Abstract
Rationale:
Primary spinal glioblastoma multiforme (GBM) is a rare clinical entity with an aggressive course and an invariably dismal prognosis. Its clinical characteristics, radiologic and pathologic findings, and treatment protocols have been discussed in a few cases.
Patient concerns:
A 15-year-old female was admitted to the neurology department with a chief complaint of progressive numbness and weakness in her left upper extremity for 3 months and neck pain for 1 month.
Diagnoses:
Spinal magnetic resonance imaging showed an intramedullary expansile mass localized between C4 and C7. The diagnosis of GBM was determined on the basis of the histopathological findings after operation.
Interventions:
Laminotomy and laminoplasty between C4 and C7 were performed, and the tumor was partially resected. The patient was administered focal adjuvant radiotherapy concomitantly with oral chemotherapy following the surgery.
Outcomes:
With severe neurologic deficits at 13 months after the diagnosis, the patient expired.
Lessons:
Although therapeutic options have been improving, the prognosis of the primary spinal GBM remains poor. The treatment of primary spinal GBM entered into a central registry and multiple-center cooperation is important in establishing future therapeutic strategies.
Keywords: chemotherapy, glioblastoma multiforme, radiotherapy, spinal cord, surgery
1. Introduction
Spinal cord tumors represent 6% to 8% of all central nervous system tumors combined and are relatively rare compared to intracranial neoplasms.[1] Primary spinal glioblastoma multiforme (GBM), World Health Organization (WHO) classification grade IV, is a highly malignant tumor with median survival rates around only 14 months from diagnosis with comprehensive treatment.[2,3] This malignant tumor type accounts for approximately 7.5% of all intramedullary gliomas and only 1.5% of all spinal cord tumors.[4–6] Primary spinal GBM has a predilection to develop at the cervical and thoracic regions; however, conus medullaris tumors do infrequently occur.[7,8]
Up until now, perhaps because of the low incidence of primary spinal cord GBM, fewer than 200 cases have been reported in the medical literature. Reports are necessarily limited to single-case studies and small-number case series. It is, therefore, not surprising that little is known about the clinical characteristics and treatment protocols of this devastating malignancy. The aim of this paper is to present a case of primary spinal GBM as well as to summarize and, via review of the relevant literature, update information on the clinical characteristics, radiologic features, and treatment protocols for this rare disease.
2. Case report
A 15-year-old girl presented in clinic, reporting a 3-month history of progressive numbness and weakness in her left upper extremity as well as neck pain for 1 month. On neurological examination, cranial nerves were intact. Her motor examination showed 5/5 strength throughout with the exception of the left upper extremity, which was 3/5 at the flexors and extensors. Sensory examination was significant for decreased sensation in the upper extremities along the C5, C6, and C7 dermatomes. There were no neurologic abnormalities of the lower extremities. The patient's medical history did not reveal any notable events.
The Ethics Committee of the Union Hospital of Fujian Medical University approved this study. The analysis was performed in accordance with the ethical standards of the hospital and the tenets of the Declaration of Helsinki. The patient reported in this study has provided written consent by the patient's legal guardian.
Spinal magnetic resonance imaging (MRI) revealed a large intramedullary tumor extending from C4 down to C7 with inhomogeneous enhancement of the tumor area (Fig. 1). Two days after the presumptive diagnosis of primary spinal GBM was made, the patient underwent laminotomy and laminoplasty between C4 and C7. Upon opening the dura, the spinal cord evidenced notable expansion. The visible tumor mass was brownish-red and richly vascular. It was very difficult to distinguish the tumor margin from the spinal cord. Frozen section confirmed GBM. Therefore, microsurgical subtotal excision was performed with intraoperative electrophysiological monitoring. Motor-evoked potentials were stable and showed no change throughout the excision of the tumor mass and at termination of the operation.
Figure 1.
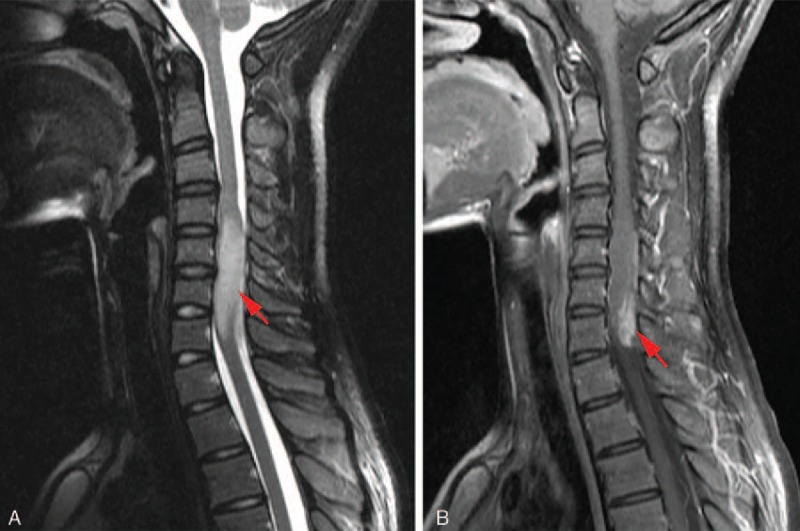
Preoperative magnetic resonance imaging (MRI): (A) sagittal T2-weighted MRI, which shows a high-signal intramedullary lesion extending from C4 to C7, filling the involved spinal canal. (B) Postcontrast sagittal T1-weighted MRI, showing a heterogeneous enhancement intramedullary lesion. Red arrows indicate the lesion.
