Abstract
Laparoscopic sleeve gastrectomy (LSG) is a therapeutic option in severely obese patients. The aim of this study was to evaluate the presence of Helicobacter pylori (HP) gastritis and non-Helicobacter gastritis in the gastrectomy specimens, and its association to other variables.
One hundred six sleeve gastrectomy specimens were examined histopathologically for the presence of gastritis and its relation to other factors like ethnicity, glycemic control, and postoperative complications.
Twelve patients had HP gastritis, 39 had non-HP gastritis, and 55 had normal mucosa. There was a statistical difference between the Arab and Jewish Israeli patients in our study. Twenty-eight of the Arab patients had HP gastritis and 48% had non-HP gastritis. In the Jewish population 6% had HP gastritis and 34% had non-HP gastritis. The preoperative glycemic control was worse in the gastritis group with a mean HbA1c of 8.344% while in the normal mucosa group the mean HbA1c was 6.55. After operation the glycemic control reverted to normal in most the diabetic patients. There were few postoperative complications however, they were not related to HP.
There is a high incidence of gastritis in obese patients. The incidence of gastritis in the Arab population in our study was higher than that in the Jewish population. The glycemic control before surgery was worse in patients with gastritis than in the normal mucosa group. HP bares no risk for postoperative complications after LSG and does not affect weight loss. However a larger cohort of patients must be studied to arrive at conclusive results.
Keywords: gastritis, glycemic control, Helicobacter pylori, laparoscopic sleeve gastrectomy, obesity
1. Introduction
Obesity is considered to be one of the most important chronic diseases facing our society. Bariatric surgery is a therapeutic option in severely obese patients, where lifestyle/medication has not been effective.[1] Laparoscopic sleeve gastrectomy (LSG) has become one of the successful bariatric surgeries for obesity. It is putatively a purely restrictive operation that reduces the size of the gastric reservoir to 60 to 100 mL, permitting the intake of only small amounts of food and imparting a feeling of satiety earlier during a meal. More recently, however, it has been suggested that attenuation of endogenous Ghrelin levels which is thought to be a hunger-regulating peptide, may also contribute to the success of LSG.[2] Ghrelin, hormone, is mainly produced in the fundus of the stomach.[2] By resecting the fundus in LSG, the majority of Ghrelin-producing cells are removed, thus reducing plasma Ghrelin levels and subsequently hunger.
The surgical specimens of the resected stomachs in LSG are sent routinely for pathological examination. The spectrum of the pathological changes seen in these cases is not well documented. However there are some reports of high incidence of gastritis and of Helicobacter pylori (HP) infestation in obese patients.[3,4] The rationale of the study was to find out what can be learnt from the meticulous histopathological examination of the gastric mucosa. The following items will be discuss the prevalence of HP and non-HP gastritis, its correlation with ethnicity, preoperative glycemic control, the histology of the inflammatory infiltrate and the postoperative complications rate.
2. Materials and methods
2.1. Patients
One hundred six patients underwent LSG during the 2010 to 2012. The medical records were checked for previous operations, concomitant diseases especially diabetes and its control, simultaneous operations with the LSG and postoperative complications. The patients were followed at an interval of 1 month for 1 year. The end point of this study was at 12 months after operation. Patients that did not come to this follow-up examination were contacted by phone and their general practitioner was asked for the weight and the HbA1c% result. The research was approved by the Ethical committee of Rabin Medical Center No. 7169.
2.2. Pathology examination
The sleeve gastrectomy specimens were examined in the pathology department. Four samples were taken from each specimen, 1 from each surgical margin and 2 random samples. If macroscopic alterations were noticed, an extra sample was taken from these areas. The specimens were fixed in 4% buffered formalin and embedded in paraffin. The sections were subsequently stained with hematoxylin and eosin. Immunohistochemical staining (Biotest, Florida) was used to detect the presence of HP. Accordingly, the following histopathologic variables were examined on each case: HP presence and density, degree of chronic inflammation, presence of lymphoid follicles, presence and density of plasma cells, polymorphonuclear neutrophil activity and presence and density of eosinophils. Each variable was graded as mild, moderate, or severe using Dixon et al[5] visual analog scale. HP density was graded as none, mild when few microorganism were present, moderate when bacteria were present in separate foci, and severe when near complete or complete surface layering with HP was observed.
2.3. Statistical analysis
The results were analyzed by SPSS v21.0 (IBM, New York). Differences in nominal variables were evaluated by Chi-square test. To compare between 2 groups we have utilized T test for independent variables. All tests were 2-tailed and a significant results considered when the P-value < .05.
