Abstract
The increased awareness of asplenia-related life-threatening complications has led to the development of parenchyma sparing splenic resections in past few years. The aim of this study is to retrospectively analyze the feasibility and safety of laparoscopic partial splenectomy (LPS) in selected emergency patients.
From January 2013 to December 2015, there were 46 emergency patients, diagnosed with splenic rupture, admitted in our department. Selection criteria for LPS: (1) Preoperative CT scan revealed single pole rupture without spleen pedicle injury; (2) BP>90/60 mm Hg and heart rates <120 bpm; (3) No sigh of multiple organ injury. Eventually, LPS was performed in 21 patients (Group LPS), while laparoscopic splenectomy (LS) was performed in 20 patients (Group LS).
The main cause of splenic rupture was traffic accident, followed by blunt injury and high falling injury. Abdominal CT scan showed the mean longitudinal diameter of spleen of group LPS was 14.2 ± 1.8 cm (range 12–17 cm), while the size of remnant spleen was 5.5 ± 1.2 cm. Between 2 groups, operation time (LPS: 122.6 ± 17.2 min vs LS: 110.5 ± 18.7 minutes, P = .117), and intraoperative blood loss (LPS: 174 ± 22 mL vs LS: 169 ± 29 mL, P = .331) were similar. There were 2 patients suffered subsequent unstable vital sign altering during mobilization when performing LPS. Conversion to LS (2/21, 9.52%) was decided and successfully completed. Although there was no patient suffered postoperative OPSI or thrombocytosis events in both groups after 6-month follow-up, the mean platelets and leukocyte count were significantly lower in group LPS. Splenic regrowth was evaluated in 20 patients of group LPS. And the mean regrowth of splenic volume reached 19% (10%-26%).
Due to its minimal invasive effect and functional splenic tissue preservation, LPS may be a safe and feasible approach for emergency patients. And prospective trials with clear inclusion criteria are needed to proof the benefit of LPS.
Keywords: emergency surgery, laparoscopic partial splenectomy, splenic rupture
1. Introduction
Traditionally, laparotomy is the primary choice for emergency patients with splenic rupture who need splenectomy. Nowadays, due to the improved laparoscopic techniques and skills, more and more laparoscopic splenectomy (LS) cases are successfully performed. However, the increased awareness of asplenia-related life-threatening complications such as overwhelming postsplenectomy infection (OPSI), thromboembolic events, enhanced arteriosclerosis, and pulmonary hypertension[1–3] have led to the development of parenchyma sparing splenic resections. Infections after total splenectomy are the most concerns for patients, especially for the younger and infant ones. Although effective vaccination and antibiotic prophylaxis have been used to reduce the risk of overwhelming postsplenectomy infections (OPSI), concerns still persist about penicillin resistant pneumococcal strains, serotypes not represented in vaccines, and lifelong compliance.[4–9] Recent studies have also raised concerns about high incidence of pulmonary hypertension, atherosclerotic, and thrombotic events in splenectomized patients.[3,9–11] Previous studies have demonstrated that preservation of 25% of the splenic parenchyma allows an appropriate immunological response to antigen stimulus.[12,13] Laproscopic partial splenectomy (LPS) has been successfully performed in over 30 cases with splenic lesions in our hospital in the past 5 years. And from January 2013, we began to adopt LPS in selected emergency patients with splenic rupture. The aim of this study is to demonstrate the feasibility and safety of LPS in selected emergency patients.
2. Methods
2.1. Patients
We retrospectively collected and analyzed patients who diagnosed with splenic rupture, admitted in our department in emergency from January 2013 to December 2015. Selection criteria for LPS: (1) Preoperative CT scan revealed single pole rupture without spleen pedicle injury; (2) BP>90/60 mm Hg and heart rates <120 bpm; (3) no sigh of multiple organ injury. Patients who could not tolerate pneumoperitoneum were excluded. All the patients were divided into 2 groups: Group LPS and Group LS. The perioperative data were retrospectively collected and analyzed. The remnant part and regrowth of spleen were evaluated by comparison of CT scan between 7 days after surgery and 6 months later. The blood routine test and the incidence of infections were also recorded during 6-month follow-up period.
