Abstract
Intravitreal ranibizumab (IVR) has been approved for treating diabetic macular edema (DME), and is used in daily clinical practice. However, the treatment efficacies of IVR monotherapy in real-world clinical settings are not well known.
The medical records of 56 eyes from 38 patients who received their first IVR for DME between April 2014 and March 2015, and were retreated with IVR monotherapy as needed with no rescue treatment, such as laser photocoagulation, were retrospectively reviewed. The clinical course, best-corrected visual acuity (BCVA), and fundus findings at baseline, before the initial IVR injection, and at 12 months, were evaluated.
Twenty-five eyes from 25 patients (16 men; mean age 68.7 ± 9.8 years) who received IVR in the first eye, or unilaterally, without any other treatments during follow-up were included. After 12 months, mean central retinal thickness (CRT), which includes edema, was reduced (P = .003), although mean BCVA remained unchanged. There was a negative correlation between individual changes in BCVA (r = −0.57; P = .003) and CRT (r = −0.60; P = .002) at 12 months compared with baseline values. BCVA changes were greater in individuals with a history of pan-retinal photocoagulation at baseline (P = .026). After adjusting for age and sex, CRT improvement >100 μm at 12 months was associated with a greater CRT at baseline (OR 0.87 per 10 μm [95% CI 0.72–0.97]; P = .018) according to logistic regression analyses; however, better BCVA and CRT at 12 months were associated with a better BCVA (r = 0.77; P < .001) and lower CRT (r = 0.41; P = .039) at baseline, respectively, according to linear regression analyses.
IVR monotherapy suppressed DME, and the effects varied according to baseline conditions. Eyes that had poorer BCVA or greater CRT, or a history of pan-retinal photocoagulation at baseline, demonstrated greater improvement with IVR monotherapy. In contrast, to achieve better outcome values, DME eyes should be treated before the BCVA and CRT deteriorate. These findings advance our understanding of the optimal use of IVR for DME in daily clinical practice, although further study is warranted.
Keywords: anti-VEGF therapy, diabetic retinopathy, macular edema, monotherapy, predictors, prognosis, retina
1. Introduction
Diabetes mellitus has affected more than 380 million patients and its incidence is increasing worldwide.[1] Diabetic macular edema (DME) is a phenotype of diabetic retinopathy, with a reported prevalence ranging from 7.5%[1] to 15.7%[2] among patients with diabetes. Since DME directly influences the function of the macula (the center of the retina), it causes central visual dysfunction, which affects activities of daily living including reading and driving. As the number of patients with diabetes is increasing with the aging population, DME is now becoming a societal health issue.[3]
To date, treatment of DME has involved focal and/or grid laser photocoagulation,[4] local steroid injection,[5] and pars plana vitrectomy.[6,7] In addition, vascular endothelial growth factor (VEGF) levels were increased in the vitreous of patients with DME,[8] and recent progress in medical and molecular science has developed new therapies using anti-VEGF agents. The drugs were approved after several sponsor-initiated clinical studies designed with strict inclusion and exclusion criteria.[9–12] However, data reflecting daily, real-world clinical encounters involving patients with various backgrounds are required.
Most previous clinical trials have involved rescue laser treatment during the study period if the anti-VEGF treatment was not sufficiently effective.[9–12] However, this additional therapy may modify the treatment effect of anti-VEGF therapy. Because vascular permeability[13] and vascular endothelial proliferation[14,15] can be accelerated by VEGF, it is reasonable to assume that anti-VEGF treatment is at least, in part, effective for DME. However, DME could also be caused by other multiple factors, such as inflammatory cytokines,[16] and vitreous modifications and traction.[7] In fact, risk factors for DME may not only include the duration of hyperglycemia and diabetes,[17,18] but also dyslipidemia[18–20] and inflammation,[8] and underlying mechanisms may involve multiple pathways rather than VEGF alone. On the contrary, therapeutic mechanisms of rescue laser photocoagulation may involve suppression of multiple pathological factors produced by the retinal tissue, and not only VEGF by destroying the retinal tissue that induces these pathological factors. Thus, results of clinical studies do not necessarily reflect the efficacies of anti-VEGF therapy.
Consequently, analysis and understanding of the therapeutic effects of anti-VEGF monotherapy for DME in various types of patients encountered in daily clinical practice is important to develop future monotherapy or combination treatment protocols.