3. Results
3.1. Clinical data
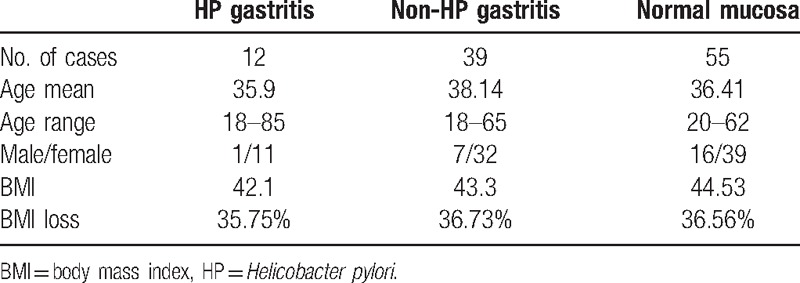
According to the histological results the cases were divided into 3 groups: HP gastritis (12 patients) when HP was found in the histological sections, non-HP gastritis (39) when inflammatory infiltrate was present without HP and normal mucosa (55) with no HP and no inflammatory infiltrate in the gastric mucosa. Their clinical data are summarized in Table 1. The weight at operation was 91 to 169 kg, mean 120.5 kg. At 12 months postoperatively the weight was 51 to 110 kg mean 80.2 kg. There were no statistical differences in the groups according to age, BMI before operation and BMI loss.
Table 1.
Clinical data.
3.2. Ethnicity
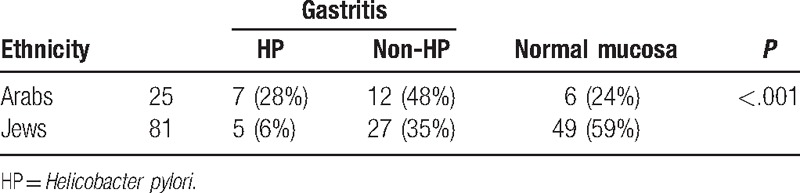
Of the 25 Arab patients 28% had HP gastritis, 48% had non-HP gastritis and only 24% had normal mucosa. In The Jewish population 6% had HP gastritis, 34% had non-HP gastritis, and 60% had normal mucosa. When taking the HP and non-HP gastritis versus the normal mucosa the Chi-square statistic is 10.1915 (P < .001) (Table 2).
Table 2.
Ethnicity.
3.3. Glycemic control
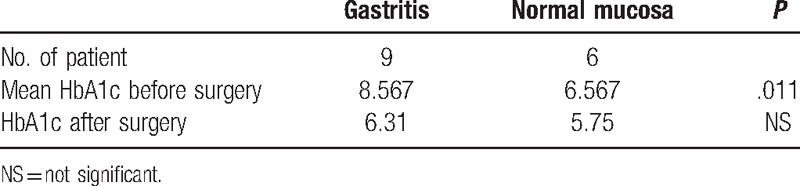
HbA1c before surgery was high in the gastritis group, mean 8.567%. Six patients had HbA1c higher than 7% and 3 reached adequate glycemic control. The patients with normal mucosa had a mean HbA1c of 6.587%. One had HbA1c of 8.3% and another of 7.1%. The other 4 had adequate glycemic control. The difference was statistically significant (P < .011). After surgery most patients reached the target of glycemic control (Table 3).
Table 3.
HbA1c in the diabetic patients before and after surgery.
3.4. The Histology of the inflammatory infiltrate
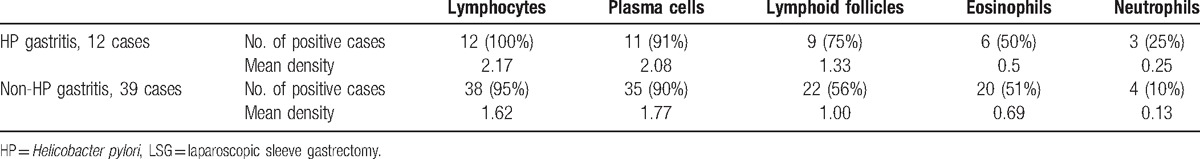
Lymphocyte were found in all the HP gastritis cases and the mean density was 2.17, in the non-HP gastritis there was 1 case without lymphocytes and the mean density was 1.61 (Table 4). Plasma cells were found in 11 cases of HP gastritis, mean density of 2.08. In the non-HP gastritis plasma cells were found in 35 cases with a mean density of 1.77. Lymphoid follicles were found in 75% of HP gastritis with a mean density of 1.33. In the non-HP gastritis lymphoid follicles were found in 22 cases (56%) with a mean density of 1.00. Eosinophils were seen in 50% of the cases, usually of low density, except for 1 case with grade 3 density in the non-HP gastritis group. Neutrophils were seen in only few cases. In general the inflammatory infiltrate was somewhat denser in the HP group than in the non-HP group.
Table 4.
Histopathological findings in the LSG specimens with inflammation.