Results of the preoperative evaluation were presented to the ethics committee of West China Hospital, Sichuan University. The surgical team stated that LPS had previously been performed in our medical center. The feasibility and advantages of LPS, as well as the risks and possibility of conversion to LS or laparotomy, had been explained to the patient or/and his family. Informed consents were obtained from the patient himself or/and his parents after a full explanation of the potential risks of LPS. The West China Hospital administration and the ethics committee authorized the surgery.
2.2. Surgical procedure
All the patients were placed in a right semidecubitus position with the left side of the body elevated under general anesthesia. The operating table tilted slightly in a reverse Trendelenburg position. Laparoscopy was performed under CO2 pneumoperitoneum with 13 mm Hg insufflation pressure. Four ports were inserted into abdominal cavity: (1) A 10 mm port (for the 30° rigid camera) was placed in the upper umbilicus; (2) A 12 mm port (for laparoscopic dissector or stapler) was placed at the left midclavicular line below the inferior margin of the costa; (3) A 5 mm port was placed at the subxiphoid for laparoscopic grasper; (4) A 5 mm port was placed at the left axillary line below the lower pole of the spleen for laparoscopic grasper or aspiration.
For LPS: Ultrasonic shears (Harmonic scalpel, Ethicon Endo-Surgery, Inc., Cornelia, GA) was used to mobilize the resecting part of spleen; for the resection of upper part of spleen, the lower branches of gastroepiploic vessels and perisplenic ligament were preserved and vice versa. The branches of the splenic artery and vein which supply the resecting part of spleen were mobilized and transected. Demarcation area was well-defined and the safe demarcation margin was 1 cm during LPS. Ultrasonic shears was used to transect the splenic parenchyma 1 cm away from the demarcation line. There was little bleeding from the parenchyma besides some mild venous ooze. Bipolar (Erbe Elektromedizin, Germany) was used for hemostasis. The remnant spleen was not routinely fixed or sutured. The specimen was put into a retrieval bag and removed from the incision of 12 mm port. Finally, the surgical site was carefully checked and an abdominal drainage was placed next to the remnant spleen (Fig. 1).
Figure 1.
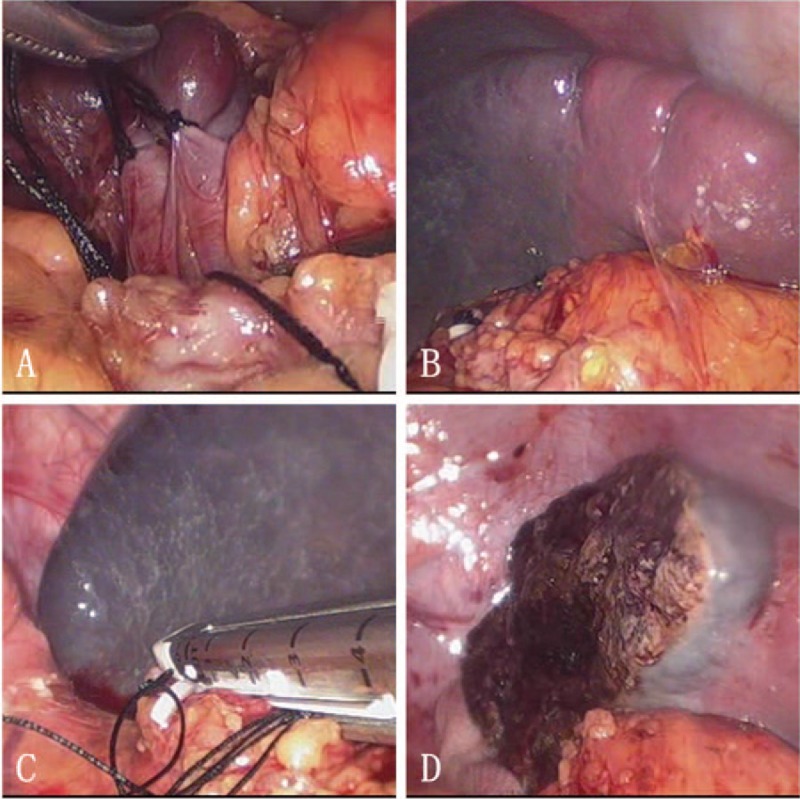
Laparoscopic partial splenectomy: (A) ligate the branches of splenic artery and vein; (B) ischemic demarcation line; (C) transect arterial and venous branches by linear stapler; (D) transect splenic parenchyma 1 cm away from the ischemic demarcation line.
