Supplemental Digital Content is available in the text
Keywords: aerobic metabolism, cardiac rehabilitation, exercise physiology, muscle strength, myokine
Abstract
Patients with coronary heart disease or acute myocardial infarction after cardiac catheterization with stenting referred for phase II cardiac rehabilitation (CR) were grouped according to their preference. Cardio-pulmonary exercise testing (CPET) was used to determine oxygen uptake ( ) at peak exercise and anaerobic threshold (AT). The control patients received counseling only while the experiment group received 36 sessions of CR in 3 to 6 months. Exercise physiology parameters and serum myokines (myostatin, insulin-like growth factor-1 (IGF-1), and interleukin-6 (IL-6) were measured pre- and postrehabilitation.
) at peak exercise and anaerobic threshold (AT). The control patients received counseling only while the experiment group received 36 sessions of CR in 3 to 6 months. Exercise physiology parameters and serum myokines (myostatin, insulin-like growth factor-1 (IGF-1), and interleukin-6 (IL-6) were measured pre- and postrehabilitation.
There were 29 patients in the experiment group and 10 in the control group, with no significant differences in baseline parameters. The experiment group had prominent progress in aerobic capacity and body composition after CR, but their serum myokine concentrations did not change significantly. Serum myostatin is positively correlated to peak  pre- and post-training, and pretraining AT
pre- and post-training, and pretraining AT  , after adjusting for age, sex, and body composition. Serum IGF-1 is positively correlated with grip strength before training.
, after adjusting for age, sex, and body composition. Serum IGF-1 is positively correlated with grip strength before training.
Serum myostatin level is positively correlated to aerobic capacity, and IGF-1 level is positively correlated to grip strength in cardiac patients receiving CR.
1. Introduction
The skeletal muscle has a complex system of homeostasis. Its growth and development are greatly influenced by related myokines such as myostatin, insulin-like growth factor-1 (IGF-1), and interleukin-6 (IL-6). Myostatin, a member of the TGF-β super-family, is a potent negative regulator of skeletal muscle mass.[1,2] Along with IGF-1, it is proposed as a counter-regulatory molecule for muscle hypertrophy.[3] IGF-1 is an age-related serum protein with insulin-like metabolic activity and growth-control ability.[4] It affects the development of several systems, the musculoskeletal being one of the most important.[5] IGF-1 and IL-6 share common pathways in physiologic regulation.[6] IL-6 can induce the proliferation of muscle stem cells after mechanical stimuli or injury.[7] Some preliminary trials report inconclusive results regarding the immediate effects of exercise-induced changes in these myokines.[8,9] How exercise influences serum levels of these myokines while increasing strength and possible muscle hypertrophy in the long-term remains controversial.
Recent researches indicate that not only the size and mass but also energy system of skeletal muscle are regulated by myostatin. Muscle fiber conversion and oxidative mitochondrial capacity in myostatin-deficient mice suggest a shift towards anaerobic metabolism in the muscle.[10,11] Myostatin-deficient mice also have decreased exercise endurance, energy efficiency, and oxygen uptake in vivo.[12] Whether this is due to the development of different muscle types because of congenital myostatin deficiency or if the energy system and aerobic capacity can be directly influenced by serum myokines warrant further elucidation. If the latter is true, a correlation between aerobic capacity and myokines is expected.
Patients with heart diseases often have generalized decreased muscle strength and muscle atrophy, and reduced aerobic capacity, possibly due to de-conditioning and cytokine effect.[13,14] Exercise, especially strengthening exercise, can maintain the muscle bulk and even reverse the process of muscle atrophy in patients with chronic diseases.[15,16] Cardiac rehabilitation (CR) is mainly comprised of targeted aerobic conditioning and strengthening exercise training, and is a widely accepted standard treatment for cardiac patients.[17] Patients receiving CR have improved aerobic capacity and muscle strength.[18,19]
This study aimed to determine whether the training effects are reflected by changes in serum myokine concentration in a prospective cohort receiving phase II CR. The hypothesis is that myokine concentrations are elevated after CR and that there is direct correlation between myokine concentration and aerobic capacity and muscle strength.
2. Methods
2.1. Patient eligibility
Consecutive cardiac patients referred to the Department of Physical Medicine and Rehabilitation of National Taiwan University Hospital after completing phase I CR program were invited. The inclusion criteria were age >20 years; coronary heart disease diagnosed based on clinical history, electrocardiography, and diagnostic cardiac catheterization; or acute myocardial infarction after cardiac catheterization with stenting. Patients with congested heart failure (CHF), atrial fibrillation/flutter, ventricular bigeminy, active inflammatory disease, malignancy, and cardiac pacemakers were excluded. The diagnosis of CHF was made clinically by a cardiologist based on the Framingham criteria.[20]
The experiment group underwent 36 sessions of hospital-based phase II CR in 3 to 6 months, while the control patients received counseling for exercise as home program only. Both groups received cardio-pulmonary exercise testing (CPET) twice, 3 to 6 months apart. The assignment was according to patient's choice.
The hospital's Research Ethic Committee approved the study protocol and all of the participants provided written informed consent. The authors certify that they comply with the ethical standards of the journal.[21]
2.2. Assessments
A CPET was performed and serum myostatin, IGF-1, and IL-6 concentrations were measured before and after the phase II CR program. The blood samples were drawn immediately before and after CPET. Demographic, hemodynamic, and body composition parameters were obtained when the patient received CPET. Measured body habitus and composition parameters included height, weight, body mass index (BMI), lean body mass, and fat percentage. Height was measured to the nearest 0.1 cm and weight to the nearest 0.1 kg on an electronic scale. Body composition parameters were measured and calculated with a bio-impedance analyzer (Bio Scan 920, Maltron, UK) using standardized protocol.