Two anti-VEGF drugs, ranibizumab and aflibercept, have been approved for the treatment of DME. These drugs have also been used for age-related macular degeneration,[21] myopic choroidal neovascularization,[22] and retinal vascular occlusion,[23] as these diseases also involve VEGF-related mechanisms in their pathogenesis. In the current case series, we evaluated the efficacy of intravitreal ranibizumab (IVR) monotherapy for patients with DME in daily practice for 12 months, and analyzed the predictive factors for better outcomes in terms of best-corrected visual acuity (BCVA) and central retinal thickness (CRT), which reflect the level of macular edema.
2. Methods
This retrospective case series was based on a detailed medical chart review, followed the tenets of the Declaration of Helsinki, was approved by the Ethics Committee of Keio University School of Medicine (Tokyo, Japan) (No. 2010003), and was registered with UMIN-CTR (UMIN000007649).
2.1. Participants
In total, 56 consecutive eyes from 38 patients who were diagnosed with DME-induced visual loss and received initial IVR (0.5 mg) monotherapy at the Vitreo-Retina Division Clinic of the Department of Ophthalmology, Keio University Hospital between April 2014 and March 2015, were included. During this period, aflibercept, which was first approved for DME in Japan in November 2014, and another off-label anti-VEGF drug, bevacizumab, were not used at this hospital for treating DME. Thus, all patients with DME who required anti-VEGF therapy were treated with IVR. Patients who had received any other treatment for DME in the past month and/or within 12 months after the initial IVR were excluded. In patients who underwent IVR in both eyes, the eye first treated was analyzed. All patients provided informed consent for IVR treatment and for the use of their data for research purposes.
2.2. Ophthalmologic examinations
All patients underwent BCVA measurements using refraction tests, slit-lamp examinations, and binocular indirect ophthalmoscopy after pupil dilation using 0.5% tropicamide throughout the study period. BCVA was measured in decimal values and converted to logMAR scores.
2.3. Fluorescein angiography
Fluorescein angiography was performed to diagnose DME using a retinal camera (Topcon TRC 50DX, Topcon Corporation, Tokyo, Japan).
2.4. Optical coherence tomography
Optical coherence tomography (OCT) was performed at each follow-up visit using an OCT system (Heidelberg Spectralis, Heidelberg Engineering GmbH, Dossenheim, Germany). The OCT images were used to evaluate CRT. CRT was defined as the distance between the internal limiting membrane and the presumed retinal pigment epithelium at the fovea. Measurements were obtained using the scale bars of the OCT system as a reference.
2.5. IVR monotherapy and follow-up
Ranibizumab (0.5 mg/0.05 mL) was intravitreally injected via the pars plana under sterile conditions once per month. It was recommended that injections be resumed if follow-up OCT and fundus findings showed any evidence of fluid build-up in the macula (identified as macular edema or subretinal fluid), or if the BCVA deteriorated, and that the injections be repeated monthly until no further resolution of the fluid or further improvement in BCVA was observed for 2 months. Injections were performed only when informed consent was obtained, thus, patients who refused the treatment were allowed to postpone additional injections and continue observation at each visit. Follow-up visits were generally conducted every month after therapy initiation; however, in cases in which no changes in fluid level or hemorrhage were detected and no injections were required for more than 2 months, the interval was extended. At each follow-up visit, BCVA was measured and other ophthalmological examinations, including OCT, were performed.
2.6. Statistical analysis
Data are expressed as the mean ± standard deviation (SD). Commercially available software (SPSS version 23, SPSS Japan, Tokyo, Japan) was used for all statistical analyses. The Mann–Whitney U test, chi-squared test, and Pearson correlation analysis were performed to compare between 2 groups. The trends of changes in BCVA and CRT before IVR, and at 3, 6, 9, and 12 months after IVR were compared using a linear mixed-effects model. This model included the change from baseline at each time point and month as a fixed-effect, and the patient as a random-effect. A forced entry method was used to determine which factors were possibly associated with CRT improvement in excess of 100 μm at 12 months compared with those at baseline. Adjusted odds ratios (ORs) and 95% confidence intervals (CIs) were estimated using logistic regression models to examine the effects of age and sex on the unadjusted results. To investigate the association between clinical factors and BCVA and CRT values at 12 months, the BCVA and CRT values were evaluated independently in each patient using a linear regression model that was adjusted for age and sex. There were no missing data from any patient, and P < .05 was considered statistically significant.