For LP: Ultrasonic shears (Harmonic scalpel, Ethicon Endo-Surgery, Inc., Cornelia, GA) was used to mobilize the spleen. The branches of gastroepiploic vessels were transected by laparoscopic LigaSure (LigaSure 5 mm BluntTip, Covidien, Boulder, CO). The splenic artery was dissected and clapped by Hem-O-Lok. The laparoscopic linear stapler (United States Surgical, Glover Avenue Norwalk, CT) with 45 mm white cartridge was used to transect the splenic pedicle. The specimen was put into a retrieval bag and removed from the incision of 12 mm port. Finally, the surgical site was carefully checked and an abdominal drainage was placed next to the remnant spleen.
3. Statistical analysis
Statistical analyses were performed using SPSS version 16.0 (SSPS Inc, Chicago, Ill). P values < .05 were considered to be significant. The differences between groups were analyzed by independent sample student's t test for quantitative descriptive variables and by chi-square test or Fisher's exact text for categorical variables.
4. Results
4.1. Demographics data
From January 2013 to December 2015, there were 46 patients diagnosed with splenic rupture. The etiology included traffic accident, high falling injury and blunt injury. According to our selection criteria, a total of 23 patients were selected to perform LPS under emergency, while LS were performed in 18 patients. But due to subsequent unstable vital sign during LPS, there were 2 patients in LPS converted into laparoscopic splenectomy. Eventually, there were 21 patients in Group LPS, while 20 patients including the 2 converted patients in Group LS. The characteristics of 2 groups were summarized in Table 1. The main cause of splenic rupture was traffic accident, followed by blunt injury and high falling injury. There were 16/21 (76.2%) men in group LPS, while 16/20 (80.0%) men in group LS. The average age was 36.0 ± 9.7 in group LPS, while 35.5 ± 9.9 in group LS. Abdominal CT scan showed the mean longitudinal diameter of spleen between groups were LPS: 14.2 ± 1.8 cm (range 12–17 cm) versus LS: 13.9 ± 1.6 cm (range 12–16.5 cm). And in group LPS, the size of remnant spleen was 5.5 ± 1.2 cm. There were 12/21 (57.1%) patients with upper pole rupture in group LPS, while 8/20 (40%) patients in group LS.
Table 1.
Demographics of 2 groups.

4.2. Peri-operative analysis
Between 2 groups, operation time (LPS: 122.6 ± 17.2 minutes vs LS: 110.5 ± 18.7 minutes, P = .117), intraoperative blood loss (LPS: 174 ± 22 mL vs LS: 169 ± 29 mL, P = .331), autologous blood transfusion (LPS: 221 ± 36 mL vs LS: 206 ± 27 mL, P = .078), allogeneic blood transfusion (LPS: 125 ± 25 mL vs LS: 150 ± 30 mL, P = .878) and conversion to laparotomy (LPS: 0 vs LS: 0, P = 1.000) were similar (Table 2). There were 2 patients suffered subsequent unstable vital sign altering during mobilization when performing LPS. Conversion to LS (2/21, 9.52%) was decided and successfully completed.
Table 2.
Intraoperative data.