Hemodynamic parameters other than heart rate and blood pressure were detailed in the following section. The maximal grip strength of bilateral hand of each participant was measured using a digital hand grip dynamometer (Takei Digital Grip Strength Dynamometer, Takei Scientific Instruments Co., Ltd, Niigata, Japan) in a standardized arm and hand position. Three trials were given for familiarization to the instrumentation and technique. The strongest value was adopted for analysis.[22]
2.3. Cardio-pulmonary exercise testing (CPET)
The CPET was performed at least 2 hours after a meal. The test began with 2 minutes of baseline data collection with the patient sitting on the cycle ergometer (Corival, Lode B.V., Zernikepark 16, Groningen, the Netherlands), followed by 2 minutes warm-up cycling with no resistance, and then with load increased by 10 W/min in a ramp protocol. The patient was asked to cycle at 60 to 70 revolutions per min while the workload was gradually increased until volitional exhaustion or termination according to the guidelines of American College of Sports Medicine. The CPET was conducted by a physiatrist.
Blood pressure (TANGO, SunTech Medical Instruments, NC) and continuous 12-lead electrocardiogram were monitored during exercise. Computerized breath-by-breath metabolic system (Quark b2 system, Cosmed s.r.l., Rome, Italy, or Cortex MetaMax 3B system, Leipzig, Germany) was used to analyze the expired air. Exercise cardio-pulmonary parameters, including workload, minute ventilation (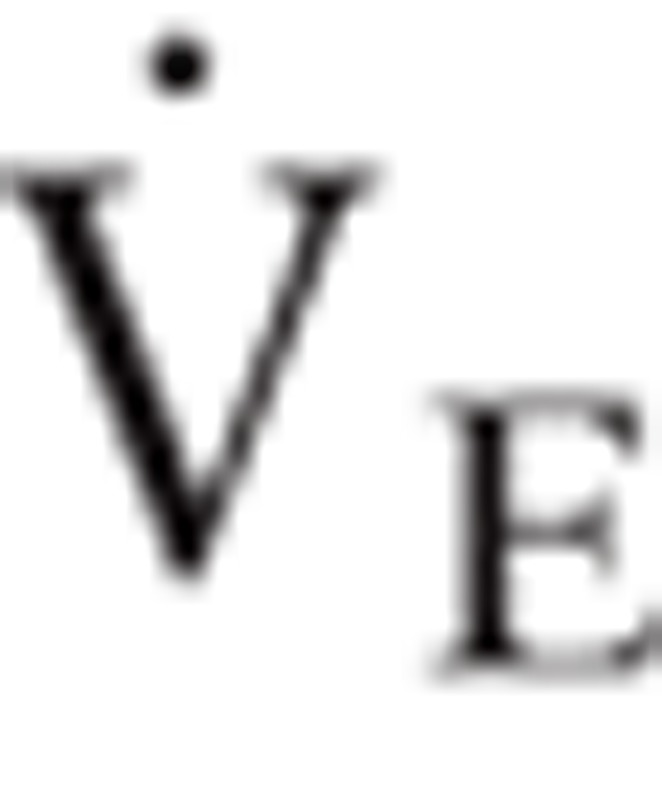 ), oxygen uptake (
), oxygen uptake ( ), carbon dioxide production (
), carbon dioxide production (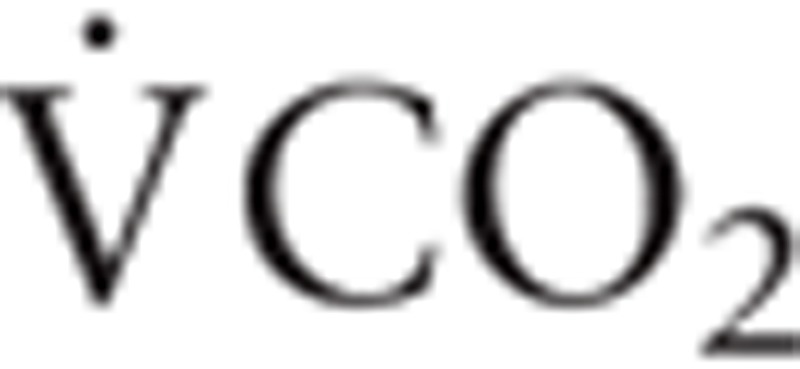 ), oxygen pulse, ventilator equivalent for oxygen (
), oxygen pulse, ventilator equivalent for oxygen (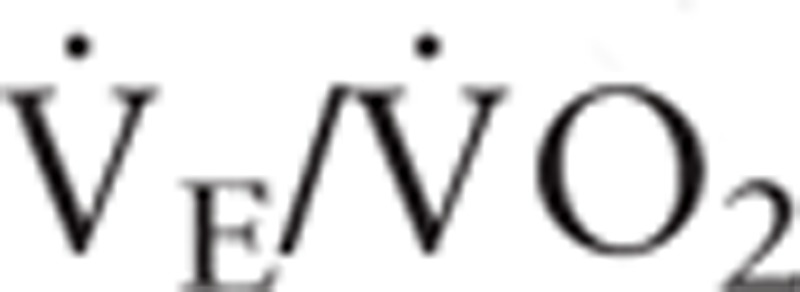 ), and ventilatory equivalent for carbon dioxide (
), and ventilatory equivalent for carbon dioxide (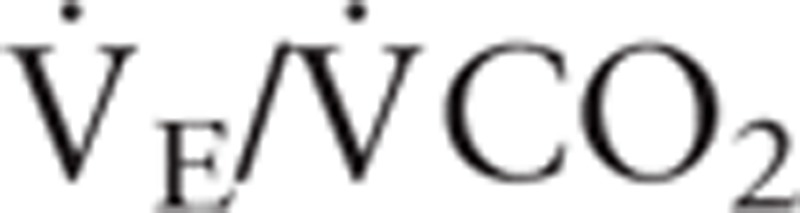 ), were processed.
