are innate immune receptors for internalized Streptococcus pneumoniae. The Journal of biological chemistry. 2004;279(35):36426–32. doi: 10.1074/jbc.M403861200 [DOI] [PubMed] [Google Scholar]
- 11.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–8. doi: 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 12.Dvinge H, Bertone P. HTqPCR: high-throughput analysis and visualization of quantitative real-time PCR data in R. Bioinformatics. 2009;25(24):3325–6. PubMed Central PMCID: PMC2788924. doi: 10.1093/bioinformatics/btp578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic acids research. 2015;43(7):e47 PubMed Central PMCID: PMC4402510. doi: 10.1093/nar/gkv007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krull M, Kramp J, Petrov T, Klucken AC, Hocke AC, Walter C, et al. Differences in cell activation by Chlamydophila pneumoniae and Chlamydia trachomatis infection in human endothelial cells. Infection and immunity. 2004;72(11):6615–21. PubMed Central PMCID: PMC523009. doi: 10.1128/IAI.72.11.6615-6621.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim JK, Yuk JM, Kim SY, Kim TS, Jin HS, Yang CS, et al. MicroRNA-125a Inhibits Autophagy Activation and Antimicrobial Responses during Mycobacterial Infection. Journal of immunology. 2015;194(11):5355–65. [DOI] [PubMed] [Google Scholar]
- 16.Chen K, He H, Xie Y, Zhao L, Zhao S, Wan X, et al. miR-125a-3p and miR-483-5p promote adipogenesis via suppressing the RhoA/ROCK1/ERK1/2 pathway in multiple symmetric lipomatosis. Scientific reports. 2015;5:11909 PubMed Central PMCID: PMC4493643. doi: 10.1038/srep11909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ninio-Many L, Grossman H, Levi M, Zilber S, Tsarfaty I, Shomron N, et al. MicroRNA miR-125a-3p modulates molecular pathway of motility and migration in prostate cancer cells. Oncoscience. 2014;1(4):250–61. PubMed Central PMCID: PMC4278297. doi: 10.18632/oncoscience.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yin F, Zhang JN, Wang SW, Zhou CH, Zhao MM, Fan WH, et al. MiR-125a-3p regulates glioma apoptosis and invasion by regulating Nrg1. PloS one. 2015;10(1):e0116759 PubMed Central PMCID: PMC4283963. doi: 10.1371/journal.pone.0116759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Szklarczyk D, Morris JH, Cook H, Kuhn M, Wyder S, Simonovic M, et al. The STRING database in 2017: quality-controlled protein-protein association networks, made broadly accessible. Nucleic acids research. 2017;45(D1):D362–D8. PubMed Central PMCID: PMC5210637. doi: 10.1093/nar/gkw937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Staedel C, Darfeuille F. MicroRNAs and bacterial infection. Cellular microbiology. 2013;15(9):1496–507. doi: 10.1111/cmi.12159 [DOI] [PubMed] [Google Scholar]
- 21.Abshire CF, Dragoi AM, Roy CR, Ivanov SS. MTOR-Driven Metabolic Reprogramming Regulates Legionella pneumophila Intracellular Niche Homeostasis. PLoS pathogens. 2016;12(12):e1006088 PubMed Central PMCID: PMC5179073. doi: 10.1371/journal.ppat.1006088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Du Bois I, Marsico A, Bertrams W, Schweiger MR, Caffrey BE, Sittka-Stark A, et al. Genome-wide Chromatin Profiling of Legionella pneumophila-Infected Human Macrophages Reveals Activation of the Probacterial Host Factor TNFAIP2. The Journal of infectious diseases. 2016;214(3):454–63. doi: 10.1093/infdis/jiw171 [DOI] [PubMed] [Google Scholar]
- 23.Schulte LN, Westermann AJ, Vogel J. Differential activation and functional specialization of miR-146 and miR-155 in innate immune sensing. Nucleic acids research. 2013;41(1):542–53. PubMed Central PMCID: PMC3592429. doi: 10.1093/nar/gks1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schnitger AK, Machova A, Mueller RU, Androulidaki A, Schermer B, Pasparakis M, et al. Listeria monocytogenes infection in macrophages induces vacuolar-dependent host miRNA response. PloS one. 2011;6(11):e27435 PubMed Central PMCID: PMC3219661. doi: 10.1371/journal.pone.0027435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cantor JR, Stone EM, Georgiou G. Expression and biochemical characterization of the human enzyme N-terminal asparagine amidohydrolase. Biochemistry. 2011;50(14):3025–33. PubMed Central PMCID: PMC3085321. doi: 10.1021/bi101832w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sijts AJ, Pilip I, Pamer EG. The Listeria monocytogenes-secreted p60 protein is an N-end rule substrate in the cytosol of infected cells. Implications for major histocompatibility complex class I antigen processing of bacterial proteins. The Journal of biological chemistry. 1997;272(31):19261–8. [DOI] [PubMed] [Google Scholar]
- 27.Moors MA, Auerbuch V, Portnoy DA. Stability of the Listeria monocytogenes ActA protein in mammalian cells is regulated by the N-end rule pathway. Cellular microbiology. 1999;1(3):249–57. [DOI] [PubMed] [Google Scholar]
- 28.Franco IS, Shuman HA, Charpentier X. The perplexing functions and surprising origins of Legionella pneumophila type IV secretion effectors. Cellular microbiology. 2009;11(10):1435–43. doi: 10.1111/j.1462-5822.2009.01351.x [DOI] [PubMed] [Google Scholar]
- 29.Kubori T, Shinzawa N, Kanuka H, Nagai H. Legionella metaeffector exploits host proteasome to temporally regulate cognate effector. PLoS pathogens. 2010;6(12):e1001216 PubMed Central PMCID: PMC2996335. doi: 10.1371/journal.ppat.1001216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Z, Filgueiras LR, Wang S, Serezani AP, Peters-Golden M, Jancar S, et al. Leukotriene B4 enhances the generation of proinflammatory microRNAs to promote MyD88-dependent macrophage activation. Journal of immunology. 2014;192(5):2349–56. PubMed Central PMCID: PMC3943984. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PCR was performed to validate the WT and MyD88-/- genotype of the used BMMs. The primer pair for the MyD88 gene (A+B) yielded a product only in the WT cells, while the primer pair designed to detect the inserted neomycin resistance cassette (B+C) only yielded a product in the MyD88-/- cells. A no-template control (NTC) was included to ensure method fidelity.
(TIF)
Data Availability Statement
All Taqman Low Density Array data are available from the ncbi database: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?token=ajqpcogehvapzwb&acc=GSE92600.