Abstract
Background:
Malignant catatonia (MC) is a disorder consisting of catatonic symptoms, hyperthermia, autonomic instability, and altered mental status. Neuroleptic malignant syndrome (NMS) caused by antipsychotics is considered a variant of MC. Benzodiazepine (BZD) medications are safe and effective treatments providing rapid relief from MC. This case study reports a detailed clinical course of a case of MC associated with schizophrenia initially diagnosed as NMS that responded successfully to BZDs but not to dantrolene.
Case presentation:
A 53-year-old man with schizophrenia was admitted to the psychiatric hospital because of excitement, monologue, muscle rigidity, and insomnia. In the 3 days before admission, the patient had discontinued his medications after his family member's death. He presented with hyperthermia, tachycardia, hypertension, excessive sweating, and an elevated serum creatine phosphokinase (CPK) level. On the basis of these features, he was suspected to have NMS. The patient was treated with dantrolene for 7 days without improvement despite having a normalized serum CPK level. The patient was transferred to our university hospital for an in-depth examination and treatment of his physical status. Infection and pulmonary embolism were excluded as possible causes. To treat his excitement and auditory hallucination, an intravenous drip (IVD) of haloperidol was initiated, but this treatment increased the patient's catatonic and psychotic symptoms, although his serum CPK level had remained within a normal range. As a result, the treatment was changed to diazepam. After an IVD of diazepam, the patient's symptoms rapidly improved, and the IVD was subsequently replaced with oral administration of lorazepam. Eventually, the patient was diagnosed with MC associated with schizophrenia. BZD therapy was dramatically effective.
Conclusion:
Catatonia, MNS, and MC may be due to a common brain pathophysiology and these conditions may be in a spectrum, although uncertainty in the boundaries among conditions, and the BZD treatment may be useful. Most importantly, catatonia has not been described as a subtype of schizophrenia on the basis of the Diagnostic and Statistical Manual of Mental Disorders (DSM)-5 criteria, and the medications for catatonia and schizophrenia are different. Antipsychotics are not effective in relieving catatonia, or they may induce NMS, whereas BZDs are effective for treating both MC and NMS.
Keywords: benzodiazepine, catatonia, DSM-5, malignant catatonia, neuroleptic malignant syndrome, schizophrenia
1. Introduction
In 1874, Kahlbaum proposed that catatonia is a syndrome of motor dysfunction that includes mutism, immobility, staring gaze, negativism, stereotyped behavior, waxy flexibility, and verbigeration.[1] Catatonia is found in 10% of psychiatric inpatients and is more common in patients with mood disorders, especially mania, than in those patients with schizophrenia.[2] In addition, catatonia can also occur in a wide range of illnesses, including drug intoxications, neurological disorders, and other general medical conditions. Therefore, catatonia is not an exclusive syndrome related to a specific psychiatric disorder. In particular, malignant catatonia (also known as lethal or pernicious catatonia) is a rare but life-threatening disorder that was first reported by Stauder in 1934, long before the era of antipsychotic drugs.[3] Malignant catatonia is characterized by excitement, altered mental status (altered level of consciousness), hyperthermia and autonomic instability as well as catatonic symptoms.[4,5] In contrast, neuroleptic malignant syndrome (NMS) associated with the use of antipsychotic medications is considered to be a variant of malignant catatonia.[4–7] The diversity of symptoms often leads to a delay in diagnosis. The mortality rate associated with malignant catatonia has declined but remains high (10%–20%). Although the pathophysiology of malignant catatonia has not still been clarified, the most likely hypothesis is dopamine hypoactivity in the brain, specifically in the basal ganglia and the hypothalamus.[5]
Persistent catatonic symptoms produce risks of physical complications including fatal pulmonary embolism, which is associated with high morbidity and mortality.[8,9] Thus, to prevent the complications associated with catatonia, early recognition and rapid treatment are clinically important. Electroconvulsive therapy (ECT) is crucial to improve morbidity and mortality. ECT is the first-choice treatment for malignant catatonia, on the basis of previous case reports.[5,10] Patient response rates to ECT are higher and more rapid than those with benzodiazepines (BZDs) alone. However, BZDs can also relieve catatonia and cause less severe complications,[11] whereas ECT causes severe complications, including status epilepticus, prolonged seizure, postictal agitation, cardiovascular disorders, and pulmonary embolism.[12] Among different BZDs, lorazepam and diazepam have roles as first-line medications for treatment of acute catatonia and the maintenance treatment to prevent relapse.[13–18] However, an effective treatment for NMS is dantrolene, which inhibits contraction and heat production in skeletal muscle.[19]
In this paper, we report a clinical course of a patient with malignant catatonia who was initially diagnosed with NMS and who responded successfully to BZD administration. The patient was a 53-year-old male with a 29-year history of schizophrenia who required a rapid cure to prevent the risks of physical complications with high morbidity and mortality. We suggest that malignant catatonia may have a different etiology from that of schizophrenia and that the necessary treatments may differ between the disorders.
