Abstract
To evaluate intraoperative decentration from pupil center and kappa intercept during small incision lenticule extraction (SMILE) and its impact on visual outcomes.
This was a retrospective noncomparative case series. A total of 164 eyes that underwent SMILE at the Singapore National Eye Center were included. Screen captures of intraoperative videos were analyzed. Preoperative and 3 month postoperative vision and refractive data were analyzed against decentration.
The mean preoperative spherical equivalent (SE) was −5.84 ± 1.77. The mean decentration from the pupil center and from kappa intercept were 0.13 ± 0.06 mm and 0.47mm ± 0.25 mm, respectively. For efficacy and predictability, 69.6% and 95.0% of eyes achieved a visual acuity (VA) of 20/20 and 20/30, respectively, while 83.8% and 97.2% of eyes were within ±0.5D and ±1.0D of the targeted SE. When analyzed across 3 groups of decentration from the pupil center (<0.1 mm, 0.1–0.2 mm, and >0.2 mm), there was no statistically significant association between decentration, safety, efficacy, and predictability. When analyzed across 4 groups of decentration from kappa intercept (<0.2 mm, 0.2–<0.4 mm, 0.4–<0.6 mm, and ≥0.6 mm), there was a trend toward higher efficacy for eyes with decentration of kappa intercept between 0.4 and <0.6 mm (P = .097). A total of 85.4% of eyes in the 0.4 to <0.6 mm group had unaided distance VA of 20/20 or better, as compared to only 57.8% of eyes in ≥0.6 mm group.
Decentration of 0.13 mm from the pupil center does not result in compromised visual outcomes. Decentration of greater than 0.6 mm from the kappa intercept may result in compromised visual outcomes. There was a trend toward better efficacy in eyes which had decentered treatment from 0.4 to <0.6 mm from the kappa intercept. Patients with a large kappa intercept (>0.6 mm) should have their lenticule created 0.4 to 0.6 mm from the kappa intercept and not close to the pupil.
Keywords: centration, refractive surgery, SMILE
1. Introduction
Laser in situ keratomileusis (LASIK) is currently the most popular form of laser refractive procedure for the treatment of myopia and myopic astigmatism.[1] It provides rapid postoperative visual improvement and refractive stability with minimal patient discomfort.[1] The use of the femtosecond laser in femtosecond-assisted LASIK (FS-LASIK) has further revolutionized corneal refractive surgery by increasing safety, precision, and predictability over traditional microkeratome-assisted LASIK.[2] Small incision lenticule extraction (SMILE, Carl Zeiss Meditec AG) is a relatively new technique in corneal refractive surgery and has gained acceptance due to its predictability, safety, and efficacy.[3,4] It has also been reported to result in less postoperative dry eye syndrome[4] and eliminates the risk of any flap associated complications.[5]
Centration of the treatment zone during corneal refractive surgery is key to optimized visual outcomes. Decentered treatments can be associated with reduced visual acuity, irregular astigmatism, halos, glare,[6] reduced contrast sensitivity,[7] and monocular diplopia.[8] In LASIK, the centration of the photoablation is targeted to the pupil center or to the coaxial corneal light reflex (CCLR) or in between, depending on the type (eg, myopia/hyperopia) and amount of refractive error.[9] The alignment of the photoablation in LASIK can be controlled by the surgeon and has been shown to be improved with the use of eye trackers.[10]
Unlike LASIK, no active eye-tracker is available in SMILE, and laser centration depends on the patient, who must fixate during surgery on a blinking green light while the surgeon controls the docking of the suction interface cone. Because the eye is held by a low level of suction during the docking, the patient does not suffer from fixation fatigue; however, it does rely on some degree of patient compliance. The patient is able to fixate on the green light after docking since the intraocular pressure rise following SMILE has been shown to be below that necessary to occlude the posterior segment vasculature.[11] The intraocular pressure rise has also been shown to be stable throughout the lenticule creation process, but loss of fixation can occur when the lenticule creation crosses the visual axis, that is, during the center of the posterior lenticule creation.[12] Hence, the options for centration of the lenticule can be on the pupil center, line of sight (fixation), or on the corneal vertex.[9]
There are only a few reported studies on the effect of treatment decentration and its effect on visual outcomes. Li et al[13] measured decentration from the corneal vertex postoperatively and found good visual outcomes despite mild decentration. Liu et al[14] also evaluated decentration from pupil center and the corneal vertex against visual outcomes. Better refractive outcomes were achieved when the lenticule center was closer to the corneal vertex. Reinstein et al[15] measured decentration from corneal vertex in SMILE, using pre- and postoperative Atlas 9000 (Carl Zeiss Meditec AG) topography, and compared the result with eyes undergoing LASIK. They found there was no statistically significant difference in mean centration offset between the 2 groups. Reinstein et al also reported that the mean center of the optical zone was 0.34 ± 0.17 mm from the corneal vertex measured on corneal topography after femtosecond LASIK and femtosecond lenticule extraction (Relex Flex) in a separate study.[16] However, these studies relied on comparison with topography scans following patient treatment.
Lenticule decentration can be accurately assessed postoperatively by comparing topography profiles as previously reported. However, assessment intraoperatively would allow the surgeon to alter the lenticule creation before starting the photo-disruption process which is important in SMILE. Hence, the aim of this study was to evaluate intraoperative decentration from the pupillary center and the kappa intercept during SMILE for the correction of myopia and myopic astigmatism, and to investigate its impact on predictability, efficacy, and safety outcomes.
2. Methods
2.1. Patients
This study was a retrospective audit of consecutive eyes that underwent SMILE between March, 2012 and July, 2014, at a single tertiary referral center (Singapore National Eye Center [SNEC]). The study followed the principles of the Declaration of Helsinki, with the local institutional review board providing ethics approval.