3. Results
Fifty-six eyes from 38 patients were treated with IVR, who had no previous treatment for their DME during the preceding month. Among these, 18 patients were treated bilaterally; thus, 38 eyes from 38 patients were treated as the first or unilaterally treated eye. These eyes included 1 that was treated with focal retinal photocoagulation, 6 that underwent pan-retinal photocoagulation (PRP), 4 that underwent cataract surgery, and 1 that underwent pars plana vitrectomy due to proliferative diabetic retinopathy with cataract surgery, after the initial IVR treatment up to month 12. Thus, these eyes were excluded. One patient dropped out from the study before the 12-month follow-up visit, and was excluded. Therefore, therapeutic responses to IVR were analyzed in 25 eyes from 25 patients with DME who had been treated only with IVR after the initial injection, and followed-up for more than 12 months. Sixteen men and 9 women, ranging in age from 47 to 81 years (68.7 ± 9.8 years, mean ± SD), were included (Table 1). The mean number of injections during the 12-month follow-up was 4.1 ± 1.9.
Table 1.
Baseline characteristics.
The mean BCVA score at baseline was 0.26 ± 0.29, and 0.28 ± 0.28, 0.21 ± 0.23, 0.21 ± 0.25, 0.26 ± 0.33, and 0.22 ± 0.24 at 1, 3, 6, 9, and 12 month follow-up visits, respectively (Fig. 1). There was no significant difference between the BCVA at each time point and baseline according to the linear mixed-effects model (P values between baseline and 1, 3, 6, 9, and 12 months were 0.55, 0.17, 0.13, 0.83, and 0.21, respectively). The mean change in BCVA at 12 months compared with baseline was −0.047 ± 0.19 (Fig. 2).
Figure 1.
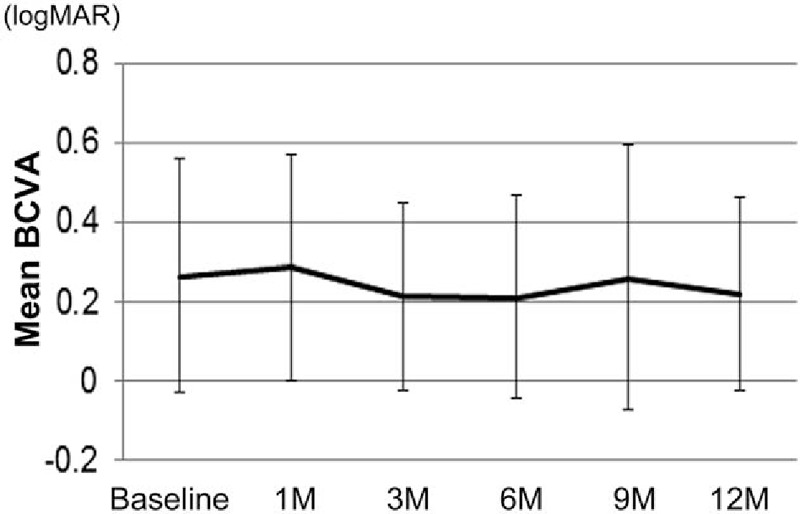
Mean BCVA after intravitreal ranibizumab injection. The mean BCVA at each time point is shown in logMAR. There were no significant changes between mean BCVA at baseline and at each time point, based on a linear mixed-effects model. Values represent the mean ± SD. BCVA = best-corrected visual acuity, SD = standard deviation.
Figure 2.
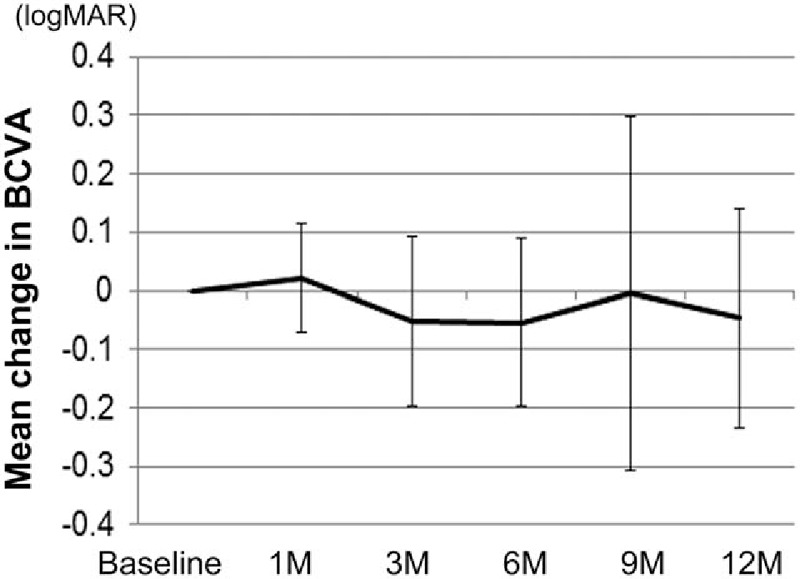
Mean changes in BCVA at each time point compared with baseline after intravitreal ranibizumab injection. Mean BCVA changes at each time point compared with BCVA at baseline are shown. Values represent the mean ± SD. BCVA = best-corrected visual acuity, SD = standard deviation.
