Abstract
Background:
Osteonecrosis of the femoral head (ONFH) is a progressive disease, which mainly affects young adults and often necessitates total hip arthroplasty (THA), so early interventions are critical to successfully protect hip joint from THA. In this review, our purpose was to determine the effects of anticoagulants for preventing and treating the primary and secondary ONFH, respectively, before the collapse stage or before the pathology of necrosis.
Methods:
We searched PubMed, Embase, Web of Science databases for relevant studies. Any observational or experimental studies that evaluated anticoagulants and ONFH were our goal of searching the electric databases.
Results:
Four studies including a total of 218 hips were identified in this review, 2 of them were prospective studies which performed by 1 group, 1 was a retrospective study, and the last was a prospective comparative study.
Conclusions:
Our findings supported that the anticoagulants could be used for primary ONFH. However, anticoagulants cannot play a protective role on secondary ONFH. Moreover, there were no serious adverse effects reported in the studies after anticoagulants treatment. Nevertheless, our present study with some limitations such as the limited sample size only provided limited quality of evidence; confirmation from further systematic review or meta-analysis with large-scale, well-designed randomized control trials is required.
Keywords: anticoagulants, osteonecrosis of the femoral head, systematic review
1. Introduction
Osteonecrosis of the femoral head (ONFH), which is also called avascular necrosis of femoral head or aseptic necrosis of femoral head, is a progressive disease that can be divided into 2 categories. Primary (idiopathic) osteonecrosis of hips is commonly associated with inherited hrombophilia or hypofibrinolysis or antiphospholipid antibodies syndrome,[1–8] whereas ONFH can also be secondary to multiple risk factors such as high-dose or long-term corticosteroids, alcoholism, decompression sickness, hip trauma including femoral neck fractures and hip dislocations, and so on.[1,9,10] Although the pathogenesis of this multifactorial disease has not been completely elucidated, a sequence for the development of ONFH has been postulated by some scholars: osseous venous outflow obstruction is caused by venous thrombosis because of thrombophilia-hypofibrinolysis, circulating lipids, nitrogen bubble formation or direct interruption, leading to reduced arterial flow, ischemia, bone death, and collapse of the articular surface[1,11–17] (Fig. 1). And there were also some animal experiments or other experimental models of osteonecrosis confirming venous occlusion as a primary event.[10,17–20] As this devastating disease mainly affects adults in their 3rd, 4th, or 5th decade of life, and often necessitates total hip arthroplasty (THA) at a young age,[21] it is increasingly essential to find a feasible and less-invasive therapeutic regimen to save the hips before collapse (Ficat stages I or II) or before the pathology of necrosis[11,22,23] (Table 1). Various options for the management of ONFH to prevent surgical procedures have been applied, including bisphosphonates, vasodilators, and biophysical modalities.[11,24,25] Recently, some scholars have investigated the effects of anticoagulants on relieving or reversing venous occlusion. Anticoagulant drugs, which prevent blood coagulation by participating in physiological coagulation, could be classified into 4 kinds: indirect thrombin inhibitor (heparin and enoxaparin, among others), direct thrombin inhibitor (argatroban and hirudin, among others), vitamin K antagonists (warfarin and dicoumaral, among others), and selective factor Xa inhibitors such fondaparinux and ximelagatran.[26]
Figure 1.
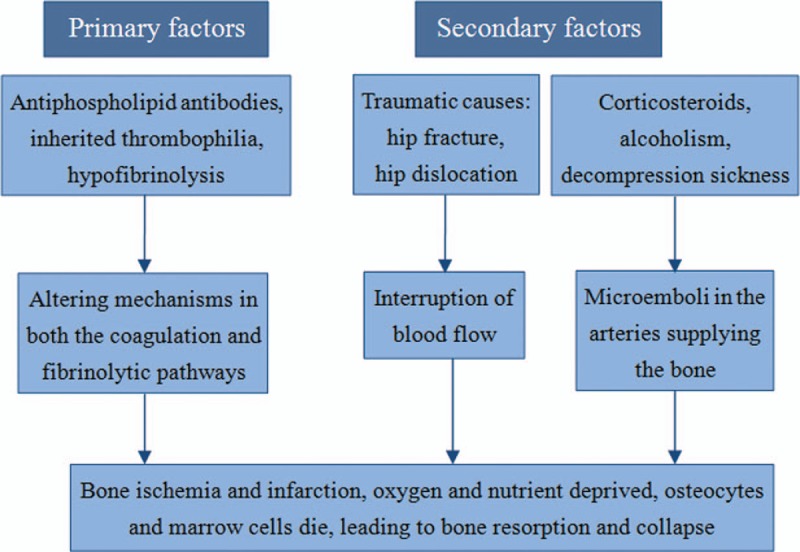
Etiology and pathogenesis of osteonecrosis of the femoral head.
