Abstract
The sacroiliac joints (SIJs) are one of the most common sites involved in axial spondyloarthritis (axSpA), and there are few studies on the histopathology of the SIJ in this group of patients.
Mononuclear cell infiltrates in the bone marrow and fibrous tissue resembling a pannus formation were the pathological features of early sacroiliitis in our previous study. We undertook a further immunohistological evaluation of these features in patients with axSpA.
Biopsy specimens from the SIJ of 6 patients with established ankylosing spondylitis (AS) and 13 patients with nonradiographic axial spondyloarthritis (nr-axSpA) were analyzed. An immunohistological method was performed to examine the macrophages (CD163), T cells (CD3), and B cells (CD20).
Mononuclear cell infiltrates in the bone marrow were observed in only 6 patients with nr-axSpA. Fibrous tissue was observed in all patients with established AS and 9 patients with nr-axSpA. Macrophage, T cell, and B cell infiltrates could be detected in both the bone marrow and fibrous tissue. All bone marrow specimens from 6 nr-axSpA patients exhibited CD163+ macrophage infiltrates; of these, 5 exhibited CD20+ B cell infiltrates and 3 exhibited CD3+ T cell infiltrates. Among the fibrous tissue specimens, all exhibited macrophage infiltrates, 9 exhibited B cell infiltrates, and 4 exhibited T cell infiltrates.
In addition to macrophages and T cells, B cells are also involved in active sacroiliitis in patients with axSpA.
Keywords: ankylosing spondylitis, pathology, sacroiliac joints, spondyloarthritis
1. Introduction
The sacroiliac joints (SIJs) are one of the most common sites involved in ankylosing spondylitis (AS), and sacroiliitis is crucial for the diagnosis of AS.[1] The classification criteria for axial spondyloarthritis (axSpA) were developed due to the diagnostic limitations in early disease according to the modified New York criteria.[2,3] axSpA includes the established AS that met the modified New York criteria, and nonradiographic axSpA (nr-axSpA), without definite radiographic sacroiliitis. Early active sacroiliitis can be detected both by magnetic resonance imaging (MRI) and by needle biopsy of SIJ.[4,5]
The location of the SIJ is poorly accessible; therefore, there are only a few studies on the histopathology of SIJ in this group of patients, showing inconsistent findings.[6–9] Shichikawa [8] observed that subchondral granulation tissue but not inflammatory cells is the feature of sacroiliitis in open biopsies of 5 patients with AS. However, inflammatory cell infiltrates were demonstrated in the SIJ of 5 patients with active AS in another study using needle biopsy.[6] Moreover, there is a paucity of data on the immunohistology of SIJ in patients with AS, particularly of early cases.[5,10] T cells and macrophages are the most frequent cells in active sacroiliitis.[5,6,10]
These key features of early sacroiliitis, the presence of mononuclear cell infiltrates in the bone marrow, and the invasion of the subchondral bone plate and cartilage by fibrous tissue, resembling a pannus, also have been described in our previous paper.[9] In the present study, we applied immunohistochemical methods to further characterize these findings.
2. Patients and methods
2.1. Patients
Nineteen patients who met the Assessment of SpondyloArthritis international Society classification criteria for axSpA were recruited between 2003 and 2013 by our department.[2] Only patients with SIJ biopsy sections of >3 mm2 were recruited. Six patients with established AS, which met the revised New York criteria,[3] and 13 nr-axSpA patients with the absence of definite radiographic sacroiliitis (3 of them met the clinical arm of axSpA). Clinical, laboratory, and imaging data of the SIJ were collected. This study was approved by the ethics committee of the first Affiliated Hospital of Shantou University Medical College. All patients signed an informed consent.
2.2. Materials
A computed tomography (CT)-guided needle biopsy of the SIJ was performed as previously described.[11] At the same time of sacroiliac biopsy, an intra-articular corticosteroid injection was performed. SIJ needle biopsy specimens were obtained from both sides from the patients, but only the specimens from one side met the inclusion criteria. Sections of biopsy tissue with a thickness of 4 to 5 μm were cut for a routine hematoxylin and eosin (H&E) staining for the diagnosis of early sacroiliitis.