Eyes were included if they had stable refraction for at least 6 months, no soft contact lens wear for 1 week before or rigid contact lens wear for 2 weeks before surgery, and no other ocular disease or previous ocular surgery. Patients were excluded if they had keratoconus or suspected keratoconus, active ocular or systemic disease likely to affect corneal wound healing (eg, autoimmune conditions), severe dry eyes, or a calculated postoperative corneal residual bed thickness of less than 250 μm. Patients on immunosuppression therapy were also excluded.
All eyes had a standard preoperative evaluation that included slitlamp and dilated fundus examinations, uncorrected distance visual acuity (UDVA), corrected distance visual acuity (CDVA), manifest refraction, and corneal topography (OrbscanIIz, Bausch & Lomb). Trained refractive optometrists and technicians performed all measurements and refraction to ensure accuracy and reproducibility.
2.2. Surgical technique
All surgeries were performed using a VisuMax femtosecond laser system (Carl Zeiss Meditec AG). Each SMILE was performed using a previously described technique.[3,4,17] After application of topical anesthesia, standard sterile draping, and insertion of the speculum, patients were asked to fixate on the internal target light, which was mounted coaxial with the femtosecond laser beam. The eye was then docked with the curved interface cone before suction fixation was applied. Centration was attempted on the pupil center. In all eyes, the S-size contact glass was used and the laser was activated for photodissection in the following sequence.[18] The posterior surface of the refractive lenticule (spiral in) was created, after which the lenticule border was created. The anterior surface of the refractive lenticule (spiral out) was formed, extending beyond the posterior lenticule diameter by 0.5 mm to form the anterior flap. This was followed by a rim cut. The following femtosecond laser parameters were used: 120 μm cap thickness, 7.5 mm cap diameter, 6.5 mm optical zone of lenticule, and 145 nJ power with side-cut angles at 90°. The spot distance and tracking spacing were, respectively, 3.0 and 3.0 μm for the lenticule, 2.5 and 2.5 μm for the lenticule sidecut, 3.0 and 3.0 μm for the cap, and 2.0 and 2.0 μm for the cap side cut. After suction release, a SMILE lamellar dissector (Asico, LLC) was inserted through the side cut over the anterior surface of the refractive lenticule, dissecting this plane and then the posterior plane of the lenticule. The lenticule was then grasped and removed through the small incision using a nontoothed serrated forceps. The intrastromal space was flushed with a balanced salt solution using a standard irrigating cannula and the anterior cornea gently wiped to massage any Bowman folds toward the periphery and to aid removal of irrigation fluid.[19]
2.3. Measuring decentration
The pupillary axis is an anatomically defined axis; it is the line perpendicular to the cornea that passes through the center of the entrance pupil.[20,21] The visual axis is a line that connects the point of fixation with the 1st and 2nd nodal points and the fovea. It travels from the fixation point to the 1st nodal point, exits from the 2nd nodal point with the same angle, and intersects the fovea when the patient is fixating.[20] Angle kappa is defined in the literature as the angle between the pupillary axis and visual axis.[21,22] The subject-fixated CCLR is formed by the reflection of light from the anterior corneal surface. It is the virtual image of the object of fixation which is also known as the first Purkinje–Sanson image.[23]
Screen captures of intraoperative videos were obtained after suction fixation was applied and just before the commencement of photodissection. Decentration was measured using Adobe Illustrator CS6 Version 16.0 (Adobe Systems Incorporated) on these screen captures of intraoperative videos. The pupil center was located automatically by the software, after the pupil was outlined using the ellipse tool. Decentration from the pupillary center was defined as the distance from the pupillary center to the green fixation light, as seen on the intraoperative screen captures. The maximum horizontal and vertical pupil size were also recorded (Fig. 1).
Figure 1.
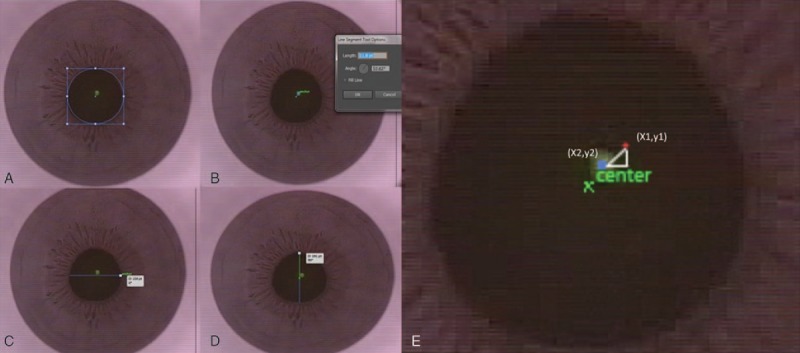
Intraoperative screen captures. (A) Pupil outlined with ellipse tool and center of pupil identified. (B) Decentration from pupil center measurement. Small blue dot = pupil center. Green dot = fixation light. (C) Horizontal pupil diameter measurement. (D) Vertical pupil diameter measurement. (E) Red dot = kappa intercept derived from Orbscan. Distance between fixation light and kappa intercept is calculated by trigonometry.
The kappa intercept was derived from angle kappa and is the position in which the visual axis intersects the anterior corneal surface expressed in Cartesian coordinates. The angle kappa value and kappa intercept values for each eye were obtained preoperatively from the corneal topography (OrbscanIIz, Bausch, and Lomb). Fixation was checked during Orbscan acquisition by our ophthalmic technician using the fixation marker and scans with poor fixation/centration were rejected and repeated. The polar co-ordinate values were then converted to Cartesian co-ordinates with the following trigonometric functions x = r cos α and y = r sin α, x and y representing the co-ordinates on the x-axis and y-axis respectively, r representing the radial co-ordinate value, and α representing the angular co-ordinate.[24] The kappa intercept's distance from the green fixation light was then calculating using the following trigonometric function
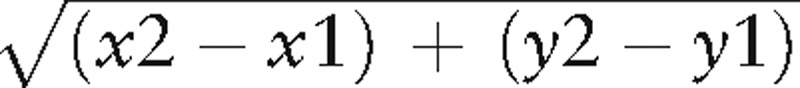 |
, with (x1,y1) representing the x-axis and y-axis co-ordinates of the kappa intercept, and (x2,y2) representing the x-axis and y-axis co-ordinates of the green fixation light.