After surgery the patient's clinical status was unchanged. Histopathological study and immunohistochemical staining confirmed the diagnosis of primary spinal cord GBM (Fig. 2). Serial follow-up spinal MRIs were performed at 1-week, 3-month, and 6-month intervals postsurgery (Fig. 3). After the histologic diagnosis, the patient was administered adjuvant therapy consisting of focal radiotherapy (RT) to the C4 to C7 lesion (5000 cGy delivered in 25 fractions) and administration of chemotherapy (CT) with temozolomide (TMZ). TMZ was given as a daily dose of 75 mg/m2 during the irradiation period, followed by a daily dose of 150 mg/m2 for 5 days. Then, 21 days after combined therapy, TMZ was continued at an increased dose of 200 mg/m2 for 5 days and repeated every 28 days for subsequent cycles.[9] During the early stage of these adjuvant treatments, the patient experienced no associated severe hematotoxic effects, and she required no interruptions or delays in treatment because of hematotoxicity.
Figure 2.
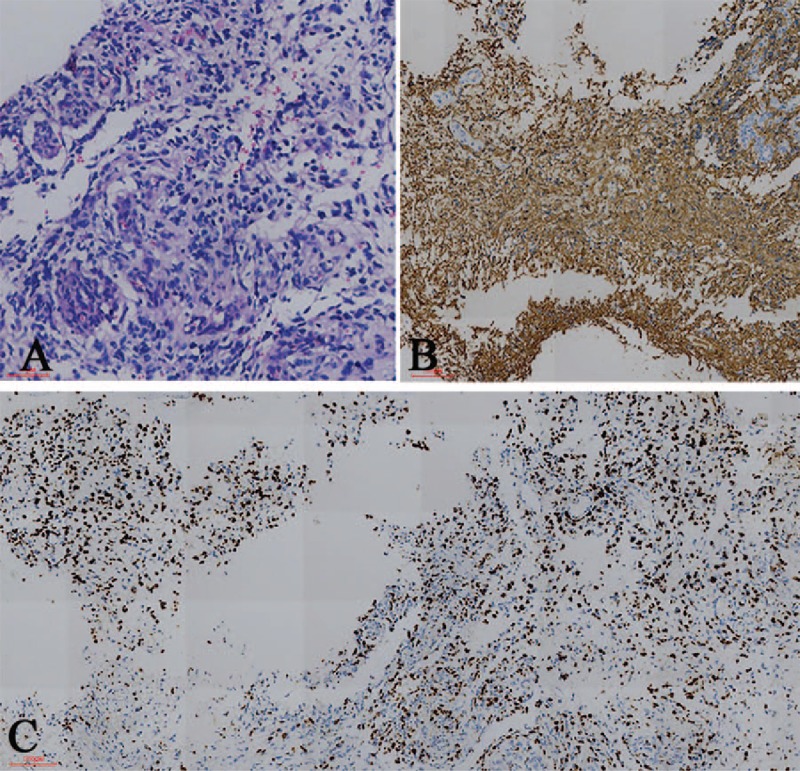
Histopathological and immunochemical findings. (A) Hematoxylin- and eosin-stained section revealed that the tumor is composed of cells with prominent eosinophilic cytoplasm, nuclear atypia, and microvascular proliferation. (B) Immunohistochemical staining shows positivity for glial fibrillary acidic protein. (C) The proliferation index (anti-Ki67) is 60% [magnification (A) 200×, (B), and (C) 100×].
Figure 3.
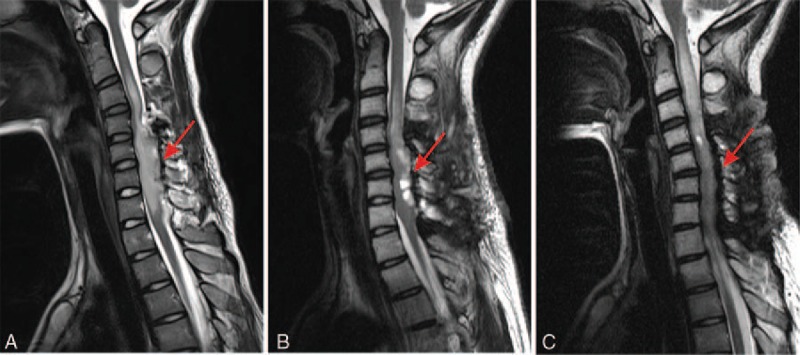
Postoperative magnetic resonance imaging (MRI): sagittal T2-weighted imaging MRI of the cervical spine shows a subtotal resection of the primary lesion. Showing the T2 appearances of the lesion at (A) 1 week, (B) 3 months, and (C) 6 months after surgery. Red arrows indicate the lesion site.