4.3. Postoperative outcomes
It was not significantly different when using Clavien–Dindo classification to grade the severity of postoperative complications (LPS: 18.2% vs LS: 20.0%, P = 1.000). There were 4 patients in group LPS and 4 patients in group LS suffered postoperative complications. Postoperative fluid collection (LPS: n = 4, LS: n = 2), and splenic venous thrombosis (LPS: n = 0, LS: n = 2) were seen in both groups. For fluid collection, antibiotics were used in 4 patients in LPS and 1 patient in LS, while ultrasonography-guided percutaneous drainage under local anesthesia was adopted in 1 patient in LS. For splenic vein thrombosis, anticoagulant drugs were used in 2 patients in LS. There was no patient suffered postoperative intraperitoneal bleeding. The length of hospital stay was similar (P > .05) (Table 3). There was no mortality during hospitalization. After a 6-month follow-up, although there was no significant difference in susceptibility to infection (cough, sore throat or cold) between groups (LPS: 0/21 vs LS: 3/20, P = .107), the counts of platelet (LPS: 147 ± 48 × 109 vs LS: 282 ± 61 × 109, P = .031) and leukocyte (LPS: 6.7 ± 1.1 × 109 vs LS: 8.9 ± 1.9 × 109, P = .017) were significantly different.
Table 3.
Postoperative complications and clinical outcome.

4.4. Splenic remnant regrowth
The splenic volume was evaluated by abdominal CT scan. Splenic remnant regrowth was observed in 20 patients 6 months after LPS in the study (1 was lost because of traveling aboard). The mean regrowth of splenic volume reached 19% (range from 10% to 26%).
5. Discussion
Total splenectomy is traditionally considered the primary choice for splenic diseases. But followed by the recognition of the spleen as an important organ of the human immune system and the potential threat of postoperative complications, more and more surgeons have considered to perform partial splenectomy in selected patients. Nowadays, due to the improved laparoscopic techniques and skills, more and more laparoscopic splenectomy (LS) cases have been successfully performed. We are experienced in such procedure with over 800 patients in the past 10 years. Although autologous splenic tissue transplantation is an option for preserving the splenic function, it is inferior to partial splenectomy in terms of regeneration, blood flow, reticuloendothelial clearance, and immunologic function.[14,15] Although open partial splenectomy is also a feasible and safe option for patients with different splenic diseases such as focal splenic lesion, and hereditary spherocytosis,[16] there may be much severer body pain and cosmetic disadvantages. We successfully performed our first LPS for patient with focal splenic lesion in 2011 and the first emergency case of LPS in January 2013. As a result, we retrospectively collected and compared the perioperative data between LPS and LS to evaluate the feasibility and safety of performing LPS in selected emergency patients.
Generally, LPS is mostly often performed in patients with focal splenic lesions, including splenomegaly of unknown origin for biopsy, spleen tumors, solitary metastasis, splenic infarcts, hereditary spherocytosis, and so on.[17,18] In our previous study, considering the splenomegaly, perisplenic inflammation, and adhesion in some patients would increase difficulty and risk of the surgery, nonparasitic cyst was the most common indication for LPS.[19] Poulin et al[20] reported the first case of LPS for ruptured spleen in 1995. Partial splenic artery embolism was performed to control bleeding before parenchymal transection. Huscher et al[21] reported the largest series of patients treated laparoscopically for a splenic injury, including 6 patients of splenectomy, 1 case of upper polar resection, 3 patients of polyglycolic mesh wrapping, and 1 patient of splenic hemostasis using argon beam coagulation. As a result, it was technically feasible to perform laparoscopic splenic surgery for ruptured spleen. The control of intraoperative bleeding is the most important concern during this complex procedure. Due to its specific anatomy, the blood supply of spleen is mainly consisted of 2 separate lobes, which are further divided into 3 to 5 segments, with the interlobar and intersegmental planes being avascular, and with the improvement of laparoscopic surgical instruments, intraoperative bleeding risk is significantly reduced.[19,22] Based on previous studies, hand-assisted, robotic-assisted, and radiofrequency ablation-assisted LPS are 3 feasible approaches.[23–25] But extra incision in upper abdominal wall, expensive cost uncovered by medical insurance in China, and possibility of septic complications hinder the popularization of these techniques.[26] In our LPS for splenic lesions, we routinely dissect and transect the branches of the splenic artery and vein which supply the resecting part of spleen prior to splenic parenchyma transection. Different instruments are adopted during splenic parenchyma transection. The ultrasonic harmonic scalpel is used during LPS. But we agree that placing the parenchymal transection line inside the ischemia demarcation limit is more important than the type of instruments employed in minimizing blood loss during LPS.[27] The safe splenic parenchymal transection line adopted in this study is 1 cm inside the ischemia demarcation line. Bipolar coagulator is used to hemostasis for the cut edge.