2.4. Statistical analysis
The primary outcome measures were the mean decentration values of the green fixation light to the pupillary center, and the mean decentration of the green fixation light from the kappa intercept. The secondary outcome measures were the postoperative outcomes in relation to the centration values as mentioned above.
Predictability was defined as the proportion of eyes achieving a postoperative spherical equivalent (SE) within ±0.50 Diopter (D) and ±1.00D of the intended target. Efficacy was defined as the proportion of eyes achieving an UDVA of 20/20 and 20/40 or better postoperatively. Safety was defined as the proportion of eyes that lost or gained 1 or more lines of postoperative CDVA relative to preoperative CDVA. The generalized estimating equation model is an extension of the general linear regression model. It provides an appropriate statistical approach to account for the presence of correlated observation in our data (ie, paired eye data). Data analyses were performed with IBM SPSS Statistics (Version 22, IBM Corp). Whenever appropriate, descriptive data were represented as mean ± standard deviation. Significance level was set at P < .05.
3. Results
3.1. Demographics, centration, and pupil measurements
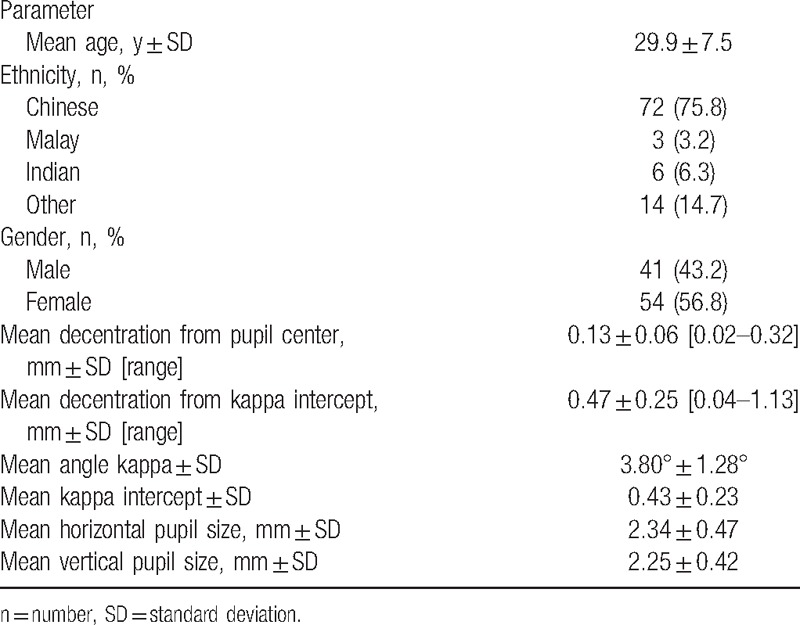
A total of 164 eyes that underwent SMILE were enrolled in the study. All eyes had postoperative follow-up period of at least 3 months. The mean age of the patients was 29.9 ± 7.5 years, 54 (56.8%) were women, and 72 (75.8%) were of Chinese ethnicity. The mean decentration from the pupillary center was 0.13 ± 0.06 mm (range 0.02–0.32 mm). Figure 2 shows the distribution of the decentration values from the pupillary center. The mean angle kappa was 3.80° ± 1.28°, the mean kappa intercept was 0.43 ± 0.23, and the mean decentration from the kappa intercept was 0.47 ± 0.25 mm (range 0.04–1.13 mm). Figure 3A and B illustrates the distribution of kappa intercept and decentration from the kappa intercept, respectively. Patient demographics and the primary outcome measurements are shown in Table 1.
Figure 2.
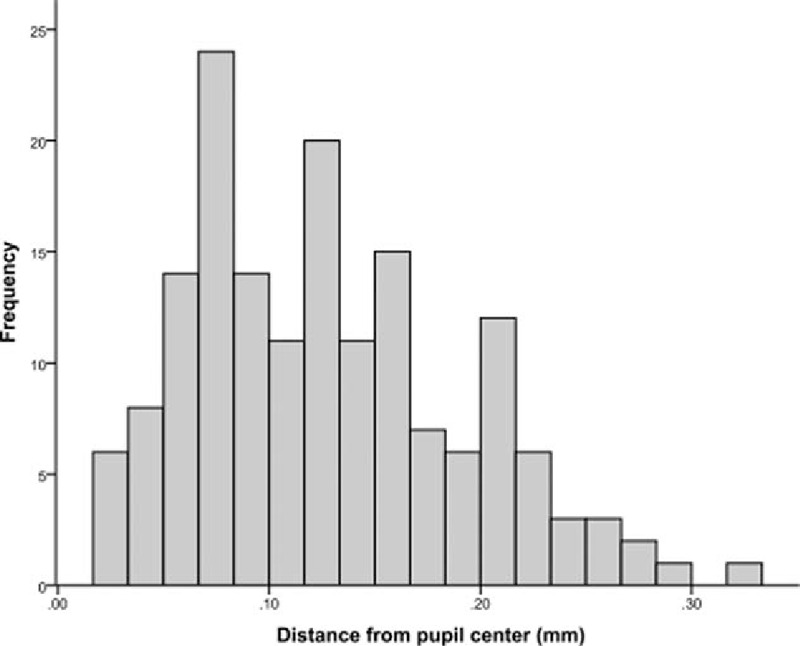
Distribution of decentration from the pupillary center.