The mean CRT at baseline was 492 ± 144 μm, and 458 ± 134 μm, 388 ± 155 μm, 389 ± 123 μm, 372 ± 140 μm, and 374 ± 132 μm at the 1, 3, 6, 9, and 12 month follow-up visits, respectively (Fig. 3). The mean CRT significantly improved at 3, 6, 9, and 12 months compared with baseline (P = .004, .003, <.001, and <.001, respectively). The mean change in CRT at 12 months compared with baseline was −119 ± 151 μm (Fig. 4). There were no eyes that had obvious nonperfusion area in the macula at baseline in the current study (data not shown).
Figure 3.
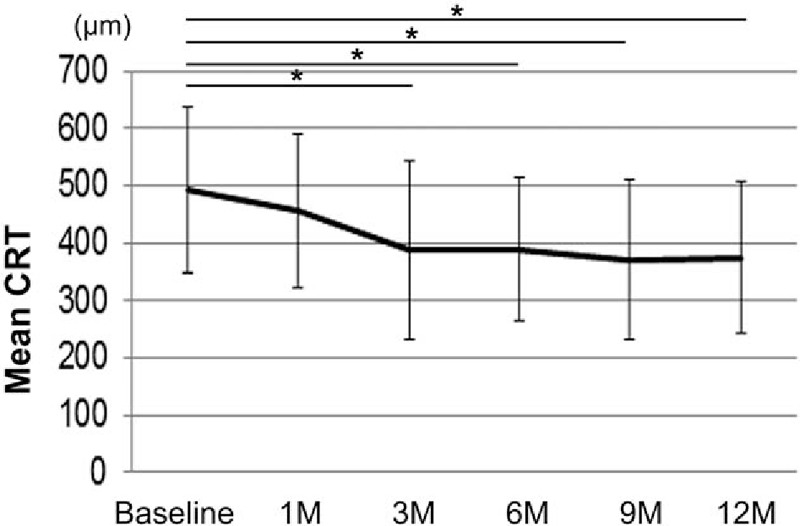
Mean CRT after intravitreal ranibizumab injection. The mean CRT at each time point is shown. Mean CRT was significantly improved at 3, 6, 9, and 12 months compared with baseline measurements, based on a linear mixed-effects model. Values represent the mean ± SD. ∗P < .05. CRT = central retinal thickness, SD = standard deviation.
Figure 4.
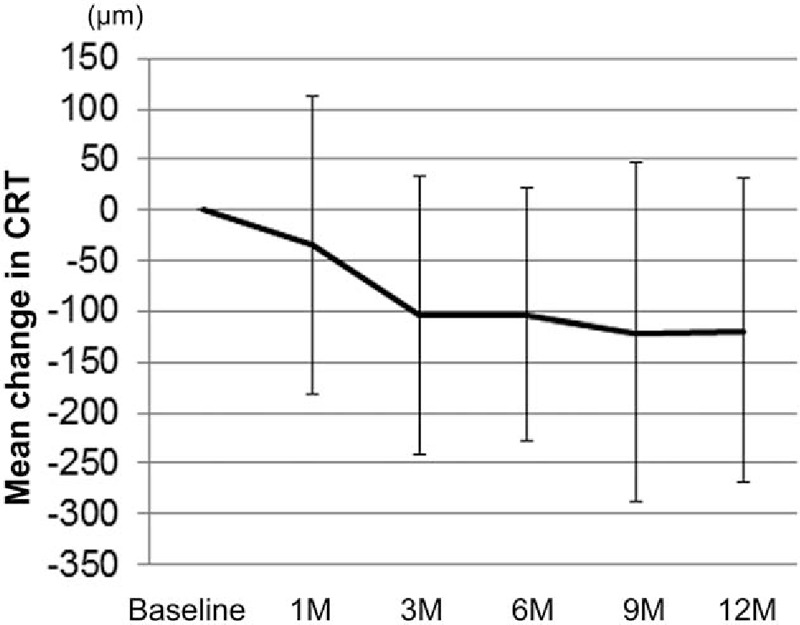
Mean changes in central retinal thickness at each time point compared with baseline after intravitreal ranibizumab injection. The mean CRT changes at each time point as compared with CRT at baseline are shown. Values represent the mean ± SD. CRT = central retinal thickness, SD = standard deviation.