Over the 12 months following surgery, the patient's neurological examination findings continued to deteriorate, as did her overall general condition. Considering the definitive pathological findings in this patient and the poor prognosis associated with the disease, we did not consider further treatment. The patient's condition deteriorated over the following 3 weeks, and the patient died 13 months after the diagnosis.
3. Discussion
3.1. Clinical characteristics
We performed a review of the literature, and, to date, a total number of 165 primary spinal GBM cases have been reported since 1938, including the present case. We performed an electronic search on PubMed, Web of Science, and Google Scholar using the predefined search terms “glioblastoma” and “spinal cord.” The literature search was continued through October 2016. All studies were published in extenso and reported in the German, French, Spanish, Italian, or English language.
From the eligible articles, data relating to several variables, including survival time, age, gender, and tumor location, were recorded on a standard data extraction form (Table 1). The prognoses of these cases proved exceptionally dismal, with a mean average survival of 14.3 (median 16.0) months from diagnosis, and only 14.1% of the patients were still alive 24 months after initial diagnosis (Fig. 4). Tumors occurred in relatively young individuals (mean 26.0 years), with a slight male predominance (57.1%). We found that spinal GBM occurred most frequently within either thoracic spinal cord or cervical area (28.5% combined), followed by the cervicothoracic region (14.5%), lumbar area (9.7%), and least frequently in the conus medullaris (5.5%). Tumors in the thoracic region proved to have a more favorable prognosis in comparison to those in the cervical region. Our results were similar to those of previous investigators.[10,11]
Table 1.
Characteristics of patients with primary spinal GBM.
Figure 4.
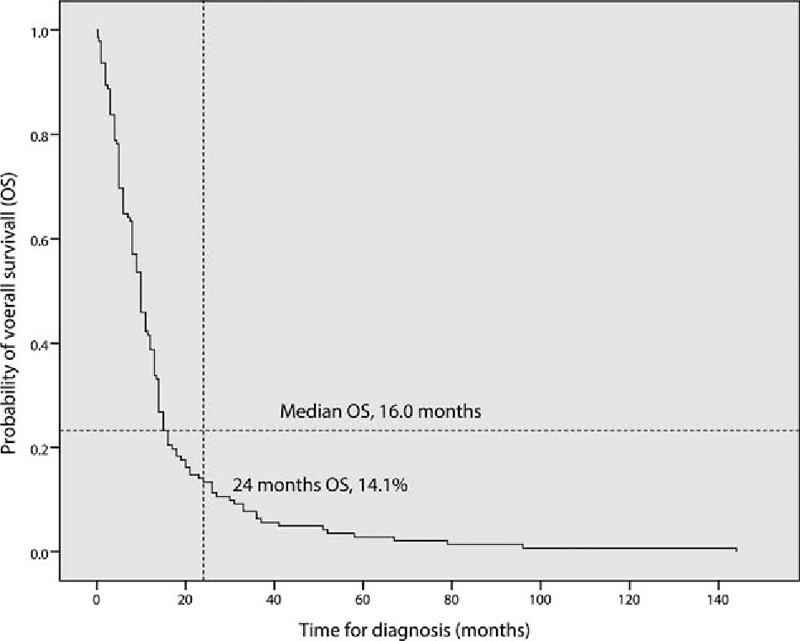
Overall survival of 142 patients with primary spinal glioblastomas reported in literature. In 23 of the 165 patients (13.9%), survival data were not available.
3.2. Diagnosis
MRI is considered the gold standard imaging modality to diagnose spinal intramedullary tumors.[12,13] The majority of primary spinal GBM lesions appear as infiltrative, expansile masses with high T2 signal, and heterogeneous enhancement on postcontrast T1-weighted sequences.[14–16] However, differentiation of primary spinal GBM from other spinal diseases, such as transverse myelitis or other intramedullary tumors, is difficult, since primary spinal GBM tumors sometimes show ambiguous MRI characteristics. In such instances, it might be suggested that F-18-fluoro-deoxy-glucose positron emission tomography be performed for further delineation. However, the risk that this modality may significantly increase the rate of glycolysis in malignant lesions must be considered.[17] Therefore, the favored means of establishing definitive diagnosis is with tissue biopsy and histopathological evaluation.