Figure 3.
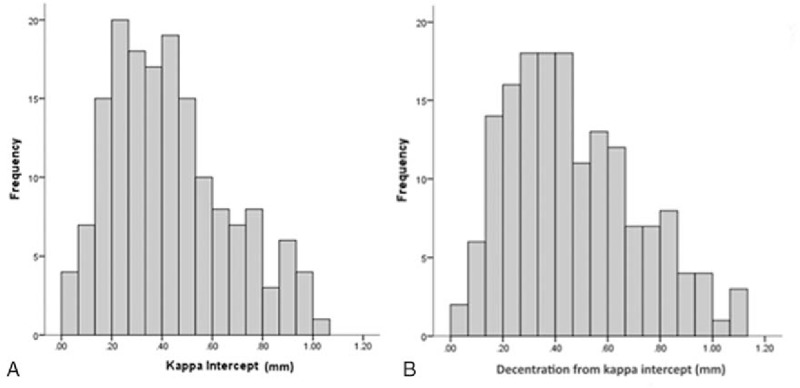
(A) Distribution of kappa intercept. (B) Distribution of decentraton from kappa intercept.
Table 1.
Patient demographics, centration, and pupil measurements.
There was a statistically significant positive relationship between angle kappa and decentration from pupil centre (Pearson correlation coefficient of r = 0.196, P = .013) (Fig. 4A). There was a statistically significant relationship between angle kappa and decentration from the kappa intercept (r = 0.286, P < .001) (Fig. 4B). The mean vertical and horizontal pupil size were 2.34 ± 0.47 mm and 2.25 ± 0.42 mm, respectively. There was no statistically significant relationship between maximum pupil size and decentration from kappa intercept (r = −0.07, P = .37) (Fig. 4C). There was no obvious trend between pupil size and decentration from pupillary center (r = 0.07, P = .35) (Fig. 4D). There was a statistically significant positive relationship between decentration from pupil center and decentration from kappa intercept (r = 0.17, P = .03) (Fig. 4E).
Figure 4.
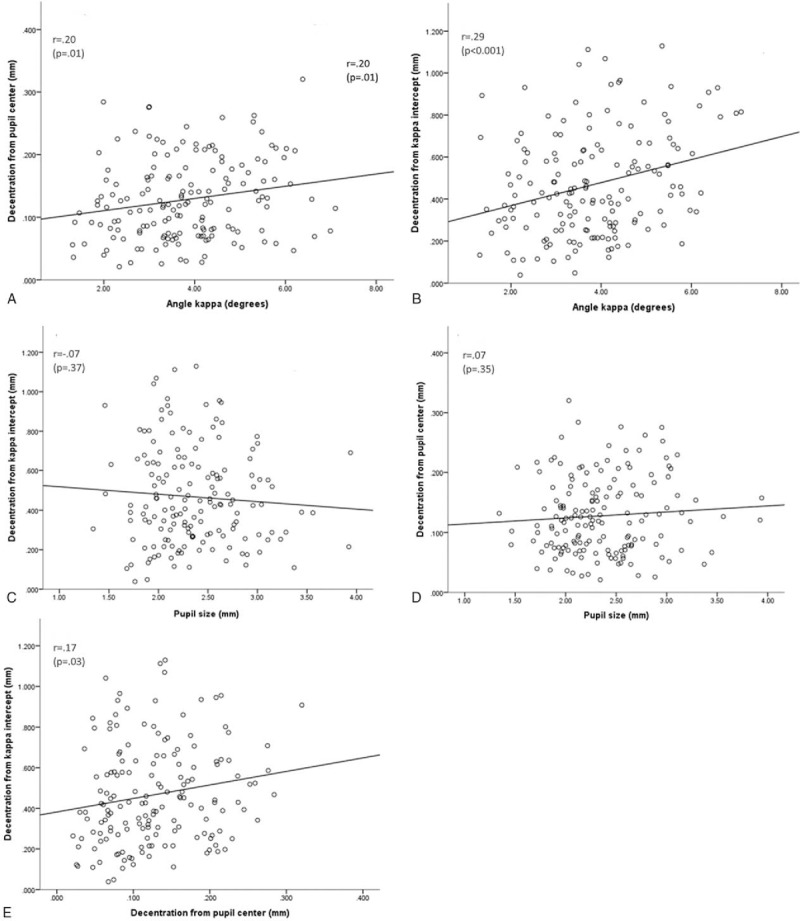
(A) Plot of decentration from pupil center against angle kappa. (B) Plot of decentration from kappa intercept against angle kappa. (C) Plot of maximum pupil size against decentration from kappa intercept. (D) Plot of maximum pupil size against decentration from pupil center. (E) Plot of decentration from pupil center against decentration from kappa intercept. r = Pearson correlation coefficient.
3.2. Efficacy, predictability, and safety
The mean preoperative SE was −5.84 ± 1.77. The mean postoperative SE at 3 months was 0.05 ± 0.47. With regards to efficacy, 112 (69.6%) eyes and 159 (99.0%) eyes achieved a VA of 20/20 and better than 20/40, respectively. With regards to predictability, 119 (83.8%) of eyes and 138 (97.2%) of eyes were within ±0.5D and ±1.0D of the targeted SE, respectively. There were no major intraoperative or postoperative complications that affected visual outcomes. Two (1.4%) eyes lost more than 2 line of CDVA at 3 months postoperatively.