To identify factors that determine therapeutic response to IVR at 12 months, correlations between changes in BCVA from baseline to month 12, and baseline characteristics in individual patients (Table 2) were evaluated. The change in BCVA was negatively correlated with baseline BCVA; thus, patients who had worse BCVA at baseline showed greater improvement (r = −0.57; P = .003). In addition, 13 eyes from 13 patients that had been treated with PRP at baseline due to ischemic changes showed greater improvement in BCVA at 12 months than those that underwent no previous PRP (post-PRP, −0.10 ± 0.17 vs non-PRP, 0.0015 ± 0.19; P = .026) (data not shown).
Table 2.
Association between clinical factors and changes in either BCVA or CRT at month 12.
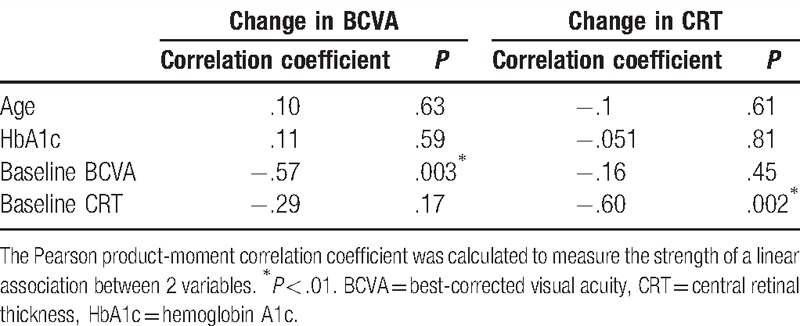
Correlations between changes in CRT at 12 months and at baseline (Table 2) were further evaluated. The changes in CRT were also negatively correlated with baseline CRT; thus, those who had a thicker CRT at baseline showed greater improvement (r = −0.60; P = .002). There was no correlation between changes in CRT and PRP (post-PRP, −135 ± 157 μm vs non-PRP, −100 ± 140 μm; P = .65) (data not shown).
There was no correlation between age, sex, glycated hemoglobin A1c (HbA1c), systolic and diastolic blood pressures, type of DME,[24] and the presence or absence of hard exudates within 500 μm from the fovea, and changes in the BCVA or CRT (Table 2, some data not shown).
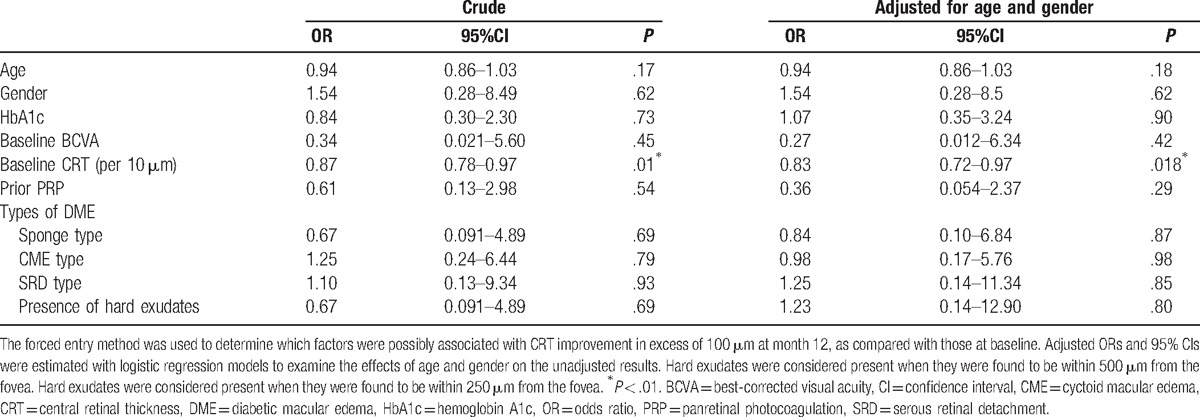
Factors associated with CRT improvement in excess of 100 μm at 12 months are shown in Table 3. Baseline CRT was significantly associated with improvement (OR 0.83 per 10 μm [95% CI 0.72–0.97]; P = .018).
Table 3.
Predictive factors for improvement of central retinal thickness more than 100 μm at month 12.