2.3. Grading of radiographic sacroiliitis
Radiographic sacroiliitis was graded according to the revised New York criteria for AS.[3]
2.4. Scoring of bone marrow edema (BME) on magnetic resonance imaging (MRI)
BME on MRI was defined according to the Assessment of SpondyloArthritis International Society (ASAS)/Outcome Measures in Rheumatology (OMERACT) MRI Group.[4] BME scoring was based on the Spondyloarthritis Research Consortium of Canada (SPARCC) criteria.[12]
2.5. Immunohistochemical procedures
Immunohistochemistry studies were performed to detect CD3+ T cells, CD20+ B cells, and CD163+ macrophages using a 2-step polymer horseradish peroxidase (HRP) detection system. All primary antibodies (monoclonal antibodies) against T cells, B cells, and macrophages, secondary antibodies, and the Polink-2 Plus detection system Kit were purchased from Zhongshan Biotechnology Company Limited (Beijing, China).
Each specimen was cut into 4 μm thick sections, mounted on chrome alum-gelatin-coated slides, and then baked for an hour at 60°C before staining. The slides were deparaffinized in xylene, rehydrated and subjected to heat-induced antigen retrieval in a high-pressure cooker for 3 minutes (CD20 and CD163) or 5 minutes (CD3). For antigen retrieval, the slides were immersed in a Tris-EDTA buffer solution at pH 9.0. After heating, the tissues were cooled to room temperature, incubated with the peroxide block for 10 minutes to block endogenous peroxidase activity, washed in phosphate-buffered saline (pH 7.4), and incubated with the primary antibodies overnight at 4°C. The primary antibodies included monoclonal antibodies against CD3 (clone EP41, dilution 1 : 50), CD20 (EP7, dilution 1 : 50), and CD163 (10D6, dilution 1 : 300). A 2-step polymer HRP detection system was used for detection. Fresh 3, 3′-diaminobenzidine (DAB) (prepared in a ratio of 1 : 20) was used as the chromogen.
The number of lymphocyte infiltrates per high-power field [HPF (400×)] was counted. Lymphocyte aggregates were defined as ≥50 T cells or B cells per HPF.
2.6. Statistical analysis
All data were analyzed using SPSS software (version 20.0 for Windows; IBM Corp., Armonk, NY) The Mann–Whitney U test was used to compare the differences between groups. P values less than .05 were considered statistically significant.
3. Results
3.1. Patient characteristics
The demographic and clinical characteristics of 19 axSpA patients are summarized in Table 1. Approximately 68.4% of the patients were HLA-B27-positive. The mean age was 22.5 ± 8.7 years (range: 12.0–49.0 years), and the mean age of onset was 18.2 ± 6.7 years (range: 8.0–33.0 years). The mean disease duration was 4.3 ± 5.8 years (median 2.0 years; range: 0.3–25.0 years). Uveitis, psoriasis, and inflammatory bowel disease were not found in this group of patients, and the other SpA features are summarized in Table 1. The laboratory evaluations showed that the mean erythrocyte sedimentation rate (ESR) was 42.2 ± 28.5 mm/h and the mean C-reactive protein (CRP) was 22.5 ± 23.5 mg/L. According to the radiographic sacroiliitis grading, 5 patients had radiographic sacroiliitis Grade 0, 7 had Grade 1, 4 had Grade 2, and 3 had Grade 3. MRI of the SIJ was performed on 14 of the 19 patients, all of whom had BME on MRI. The mean score was 15.8 ± 10.6 (median 14.0, bilateral). None of the patients received antitumor necrosis factor (anti-TNF) α therapies.
Table 1.
Demographic and clinical characteristics of 19 patients with axSpA.
3.2. Histopathological features of SIJ
Mononuclear cell infiltrates in the bone marrow were observed in only 6 of the patients with nr-axSpA, but not in the patients with AS. Fibrous tissue was observed in both nr-axSpA patients (n = 9) and AS patients (n = 6). (Histopathological features of SIJ are shown in Fig. 1). In 4 patients with nr-axSpA, only mononuclear cell infiltrates were observed in the bone marrow sections. The mean BME score on MRI of these patients was 3.0 (median). In 13 patients with AS (n = 6) and nr-axSpA (n = 7), only fibrous tissue that invaded the subchondral bone plate and cartilage was observed in the sections. The mean BME score on MRI was 14.0, which was significantly higher than the group with inflammatory cell infiltrates (P = .002). Both pathological features of SIJ were observed in 2 patients with nr-axSpA. The mean BME score on MRI was 4.5 (Table 2).