), were processed.
The anaerobic threshold (AT) was determined by at least two of the following criteria: 1) the 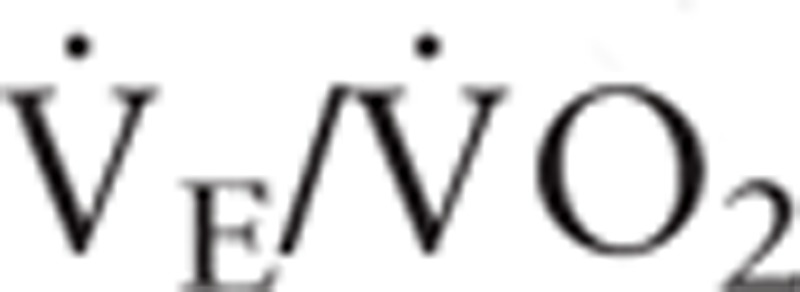 began to increase systematically without a corresponding increase in
began to increase systematically without a corresponding increase in 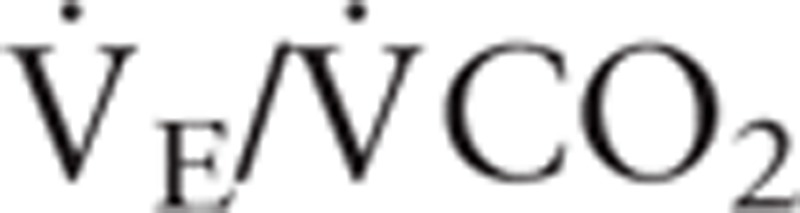 ; 2) the end tidal PO2 began to increase without a decrease in the end tidal PCO2; and 3) departure from linearity for minute ventilation. Two independent observers with experience in CPET determined the ventilatory threshold. The peak oxygen uptake (peak
; 2) the end tidal PO2 began to increase without a decrease in the end tidal PCO2; and 3) departure from linearity for minute ventilation. Two independent observers with experience in CPET determined the ventilatory threshold. The peak oxygen uptake (peak  ) indicated the highest
) indicated the highest  value recorded using CPET and was used as the main outcome after CR.
value recorded using CPET and was used as the main outcome after CR.
2.4. Cardiac rehabilitation (CR)
The phase I CR program included breathing exercises, splinted cough, range of motion exercises, chest physical therapy, bed mobility exercises, and ambulation training. After discharge, the 3-month phase II CR program was started according to the results of CPET. The exercise sessions were performed 2 to 3 times a week at the outpatient clinic. Each session consisted of both aerobic and resistive exercises as suggested by the American Heart Association on phase II CR[17] Aerobic training included a 5-minutes warm-up period, a 30-minutes bicycle ergometer and a 5-minutes cool-down period. The workload of training intensity was set at 55% to 70% of the peak  or near the level of the AT obtained from the previous CPET, while the perceived exertion rating was maintained at 12 to 13 on the Borg scale during exercise. The resistance training included 8 to 10 exercises covering the major muscle groups; chest, shoulders, arms, back, abdomen, thigh, lower legs; using a weight that can be lifted for 8 to 10 repetitions[23] The training course was supervised by a physical therapist and monitored by continuous ECG and blood pressure measurements.
or near the level of the AT obtained from the previous CPET, while the perceived exertion rating was maintained at 12 to 13 on the Borg scale during exercise. The resistance training included 8 to 10 exercises covering the major muscle groups; chest, shoulders, arms, back, abdomen, thigh, lower legs; using a weight that can be lifted for 8 to 10 repetitions[23] The training course was supervised by a physical therapist and monitored by continuous ECG and blood pressure measurements.
2.5. ELISA of myostatin, IGF-1 and IL-6
Serum myostatin levels were measured using competitive immunoassay kits according to the manufacturer's protocol (Immunodiagnostik AG, Bensheim, Germany). The full-length myostatin peptide was measured with high specificity. The test sensitivity was 270 pg/mL, while the intra- and interassay variabilities were < 10% and < 15%, respectively.[24] Briefly, the serum samples were thawed and diluted five times with the dilution buffer provided. After mixing with competitive myostatin antibody solution, the samples were incubated in coated wells for 2 hours at room temperature. After washing, secondary antibody conjugated with peroxidase was added and the mixture was incubated for 1 hour.