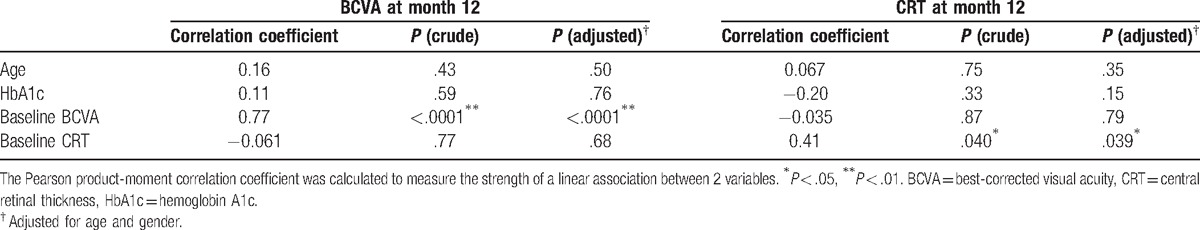
We also evaluated possible predictive factors for better BCVA and CRT values at 12 months. Table 4 shows the association between baseline characteristics and either BCVA or CRT at 12 months. The mean BCVA and CRT at 12 months was positively correlated with BCVA at baseline (r = 0.77; P < .001) and CRT at baseline (r = 0.41; P = .039), respectively, in the multivariate analyses adjusted for age and sex (Table 4).
Table 4.
Association between clinical factors and either BCVA or CRT at month 12.
4. Discussion
We reported the therapeutic effects of monotherapy with an anti-VEGF drug, ranibizumab, without rescue treatment for DME in various types of patients encountered in daily clinical practice. Although the mean BCVA remained unchanged, the mean CRT was significantly reduced by IVR in patients with DME included in the present study. Those who had a poorer BCVA or greater CRT at baseline, and who had already undergone PRP before the initial IVR injection due to the progress of diabetic retinopathy, achieved greater improvements at 12 months. A CRT improvement of more than 100 μm was associated with a greater CRT at baseline. However, BCVA and CRT at 12 months were positively correlated with baseline measurements, and better BCVA and CRT values were achieved at 12 months in patients who had better BCVA and a milder CRT increase at baseline.
Poor BCVA and greater CRT, and a history of PRP may reflect a more progressed lesion at baseline. Thus, those who already have advanced lesions may not necessarily need to abandon treating DME and may see better improvements with IVR monotherapy. The association between greater improvement in BCVA and poor BCVA at baseline was consistent with the previous sponsor-initiated clinical studies, RISE and RIDE, in which monthly IVR and sham injection groups were compared.[25] The RISE and RIDE studies included macular laser photocoagulation for treating DME in patients who experienced insufficient treatment effects of monthly IVR; thus, the result did not exclusively represent the effect of the anti-VEGF drug monotherapy.[26] In contrast, the current study reflects the efficacy of IVR monotherapy. Moreover, the number of injections during the first year in the current study was 4.1 ± 1.9, while in the RISE and RIDE studies, IVR injection was performed monthly for the ranibizumab groups. Collectively, the results of the present study demonstrated a direct effect of IVR monotherapy in a real world-clinic and appear to result in a better outcome.
In terms of previous PRP treatment in improvement in BCVA, this may be because previous PRP may have reduced VEGF expression in the retina,[27] and IVR may have sufficiently suppressed residual VEGF. It may be more advantageous to treat DME under conditions in which ischemic diabetic retinopathy, which induces VEGF expression, is stabilized by PRP. However, our findings were in contrast to those of a previous study,[28] that was limited by the fact that the pathological grade of lesions was not confirmed by an independent reading center. Further study is required to obtain a definitive conclusion.
Importantly, better BCVA and CRT values at 12 months were associated with better BCVA and CRT at baseline, respectively. The better BCVA after IVR treatment in patients who had better values at baseline corresponded with the results from a previous study.[25] In addition, low level of CRT after IVR treatment was also associated with a smaller increase in CRT, representing less edema at baseline, in the current study. Those who had lower CRT at baseline were less likely to have a history of any treatment interventions for retinopathy (P = .013, data not shown). These results suggest that early treatment of DME with IVR may be recommended for better prognosis.