Figure 1.
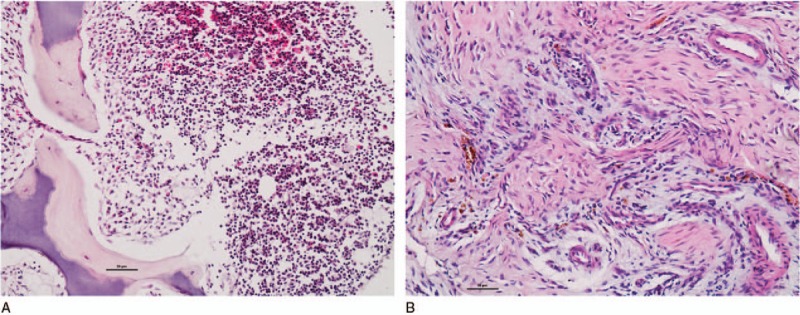
(A) Mononuclear cell infiltrates, SIJ biopsy sections of nr-axSpA patients, a 15-year-old man, with HLA-B27+, with a disease duration of 5 months and severe unilateral hip pain. (B) Fibrous tissue resembling a pannus formation. SIJ biopsy sections of a nr-axSpA patient, a 24-year-old woman, who was HLA-B27+, with a disease duration of 8 months and alternating buttock pain. Bars = 50 μm.
Table 2.
Distribution of the histopathological features, BME scores on the MRI, and grade of radiographic sacroiliitis of 19 axSpA patients.
3.3. Immunohistological features
We applied an immunohistochemical method to further characterize these pathological features of the SIJ. Lymphocyte aggregates were defined as clusters of ≥50 T cells or B cells per HPF (400×).
3.3.1. Mononuclear cell infiltrates in bone marrow
In all 6 specimens from the nr-axSpA patients, CD163+ macrophages were observed as a dense cellular infiltrate in the bone marrow. Both CD3+ T cell and CD20+ B cell aggregates were observed in 2 specimens. Interstitial CD20+ B cell infiltrates were observed in 3 of the 6 specimens, and interstitial CD3+ T cell infiltrates were seen in 1 of the 6 specimens. The numbers of B cell and T cell infiltrates were 30.3 ± 6.3 per HPF (400×) and 40.0 per HPF (400×), respectively (Representative immunohistochemical staining of bone marrow is showed in Fig. 2).
Figure 2.
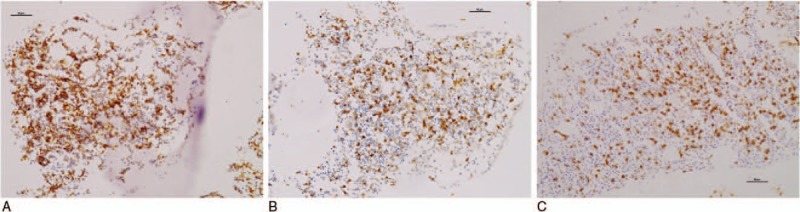
The same patients as shown in Fig. 1A (A–C). Immunohistochemical staining of bone marrow. (A) Dense CD163+ macrophage infiltrates. (B) CD3+ T cell aggregates. (C) CD20+ B cell aggregates. Bars = 50 μm.
3.3.2. Fibrous tissue
CD163+ macrophages were also observed as a dense cellular infiltrate in the fibrous tissue in all 15 specimens. Due to the limited biopsy material, only 13 of the 15 specimens that contained fibrous tissue could be stained for CD3 and CD20. CD20+ B cells were detected in 9 of the 13 specimens; these B cells were detected as lymphocyte aggregates in three specimens, and the number of the cells in the other 6 specimens was 30.3 ± 6.3 per HPF (400×). CD3+ T cells were detected in 4 specimens; these cells were detected as lymphocyte aggregates in 2 specimens, and the number of cells in the other specimens was 25.0 per HPF (400×) (Table 3, and representative immunohistochemical staining of fibrous tissue is showed in Fig. 3).