LPS can be safely performed in selected emergency patients. Based on the experience gained before, the selection criteria included: (1) patients were diagnosed with splenic rupture under stable hemodynamics (BP>90/60 mm Hg and heart rates <120 bpm); (2) the location of ruptured spleen was limited to 1 pole of the spleen (upper or lower pole), without splenic pedicle injury; (3) no sign of multiple organ injury. Patients with unstable hemodynamics should be performed with LS or even open splenectomy without any hesitation. In this study, there were 2 patients (2/21, 9.52%) suffered unstable vital sign altering during mobilization when we attempted to perform LPS. Conversion to LS was decided and successfully completed. A conversion from LPS to LS was still under laparoscopic procedure. Based on this, the relatively higher conversion rate should be acceptable. During LPS, the blood clot that covered the rupturing location should not be removed as it was helpful to control the hemorrhage from the splenic wound. And the autologous transfusion should also be adopted after the exclusion of hollow organ rupture or liver rupture.
It is clearly established that individuals with anatomical or functional asplenia are at high risk of developing severe and potentially fatal infections caused by encapsulated bacteria (OPSI).[27] Phagocyticactivity of splenic macrophages and synthesis of antipolysaccharide antibodies by splenic B-lymphocytes play important roles in defending against infections.[5] The reported prevalence of OPSI after total splenectomy is around 4%, and the overall mortality is 2%.[5] Current guidelines and most authorities recommend long term, some even lifelong administration of prophylactic antibiotics.[6,9] In asplenic patients, thrombocytosis may affect the development of postoperative pulmonary hypertension. Therefore, the functional remnant volume of spleen is important for the long-term results. Previous studies suggested a preservation of 25% of the spleen with adequate perfusion would be sufficient to maintain a normal splenic function.[12,13] All LPS patients in this study preserved at least 25% of splenic volume with normal blood supply. And it was comparable in postoperative mild infection rate between 2 groups. Although there was no patient suffered postoperative OPSI or thrombocytosis events in both groups after 6-month follow-up, the mean count of platelets and leukocyte were significantly different. This might potentially affect long-term results in longer observation.
Theoretically, it is possible to develop postoperative pedicle torsion after LPS. In this study, we only mobilized the ligaments of the resection part and preserved the ligaments of remnant part. And due to the quick adhesion formation after LPS, splenopexy was not routinely performed. There was no patient suffered this complication in this study. In addition, there is a possibility of splenic remnant regrowth after partial splenectomy.[28] Our results showed the mean regrowth of splenic volume reached 19% during follow-up period, which was consistent with previous data.[12]
There might be several limitations in this study: (1) this was a retrospective study; (2) all the conclusions were based on the selection criteria; (3) the sample size was relatively small. According to our experience, a laparoscopic surgeon who attempts to perform LPS for splenic lesion should have successfully performed over 50 LS cases before. And he should have completed at least 30 ordinary LPS cases before trying the emergency LPS. As a result, the experience might be another limiting factor.
In conclusion, this study firstly compared the perioperative outcomes between LPS and LS. Because of its minimal invasive effect and functional splenic tissue preservation, LPS may benefit emergency patients and does not increase perioperative risks. Prospective trials with clear inclusion criteria are needed to proof the benefit of LPS.