The substrate 3,3′,5,5′-tetramethylbenzidine for peroxidase was then added. The absorption of each well was read using VersaMAX tunable microplate reader (Molecular Devices, Sunnyvale, CA) at 450 nm against 620 nm as reference. The 4-parameter logistic regression model was employed to calculate the concentrations with OD values.[25]
Serum IGF-1 levels were measured using ELISA kits according to the manufacturer's protocol (Mediagnost, Reutlingen, Germany). Sensitivity was 0.09 ng/mL, and the inter- and intra-assay coefficient of variations were 6.8% and 6.7%, respectively.[26] The IL-6 levels were analyzed using sandwiched-type ELISA according to the manufacturer's instructions (R&D Systems). The inter- and intra-assay coefficient of variations were <10%.[27]
2.6. Statistical analysis
Differences between the experiment and control groups were analyzed using the Mann–Whitney test for ordinal data and Chi-square test for categorical data (sex). The Wilcoxon matched pairs test was employed to analyze the progress of parameters pre- and post-CR.
Data from experiment and control groups were pooled together for analysis of the association between serum myokines and aerobic capacity or muscle strength. Spearman's correlation coefficient was used to test the correlation. The variables analyzed included pre- and post-training serum myokines levels, as well as increments of levels, peak  , AT
, AT  , and grip strength. Subgroup analysis was also performed for the experiment group only.
, and grip strength. Subgroup analysis was also performed for the experiment group only.
Parameters with significant correlations were further analyzed by linear regression model adjusted for age, sex, and body composition. Possible determinants of peak  , AT
, AT  , and mean bilateral grip strength were identified. The independent variables were myostatin, IGF-1, and IL-6. Significance level was set at P < .05. All statistical tests were performed using the SPSS 11.5 (SPSS Inc. Chicago, Illinois).
, and mean bilateral grip strength were identified. The independent variables were myostatin, IGF-1, and IL-6. Significance level was set at P < .05. All statistical tests were performed using the SPSS 11.5 (SPSS Inc. Chicago, Illinois).
3. Results
3.1. Baseline data
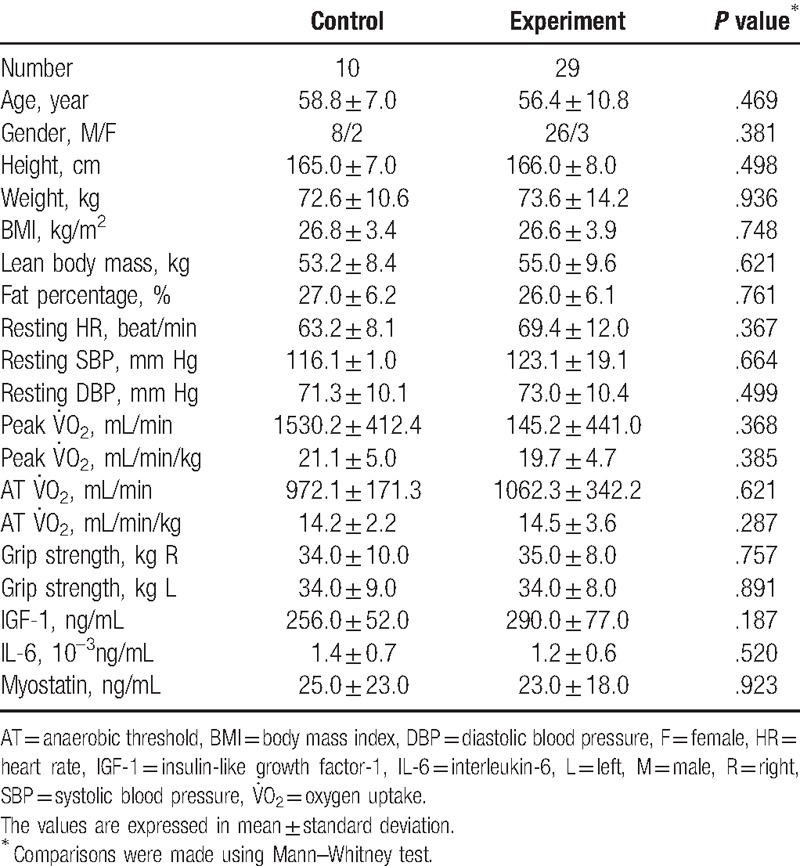
Of the 39 patients with coronary heart disease included, nine had a history of acute myocardial infarction. The time from cardiac catheterization and stenting to enrollment ranged from 45 days to 1 year. The control group (n=10) and the experiment group (n = 29) had no differences in age, sex, ratio, body composition, exercise physiology, and serum myokine concentrations at baseline (Table 1, Supplemental Data).
Table 1.
Characteristics of the experiment group receiving phase II cardiac rehabilitation and the control group at baseline.