3.5. Postoperative complications
There were no complications in the HP group. In the non-HP gastritis there were 2 patients who underwent cholecystectomy and 1 case each of intraoperative symptomatic bleeding, suture bleeding, and wound infection. In the normal mucosa group there were 4 cases of cholecystectomy and 1 case each of suture bleeding and wound infection. Cholecystectomy was performed simultaneously with the LSG in 3 patients and 7 patients had a history of previous cholecystectomy. In total 16 out of 106 patients underwent cholecystectomy
4. Discussion
4.1. HP and non-HP gastritis in obese population
Our study confirms the occurrence of gastritis in obese patients. Franklin et al[6] found 5% of HP gastritis and 40% had non-HP gastritis. Clapp[4] found a 44% rate of chronic gastritis. After the operation the rate of gastritis was reduced. Czeczko et al[10] found gastritis in 51.8% of patients who underwent Roux-EN-Y Gastro-Jejuno bypass, which decreased to 5.5% after operation. Onzi et al[9] found chronic gastritis with inflammatory activity associated with HP in 33.3% of the patients, along with foveolar hyperplasia at 58.3%. This was reduced by 16.7% and 33.3% respectively. The reduction of gastritis after operation and weight loss might indicate that obesity in itself might be one of the causes of gastritis.
4.2. The prevalence of gastritis in different ethnic groups
Our study included 25 Arab patients and 81 Jewish patients (Table 2). In the Jewish population 6% had HP gastritis and 34% had non-HP gastritis. This is in keeping with previous studies of Czeczko et al[10] who found 44.7% of gastritis in their Brazilian patients, and of Lauti et al[11] from New Zealand investigated 976 laparoscopic sleeve gastrectomies and found HP infection in 8.6%, and gastritis in 38.9%. Among the 25 Arab patients 76% had some type of gastritis and only 24% had normal mucosa. A similar rate was found by Almazeedi et al[3] who found a 74.4% rate of chronic gastritis in their Kuwait population. This is a high percent of gastritis compared to other studies. This could possibly be related to the different diet. In the tropics the diet includes high cholesterol, highly spiced foods, alcohol, nicotine, and caffeine which provoke gastric acid secretions dyspepsia and heartburn.[12] This type of diet (except for alcohol) is also be prevalent in the Arab population in Israel and in Kuwait. On the other hand Makki et al[7] found in a study of Saudi Arabian patients 8.5% of HP gastritis and only 24% of non-HP gastritis. This contradicts our hypothesis on the dietary impact. Miller et al[13] from Queensland, Brisbane, Australia found only 7.2% non-Helicobacter associated chronic gastritis, 6.8% HP associated gastritis, which is much lower than other studies. This leads to the question if there is a geographical distribution of gastritis in the obese population and what causes the difference.
4.3. Glycemic control
In our study there was 1 diabetic patient with HP gastritis, his HbA1c was 9.6% and 8 patients with non-HP gastritis with a mean HbA1c of 8.2 which is higher than the desired target of 7%. On the other hand our patients with normal mucosa had better glycemic control. The difference between the glycemic control in the gastritis group versus the normal mucosa seems statistically significant. There are some articles about the effect of HP on the glycemic control. Dai et al[14] in a meta-analysis of 11 studies with 513 patients with diabetes mellitus (DM) showed significantly lower HbA1c levels in the HP-negative than HP-positive DM participants. We showed that also non-HP gastritis had negative influence on the glycemic control; however, we could not find any studies related to this question. De Luis et al[15] found that a delay in gastric emptying was observed with eradication of the HP and disappearance of the gastritis, and patients had better glucose levels. Ukarapol et al[8] found that there was an association between Helicobacter felis induced gastritis and elevated HbA1c levels in a mouse model of type I diabetes. HbA1c levels were significantly higher in infected mice with gastritis (11.6%; n = 6) than in infected mice without gastritis (8.4%; n = 4) or uninfected mice (7.6%; n = 10). In our study the obese patients without gastritis had better control of their DM, so it is tempting to hypothesize that the gastritis interferes with the glycemic control in these obese patients, possibly by its influence on the gastric emptying.
5. Conclusions
-
1.
We found a high prevalence of gastritis in obese patient and we support the suggestion of Yamamoto et al[16] that obesity-related gastritis might become a new category of gastritis.
-
2.
We found a higher prevalence of gastritis in the Arab population than in the Jewish population in our study group, which leads to the question if there is an ethnic distribution of gastritis in the obese population and what might causes the difference. This is a question for further investigations in a larger cohort and in different settings.
-
3.
We found that patients with gastritis had poorer glycemic control than the patients with normal mucosa. Because the number of cases in our study is small it could be interesting to investigate the effect of gastritis on the glycemic control and the gastric emptying half time in a larger scale study.
-
4.