Acknowledgments
We thank Shawna Williams for her editing assistance in the preparation of this manuscript.
Footnotes
Abbreviations: CT = computed tomography, LPS = laparoscopic partial splenectomy, LS = laparoscopic splenectomy.
Disclosure of Financial Interests and Potential Conflicts of Interest: The authors have no benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
Funding: This work was supported by grant from the Science and Technology Department of Sichuan Province of China (Project No. 2017FZ0043).
The authors have no conflicts of interest to disclose.
References
- [1].Lynch AM, Kapila R. Overwhelming postsplenectomy infection. Infect Dis Clin North Am 1996;10:693–707. PubMed PMID: 8958164. [DOI] [PubMed] [Google Scholar]
- [2].Robinette CD, Fraumeni JF., Jr Splenectomy and subsequent mortality in veterans of the 1939–45 war. Lancet 1977;2:127–9. PubMed PMID: 69206. [DOI] [PubMed] [Google Scholar]
- [3].Schilling RF. Spherocytosis, splenectomy, strokes, and heat attacks. Lancet 1997;350:1677–8. PubMed PMID: 9400518. [DOI] [PubMed] [Google Scholar]
- [4].Holdsworth RJ, Irving AD, Cuschieri A. Postsplenectomy sepsis and its mortality rate: actual versus perceived risks. Brit J Surg 1991;78:1031–8. PubMed PMID: 1933181. [DOI] [PubMed] [Google Scholar]
- [5].Di Sabatino A, Carsetti R, Corazza GR. Post-splenectomy and hyposplenic states. Lancet 2011;378:86–97. PubMed PMID: 21474172. [DOI] [PubMed] [Google Scholar]
- [6].Davidson RN, Wall RA. Prevention and management of infections in patients without a spleen. Clin Microbiol Infect 2001;7:657–60. PubMed PMID: 11843905. [DOI] [PubMed] [Google Scholar]
- [7].Cullingford GL, Watkins DN, Watts AD, et al. Severe late postsplenectomy infection. Brit J Surg 1991;78:716–21. PubMed PMID: 2070242. [DOI] [PubMed] [Google Scholar]
- [8].Brivet F, Herer B, Fremaux A, et al. Fatal post-splenectomy pneumococcal sepsis despite pneumococcal vaccine and penicillin prophylaxis. Lancet 1984;2:356–7. PubMed PMID: 6146903. [DOI] [PubMed] [Google Scholar]
- [9].Dahyot-Fizelier C, Debaene B, Mimoz O. Management of infection risk in asplenic patients. Ann Fr Anesth Reanim 2013;32:251–6. PubMed PMID: 23538102. Gestion du risque infectieux chez le splenectomise. [DOI] [PubMed] [Google Scholar]
- [10].Rezende AB, Neto NN, Fernandes LR, et al. Splenectomy increases atherosclerotic lesions in apolipoprotein E deficient mice. J Surg Res 2011;171:e231–6. PubMed PMID: 21962813. [DOI] [PubMed] [Google Scholar]
- [11].Crary SE, Buchanan GR. Vascular complications after splenectomy for hematologic disorders. Blood 2009;114:2861–8. PubMed PMID: 19636061. Pubmed Central PMCID: 2756197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Bader-Meunier B, Gauthier F, Archambaud F, et al. Long-term evaluation of the beneficial effect of subtotal splenectomy for management of hereditary spherocytosis. Blood 2001;97:399–403. PubMed PMID: 11154215. [DOI] [PubMed] [Google Scholar]
- [13].Goldthorn JF, Schwartz AD, Swift AJ, et al. Protective effect of residual splenic tissue after subtotal splenectomy. J Pediatr Surg 1978;13:587–90. PubMed PMID: 731357. [DOI] [PubMed] [Google Scholar]