Table 3.
Cellular infiltrates of sacroiliitis.
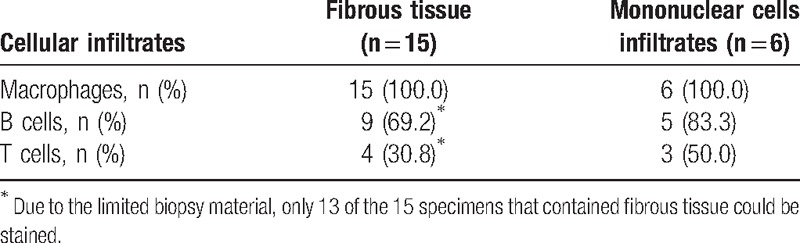
Figure 3.
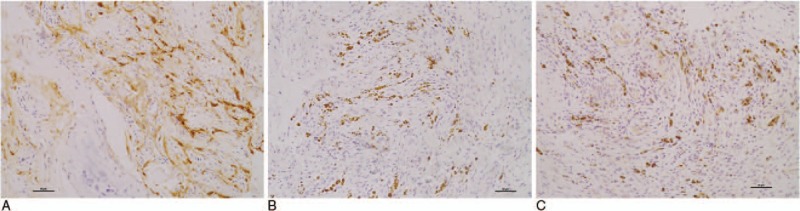
The same patients as shown in Fig. 1B (A ∼ C). Immunohistochemical staining of fibrous tissue. (A) Dense CD163+ macrophage infiltrates. (B) CD3+ T cell infiltrates. (C) CD20+ B cell infiltrates. Bars = 50 μm.
4. Discussion
This study shows that in addition to macrophages and T cells, B cells are also involved in SIJ inflammation in patients with AS or nr-axSpA. A large amount of CD20+ B cell infiltrates were detected in the bone marrow, and CD20+ B cell infiltrates were also observed in fibrous tissue resembling a pannus.
There are limited reports of immunohistological staining for B cells in axSpA patients. Appel et al[13] first identified a significantly increased number of CD20+ B cells in advanced AS patients with persistent inflammation in the zygapophyseal joints. This result supported our finding that CD20+ B cell is an important cell subset in active sacroiliitis in AS or axSpA. In another previous study,[5] researchers performed immunohistological staining for B cells in SIJ biopsy specimens from 18 AS and 12 undifferentiated spondyloarthritis (SpA) patients; however, it seems that the number of B cells was not impressive. The difference in this finding from our study may be due to the different detection systems used in the 2 reports. In our study, the Polink-2 Plus detection system was used that was more sensitive than the alkaline phosphatase-anti-alkaline phosphatase technique (APAAP) used in the previous study. Another reason for the difference might be the difficulty in accessing the SIJ to obtain representative biopsy material. In fact, some of the specimens in our study also showed negative staining for CD3 or CD20 in the fibrous tissue.
The role of B cells in axSpA patients remains unclear. Although rituximab was effective in patients with SpA who were TNF blocker-naive, there was no clear response for whom TNF blockers had failed.[14,15] Unlike rheumatoid arthritis or other connective tissue diseases, no specific autoantibodies were detected in patients with axSpA. B cells could be antigen-presenting cells, as well as precursors of antibody-secreting plasma cells, which can enhance the activation of T cells.[16] This possibility will require more exploration in the future to determine the role of B cells in the pathogenesis of AS.