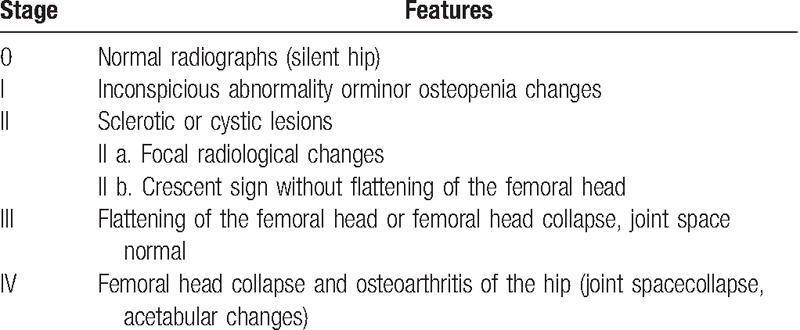
Table 1.
Ficat and Arlet classification system.
Given this process characterized by bone death and reduced local blood flow, we performed a study with a purpose of determining the efficacy of anticoagulants for preventing and treating the primary and secondary ONFH on its precollapse stage to improve the hip prognosis.
2. Materials and methods
This meta-analysis was carried out in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses reporting guidelines for the meta-analysis of intervention trials.[27] Ethical approval for this study was unnecessary because it was a review of existing literature and did not involve any handling of individual patient data.
2.1. Search strategy
Three electronic databases (PubMed, EMBASE, and Web of Science) were searched and we used terms and Boolean operators as follows: “(anticoagulants OR anticoagulant drugs) AND (avascular necrosis OR aseptic necrosis OR osteonecrosis) AND (femoral head)”. We did not limit the year of publication, publication status, or language, and there was also no limitation on any particular study design: randomized or nonrandomized clinical trials, cohort, and case-control studies. In addition, we also checked the references of the articles manually to identify other potentially relevant publications. We did not seek unpublished articles. The study selection was then independently performed by 2 of the authors, and any different opinions were resolved through discussion.
2.2. Eligibility criteria
Studies were considered eligible if they met the following criteria: the study enrolled patients with ONFH; the intervention considered in the study was the use of anticoagulants saving the hips before collapse (Ficat stages I or II) or before the pathology of necrosisresults; the efficiency before and after the treatment were at least evaluated by Ficat or Arlet Stage. Articles that reported at least 1 outcome were included and those without the outcome measures of interest were excluded. Letters, comments, editorials, and practice guidelines were excluded. Two authors independently reviewed the titles and abstracts of potentially relevant studies. Any discrepancy was resolved by consensus with a 3rd author.
2.3. Data extraction
We extracted the following details from each article: the first author's name, publication year, study design, the type of ONFH, study population (hips/patients), patient's sex and age, follow-up time, interventions, outcomes, and adverse effects.
2.4. Statistical analysis
Review Manager Software (Revman v5.3) was used to analyze experimental data from the included trials. Odds ratios of successful treatments were assessed for dichotomous data. Continuous outcomes were expressed as the mean ± standard deviation. Heterogeneity among studies was estimated using the I2 statistics; substantial heterogeneity was reflected by I2 >50%. A fixed-effects model was used when I2 < 50%; otherwise, the random-effects model was adopted. P < .05 was considered statistically significant.