3.2. Progress after CR
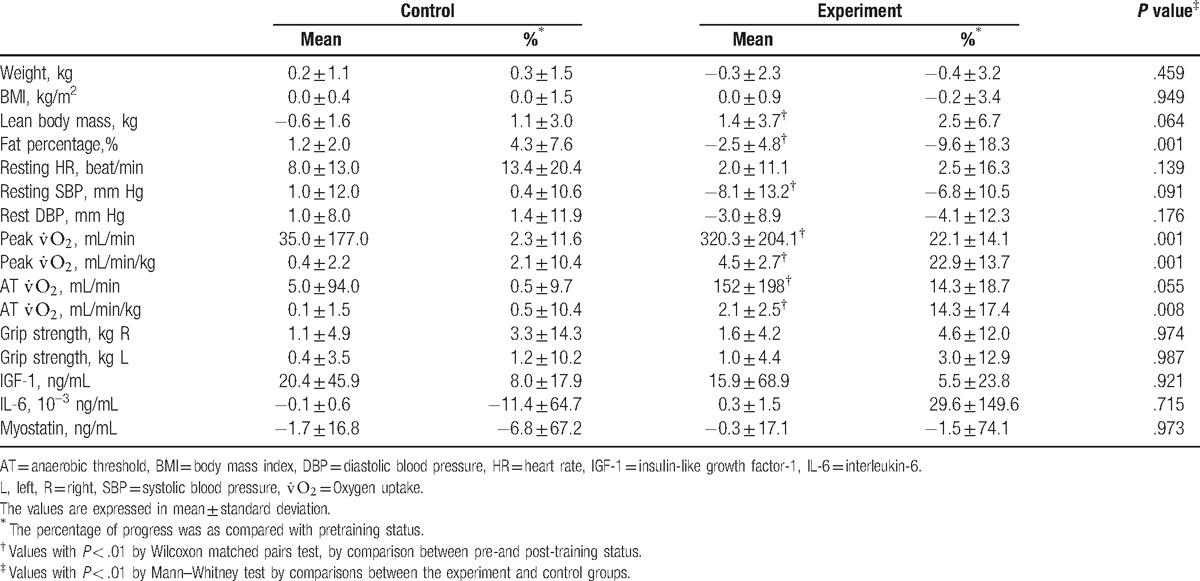
After CR, the experiment group had significantly more progress in peak  , AT
, AT  , and body fat percentage compared to the control group. However, serum myokine concentrations did not significantly change after CR in both groups (Table 2, Supplemental Data). The experiment group showed >20% improvement in peak
, and body fat percentage compared to the control group. However, serum myokine concentrations did not significantly change after CR in both groups (Table 2, Supplemental Data). The experiment group showed >20% improvement in peak  and 14% improvement in AT
and 14% improvement in AT  regardless of adjustment by weight. Decreased body fat percentage and increased lean body mass were also noted. There was a trend towards progress in grip strength but this did not reach statistical significance (P = .093 and .064 for the right and left sides, respectively).
regardless of adjustment by weight. Decreased body fat percentage and increased lean body mass were also noted. There was a trend towards progress in grip strength but this did not reach statistical significance (P = .093 and .064 for the right and left sides, respectively).
Table 2.
Progress of parameters in body habitus and composition, exercise physiology, and myokines concentration in the experiment (after hospital-based cardiac rehabilitation) and control (after home program) groups.
3.3. Correlations between serum myokines and aerobic capacity or muscle strength
Pooling the data of the experiment and control groups revealed significant positive unadjusted correlation between pretraining myostatin and pretraining peak  (P = .018) and increased peak
(P = .018) and increased peak  (P = .021); between pre- or post-training IGF-1 and increased AT
(P = .021); between pre- or post-training IGF-1 and increased AT  (P = .013 and .040, respectively). Furthermore, there was a significant positive correlation between pre- or post-training IGF-1 and grip strength (P = .001 and.032, respectively). There were no significant correlations between myokines and other parameters of exercise physiology.
(P = .013 and .040, respectively). Furthermore, there was a significant positive correlation between pre- or post-training IGF-1 and grip strength (P = .001 and.032, respectively). There were no significant correlations between myokines and other parameters of exercise physiology.
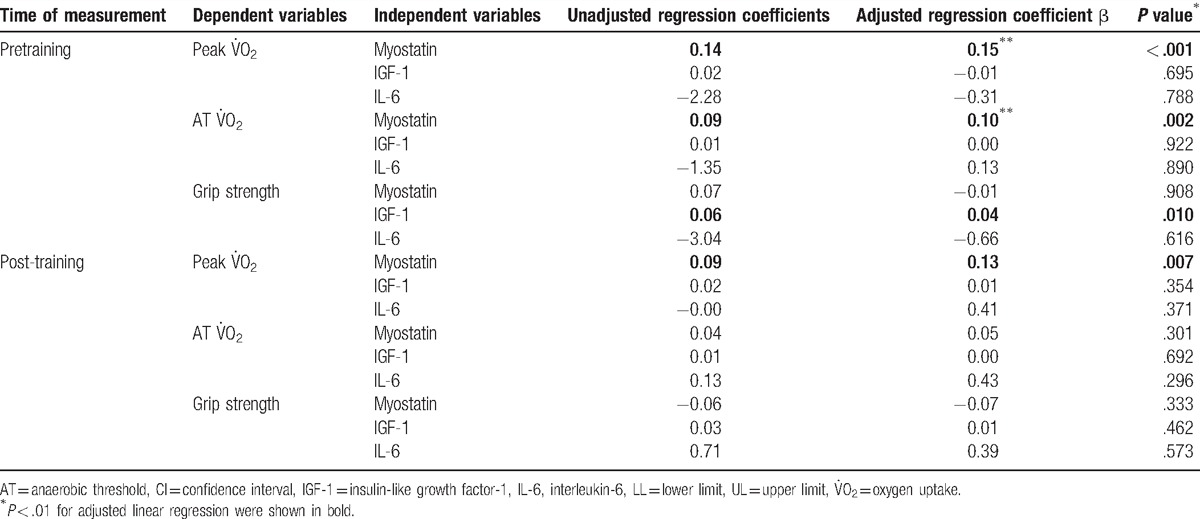
By the linear regression model adjusted for age, sex, and body composition (percentage of body fat), myostatin was a determinant of pre- and post-training peak  (Table 3). The trend for pretraining myostatin to predict increased peak
(Table 3). The trend for pretraining myostatin to predict increased peak  and AT
and AT  was less significant (P = .078, and .055, respectively). IGF-1 was the determinants of pretraining status of mean bilateral grip strength. Subgroup analysis performed for the experiment group did not contradict the findings (data not shown).
was less significant (P = .078, and .055, respectively). IGF-1 was the determinants of pretraining status of mean bilateral grip strength. Subgroup analysis performed for the experiment group did not contradict the findings (data not shown).