In a study on the synovium in active SpA patients (half of which were AS), 12 weeks of treatment with anti-TNFα therapies (infliximab) induced a reduction in inflammatory cell infiltration by macrophages and T cells, but not B cells.[17] In a study on peripheral blood B cell subsets in patients with AS, the number of CD19+ B cells in the active AS patients was increased and positively correlated with the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI). After 12 weeks of treatment with anti-TNFα therapies (etanercept), the high percentage of CD19+ B cells in the active AS patients could not be downregulated.[18] It seems that anti-TNFα therapies are ineffective in reversing the inflammation induced by B cells. The development of anti-TNF-α (anti-TNFα) therapies is a milestone in the treatment of AS. It is unusual that the 3 major anti-TNFα inhibitors (etanercept, infliximab, and adalimumab) did not show an effect on radiographic progression in patients with AS after 2 years of treatment.[19–21] The resistance of B cells to anti-TNFα therapies might explain why radiographic progression continued in AS after 2 years of treatment. Future studies need to explore whether a combination of rituximab and TNF blockers will achieve further improvement for AS or axSpA.
In patients with AS, only fibrous tissue was observed in the specimens. Inflammatory cell infiltrates were only observed in patients with nr-axSpA. This finding is consistent with a previous report.[8] Shichikawa[8] described the pathology of the SIJ in open biopsies of 5 patients with AS. All the cases showed subchondral granulation tissue, but did not show inflammatory cells. In this study, we found that fibrous tissue contained not only abundant blood vessels but also a large amount of infiltrated inflammatory cells. Interestingly, the mean BME score on MRI was significantly higher in patients with fibrous tissue than in patients who only showed inflammatory cell infiltrates. It seems that fibrous tissue might be correlated with the BME on MRI.
There are some limitations to this study. First, the sample size of this study is small, because most of the remaining needle biopsy material is not sufficient for the immunohistological analysis. The location of the SIJ is poorly accessible; thus, it is very difficult to obtain a large piece of tissue. Second, the nr-axSpA patients in this study were lost to follow-up. Only 2 of them had progressed to AS after 5 years.
In conclusion, the present study confirms that in addition to macrophages and T cells, B cells are also involved in active sacroiliitis in patients with axSpA. Fibrous tissue with abundant inflammatory cells infiltration might be correlated with the BME on MRI.
Acknowledgment
We thank Professor Qingyu Zeng (Director of the Department of Rheumatology, Shantou University Medical College) for providing guidance throughout the study.
Footnotes
Abbreviations: axSpA = axial spondyloarthritis, nr-axSpA = nonradiographic axial spondyloarthritis, SIJ = sacroiliac joints.
JP and YG contributed equally to this work.
Authorship: All the authors were involved in drafting the article or critically revising it for important intellectual content, and all the authors approved the publication of the final version. Dr Xiao had full access to all data in the study and took responsibility for the integrity of these data and the accuracy of the data analysis.
Study conception and design: Peng, Gong, and Xiao. Data acquisition: Peng and Gong. Data analysis and interpretation: Peng, Gong, Zhang, Wang, and Xiao.
Funding/support: This work was sponsored by Shantou University Medical College Clinical Research Enhancement Initiative.
The authors report no conflicts of interest.
References
- [1].Braun J, Sieper J. Ankylosing spondylitis. Lancet 2007;369:1379–90. [DOI] [PubMed] [Google Scholar]
- [2].Rudwaleit M, Landewé R, van der Heijde D, et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part I): classification of paper patients by expert opinion including uncertainty appraisal. Ann Rheum Dis 2009;68:770–6. [DOI] [PubMed] [Google Scholar]
- [3].van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnosis criteria for ankylosing spondylitis: a proposal for modification of the New York criteria. Arthritis Rheum 1984;27:361–8. [DOI] [PubMed] [Google Scholar]