3.3. Comparison of outcomes against decentration
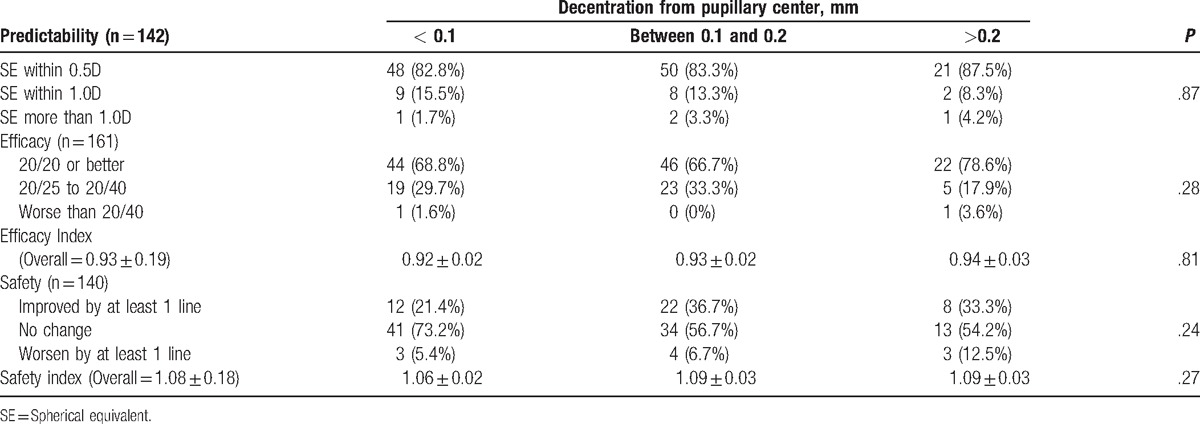
When analyzed across 3 groups of decentration from the pupillary center (<0.1 mm, 0.1–0.2 mm, and >0.2 mm), there was no statistically significant association between decentration from pupillary center and outcomes of predictability, efficiency, and safety (Table 2)
Table 2.
Correlating outcomes with decentration from pupillary centre.
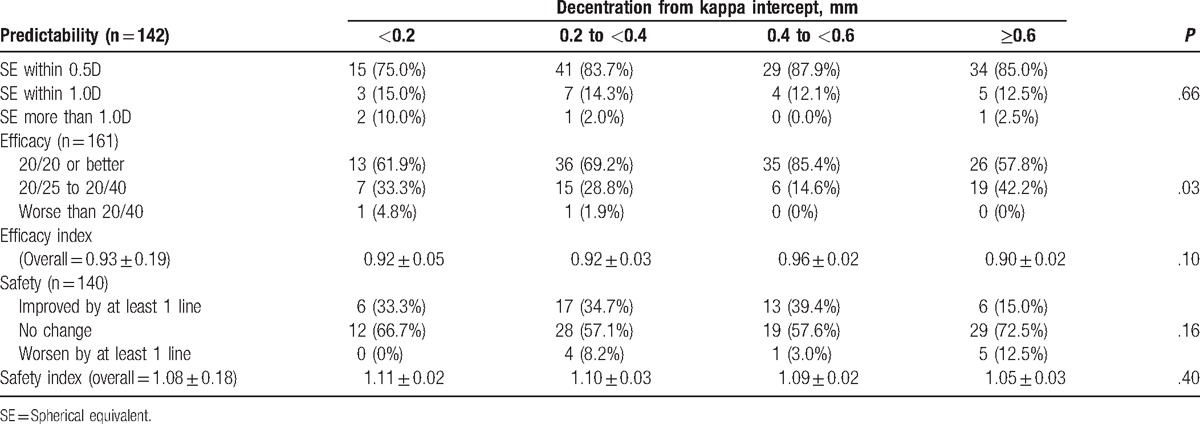
When analyzed across 4 groups of decentration from kappa intercept (<0.2 mm, 0.2–<0.4 mm, 0.4–<0.6 mm, and ≥0.6 mm), there was a trend toward higher efficacy for eyes with decentration of kappa intercept between 0.4 and <0.6 mm (P = .10, generalized estimating equation) 0.85.4% of eyes in 0.4 to <0.6 mm group had UDVA 20/20 or better, as compared to only 57.8% of eyes in ≥0.6 mm group. Subgroup analysis of using Pearson Chi-square test indicated a statistically significant association between decentration from kappa intercept and kappa intercept values. Eyes that had greater decentration from kappa intercept had larger kappa intercepts (P < .001). In particular, 71.7% of eyes with decentration from kappa intercept of ≥0.6 had kappa intercept of at least 0.6 mm while 90.5% of eyes with decentration from kappa intercept of 0.4 to <0.6 mm had kappa intercept less than 0.6 mm.
One-way ANOVA analysis indicated statistically significant safety differences among the 4 groups (P = .04). Post-hoc pairwise comparisons, with Bonferroni correction applied, showed that the group with greater than 0.6 mm decentration from the kappa intercept had a lower safety index compared to the group with 0.4 to <0.6 distance (mean difference −0.11, 95% CI −0.219–0.003, adjusted P = .06). There was no statistically significant association between decentration from kappa intercept with predictability (Table 3).
Table 3.
Correlating outcomes with decentration from kappa intercept.
There was no statistically significant relationship between angle kappa and postoperative month 3 UDVA, Spearman rho correlation coefficient = −0.46 (P = .56). There was no statistically significant relationship between kappa intercept and postoperative month 3 UDVA, Spearman rho correlation coefficient = 0.19, P = .82.