3.3. Therapeutic strategy
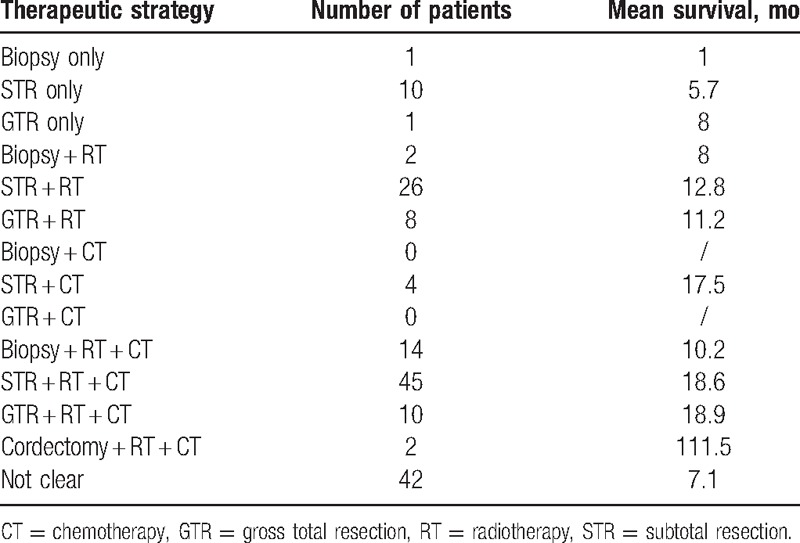
Treatment protocol choices for primary spinal GBM remain controversial but usually include surgery, RT, and CT. Among surgical options are gross total resection (GTR), subtotal resection (STR), and biopsy. The main difference among the surgical approaches is the scope of resection; however, the relationship between scope of resection and survival is yet to be established.[18] Postoperative adjuvant treatment has generally combined RT and CT, similar to the treatment strategy for intracranial GBM. However, adjuvant treatment effects in primary spinal GBM are still not clear. In a lot of clinical literature, the data were incompletely recorded. In order to better analyze, independent statistics were carried out for various treatment protocols (Table 2). From this analysis, various therapeutic strategies have yielded varying results. A summary of patient survival data for different therapeutic strategies was also recorded (Table 3).
Table 2.
Treatment characteristic of patients with primary spinal GBM.
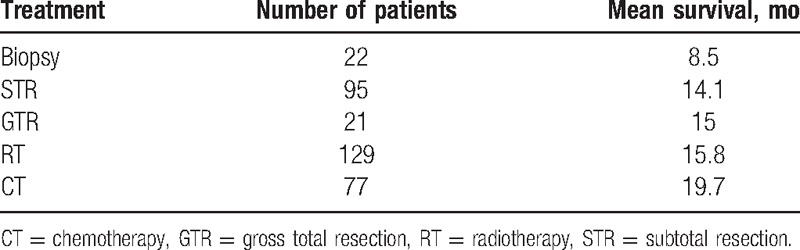
Table 3.
Patient survival in different treatment groups.
3.3.1. Surgery
Once MRI establishes that a patient is likely to have a primary spinal GBM, surgical intervention is usually recommend to provide a definitive diagnosis and, if possible, to remove the mass in toto.[19–21] GTR is recommended whenever feasible. Several studies have presented that GTR improved mortality in patients who have had primary malignant spinal cord tumors,[16,18,22] while a few studies found that GTR did not have any benefit and could even be associated with higher mortality.[5,23] Since primary spinal GBMs tend to be infiltrative and there is no distinct margin between tumor and surrounding normal cord tissue,[24] a STR or biopsy that does not cause further neurological dysfunction is appropriate in some cases. According to our review findings, STR was performed to establish a histologic diagnosis in most patients. STR was performed in 57.6% of cases, whereas GTR could be achieved in only 12.7% of patients. Biopsy only was performed in 13.3% of cases. According to the Kaplan–Meier curve (Fig. 5), extent of resection did not influence overall median survival (log-rank P = 0.16). Cordectomy, the most radical surgical approach, is recommended in patients whose tumors occupy the lower lumbar or sacral segments.[25] However, this technique cannot be utilized in patients with primary spinal GBM with cervical or holocordal involvement.[26] So far, a few reports have suggested that cordectomy may achieve longer patient survival.[27,28] All of the cordectomy cases achieved good results (mean survival 111.5 months), but there are too few cases to judge the ultimate clinical efficacy of cordectomy.
Figure 5.