- [4].Rudwaleit M, Jurik AG, Hermann KG, et al. Defining active sacroiliitis on magnetic resonance imaging (MRI) for classification of axial spondyloarthritis: a consensual approach by the ASAS/OMERACT MRI group. Ann Rheum Dis 2009;68:1520–7. [DOI] [PubMed] [Google Scholar]
- [5].Bollow M, Fischer T, Reisshauer H, et al. Quantitative analyses of sacroiliac biopsies in spondyloarthropathies: T cells and macrophages predominate in early and active sacroiliitis cellularity correlates with the degree of enhancement detected by magnetic resonance imaging. Ann Rheum Dis 2000;59:135–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Braun J, Bollow M, Neure L, et al. Use of immunohistologic and in situ hybridization techniques in the examination of sacroiliac joint biopsy specimens from patients with ankylosing spondylitis. Arthritis Rheum 1995;38:499–505. [DOI] [PubMed] [Google Scholar]
- [7].François RJ, Gardner DL, Degrave EJ, et al. Histopathologic evidence that sacroiliitis in ankylosing spondylitis is not merely enthesitis. Arthritis Rheum 2000;43:2011–24. [DOI] [PubMed] [Google Scholar]
- [8].Shichikawa K. Histopathology of early sacroiliitis and enthesitis in ankylosing spondylitis. In: Advances in Inflammation Research, vol 9 New York: Raven Press: 1985. pp. 15–24. [Google Scholar]
- [9].Gong Y, Zheng N, Chen SB, et al. Ten years’ experience with needle biopsy in the early diagnosis of sacroiliitis. Arthritis Rheum 2012;64:1399–406. [DOI] [PubMed] [Google Scholar]
- [10].François RJ, Neure L, Sieper J, et al. Immunohistological examination of open sacroiliac biopsies of patients with ankylosing spondylitis: detection of tumour necrosis factor alpha in two patients with early disease and transforming growth factor beta in three more advanced cases. Ann Rheum Dis 2006;65:713–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Liu Y, Wang QW, Xiao ZW, et al. Effect of CT-guided biopsy and intra-articular injection technique in sacroiliac joint on early diagnosis and treatment of ankylosing spondylitis. Chin J Clin Rehabil 2004;8:5172–4. [Google Scholar]
- [12].Maksymowych WP, Inman RD, Salonen D, et al. Spondyloarthritis Research Consortium of Canada Magnetic Resonance Imaging Index for assessment of sacroiliac joint inflammation in ankylosing spondylitis. Arthritis Rheum 2005;53:703–9. [DOI] [PubMed] [Google Scholar]
- [13].Appel H, Kuhne M, Spiekermann S, et al. Immunohistologic analysis of zygapophyseal joints in patients with ankylosing spondylitis. Arthritis Rheum 2006;54:2845–51. [DOI] [PubMed] [Google Scholar]
- [14].Song IH, Heldmann F, Rudwaleit M, et al. Different response to rituximab in tumor necrosis factor blocker-naive patients with active ankylosing spondylitis and in patients in whom tumor necrosis factor blockers have failed: a twenty-four-week clinical trial. Arthritis Rheum 2010;62:1290–7. [DOI] [PubMed] [Google Scholar]
- [15].Wendling D, Dougados M, Berenbaum F, et al. Rituximab treatment for spondyloarthritis. A nationwide series: data from the AIR registry of the French Society of Rheumatology. J Rheumatol 2012;39:2327–31. [DOI] [PubMed] [Google Scholar]
- [16].Kouskoff V, Korganow AS, Duchatelle V, et al. Organ-specific disease provoked by systemic autoimmunity. Cell 1996;87:811–22. [DOI] [PubMed] [Google Scholar]
- [17].Kruithof E, Baeten D, Van den Bosch F, et al. Histological evidence that infliximab treatment leads to downregulation of inflammation and tissue remodelling of the synovial membrane in spondyloarthropathy. Ann Rheum Dis 2005;64:529–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lin Q, Gu JR, Li TW, et al. Value of the peripheral blood B-cells subsets in patients with ankylosing spondylitis. Chin Med J (Engl) 2009;122:1784–9. [PubMed] [Google Scholar]
- [19].van der Heijde D, Landewé R, Baraliakos X, et al. Radiographic findings following two years of infliximab therapy in patients with ankylosing spondylitis. Arthritis Rheum 2008;58:3063–70. [DOI] [PubMed] [Google Scholar]
- [20].van der Heijde D, Landewé R, Einstein S, et al. Radiographic progression of ankylosing spondylitis after up to two years of treatment with etanercept. Arthritis Rheum 2008;58:1324–31. [DOI] [PubMed] [Google Scholar]
- [21].van der Heijde D, Salonen D, Weissman BN, et al. Assessment of radiographic progression in the spines of patients with ankylosing spondylitis treated with adalimumab for up to 2 years. Arthritis Res Ther 2009;11:R127. [DOI] [PMC free article] [PubMed] [Google Scholar]