4. Discussion
The centration of ablation in LASIK has been extensively studied but controversy still exists regarding optimal centration. It has been shown that photoreceptors are oriented toward the pupil center.[25] However, it is known that the center of the pupil shifts when the pupil size changes.[26] Pande and Hillman[27] found that the CCLR was the closest measurable point to the visual axis and should be used for centration. Angle kappa values have been reported to be larger in hypermetropes[28] and emmetropes[29] compared to myopes, and therefore adjusting laser centration for angle kappa is more important in eyes with hyperopia,[28] low myopic astigmatism, and mixed astigmatism. Several studies have demonstrated the benefits of moving centration for hyperopic LASIK to the CCLR to adjust for a large angle kappa.[30] Centering over a point half of the distance between the CCLR and the pupil center has also been found to be effective in patients with large angle kappa.[31] With regards to SMILE, good visual results have been reported with treatment centered on the pupil center,[32] CCLR,[33] and corneal vertex.[13,14] However, there are no comparative studies on visual outcomes between different methods of treatment centration.
There are currently 3 published studies that have evaluated SMILE treatment decentration and its effect on visual outcomes. Liu et al evaluated decentration from pupil center and the corneal vertex against visual outcomes. Decentration was measured using preoperative corneal wavefront analysis (WaveLightOculyzer II; Alcon) and intraoperative calibrated video capture images. The WaveLight Oculyzer was used preoperatively to measure the coordinates of the vertex normal with respect to the pupil center coordinates. Postoperatively, the pupil center and lenticule center were marked manually on calibrated video capture images. Better refractive outcomes were achieved when the lenticulecenter was closer to the corneal vertex.[14] Li et al[13] measured decentration from the corneal vertex postoperatively with a Scheimpflug topographer (Pentacam, Oculus) and found good visual outcomes despite mild decentration. Reinstein et al generated a difference map of the tangential curvature of each eye using preoperative and 3 month postoperative Atlas 9000 (Carl Zeiss Meditec AG) topography. The location of the corneal vertex was also obtained from the topography. The optical zone was defined on the tangential difference map as the central zone up to the mid-peripheral power inflection point. The best-fitting circle was superimposed on the optical zone to determine the location of the optical zone center with reference to the corneal vertex. Decentration from the corneal vertex in SMILE and LASIK eyes were compared, with no statistically significant in mean centration offset between the 2 groups.[15] All of the previous papers have relied on the use of topography evaluation either preoperative or postoperatively to assess decentration once the lenticule creation has occurred. Our study wanted to evaluate the decentration using methods that may aid the surgeon intraoperative to improve the outcomes of the lenticule creation. We analyzed the decentration from pupil center and kappa intercept since these can aid the surgeon intraoperatively prior to lenticule creation.
In our study, the mean decentration from the pupillary center was 0.13 mm. This is within the range of values of 0.10 to 0.32 mm published in earlier reports.[14,33] The mean decentration from the kappa intercept in our study was 0.47 mm. There are no other publications that have reported decentration from the kappa intercept. Our study was targeted at finding a method for intraoperative guidance, and we therefore did not measure decentration from the corneal vertex, which is not a landmark that can be assessed when the patient is undergoing laser treatment. Decentation from the corneal vertex has previously been reported to be in the range of 0.17 to 0.32 mm.[13,14,33–35] The visual outcomes of our study group in terms of predictability, efficacy, and safety were similar to earlier published data.[3,4,13,14,17,32,35–37] There was no statistical difference in postoperative visual acuity among the 3 groups of eyes with different pupillary decentration (<0.1 mm, 0.1–0.2 mm, and >0.2 mm).
Mathur et al[38] studied the change in position of pupil center in relation to pupil size changes due to luminance adjustments. The average shift of pupil center was 0.12 mm nasally, with maximum shifts of 0.5 mm. Our patients had a mean vertical and horizontal pupil size of 2.34 ± 0.47 mm and 2.25 ± 0.42 mm, respectively. There was no statistical significant relationship between pupil size and decentration from pupil center/kappa intercept. We believe that the variation of pupil size among our patients was relatively small because of the controlled low level of luminence during the docking stage and the use of infrared light to check the docking position. Therefore, pupil size was not significantly related to decentration.
The statistically significant positive relationship between angle kappa and decentration from pupil center that we report is in line with the fact that patients undergoing SMILE align their visual axis for treatment by focusing on the green fixation light. Centration will be closer to the visual axis than pupil center if patients were able to co-operate with fixation. We also found a statistically significant relationship between the size of angle kappa and decentration from the kappa intercept. This suggests that patients with larger angle kappa values are prone to have treatments centered further away from the visual axis. However, based on our findings, there was a trend toward higher efficacy for eyes with decentration 0.4 to <0.6 mm from the kappa intercept. There was a trend toward poorer visual outcomes with eyes having decentered treatment of greater than 0.6 mm from the kappa intercept. We also found that 71.7% of eyes with decentration from kappa intercept of ≥0.6 mm had kappa intercept of at least 0.6 mm, we postulate that these eyes had treatment centered nearer the pupil. Hence, the results would recommend that eyes with kappa intercept ≥0.6 mm have their treatment centered at least 0.2 mm from the pupil center or within 0.4 to 0.6 mm of the kappa intercept.
A possible limitation of our study is use of the Orbscan IIz to measure angle kappa values. At the time of the study the Orbscan IIz was used for LASIK preoperative evaluation at our center. There are a multitude of platforms which may be used for the measurement of angle kappa such as the synoptophore, Pentacam, Galilei (Ziemer Ophthalmic System AG), and the OPD-scan (Nidek Co) but there has been no comparative study among the different platforms. It can be argued that the Orbscan may not be the best tool for measuring angle kappa, but this was a retrospective study and we were limited to the method of measurement used at the time of the study. The use of the Orbscan IIz for the measurement of angle kappa has been well described.[21,28,30]
The combined use of preoperative kappa intercept values on Orbscan IIz together with intraoperative image capture values of pupil center and decentration values was another possible limitation of our study. Qi et al reported a positive correlation in horizontal and vertical components of kappa angle in preoperative sitting position measured via Pentacam and intraoperative supine position measured via the Allegretto Wavelight Excimer Laser System (WaveLight Laser Technologie AG).[38] Therefore, although our measurement values may be affected by change in posture due to the effect of cyclotortion, and also due to comparing measurements across different platforms, a positive correlation between measured values in different postures and platforms has already been previously described.[39] In retrospect, it may still be beneficial to have postoperative topography to confirm accuracy of centration analysis.