Table 3.
Linear regression model for predicting aerobic capacity and grip strength adjusted by age, sex, and body composition of the participants.
4. Discussion
This prospective cohort study reveals that patients receiving phase II CR have significantly improved resting blood pressure, body composition, and aerobic capacity. Serum myostatin, IL-6, and IGF-1 levels do not change significantly post-CR. In the cross-sectional observation part, serum myostatin level is positively correlated to aerobic capacity after adjusting for age, sex, and body composition. Myostatin level is positively correlated to pre- and post-training peak  , and pretraining AT
, and pretraining AT  . Serum IGF-1 level is positively correlated to pretraining grip strength.
. Serum IGF-1 level is positively correlated to pretraining grip strength.
Muscle growth and differentiation are known to be regulated by muscle-related cytokines. Myostatin, primarily expressed in skeletal muscles, has been proposed as a “chalone” of muscle tissue or as a counter-regulatory molecule along with IGF-1 for muscle hypertrophy.[3,28] Myostatin knockout mice had larger skeletal muscle size compared to the wild type.[1] Myostatin-inhibiting monoclonal antibodies in mdx (Duchenne muscular dystrophy) mice show improved dystrophic symptoms with increasing muscle mass and myofibril size. On the other hand, excessive transgenic myostatin in mice lead to significant cachexia.[2] Recently, accumulated evidence reveals that myostatin plays an important role in modulating the energy system of muscle aside from regulating muscle growth. There is a conversion of slow oxidative (type I) fiber to fast glycolytic (type IIB) fiber, as well as reduced oxidative mitochondrial capacity in myostatin deficient mice, suggesting a shift toward anaerobic metabolism in the muscle.[10,11] Mouisel et al[12] further indicate decreased exercise endurance, energy efficiency, and oxygen uptake in myostatin deficient mice. Whether the serum level of myostatin directly reflects aerobic capacity remain mostly unknown. To date, this is the first study that provides evidence that myostatin is positively correlated to aerobic capacity in cardiac patients receiving phase II CR.
CR can increase  by increasing stroke volume, lowering resting heart rate and blood pressure, and improving physical fitness such as increasing skeletal muscle mass and strength, with decreasing body fat percentage.[17,18] This study also shows that the patients receiving phase II CR have significantly improved parameters of exercise physiology and body composition. However, the corresponding serum myostatin, IL-6, and IGF-1 levels do not change.
by increasing stroke volume, lowering resting heart rate and blood pressure, and improving physical fitness such as increasing skeletal muscle mass and strength, with decreasing body fat percentage.[17,18] This study also shows that the patients receiving phase II CR have significantly improved parameters of exercise physiology and body composition. However, the corresponding serum myostatin, IL-6, and IGF-1 levels do not change.
Previous studies indicate myostatin changes in response to both muscle growth and exercise. In the “accelerator-brake” model or the “yin-yang” concept coined by Mak and Rotwein and Han et al,[3,28,29] myostatin and IGF-1 act as counter-regulatory molecules for muscle hypertrophy. The myostatin expression increases, as a brake, to limit the over-speeding growth of muscle tissue. On the other hand, two small trials have shown that the expression of myostatin decrease after 6 months of aerobic exercise in obese or insulin-resistant participants.[30,31] One possible reason is that aerobic exercise, in contrast to resistance training, may not be enough stimuli for muscle hypertrophy and growth. Hence, no myostatin “brake” is needed. Furthermore, aerobic exercise affects not only muscle metabolism but also body composition, which may further influence serum level of myostatin.[32] The participants in the present study received both aerobic and resistant training. Thus, changes in serum myostatin may not be explained by any of the previous observations alone, and further research is warranted for a conclusive result.
In the present study, IGF-1 and IL-6 also do not change significantly after CR. There is positive association between pretraining IGF-1 and grip strength, which is consistent with a previous report.[33] Antecedent trials about exercise-related changes of IL-6 and IGF-1 appear to be inconclusive. Some indicate short-lasting increases in circulating IL-6 from muscle after exercise.[34,35] IL-6 mRNA expression increases immediately after exercise in muscle biopsy samples,[36,37] but the long-term effect of exercise training on IL-6 level remain unknown. Regarding IGF-1 changes after exercise, some report a transient increase while others show no changes.[8,38] Trials are preliminary and include limited case numbers. In a randomized control trial with 319 participants, 16 weeks of aerobic exercise provide minimal or no effect on serum IGF-1.[39] The response in these myokines after CR has multiple confounding factors, with age and body composition as the most important. In the present study, there is no correlation between these factors and myokines pre- and post-training (data not shown). In addition, positive correlations between aerobic capacity and myostatin and between grip strength and IGF-1 remain after adjustment for these confounders.