2. Case presentation
The patient was a 53-year-old male who was diagnosed 29 years earlier with schizophrenia according to the criteria of the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5). His psychotic symptoms had been relatively stable for several years with a regimen of risperidone (7 mg/day), chlorpromazine (75 mg/day), biperidene (3 mg/day), diazepam (6 mg/day), and brotizolam (0.25 mg/day). However, the patient suddenly discontinued his entire medication regimen after his family member's death. After 3 days without medication, the patient was admitted to a private psychiatric hospital where he presented with agitation, monologue, and insomnia (Hospital day 1). At the time of admission, the patient also presented with hyperthermia, tremor, muscle rigidity, and clouded consciousness. Autonomic symptoms such as tachycardia, hypertension, and profuse perspiration were present. The patient's serum CPK level was elevated to 8559 IU/L (normal value = 68–287 IU/L), and his urine myoglobin was positive. His electroencephalography (EEG) results were unremarkable. NMS was suspected and a 20 mg/day dose of dantrolene was initiated. In addition, a 2 to 3 mg/hour intravenous (IV) infusion of midazolam was administered to treat his excitement.
By hospital day 7, the hyperthermia and autonomic dysfunctions such as tachycardia, hypertension, and profuse perspiration had not improved, although his serum CPK level had decreased to 219 IU/L. As a result, the dantrolene was discontinued. Antibiotics were started because of the need for frequent suctioning and the suspicion of aspiration pneumonia. There was no significant clinical improvement. To decrease the dose of midazolam for sedation, the patient received a single intramuscular injection (IMI) of 10 mg of diazepam on hospital day 9. The diazepam seemed to be possibly effective for the hyperthermia, despite the continued presence of other symptoms, including autonomic dysfunction and agitation. Therefore, the inflammatory focus and autonomic symptoms were suspected to be due to other infectious diseases or to a pulmonary embolism.
To closely examine and treat the patient's physical status, the patient was transferred to our psychiatric ward at Kanazawa Medical University Hospital on hospital day 11. In our hospital, catatonic symptoms such as agitation, negativism, mannerism, stereotypy, echolalia and echopraxia, auditory hallucination, persecutory delusion, and somnolence were prominent in the patient. Autonomic symptoms such as hyperthermia (37.4°C), tachycardia (102 bpm) and hypertension (144/76 mm Hg) were also present. Laboratory findings showed that the patient's serum CKP level was within normal range (152 IU/L), and his urine myoglobin was negative, even though mild inflammatory founding was evident [white blood cell (WBC): 8.1 × 104/μL, normal range 2.97–9.13 × 104/μL; C-reactive protein (CRP): 4.8 mg/dL, normal range <0.3]. A twice-daily IVD of 5 mg of haloperidol in a 500 mL fluid replacement administered over 6 hours was administered to treat the agitation, auditory hallucinations, and persecutory delusions. Assessments for pulmonary thromboembolism and deep vein thrombosis were negative. We performed an extensive infectious disease workup. Chest and abdominal computed tomography (CT) indicated a mild pulmonary infiltrative shadow and perinephric stranding; therefore, antibiotic treatment was resumed because of suspicions of aspiration pneumonia and acute pyelonephritis. However, the patient's hyperthermia (up to 40.0°C) and autonomic symptoms such as tachycardia (up to 148 bpm), hypertension (up to 178/98 mm Hg) and profuse perspiration did not improve, as shown in Fig. 1. His catatonic symptoms such as stupor or excitement, echolalia, catalepsy, mutism, and negativism were advanced and prominent (Fig. 2), although his serum CPK level remained within normal limits, and the inflammatory findings were mild. In addition, muscle rigidity, salivation, and dysphagia were also prominent. Brain CT was normal; abnormalities, such as infarcts, hemorrhages, or brain tumors, were excluded. Several days later, we were informed that the results of bacterial examinations of sputum and urine were negative. Eventually, the patient was diagnosed with malignant catatonia associated with schizophrenia.