It has been reported that DME and diabetic retinopathy are associated with poor glycemic control,[20,29] and HbA1c levels ≥64 mmol/L (8.0%) are associated with a greater risk for DME.[30] In the current study, the average baseline HbA1c level was 54 mmol/L (7.1%), and it was not associated with either BCVA or CRT at baseline (BCVA: r = 0.024 [P = .91]; CRT: r = −0.13 [P = .53], data not shown). In addition, the effect of IVR treatment was not associated with HbA1c at baseline in terms of improvement in either BCVA or CRT. This result is consistent with previous studies, which showed that the responsiveness of DME to IVR was independent of glycemic control.[28,31]
The current retrospective study was limited by the relatively small number of eyes and the less stringent reinjection criteria than sponsor-initiated clinical trials. The small sample size could induce type 1 errors in logistic regression analyses after adjustment; therefore, we have shown both the crude and adjusted data in Table 3. The results were similar, suggesting that the adjusted data were not the result of a type 1 error. In addition, for the linear regression analysis, we adjusted only age and gender to analyze the independent variables, to follow statistical practices. Because informed consent was necessary for each reinjection, patients who refused the injection, although a minority, were allowed to, despite deteriorating BCVA and/or CRT at some of their visits. However, this could reflect the clinical course in a real-world clinical setting. Eighteen of 25 patients required bilateral IVR for DME, which may have contributed to patient hesitation with undergoing IVR due to economic and/or psychological issues. However, patients in the current study received reimbursement (at least 70% of the cost will be refunded) for each injection with no limitation on the number of injections they received. Eyes that underwent other treatments, regardless of the effects of IVR on DME, were excluded from the study; 7 eyes were treated for proliferative diabetic retinopathy, 4 eyes were treated for cataract, and 1 eye was treated for DME. Although the other treatments were not performed in order to treat DME except for in 1 eye that underwent focal laser photocoagulation, the exclusion of these eyes might also have affected the results.
Because DME can also be treated by photocoagulation,[9–11] local steroid injection,[5] and pars plana vitrectomy,[6,7] the advantages and protocols of combined therapies should be investigated in the future. Results of the current study, in which eyes were treated only with IVR after the initial injection, may be valuable in designing future clinical studies for optimizing treatment protocols according to patient characteristics.
In conclusion, patients with DME who had poorer BCVA or CRT, and who had already undergone PRP due to retinal ischemia at baseline, were more likely to have a better improvement with IVR monotherapy. However, to obtain better BCVA or CRT outcomes, DME eyes should be treated with IVR before BCVA or CRT has markedly deteriorated, and before edema greatly progresses. These results were obtained using IVR monotherapy in a real-world clinical practice, and advance the understanding of the optimal use of IVR for DME. Further studies involving combination therapies are warranted to inform and develop optimal treatment protocols for individual patients.
Acknowledgments
The authors thank the help of all their colleagues, including comedical staff in the Department of Ophthalmology, Keio University Hospital.
Footnotes
Abbreviations: BCVA = best-corrected visual acuity, CRT = central retinal thickness, DME = diabetic macular edema, HbA1c = hemoglobin A1c, IVR = intravitreal ranibizumab, OCT = optical coherence tomography, VEGF = vascular endothelial growth factor.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Leon BM, Maddox TM. Diabetes and cardiovascular disease: epidemiology, biological mechanisms, treatment recommendations and future research. World J Diabetes 2015;6:1246–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Yau JW, Rogers SL, Kawasaki R, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 2012;35:556–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Poh S, Mohamed Abdul RB, Lamoureux EL, et al. Metabolic syndrome and eye diseases. Diabetes Res Clin Pract 2016;113:86–100. [DOI] [PubMed] [Google Scholar]
- [4].Aiello LP, Edwards AR, Beck RW, et al. Factors associated with improvement and worsening of visual acuity 2 years after focal/grid photocoagulation for diabetic macular edema. Ophthalmology 2010;117:946–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ercalik NY, Yenerel NM, Imamoglu S, et al. Combined intravitreal ranibizumab and sub-tenon injection of triamcinolone for the treatment of diabetic macular edema with retinal detachment. J Ocul Pharmacol Ther 2016;32:225–9. [DOI] [PubMed] [Google Scholar]