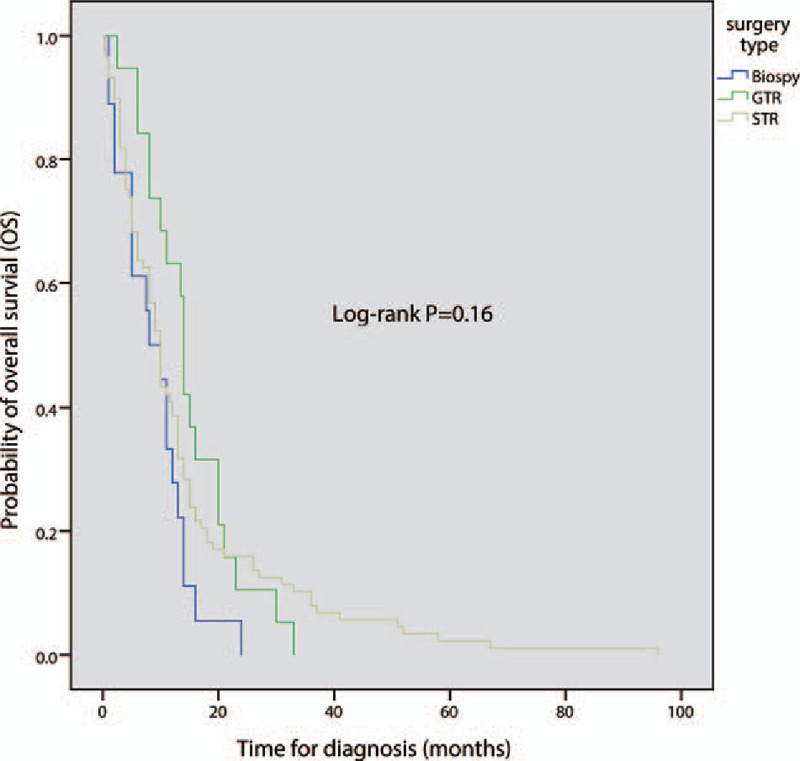
Comparison of overall survival according to the extent of resection; P < 0.05 is considered significant.
3.3.2. Radiotherapy
Adjuvant RT is commonly recommended postoperatively due to the risk of tumor recurrence, including dissemination.[24,29] Several studies found that postoperative RT significantly lengthened survival of patients with high-grade tumors (WHO Grade III-IV).[4,30,31] Another study claimed that there was no significant relationship between RT and survival outcomes.[32] Kaplan–Meier analysis (Fig. 6) showed that postoperative RT has a significant effect on overall survival (log-rank P = 0.007). Therefore, we conclude that RT can significantly prolong survival time. On the contrary, the spinal cord is sensitive to the effects of radiation.[33] Overdosage of RT treatment has been proven to lead to the occurrence of radiation-related tumors.[34–37] Considering this, overdosage must be avoided to reduce the risk of radiation-related tumors. Various radiation doses have been used, though optimal dosage remains uncertain. The accepted spinal cord tolerance level is about 5000 to 5500 cGy.[38] However, the contribution of adjuvant RT in spinal cord GBM remains unclear.
Figure 6.
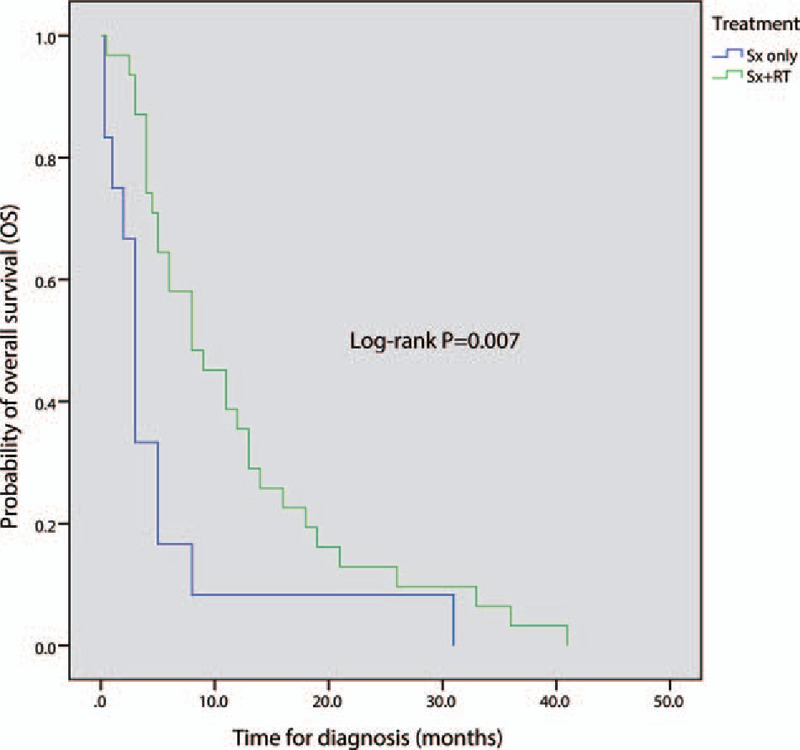
Comparison of overall survival between surgery-only group and surgery-plus-radiotherapy group; Sx: surgery (include biopsy, subtotal resection, and gross total resection); P < 0.05 is considered significant.