Our results suggests that decentration from pupil center did not affect our studied outcomes, which was also reported by Liu et al.[14] The distance from pupil center however did affect visual outcomes in terms of higher-order aberrations (HOA) in their study. In our study, the pupil decentration was much smaller than that reported by Liu et al[14] even though we report outcomes on a much larger series of eyes, than previously published, we wanted to focus our study on decentration from angle kappa and the pupil center on primary tested visual outcomes. HOA were not measured in this retrospective study as additional software such as the VOL-CT (Sarver and Associates, Inc) was not routinely available on our Orbscan IIz. We have also previously reported on HOA and contrast sensitivity outcomes following SMILE from our center,[40] which shows that refractive lenticule extraction was not associated with significant induction of HOAs.
In conclusion, the results of our study suggest that decentration values of greater than 0.6 mm from the kappa intercept may result in compromised visual outcomes. There was a trend toward better efficacy in eyes which had decentered treatment from 0.4 to <0.6 mm from the kappa intercept, in which 85.4% of eyes achieved 20/20 or better UDVA, compared to 61.9% in the <0.2 mm group, 69.2% in the 0.2 to <0.4 mm group, and 57.8% in the ≥0.6 mm group.
Acknowledgements
The authors thank Mr Mohamed Farook, senior research optometrist from the Singapore National Eye Center for his assistance with data collection.
Footnotes
Abbreviations: CCLR = coaxial corneal light reflex, CDVA = corrected distance visual acuity, HOA = higher order aberrations, LASIK = laser in situ keratomileusis, SE = spherical equivalent, SMILE = small incision lenticule extraction, UDVA = uncorrected distance visual acuity.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Sugar A, Rapuano CJ, Culbertson WW, et al. Laser in situ keratomileusis for myopia and astigmatism: safety and efficacy; a report by the American Academy of Ophthalmology (Ophthalmic Technology Assessment). Ophthalmology 2002;109:175–87. [DOI] [PubMed] [Google Scholar]
- [2].Sugar A. Ultrafast (femtosecond) laser refractive surgery. Curr Opin Ophthalmol 2002;13:246–9. [DOI] [PubMed] [Google Scholar]
- [3].Shah R, Shah S, Sengupta S. Results of small incision lenticule extraction: all-in-one femtosecond laser refractive surgery. J Cataract Refract Surg 2011;37:127–37. [DOI] [PubMed] [Google Scholar]
- [4].Sekundo W, Kunert KS, Blum M. Small incision corneal refractive surgery using the small incision lenticule extraction (SMILE) procedure for the correction of myopia and myopic astigmatism: results of a 6 month prospective study. Br J Ophthalmol 2011;95:335–9. [DOI] [PubMed] [Google Scholar]
- [5].Reinstein DZ, Archer TJ, Randleman JB. Mathematical model tocompare the relative tensile strength of the cornea after PRK, LASIK, and small incision lenticule extraction. J Refract Surg 2013;29:454–60. [DOI] [PubMed] [Google Scholar]
- [6].Fay AM, Trokel SL, Myers JA. Pupil diameter and the principal ray. J Cataract Refract Surg 1992;18:348–51. [DOI] [PubMed] [Google Scholar]
- [7].Terrell J, Bechara SJ, Nesburn A, et al. The effect of globe fixationon ablation zone centration in photorefractive keratectomy. Am J Ophthalmol 1995;119:612–9. [DOI] [PubMed] [Google Scholar]
- [8].Mulhern MG, Foley-Nolan A, O’Keefe M, et al. Topographical analysis of ablation centration after excimer laser photorefractive keratectomy and laser in situ keratomileusis for high myopia. J Cataract Refract Surg 1997;23:488–94. [DOI] [PubMed] [Google Scholar]
- [9].Lazaridis A, Droutsas K, Sekundo W. Topographic analysis of the centration of the treatment zone after SMILE for myopia and comparison to FS-LASIK: subjective versus objective alignment. J Refract Surg 2014;30:680–6. [DOI] [PubMed] [Google Scholar]
- [10].Lee YC. Active eye-tracking improves LASIK results. J Refract Surg 2007;23:581–5. [DOI] [PubMed] [Google Scholar]
- [11].Ang M, Chaurasia SS, Angunawela RI, et al. Femtosecond lenticule extraction (FLEx): clinical results, interface evaluation, and intraocular pressure variation. Invest Ophthalmol Vis Sci 2012;53:1414–21. [DOI] [PubMed] [Google Scholar]
- [12].Wong CW, Chan C, Tan D, et al. Incidence and management of suction loss in refractive lenticule extraction. J Cataract Refract Surg 2014;40:2002–10. [DOI] [PubMed] [Google Scholar]
- [13].Li M, Zhao J, Miao H, et al. Mild decentration measured by a scheimpflug camera and its impact on visual quality following SMILE in the early learning curve. Invest Ophthalmol Vis Sci 2014;55:3886–92. [DOI] [PubMed] [Google Scholar]
- [14].Liu M, Sun Y, Wang D, et al. Decentration of optical zone center and its impact on visual outcomes following SMILE. Cornea 2015;34:392–7. [DOI] [PubMed] [Google Scholar]
- [15].Reinstein D, Gobbe M, Gobbe L, et al. Optical zone centration accuracy using corneal fixation-based SMILE compared to eye tracker-based femtosecond laser-assisted LASIK for myopia. J Refract Surg 2015;31:586–92. [DOI] [PubMed] [Google Scholar]