Figure 1.
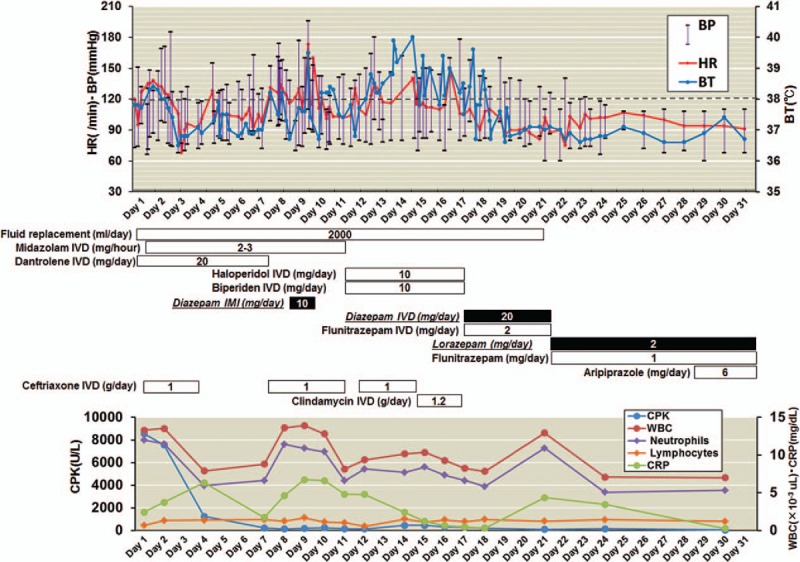
The clinical course of a 53-year-old man with malignant catatonia associated with schizophrenia. Dotted line indicates 38°C of BT. BP = blood pressure; BT = body temperature; CPK = creatine phosphokinase; CRP = C-reactive protein; HR = heart rate; IMI = intramuscular injection; IVD = intravenous dripping; WBC = white blood cell.
Figure 2.
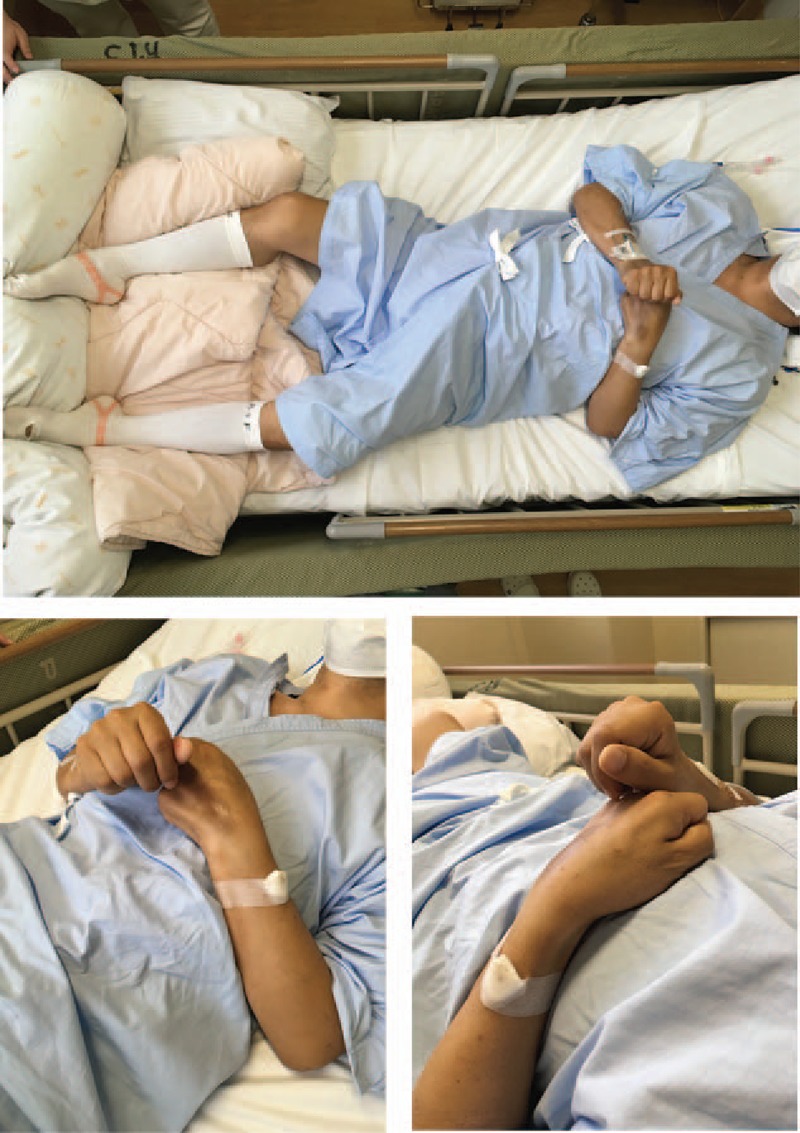
Symptoms of catatonia in the patient with severe motoric immobility. During the patient's clinical course, all 12 psychomotor features (stupor, catalepsy, waxy flexibility, mutism, negativism, posturing, mannerism, stereotypy, agitation, grimacing, echolalia, and echopraxia) in the criteria of the DSM-5 for catatonia were present.
On hospital day 17, we terminated neuroleptic medications, and a twice-daily IVD of 10 mg of diazepam in 100 mL of normal saline administered over 1 hour was administered for the next 5 days. In addition, an IVD of 2 mg of flunitrazepam administered at night was administered to decrease the patient's insomnia. After the initiation of diazepam, the patient experienced a swift resolution of symptoms. Diazepam produced muscular relaxation and relieved the salivation and dysphagia, and hyperthermia and autonomic symptoms such as tachycardia, hypertension, and profuse perspiration also improved. On hospital day 22, the IVDs of diazepam and flunitrazepam were replaced with oral administrations of 2 mg/day of lorazepam and 1 mg/day of flunitrazepam. On hospital day 29, aripiprazole (6 mg/day) was initiated to treat the patient's auditory hallucinations and delusions. The patient remained normopyretic, and his psychotic symptoms were stable.
For publication of this case report and accompanying images, written informed consent was obtained from the patient and his sister.
3. Discussion
This report describes a detailed clinical course of malignant catatonia associated with schizophrenia in a patient who was initially suspected to have NMS. His symptoms were not responsive to dantrolene sodium or antibiotics, and the patient continued to present with hyperthermia (up to 40.0°C) and autonomic symptoms such as hyperthermia, tachycardia, hypertension, profuse perspiration, and catatonic symptoms. However, these symptoms successfully and rapidly responded to administration of diazepam and lorazepam. This case was difficult to diagnose malignant catatonia from his clinical course. Once thought to be a subtype of schizophrenia according to the DSM-IV criteria, catatonia is now recognized to occur with a broad spectrum of medical and psychiatric illnesses according to the DSM-5 criteria. We suggest that the catatonia should be treated before any underlying conditions can be accurately diagnosed because the underlying conditions are complex and heterogeneous and the catatonia is associated with significant morbidity and mortality if left untreated.