3.3.3. Chemotherapy
The efficacy of concomitant TMZ with adjuvant RT in patients with intracranial GBM has been investigated in randomized phase III trials.[39,40] However, because of the rarity of primary spinal GBM, no phase III or large phase II trial has been conducted vis-à-vis patient survival. There is a lack of data to evaluate the curative effect of CT for the treatment of primary spinal GBM. To the best of our knowledge, the most commonly used chemotherapeutic agent is TMZ. Hernandez-Duran et al[41] concluded that TMZ does increase survival in primary spinal GBM patients, and they did advocate for it, though it did not reach statistical significance between patients with primary spinal GBM treated with TMZ versus those not treated with TMZ. Consistent with some reports in the literature that declared that CT provides no survival benefit for high-grade spinal cord gliomas,[18,42,43] our analysis found that postoperative CT did not play a significant role in prolonging survival of primary spinal GBM (log-rank P = 0.078; Fig. 7).
Figure 7.
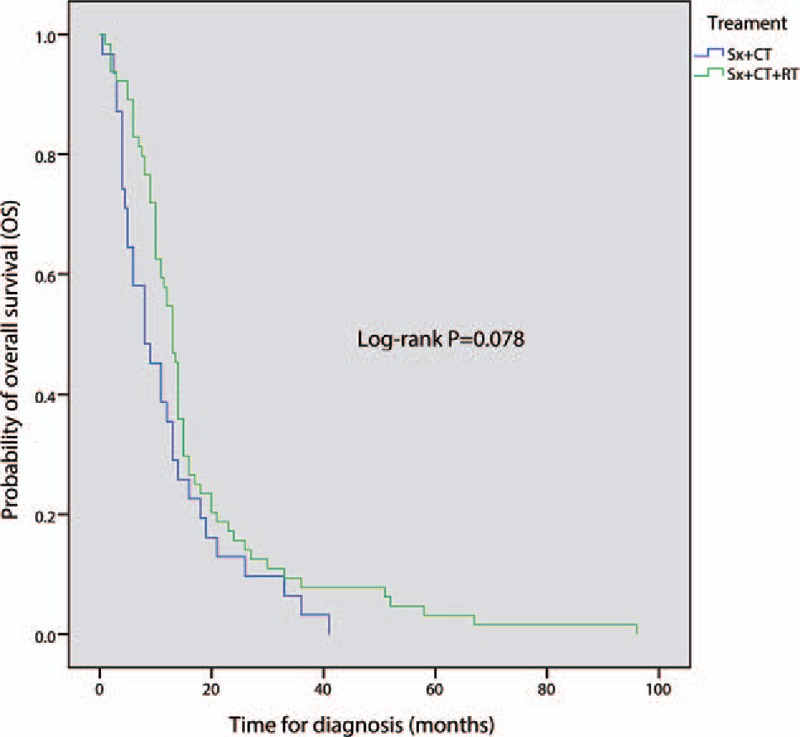
Comparison of overall survival between surgery-plus-radiotherapy (RT) group and surgery-plus-RT-and-chemotherapy group; Sx: surgery (include biopsy, subtotal resection, and gross total resection); P < 0.05 is considered significant.
Bevacizumab (BEV), a monoclonal antibody to the vascular endothelial growth factor (VEGF), is another CT drug of potential interest. One study reported that BEV may be beneficial in spinal cord high-grade gliomas.[44] However, 2 large prospective randomized trials recently declared that BEV did not improve overall survival in patients with intracranial glioblastoma.[45,46] Obviously, more preclinical studies are needed to evaluate the possible curative effect of BEV in primary spinal GBM.
4. Conclusion
Primary spinal GBM is a clinically rare entity that progresses rapidly with a dismal prognosis and a short survival time despite aggressive management. Therefore, clinical treatment trials are nonexistent. For this reason, central registry and multiple-center cooperation is needed in order to understand this rare disease and attain better therapeutic outcomes.
Acknowledgments
The authors would like to thank Clarity Manuscript Consultant LLC (Indianapolis) for their language-editing assistance.
Footnotes
Abbreviations: BEV = bevacizumab, CT = chemotherapy, GBM = glioblastoma multiforme, GTR = gross total resection, MRI = magnetic resonance imaging, RT = radiotherapy, STR = subtotal resection, TMZ = temozolomide, WHO = World Health Organization.
C-XS and J-FW—co-first authors.
Availability of data and materials: The data and material are freely available upon requests.
Please contact WZ (xing.guangweishi@163.com) for further information, who is responsible for the dataset.