- [14].Westermann J, Pabst R. Autotransplantation of splenic fragments: lymphocyte subsets in blood, lymph nodes and splenic tissue. Clin Exp Immunol 1986;64:188–94. PubMed PMID: 2942322. Pubmed Central PMCID: 1542160. [PMC free article] [PubMed] [Google Scholar]
- [15].Malangoni MA, Evers BM, Peyton JC, et al. Reticuloendothelial clearance and splenic mononuclear cell populations after resection and autotransplantation. Am J Surg 1988;155:298–302. PubMed PMID: 3257658. [DOI] [PubMed] [Google Scholar]
- [16].Buesing KL, Tracy ET, Kiernan C, et al. Partial splenectomy for hereditary spherocytosis: a multi-institutional review. J Pediatr Surg 2011;46:178–83. PubMed PMID: 21238662. [DOI] [PubMed] [Google Scholar]
- [17].Uranues S, Grossman D, Ludwig L, et al. Laparoscopic partial splenectomy. Surg Endosc 2007;21:57–60. PubMed PMID: 17031738. [DOI] [PubMed] [Google Scholar]
- [18].Hery G, Becmeur F, Mefat L, et al. Laparoscopic partial splenectomy: indications and results of a multicenter retrospective study. Surg Endosc 2008;22:45–9. PubMed PMID: 17943384. [DOI] [PubMed] [Google Scholar]
- [19].Wang X, Wang M, Zhang H, et al. Laparoscopic partial splenectomy is safe and effective in patients with focal benign splenic lesion. Surg Endosc 2014;28:3273–8. PubMed PMID: 24939157. [DOI] [PubMed] [Google Scholar]
- [20].Poulin EC, Thibault C, DesCoteaux JG, et al. Partial laparoscopic splenectomy for trauma: technique and case report. Surg Laparosc Endosc 1995;5:306–10. PubMed PMID: 7551284. [PubMed] [Google Scholar]
- [21].Huscher CG, Mingoli A, Sgarzini G, et al. Laparoscopic treatment of blunt splenic injuries: initial experience with 11 patients. Surg Endosc 2006;20:1423–6. PubMed PMID: 16736315. [DOI] [PubMed] [Google Scholar]
- [22].Liu DL, Xia S, Xu W, et al. Anatomy of vasculature of 850 spleen specimens and its application in partial splenectomy. Surgery 1996;119:27–33. PubMed PMID: 8560382. [DOI] [PubMed] [Google Scholar]
- [23].Okano K, Kakinoki K, Suto H, et al. Hand-assisted laparoscopic partial splenectomy using an endopath monopolar sealer. Surg Laparosc Endosc Percutan Tech 2011;21:e291–4. PubMed PMID: 22146174. [DOI] [PubMed] [Google Scholar]
- [24].Giulianotti PC, Buchs NC, Addeo P, et al. Robot-assisted partial and total splenectomy. Int J Med Robot 2011;7:482–8. PubMed PMID: 21954176. [DOI] [PubMed] [Google Scholar]
- [25].Gumbs AA, Bouhanna P, Bar-Zakai B, et al. Laparoscopic partial splenectomy using radiofrequency ablation. J Laparoendosc Adv Surg Tech A 2008;18:611–3. PubMed PMID: 18721016. [DOI] [PubMed] [Google Scholar]
- [26].Zacharoulis D, Katsogridakis E, Hatzitheofilou C. A case of splenic abscess after radiofrequency ablation. World J Gastroenterol 2006;12:4256–8. PubMed PMID: 16830388. Pubmed Central PMCID: 4087387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].de la Villeon B, Zarzavadjian Le Bian A, Vuarnesson H, et al. Laparoscopic partial splenectomy: a technical tip. Surg Endosc 2015;29:94–9. PubMed PMID: 24962862. [DOI] [PubMed] [Google Scholar]
- [28].Slater BJ, Chan FP, Davis K, et al. Institutional experience with laparoscopic partial splenectomy for hereditary spherocytosis. J Pediatr Surg 2010;45:1682–6. PubMed PMID: 20713220. [DOI] [PubMed] [Google Scholar]