Dantrolene sodium is a direct skeletal muscle relaxant that disrupts excitation–contraction coupling by blocking calcium efflux from the sarcoplasmic reticulum.[20] In our case, dantrolene sodium was not effective to improve the patient's symptoms. Dantrolene has been routinely used for NMS treatment,[19] while dantrolene is not universally accepted as a treatment for NMS.[20,21] If the patient is demonstrating mild to moderate symptoms of rigidity and fever, dantrolene may be effective earlier in NMS presentation.[20] In contrast, for severe cases, dantrolene used as monotherapy is not recommended for treating NMS and may actually be associated with an increase in mortality and hepatitis.[21,22]
NMS is the more common condition and widely known in clinical practice. Although there is uncertainty in the boundaries between malignant catatonia and NMS,[20,23] NMS is considered as antipsychotic medication induced form of malignant catatonia.[4–7] If history excludes exposure to an antipsychotic, the diagnosis of malignant catatonia would be apparent.[24] However, the definition may be blurred, as the majority of patients with malignant catatonia have a psychiatric history and have been treated with antipsychotics. Malignant catatonia and NMS have been suggested to have a common pathophysiology.[24–26] A sudden and massive blockade of neurotransmitters, including dopamine, has been hypothesized as the mechanism of action of both conditions,[24–26] although the definitive cause has not been clarified. An extreme elevation in temperature reflects a breakdown in central thermoregulation. Generalized rigidity, which has been described as a “lead pipe” in its most severe form and is usually unresponsive to antiparkinsonian agents such as biperiden, is a main feature of both disorders. The symptoms including catatonia associated with fever and autonomic instability are similar, and malignant catatonia may be indistinguishable from NMS.[20] In this case, the patient had discontinued all his psychiatric medications for the 3 days after his family member's death. The abrupt withdrawal of antipsychotics or BZDs and bereavement reaction may cause a sudden and massive dopamine instability in his brain, and his catatonic and autonomic symptoms developed at or after hospital admission. According to the DSM-5 criteria regarding NMS, patients have generally been exposed to a dopamine antagonist within 72 hours before symptom development. Using this criterion, the possibility that the patient had NMS can be excluded entirely. On the basis of Fink criteria of symptoms—stupor or excitement, echolalia, catalepsy, mutism and negativism, hyperpyrexia, and autonomic instability—[27] we determined that the most likely diagnosis for the patient's condition was malignant catatonia. These findings suggest that catatonia, MNS, and MC may be due to a common brain pathophysiology, and these conditions may be in a spectrum, although there is uncertainty in the boundaries among these conditions.
In our case, BZDs were effective at relieving catatonic and autonomic symptoms. Imaging studies have indicated that inhibitory neurotransmitter gamma-aminobutyric acid (GABA) receptors are decreased in the left sensorimotor cortex in catatonic patients.[28–30] Through the mechanisms of GABA agonism, BZDs have a reliable treatment efficacy for catatonic patients. However, because a sudden and massive dopamine blockade can cause catatonia (including NMS), dopamine receptor antagonists may have no general efficacy in the treatment of catatonia, or they may actually increase the symptoms.[25] In our case, the patient's symptoms might have been increased by the haloperidol that we used to treat the agitation, auditory hallucinations, and persecutory delusions. For the 14 days, the patient was free of antipsychotic medication before starting haloperidol. There is a Food and Drug Administration (FDA) warning, since 2007, regarding the administration of IV haloperidol for patients with delirium or any other psychotic disorder.[31] Although the IV haloperidol is still widely administered to manage acute psychosis in Japan, administrating IVD of 10 mg/day of haloperidol to a patient with several organic comorbidities may increase the risks of QT prolongation, torsades de pointes (TdP), or NMS. ECT is also effective as a first-line treatment strategy to relieve catatonia or as a second-line treatment if a BZD fails.[32] It has been suggested that ECT is effective in relieving catatonia after a 6 to 16 mg/day dose of lorazepam for up to 5 days has failed.[33] If the patient's symptoms had not improved after 5 days of BZD use, we would have attempted ECT to relieve his symptoms.
As described above, both BZDs and ECT are effective tools for treating both malignant catatonia and NMS.[2] Further weakening the distinction of NMS from catatonia are reports that certain non-neuroleptic drugs can also induce NMS. Therefore, the differentiation between malignant catatonia and NMS may not be meaningful.[5] A previous review of malignant catatonic cases has reported that the majority of these cases present in the classic excited form; thus, catatonia may be distinguished from NMS on the basis of the onset of hyperthermia in association with hyperactivity before the administration of neuroleptic drugs and because of the development of stupor and rigidity. However, the diagnosis of catatonia cannot be distinguished from NMS in 22% of cases.[4] Thus, it has been suggested that NMS is a neuroleptic medication-induced form of malignant catatonia.