Authors’ contributions: C-XS and J-FW drafted this manuscript. WZ, Z-WC, and R-ZC were involved in the acquisition of data and preparing the figures. C-MC conceived of the study and revised the manuscript. All authors read and approved the final manuscript.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Bowers DC, Weprin BE. Intramedullary spinal cord tumors. Curr Treat Options Neurol 2003;5:207–12. [DOI] [PubMed] [Google Scholar]
- [2].Iwata K, Nakagawa H, Hashizume Y. Significance of MIB-1, PCNA indices, and p53 protein over-expression in intramedullary tumors of the spinal cord. Noshuyo Byori 1996;13:73–8. [PubMed] [Google Scholar]
- [3].Strik HM, Effenberger O, Schafer O, et al. A case of spinal glioblastoma multiforme: immunohistochemical study and review of the literature. J Neurooncol 2000;50:239–43. [DOI] [PubMed] [Google Scholar]
- [4].Ciappetta P, Salvati M, Capoccia G, et al. Spinal glioblastomas: report of seven cases and review of the literature. Neurosurgery 1991;28:302–6. [PubMed] [Google Scholar]
- [5].Raco A, Esposito V, Lenzi J, et al. Long-term follow-up of intramedullary spinal cord tumors: a series of 202 cases. Neurosurgery 2005;56:972–81. discussion 972-81. [PubMed] [Google Scholar]
- [6].Miller DC. Surgical pathology of intramedullary spinal cord neoplasms. J Neurooncol 2000;47:189–94. [DOI] [PubMed] [Google Scholar]
- [7].Epstein FJ, Farmer JP, Freed D. Adult intramedullary astrocytomas of the spinal cord. J Neurosurg 1992;77:355–9. [DOI] [PubMed] [Google Scholar]
- [8].Sanborn MR, Pramick M, Brooks J, et al. Glioblastoma multiforme in the adult conus medullaris. J Clin Neurosci 2011;18:842–3. [DOI] [PubMed] [Google Scholar]
- [9].Vaillant B, Loghin M. Treatment of spinal cord tumors. Curr Treat Options Neurol 2009;11:315–24. [DOI] [PubMed] [Google Scholar]
- [10].Raco A, Piccirilli M, Landi A, et al. High-grade intramedullary astrocytomas: 30 years’ experience at the Neurosurgery Department of the University of Rome “Sapienza”. J Neurosurg Spine 2010;12:144–53. [DOI] [PubMed] [Google Scholar]
- [11].Ononiwu C, Mehta V, Bettegowda C, et al. Pediatric spinal glioblastoma multiforme: current treatment strategies and possible predictors of survival. Childs Nerv Syst 2012;28:715–20. [DOI] [PubMed] [Google Scholar]
- [12].Stecco A, Quirico C, Giampietro A, et al. Glioblastoma multiforme of the conus medullaris in a child: description of a case and literature review. AJNR Am J Neuroradiol 2005;26:2157–60. [PMC free article] [PubMed] [Google Scholar]
- [13].Bonde V, Balasubramaniam S, Goel A. Glioblastoma multiforme of the conus medullaris with holocordal spread. J Clin Neurosci 2008;15:601–3. [DOI] [PubMed] [Google Scholar]
- [14].Choi WC, Lee JH, Lee SH. Spinal cord glioblastoma multiforme of conus medullaris masquerading as high lumbar disk herniation. Surg Neurol 2009;71:234–7. discussion 237. [DOI] [PubMed] [Google Scholar]
- [15].Kim WH, Yoon SH, Kim CY, et al. Temozolomide for malignant primary spinal cord glioma: an experience of six cases and a literature review. J Neurooncol 2011;101:247–54. [DOI] [PubMed] [Google Scholar]
- [16].Wong AP, Dahdaleh NS, Fessler RG, et al. Risk factors and long-term survival in adult patients with primary malignant spinal cord astrocytomas. J Neurooncol 2013;115:493–503. [DOI] [PubMed] [Google Scholar]
- [17].Won KS, Kim JS, Ra YS, et al. FDG PET of primary spinal glioblastoma initially mimicking a transverse myelitis on MRI. Clin Nucl Med 2006;31:556–7. [DOI] [PubMed] [Google Scholar]
- [18].McGirt MJ, Goldstein IM, Chaichana KL, et al. Extent of surgical resection of malignant astrocytomas of the spinal cord: outcome analysis of 35 patients. Neurosurgery 2008;63:55–60. discussion 60-1. [DOI] [PubMed] [Google Scholar]
- [19].Engelhard HH, Villano JL, Porter KR, et al. Clinical presentation, histology, and treatment in 430 patients with primary tumors of the spinal cord, spinal meninges, or cauda equina. J Neurosurg Spine 2010;13:67–77. [DOI] [PubMed] [Google Scholar]
- [20].Waters JD, Peran EM, Ciacci J. Malignancies of the spinal cord. Adv Exp Med Biol 2012;760:101–13. [DOI] [PubMed] [Google Scholar]
- [21].Mechtler LL, Nandigam K. Spinal cord tumors: new views and future directions. Neurol Clin 2013;31:241–68. [DOI] [PubMed] [Google Scholar]
- [22].Choi GH, Oh JK, Kim TY, et al. The clinical features and surgical outcomes of pediatric patients with primary spinal cord tumor. Childs Nerv Syst 2012;28:897–904. [DOI] [PubMed] [Google Scholar]
- [23].Wolff B, Ng A, Roth D, et al. Pediatric high grade glioma of the spinal cord: results of the HIT-GBM database. J Neurooncol 2012;107:139–46. [DOI] [PubMed] [Google Scholar]