This study has several limitations. The positive association between exercise physiology and myokines is from cross-section observations and does not imply any causal-relationship. This study also has a small sample size and may have inadequate test power, leading to insignificance. Furthermore, there are large interindividual variations in exercise physiology parameters and myokine levels, but no repeated measurements have been done and possible circadian fluctuations are not accounted for. Little is known regarding the time frame of the “accelerator-brake” model. The time of measurement of the myokines may not reflect steady state. Serial sampling and long-term follow-up is required for further clarification. Lastly, selection bias cannot be avoided by convenient sampling in a medical center. There should be caution when making generalizations. Further large-scale prospective studies with long-term follow-up are warranted to delineate muscle-related myokine changes in response to exercise training.
5. Conclusions
Serum myostatin level is positively correlated to aerobic capacity, and IGF-1 level is positively correlated to grip strength in cardiac patients receiving CR. Serum myostatin, IL-6, and IGF-1 do not significantly change after CR, however, further studies are warranted to delineate myokines changes in response to exercise training.
Supplementary Material
Footnotes
Abbreviations: AT = anaerobic threshold, BMI = body mass index, CHF = congested heart failure, CPET = cardio-pulmonary exercise testing, CR = cardiac rehabilitation, IGF-1 = insulin-like growth factor-1, IL-6 = interleukin-6,  = carbon dioxide production,
= carbon dioxide production,  = minute ventilation,
= minute ventilation,  = oxygen uptake.
= oxygen uptake.
Authorship: Drs. D-SH and M-YH contributed equally to this work as first authors.
Funding: This study received funding from National Science Council (NSC), grant number: NSC 100–2314-B-002-014.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].McPherron AC, Lawler AM, Lee SJ. Regulation of skeletal muscle mass in mice by a new TGF-β superfamily member. Nature 1997;387:83–90. [DOI] [PubMed] [Google Scholar]
- [2].Zimmers TA, Davies MV, Koniaris LG, et al. Induction of Cachexia in mice by systemically administered myostatin. Science 2002;296:1486–8. [DOI] [PubMed] [Google Scholar]
- [3].Gaussin V, Depre C. Myostatin, the cardiac chalone of insulin-like growth factor-1. Cardiovasc Res 2005;68:347–9. [DOI] [PubMed] [Google Scholar]
- [4].Grimberg A, Cohen P. Role of insulin-like growth factors and their binding proteins in growth control and carcinogenesis. J Cell Physiol 2000;183:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Schiaffino S, Mammucari C. Regulation of skeletal muscle growth by the IGF1-Akt/PKB pathway: insights from genetic models. Skelet Muscle 2011;1:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].O’Connor JC, McCusker RH, Strle K, et al. Regulation of IGF-I function by proinflammatory cytokines: at the interface of immunology and endocrinology. Cell Immunol 2008;252:91–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].McKay BR, De Lisio M, Johnston AP, et al. Association of interleukin-6 signalling with the muscle stem cell response following muscle-lengthening contractions in humans. PLoS One 2009;4:e6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Cappon J, Brasel JA, Mohan S, et al. Effect of brief exercise on circulating insulin-like growth factor I. J Appl Physiol 1994;76:2490–6. [DOI] [PubMed] [Google Scholar]
- [9].Drenth JP, Van Uum SH, Van Deuren M, et al. Endurance run increases circulating IL-6 and IL-1ra but downregulates ex vivo TNF-alpha and IL-1 beta production. J Appl Physiol 1995;79:1497–503. [DOI] [PubMed] [Google Scholar]
- [10].Hennebry A, Berry C, Siriett V, et al. Myostatin regulates fiber-type composition of skeletal muscle by regulating MEF2 and MyoD gene expression. Am J Physiol 2009;296:C525–534. [DOI] [PubMed] [Google Scholar]
- [11].Baligand C, Gilson H, Menard JC, et al. Functional assessment of skeletal muscle in intact mice lacking myostatin by concurrent NMR imaging and spectroscopy. Gene Ther 2010;17:328–37. [DOI] [PubMed] [Google Scholar]
- [12].Mouisel E, Relizani K, Mille-Hamard L, et al. Myostatin is a key mediator between energy metabolism and endurance capacity of skeletal muscle. Am J Physiol Regul Integr Comp Physiol 2014;307:R444–54. [DOI] [PubMed] [Google Scholar]
- [13].Toth MJ, Gottlieb SS, Fisher ML, et al. Skeletal muscle atrophy and peak oxygen consumption in heart failure. Am J Cardiol 1997;79:1267–9. [DOI] [PubMed] [Google Scholar]
- [14].Mancini DM, Walter G, Reichek N, et al. Contribution of skeletal muscle atrophy to exercise intolerance and altered muscle metabolism in heart failure. Circulation 1992;85:1364–73. [DOI] [PubMed] [Google Scholar]
- [15].Zinna EM, Yarasheski KE. Exercise treatment to counteract protein wasting of chronic diseases. Curr Opin Clin Nutr Metab Care 2003;6:87–93. [DOI] [PubMed] [Google Scholar]
- [16].Pu CT, Johnson MT, Forman DE, et al. Randomized trial of progressive resistance training to counteract the myopathy of chronic heart failure. J Appl Physiol 2001;90:2341–50. [DOI] [PubMed] [Google Scholar]