4. Conclusions
Although catatonia has typically been diagnosed in patients with schizophrenia, catatonia can occur in the context of several disorders, including schizophrenia, bipolar depression, depressive disorders, and other medical conditions. According to DSM-5 criteria, catatonia has not been defined as a subtype of schizophrenia, and most importantly, medications for the treatment of catatonia and schizophrenia are different. Antipsychotics are not effective in the relief of catatonia and may induce NMS, whereas BZDs are effective in treating both malignant catatonia and NMS. Because of the severity of the physical complications, attention should be paid to the possibility that the catatonia may be attributable to malignant catatonia or NMS.
Acknowledgment
We would like to thank the patient and his family who participated in this study.
Footnotes
Abbreviations: BZD = benzodiazepine, CPK = creatine phosphokinase, CT = computer tomography, DSM = Diagnostic and Statistical Manual of Mental Disorders, ECT = electroconvulsive therapy, GABA = gamma-aminobutyric acid, IMI = intramuscular injection, IVD = intravenous dripping, MC = malignant catatonia, NMS = neuroleptic malignant syndrome.
Authorship: KO was critically involved in the collection of the data, contributed intellectually to the interpretation of the data, wrote the first draft of the manuscript, and contributed to the editing of the final manuscript. AK, TS, TY, YN, TU, and YK were highly involved in the collection of the data and contributed intellectually to the interpretation of the data. All authors read and approved the final manuscript.
Funding/support: This work was supported by a Japan Society for the Promotion of Science (JSPS) Grant-in-Aid for Young Scientists (B) (16K19784).
The authors declare that they have no competing interests.
References
- [1].Fink M, Shorter E, Taylor MA. Catatonia is not schizophrenia: Kraepelin's error and the need to recognize catatonia as an independent syndrome in medical nomenclature. Schizophr Bull 2010;36:314–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Taylor MA, Fink M. Catatonia in psychiatric classification: a home of its own. Am J Psychiatry 2003;160:1233–41. [DOI] [PubMed] [Google Scholar]
- [3].Lotstra F, Linkowski P, Mendlewicz J. General anesthesia after neuroleptic malignant syndrome. Biol Psychiatry 1983;18:243–7. [PubMed] [Google Scholar]
- [4].Mann SC, Caroff SN, Bleier HR, et al. Lethal catatonia. Am J Psychiatry 1986;143:1374–81. [DOI] [PubMed] [Google Scholar]
- [5].Philbrick KL, Rummans TA. Malignant catatonia. J Neuropsychiatry Clin Neurosci 1994;6:1–3. [DOI] [PubMed] [Google Scholar]
- [6].Tuerlings JH, van Waarde JA, Verwey B. A retrospective study of 34 catatonic patients: analysis of clinical care and treatment. Gen Hosp Psychiatry 2010;32:631–5. [DOI] [PubMed] [Google Scholar]
- [7].White DA, Robins AH. An analysis of 17 catatonic patients diagnosed with neuroleptic malignant syndrome. CNS Spectr 2000;5:58–65. [DOI] [PubMed] [Google Scholar]
- [8].McCall WV, Mann SC, Shelp FE, et al. Fatal pulmonary embolism in the catatonic syndrome: two case reports and a literature review. J Clin Psychiatry 1995;56:21–5. [PubMed] [Google Scholar]
- [9].Carroll BT. Complications of catatonia. J Clin Psychiatry 1996;57:95. [PubMed] [Google Scholar]
- [10].Boyarsky BK, Fuller M, Early T. Malignant catatonia-induced respiratory failure with response to ECT. J ECT 1999;15:232–6. [PubMed] [Google Scholar]
- [11].Huang YC, Lin CC, Hung YY, et al. Rapid relief of catatonia in mood disorder by lorazepam and diazepam. Biomed J 2013;36:35–9. [DOI] [PubMed] [Google Scholar]
- [12].Cristancho MA, Alici Y, Augoustides JG, et al. Uncommon but serious complications associated with electroconvulsive therapy: recognition and management for the clinician. Curr Psychiatry Rep 2008;10:474–80. [DOI] [PubMed] [Google Scholar]