- [24].Mayer RR, Warmouth GM, Troxell M, et al. Glioblastoma multiforme of the conus medullaris in a 28-year-old female: a case report and review of the literature. Clin Neurol Neurosurg 2012;114:275–7. [DOI] [PubMed] [Google Scholar]
- [25].Singh PK, Singh VK, Tomar J, et al. Spinal glioblastoma multiforme: unusual cause of post-traumatic tetraparesis. J Spinal Cord Med 2009;32:583–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Mori K, Imai S, Shimizu J, et al. Spinal glioblastoma multiforme of the conus medullaris with holocordal and intracranial spread in a child: a case report and review of the literature. Spine J 2012;12:e1–6. [DOI] [PubMed] [Google Scholar]
- [27].Viljoen S, Hitchon PW, Ahmed R, et al. Cordectomy for intramedullary spinal cord glioblastoma with a 12-year survival. Surg Neurol Int 2014;5:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Marchan EM, Sekula RF, Jr, Jannetta PJ, et al. Long-term survival enhanced by cordectomy in a patient with a spinal glioblastoma multiforme and paraplegia. Case report. J Neurosurg Spine 2007;7:656–9. [DOI] [PubMed] [Google Scholar]
- [29].O’Halloran PJ, Farrell M, Caird J, et al. Paediatric spinal glioblastoma: case report and review of therapeutic strategies. Childs Nerv Syst 2013;29:367–74. [DOI] [PubMed] [Google Scholar]
- [30].Minehan KJ, Brown PD, Scheithauer BW, et al. Prognosis and treatment of spinal cord astrocytoma. Int J Radiat Oncol Biol Phys 2009;73:727–33. [DOI] [PubMed] [Google Scholar]
- [31].Adams H, Avendano J, Raza SM, et al. Prognostic factors and survival in primary malignant astrocytomas of the spinal cord: a population-based analysis from 1973 to 2007. Spine 2012;37:E727–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Lam S, Lin Y, Melkonian S. Analysis of risk factors and survival in pediatric high-grade spinal cord astrocytoma: a population-based study. Pediatr Neurosurg 2012;48:299–305. [DOI] [PubMed] [Google Scholar]
- [33].Petrovich Z, Liker M, Jozsef G. Petrovich Z, et al. Radiotherapy for tumors of the spine. Combined Modality Therapy of Central Nervous System Tumors. Berlin, Heidelberg: Springer; 2003. 547–61. [Google Scholar]
- [34].Kawanabe Y, Sawada M, Yukawa H, et al. Radiation-induced spinal cord anaplastic astrocytoma subsequent to radiotherapy for testicular seminoma. Neurol Med Chir 2012;52:675–8. [DOI] [PubMed] [Google Scholar]
- [35].Obid P, Vierbuchen M, Wolf E, et al. Radiation-induced intraspinal chondrosarcoma: a case report. Global Spine J 2015;5:e74–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Falavigna A, da Silva PG, Teixeira W. Radiotherapy-induced tumors of the spine, peripheral nerve, and spinal cord: case report and literature review. Surg Neurol Int 2016;7Suppl 4:S108–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Ng C, Fairhall J, Rathmalgoda C, et al. Spinal cord glioblastoma multiforme induced by radiation after treatment for Hodgkin disease. Case report. J Neurosurg Spine 2007;6:364–7. [DOI] [PubMed] [Google Scholar]
- [38].Newton HB. Chapter 5—overview of pathology and treatment of primary spinal cord tumors. Handbook of Neuro-Oncology Neuroimaging (2nd ed) 2016;San Diego: Academic Press, 41–53. [Google Scholar]
- [39].Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 2009;10:459–66. [DOI] [PubMed] [Google Scholar]
- [40].Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005;352:987–96. [DOI] [PubMed] [Google Scholar]
- [41].Hernandez-Duran S, Bregy A, Shah AH, et al. Primary spinal cord glioblastoma multiforme treated with temozolomide. J Clin Neurosci 2015;22:1877–82. [DOI] [PubMed] [Google Scholar]
- [42].Allen JC, Aviner S, Yates AJ, et al. Treatment of high-grade spinal cord astrocytoma of childhood with “8-in-1” chemotherapy and radiotherapy: a pilot study of CCG-945. Children's Cancer Group. J Neurosurg 1998;88:215–20. [DOI] [PubMed] [Google Scholar]
- [43].Cohen AR, Wisoff JH, Allen JC, et al. Malignant astrocytomas of the spinal cord. J Neurosurg 1989;70:50–4. [DOI] [PubMed] [Google Scholar]
- [44].Kaley TJ, Mondesire-Crump I, Gavrilovic IT. Temozolomide or bevacizumab for spinal cord high-grade gliomas. J Neurooncol 2012;109:385–9. [DOI] [PubMed] [Google Scholar]
- [45].Chinot OL, Wick W, Mason W, et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med 2014;370:709–22. [DOI] [PubMed] [Google Scholar]
- [46].Gilbert MR, Dignam JJ, Armstrong TS, et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med 2014;370:699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]