- [6].Doi N, Sakamoto T, Sonoda Y, et al. Comparative study of vitrectomy versus intravitreous triamcinolone for diabetic macular edema on randomized paired-eyes. Graefes Arch Clin Exp Ophthalmol 2012;250:71–8. [DOI] [PubMed] [Google Scholar]
- [7].Yamamoto T, Hitani K, Tsukahara I, et al. Early postoperative retinal thickness changes and complications after vitrectomy for diabetic macular edema. Am J Ophthalmol 2003;135:14–9. [DOI] [PubMed] [Google Scholar]
- [8].Funatsu H, Noma H, Mimura T, et al. Association of vitreous inflammatory factors with diabetic macular edema. Ophthalmology 2009;116:73–9. [DOI] [PubMed] [Google Scholar]
- [9].Ishibashi T, Li X, Koh A, et al. The REVEAL study: ranibizumab monotherapy or combined with laser versus laser monotherapy in Asian patients with diabetic macular edema. Ophthalmology 2015;122:1402–15. [DOI] [PubMed] [Google Scholar]
- [10].Mitchell P, Bandello F, Schmidt-Erfurth U, et al. The RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology 2011;118:615–25. [DOI] [PubMed] [Google Scholar]
- [11].Wykoff CC, Hariprasad SM. DRCR protocol-T: reconciling 1- and 2-year data for managing diabetic macular edema. Ophthalmic Surg Lasers Imaging Retina 2016;47:308–12. [DOI] [PubMed] [Google Scholar]
- [12].Brown DM, Nguyen QD, Marcus DM, et al. Long-term outcomes of ranibizumab therapy for diabetic macular edema: the 36-month results from two phase III trials: RISE and RIDE. Ophthalmology 2013;120:2013–22. [DOI] [PubMed] [Google Scholar]
- [13].Senger DR, Galli SJ, Dvorak AM, et al. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science 1983;219:983–5. [DOI] [PubMed] [Google Scholar]
- [14].Leung DW, Cachianes G, Kuang WJ, et al. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science 1989;246:1306–9. [DOI] [PubMed] [Google Scholar]
- [15].Keck PJ, Hauser SD, Krivi G, et al. Vascular permeability factor, an endothelial cell mitogen related to PDGF. Science 1989;246:1309–12. [DOI] [PubMed] [Google Scholar]
- [16].Owen LA, Hartnett ME. Soluble mediators of diabetic macular edema: the diagnostic role of aqueous VEGF and cytokine levels in diabetic macular edema. Curr Diab Rep 2013;13:476–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Anderson EJ, Richardson M, Castle G, et al. Nutrition interventions for intensive therapy in the Diabetes Control and Complications Trial. The DCCT Research Group. J Am Diet Assoc 1993;93:768–72. [DOI] [PubMed] [Google Scholar]
- [18].Ding J, Wong TY. Current epidemiology of diabetic retinopathy and diabetic macular edema. Curr Diab Rep 2012;12:346–54. [DOI] [PubMed] [Google Scholar]
- [19].Sasaki M, Kawashima M, Kawasaki R, et al. Association of serum lipids with macular thickness and volume in type 2 diabetes without diabetic macular edema. Invest Ophthalmol Vis Sci 2014;55:1749–53. [DOI] [PubMed] [Google Scholar]
- [20].Group AS, Group AES, Chew EY, et al. Effects of medical therapies on retinopathy progression in type 2 diabetes. N Engl J Med 2010;363:233–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Rosenfeld PJ. Bevacizumab versus ranibizumab for AMD. N Engl J Med 2011;364:1966–7. [DOI] [PubMed] [Google Scholar]
- [22].Wang JK, Huang TL, Su PY, et al. Approved pharmacotherapy for myopic choroidal neovascularization: a review of randomized controlled trials in ranibizumab and aflibercept. Eye Sci 2015;30:198–200. [PubMed] [Google Scholar]
- [23].Sawada O, Ohji M. Retinal Vein Occlusion. Dev Ophthalmol 2016;55:147–53. [DOI] [PubMed] [Google Scholar]
- [24].Otani T, Kishi S, Maruyama Y. Patterns of diabetic macular edema with optical coherence tomography. Am J Ophthalmol 1999;127:688–93. [DOI] [PubMed] [Google Scholar]
- [25].Sophie R, Lu N, Campochiaro PA. Predictors of functional and anatomic outcomes in patients with diabetic macular edema treated with ranibizumab. Ophthalmology 2015;122:1395–401. [DOI] [PubMed] [Google Scholar]
- [26].Nguyen QD, Brown DM, Marcus DM, et al. Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology 2012;119:789–801. [DOI] [PubMed] [Google Scholar]
- [27].Aiello LP, Avery RL, Arrigg PG, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med 1994;331:1480–7. [DOI] [PubMed] [Google Scholar]
- [28].Bressler SB, Qin H, Beck RW, et al. Factors associated with changes in visual acuity and central subfield thickness at 1 year after treatment for diabetic macular edema with ranibizumab. Arch Ophthalmol 2012;130:1153–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–86. [DOI] [PubMed] [Google Scholar]
- [30].Chou TH, Wu PC, Kuo JZ, et al. Relationship of diabetic macular oedema with glycosylated haemoglobin. Eye (Lond) 2009;23:1360–3. [DOI] [PubMed] [Google Scholar]
- [31].Bansal AS, Khurana RN, Wieland MR, et al. Influence of glycosylated hemoglobin on the efficacy of ranibizumab for diabetic macular edema: a post hoc analysis of the RIDE/RISE trials. Ophthalmology 2015;122:1573–9. [DOI] [PubMed] [Google Scholar]