- [17].Leon AS, Franklin BA, Costa F, et al. Cardiac rehabilitation and secondary prevention of coronary heart disease: an American Heart Association scientific statement from the Council on Clinical Cardiology (Subcommittee on Exercise, Cardiac Rehabilitation, and Prevention) and the Council on Nutrition, Physical Activity, and Metabolism (Subcommittee on Physical Activity), in collaboration with the American association of Cardiovascular and Pulmonary Rehabilitation. Circulation 2005;111:369–76. [DOI] [PubMed] [Google Scholar]
- [18].Arthur HM, Gunn E, Thorpe KE, et al. Effect of aerobic vs combined aerobic-strength training on 1-year, post-cardiac rehabilitation outcomes in women after a cardiac event. J Rehabil Med 2007;39:730–5. [DOI] [PubMed] [Google Scholar]
- [19].Adams KJ, Barnard KL, Swank AM, et al. Combined high-intensity strength and aerobic training in diverse phase II cardiac rehabilitation patients. J Cardiopulm Rehabil 1999;19:209–15. [DOI] [PubMed] [Google Scholar]
- [20].Ho KK, Pinsky JL, Kannel WB, et al. The epidemiology of heart failure: the Framingham Study. J Am Coll Cardiol 1993;22(4 suppl A):6A–13A. [DOI] [PubMed] [Google Scholar]
- [21].Harriss DJ, Atkinson G. Ethical standards in sport and exercise science research: 2014 update. Int J Sports Med 2013;34:1025–8. [DOI] [PubMed] [Google Scholar]
- [22].Mathiowetz V, Kashman N, Volland G, et al. Grip and pinch strength: normative data for adults. Arch Phys Med Rehabil 1985;66:69–74. [PubMed] [Google Scholar]
- [23].Braith RW, Stewart KJ. Resistance exercise training: its role in the prevention of cardiovascular disease. Circulation 2006;113:2642–50. [DOI] [PubMed] [Google Scholar]
- [24].Wintgens KF, Dschietzig T, Stoeva S, et al. Plasma myostatin measured by a competitive ELISA using a highly specific antiserum. Clinica Chimica Acta 2012;413:1288–94. [DOI] [PubMed] [Google Scholar]
- [25].Jones G, Monika W, Kreissig SB, et al. Extension of the four-parameter logistic model for ELISA to multianalyte analysis. J Immunol Meth 1994;177:1–7. [DOI] [PubMed] [Google Scholar]
- [26].Haidet AM, Rizo L, Handy C, et al. Long-term enhancement of skeletal muscle mass and strength by single gene administration of myostatin inhibitors. Proc Natl Acad Sci USA 2008;105:4318–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].McGuire TR, Brusnahan SK, Bilek LD, et al. Inflammation associated with obesityr: relationship with blood and bone marrow endothelial cells. Obesity 2011;19:2130–6. [DOI] [PubMed] [Google Scholar]
- [28].Shyu KG, Ko WH, Yang WS, et al. Insulin-like growth factor-1 mediates stretch-induced upregulation of myostatin expression in neonatal rat cardiomyocytes. Cardiovasc Res 2005;68:405–14. [DOI] [PubMed] [Google Scholar]
- [29].Mak RH, Rotwein P. Myostatin and insulin-like growth factors in uremic sarcopenia: the yin and yang in muscle mass regulation. Kidney Int 2006;70:410–2. [DOI] [PubMed] [Google Scholar]
- [30].Ryan AS, Li G, Blumenthal JB, et al. Aerobic exercise + weight loss decreases skeletal muscle myostatin expression and improves insulin sensitivity in older adults. Obesity (Silver Spring) 2013;21:1350–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hittel DS, Axelson M, Sarna N, et al. Myostatin decreases with aerobic exercise and associates with insulin resistance. Med Sci Sports Exerc 2010;42:2023–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Han DS, Chu-Su Y, Chiang CK, et al. Serum myostatin is reduced in individuals with metabolic syndrome. PLoS One 2014;9:e108230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Taekema DG, Ling CH, Blauw GJ, et al. Circulating levels of IGF1 are associated with muscle strength in middle-aged- and oldest-old women. Eur J Endocrinol 2011;164:189–96. [DOI] [PubMed] [Google Scholar]
- [34].Papanicolaou DA, Petrides JS, Tsigos C, et al. Exercise stimulates interleukin-6 secretion: inhibition by glucocorticoids and correlation with catecholamines. Am J Physiol 1996;271(3 pt 1):E601–605. [DOI] [PubMed] [Google Scholar]
- [35].Pedersen BK. Special feature for the Olympics: effects of exercise on the immune system: exercise and cytokines. Immunol Cell Biol 2000;78:532–5. [DOI] [PubMed] [Google Scholar]
- [36].Hiscock N, Chan MH, Bisucci T, et al. Skeletal myocytes are a source of interleukin-6 mRNA expression and protein release during contraction: evidence of fiber type specificity. FASEB J 2004;18:992–4. [DOI] [PubMed] [Google Scholar]
- [37].Keller C, Steensberg A, Pilegaard H, et al. Transcriptional activation of the IL-6 gene in human contracting skeletal muscle: influence of muscle glycogen content. FASEB J 2001;15:2748–50. [DOI] [PubMed] [Google Scholar]
- [38].Nishida Y, Matsubara T, Tobina T, et al. Effect of low-intensity aerobic exercise on insulin-like growth factor-I and insulin-like growth factor-binding proteins in healthy men. Int J Endocrinol 2010;2010:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Arikawa AY, Kurzer MS, Thomas W, et al. No effect of exercise on insulin-like growth factor-I, insulin, and glucose in young women participating in a 16-week randomized controlled trial. Cancer Epidemiol Biomarkers Prev 2010;19:2987–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