HP gastritis is unlikely to predispose patients to complications after LSG. Having that said, in view of the known complications of HP, like ulcers or lymphoma, patients should be offered eradication treatment or periodic surveillance.
Acknowledgment
We thank Prof. Avraham Avital (The Technion, Israel) for professional review of the statistical analyses.
Footnotes
Abbreviations: DM = diabetes mellitus, HbA1c = glycated hemoglobin, HP = Helicobacter pylori, LSG = laparoscopic sleeve gastrectomy.
Authors’ contributions: LRW and RK conceived the study and participated in its design, checked the histological slides, drafted and revised the manuscript. RV collected the clinical data. GB conceived the study, interpreted the data, drafted and revised the manuscript. AT operated the patients, collected clinical data, and revised the manuscript. ER conceived the study, revised the manuscript, and helped with the statistical analysis. All authors read and approved the final version of the manuscript.
The authors have no conflicts of interest to disclose.
References
- [1].DeMaria EJ. Bariatric surgery for morbid obesity. N Engl J Med 2007;356:2176–83. [DOI] [PubMed] [Google Scholar]
- [2].Karamanakos SN, Vagenas K, Kalfarentzos F, et al. Weight loss, appetite suppression, and changes in fasting and postprandial ghrelin and peptide-YY levels after Roux-en-Y gastric bypass and sleeve gastrectomy: a prospective, double blind study. Ann Surg 2008;247:401–7. [DOI] [PubMed] [Google Scholar]
- [3].Almazeedi S, Al-Sabah S, Al-Mulla A, et al. Gastric histopathologies in patients undergoing laparoscopic sleeve gastrectomies. Arq Bras Cir Dig 2016;29:33–7. [DOI] [PubMed] [Google Scholar]
- [4].Clapp B. Histopathologic findings in the resected specimen of a sleeve gastrectomy. JSLS 2015;19:e2013.00259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Dixon MF, Genta RM, Yardley JH, et al. Classification and grading of gastritis: the updated Sydney System. International workshop on the histopathology of gastritis, Houston 1994. Am J Surg Pathol 1996;20:1161–81. [DOI] [PubMed] [Google Scholar]
- [6].Franklin AL, Koeck ES, Hamrick MC, et al. Prevalence of chronic gastritis or Helicobacter pylori infection in adolescent sleeve gastrectomy patients does not correlate with symptoms or surgical outcomes. Surg Infect 2015;16:401–4. [DOI] [PubMed] [Google Scholar]
- [7].Makki AM, Aldaqal SM, Alorabi SH, et al. Chronic gastritis in morbidly obese patients with sleeve gastrectomy. Electron Physician 2016;8:1786–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ukarapol N, Bégué RE, Hempe J, et al. Association between Helicobacter felis-induced gastritis and elevated glycated hemoglobin levels in a mouse model of type 1 diabetes. J Infect Dis 2002;185:1363–7. [DOI] [PubMed] [Google Scholar]
- [9].Onzi TR, d’Acampora AJ, de Araújo FM, et al. Gastric histopathology in laparoscopic sleeve gastrectomy: pre- and post-operative comparison. Obese Surg 2013;314–9. [DOI] [PubMed] [Google Scholar]
- [10].Czeczko LE, Cruz MA, Klostermann FC, et al. Correlation between pre and postoperative upper digestive endoscopy in patients who underwent Roux-en-y gastrojejunal bypass. Arg Bras Cir Dig 2016;29:33–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lauti M, Gormack SE, Thomas JM, et al. What does the excised stomach from sleeve gastrectomy tell us? Obes Surg 2016;26:839–42. [DOI] [PubMed] [Google Scholar]
- [12].Nneli RO, Nwafia WC, Orji JO. Diets/dietary habits and certain gastrointestinal disorders in the tropics: a review. Niger J Physiol Sci 2007;22:1–3. [DOI] [PubMed] [Google Scholar]
- [13].Miller GC, Reid AS, Brown IS. The pathological findings seen in laparoscopic sleeve gastrectomies for weight loss. Pathology 2016;48:228–32. [DOI] [PubMed] [Google Scholar]
- [14].Dai Y, Yu W, Zhu Ht, et al. Is Helicobacter pylori infection associated with glycemic control in diabetics? World J Gastroenterol 2015;21:5407–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].De Luis DA, Cordero JM, Caballero C, et al. Effect of the treatment of Helicobacter pylori infection on gastric emptying and its influence on the glycemic control in type 1 diabetes mellitus. Diabetes Res Clin Pract 2001;52:1–9. [DOI] [PubMed] [Google Scholar]
- [16].Yamamoto S, Watabe K, Takehara T. Is obesity a new risk factor for gastritis? Digestion 2012;85:108–10. [DOI] [PubMed] [Google Scholar]