- [13].Grover S, Aggarwal M. Long-term maintenance lorazepam for catatonia: a case report. Gen Hosp Psychiatry 2011;33:82.e1-e3. [DOI] [PubMed] [Google Scholar]
- [14].Manjunatha N, Saddichha S, Khess CR. Idiopathic recurrent catatonia needs maintenance lorazepam: case report and review. Aust N Z J Psychiatry 2007;41:625–7. [DOI] [PubMed] [Google Scholar]
- [15].Fricchione GL, Cassem NH, Hooberman D, et al. Intravenous lorazepam in neuroleptic-induced catatonia. J Clin Psychopharmacol 1983;3:338–42. [PubMed] [Google Scholar]
- [16].McEvoy JP, Lohr JB. Diazepam for catatonia. Am J Psychiatry 1984;141:284–5. [DOI] [PubMed] [Google Scholar]
- [17].Rosebush PI, Hildebrand AM, Furlong BG, et al. Catatonic syndrome in a general psychiatric inpatient population: frequency, clinical presentation, and response to lorazepam. J Clin Psychiatry 1990;51:357–62. [PubMed] [Google Scholar]
- [18].Ungvari GS, Leung CM, Wong MK, et al. Benzodiazepines in the treatment of catatonic syndrome. Acta Psychiatr Scand 1994;89:285–8. [DOI] [PubMed] [Google Scholar]
- [19].Caroff SN, Mann SC, Keck PE., Jr Specific treatment of the neuroleptic malignant syndrome. Biol Psychiatry 1998;44:378–81. [DOI] [PubMed] [Google Scholar]
- [20].Pileggi DJ, Cook AM. Neuroleptic malignant syndrome. Ann Pharmacother 2016;50:973–81. [DOI] [PubMed] [Google Scholar]
- [21].Reulbach U, Dutsch C, Biermann T, et al. Managing an effective treatment for neuroleptic malignant syndrome. Crit Care 2007;11:R4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Rosenberg MR, Green M. Neuroleptic malignant syndrome. Review of response to therapy. Arch Intern Med 1989;149:1927–31. [DOI] [PubMed] [Google Scholar]
- [23].Lang FU, Lang S, Becker T, et al. Neuroleptic malignant syndrome or catatonia? Trying to solve the catatonic dilemma. Psychopharmacology (Berl) 2015;232:1–5. [DOI] [PubMed] [Google Scholar]
- [24].Park J, Tan J, Krzeminski S, et al. Malignant catatonia warrants early psychiatric-critical care collaborative management: two cases and literature review. Case Rep Crit Care 2017;2017:1951965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Osman AA, Khurasani MH. Lethal catatonia and neuroleptic malignant syndrome. A dopamine receptor shut-down hypothesis. Br J Psychiatry 1994;165:548–50. [DOI] [PubMed] [Google Scholar]
- [26].Carroll BT. The universal field hypothesis of catatonia and neuroleptic malignant syndrome. CNS Spectr 2000;5:26–33. [DOI] [PubMed] [Google Scholar]
- [27].Fink M. Catatonia: a syndrome appears, disappears, and is rediscovered. Can J Psychiatry 2009;54:437–45. [DOI] [PubMed] [Google Scholar]
- [28].Northoff G, Steinke R, Czcervenka C, et al. Decreased density of GABA-A receptors in the left sensorimotor cortex in akinetic catatonia: investigation of in vivo benzodiazepine receptor binding. J Neurol Neurosurg Psychiatry 1999;67:445–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Northoff G. What catatonia can tell us about “top-down modulation”: a neuropsychiatric hypothesis. Behav Brain Sci 2002;25:555–77. discussion 578–604. [DOI] [PubMed] [Google Scholar]
- [30].Northoff G. Brain imaging in catatonia: current findings and a pathophysiologic model. CNS Spectr 2000;5:34–46. [DOI] [PubMed] [Google Scholar]
- [31].Meyer-Massetti C, Cheng CM, Sharpe BA, et al. The FDA extended warning for intravenous haloperidol and torsades de pointes: how should institutions respond? J Hosp Med 2010;5:E8–16. [DOI] [PubMed] [Google Scholar]
- [32].Rohland BM, Carroll BT, Jacoby RG. ECT in the treatment of the catatonic syndrome. J Affect Disord 1993;29:255–61. [DOI] [PubMed] [Google Scholar]
- [33].Bush G, Fink M, Petrides G, et al. Catatonia II treatment with lorazepam and electroconvulsive therapy. Acta Psychiatr Scand 1996;93:137–43. [DOI] [PubMed] [Google Scholar]