- [16].Sekundo W, Reinstein D, Blum M. Improved lenticule shape for hyperopic femtosecond lentcule extraction (Relex Flex): a pilot study. Lasers Med Sci 2016;31:659–64. [DOI] [PubMed] [Google Scholar]
- [17].Ang M, Tan D, Mehta JS. Small incision lenticule extraction (SMILE) versus laser in-situ keratomileusis (LASIK): study protocol for a randomized, non-inferiority trial. Trials 2012;13:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Riau AK, Ang HP, Lwein NC, et al. Comparison of four different Visumax circle patterns for flap creation after small incision lenticule extraction. J Refract Surg 2013;29:236–44. [DOI] [PubMed] [Google Scholar]
- [19].Liu YC, Jayasinghe L, Ang HP, et al. Effect of intraoperative corneal stromal pocket irrigation in small incision lenticule extraction. Biomed Res Int 2015;2015:928608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Schwiegerling JT. Eye axes and their relevance to alignment of corneal refractive procedures. J Refract Surg 2013;29:515–6. [DOI] [PubMed] [Google Scholar]
- [21].Hashemi H, KhabazKhoob M, Yazdani K, et al. Distribution of angle kappa measurements with Orbscan II in a population-based survey. J Refract Surg 2010;26:966–71. [DOI] [PubMed] [Google Scholar]
- [22].Pande M, Hillman JS. Optical zone centration in keratorefractive surgery. Entrance pupil center, visual axis, coaxially sighted corneal reflex, or geometric corneal center? Ophthalmology 1993;100:1230–7. [PubMed] [Google Scholar]
- [23].Arba Mosquera S, Verma S, McAlinden C. Centration axis in refractive surgery. Eye Vis 2015;2:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Cambridge University Press, Torrence BF, Torrence EA. The Student's Introduction to Mathematica. 1999. [Google Scholar]
- [25].Enoch JM, Laties AM. An analysis of retinal receptor orientation. II. Predictions for psychophysical tests. Invest Ophthalmol 1971;10:959–70. [PubMed] [Google Scholar]
- [26].Fay AM, Trokel SL, Myers JA. Pupil diameter and the principal ray. J Cataract Refract Surg 1992;18:348–51. [DOI] [PubMed] [Google Scholar]
- [27].Pande M, Hillman JS. Optical zone centration in keratorefractive surgery. Entrance pupil center, visual axis, coaxially sighted corneal reflex, orgeometric corneal center? Ophthalmology 1993;100:1230–7. [PubMed] [Google Scholar]
- [28].Basmak H, Sahin A, Yildirim N, et al. Measurement of angle kappa with synoptophore and Orbscan II in a normal population. J Refract Surg 2007;23:456–60. [DOI] [PubMed] [Google Scholar]
- [29].Giovanni F, Siracusano B, Cusmano R. The angle kappa in ametropia. New Trends Ophthalmol 1988;3:27–33. [Google Scholar]
- [30].Moshirfar M, Hoggan RN, Muthappan V. Angle Kappa and its importance in refractive surgery. Oman J Ophthalmol 2013;6:151–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kermani O, Oberheide U, Schmiedt K, et al. Outcomes of hyperopic LASIK with the NIDEK NAVEX platform centered on the visual axis or line of sight. J Refract Surg 2009;25Suppl 1:S98–103. [DOI] [PubMed] [Google Scholar]
- [32].Kamiya K, Shimizu K, Igarashi A, et al. Visual and refractive outcomes of small incision lenticule extraction for the correction of myopia: 1-year follow-up. BMJ Open 2015;5:e008268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Lazaridis A, Droutsas K, Sekundo W. Topographic analysis of the centration of the treatment zone after SMILE for myopia and comparison to FS-LASIK: subjective versus objective alignment. J Refract Surg 2014;30:680–6. [DOI] [PubMed] [Google Scholar]
- [34].Reinstein DZ, Gobbe M, Gobbe L, et al. Optical zone centration accuracy using corneal fixation-based SMILE compared to eye tracker-based femtosecond laser-assisted LASIK for myopia. J Refract Surg 2015;31:586–92. [DOI] [PubMed] [Google Scholar]
- [35].Chansue E, Tanehsakdi M, Swasdibutra S, et al. Efficacy, predictability and safety of small incision lenticule extraction (SMILE). Eye Vis 2015;2:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Hansen RS, Lyhne N, Grauslund J, et al. Small-incision lenticule extraction (SMILE): outcomes of 722 eyes treated for myopia and myopic astigmatism. Graefes Arch Clin Exp Ophthalmol 2016;254:399–405. [DOI] [PubMed] [Google Scholar]
- [37].Miao H, Tian M, Xu Y, et al. Visual outcomes and optical quality after femtosecond laser small incision lenticule extraction: an 18-month prospective study. J Refract Surg 2015;31:726–31. [DOI] [PubMed] [Google Scholar]
- [38].Mathur A, Gehrmann J, Atchison DA. Influences of luminance and accommodation stimuli on pupil size and pupil center location. Invest Ophthalmol Vis Sci 2014;55:2166–72. [DOI] [PubMed] [Google Scholar]
- [39].Qi H, Jiang JJ, Jiang YM, et al. Kappa angles in different positions in patients with myopia during LASIK. Int J Ophthalmol 2016;9:585–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Tan DK, Tay WT, Chan C, et al. Postoperative ocular higher-order aberrations and contrast sensitivity: femtosecond lenticule extraction versus pseudo small-incision lenticule extraction. J Cataract Refract Surg 2015;41:623–34. [DOI] [PubMed] [Google Scholar]