3. Results
A total of 75 articles were initially searched from the PubMed, EMBASE, and Web of Science databases. After duplicating, title screening, and abstract or full-text screening, 4 articles with a total of 218 hips were included in this review.[28–31]Figure 2 showed the process and details of our searching work. Tables 2 and 3 provided the basic information and outcomes of the 4 studies, which included 2 prospective noncontrolled studies conducted by the same group during different follow-up periods[29,30] and 2 comparative studies.[28,31] All of the studies evaluated the efficacy of anticoagulants according to Ficat or Arlet stage identified by x-ray or magnetic resonance imaging (MRI) or both. The type, doses, and duration of anticoagulants administration differed among the studies. Two articles studied the primary ONFH[28,30] and 1 was performed on secondary ONFH,[31] whereas the rest one included both of the types.[29] Enoxaparin and warfarin were used in the studies we included. Data could not be pooled because of the methodological heterogeneity and limited number of the available controlled studies.
Figure 2.
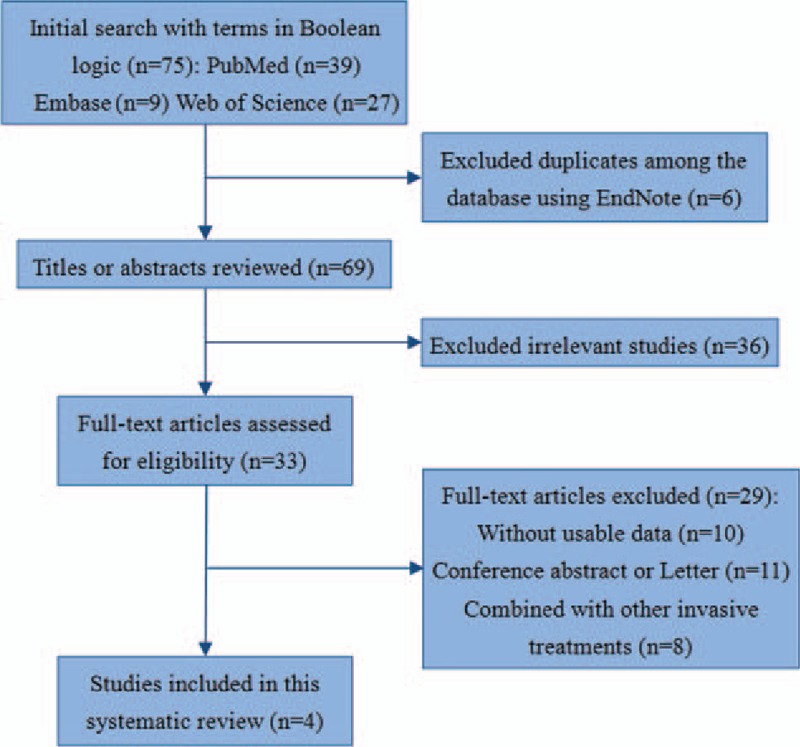
The selection process of this review.
Table 2.
Characteristics of studies of the prevention and treatment of anticoagulants for ONFH.
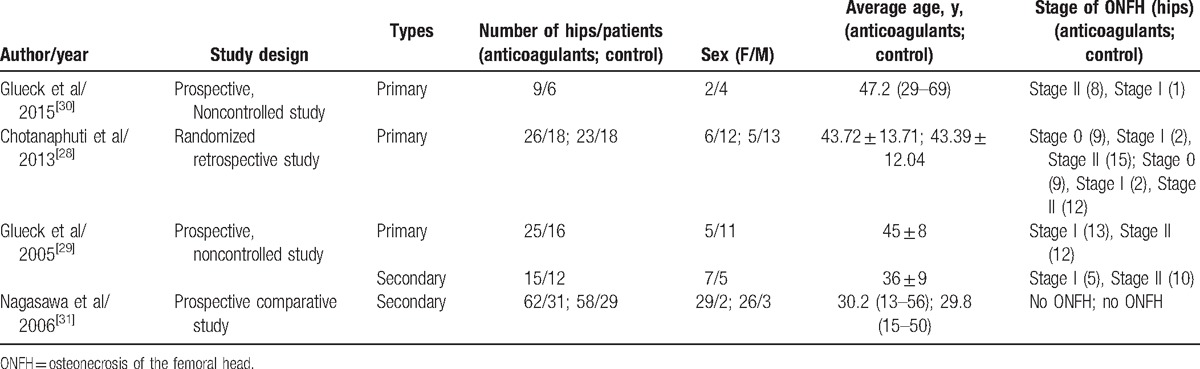
Table 3.
Methods and outcomes of studies of the prevention and treatment of anticoagulants for ONFH.
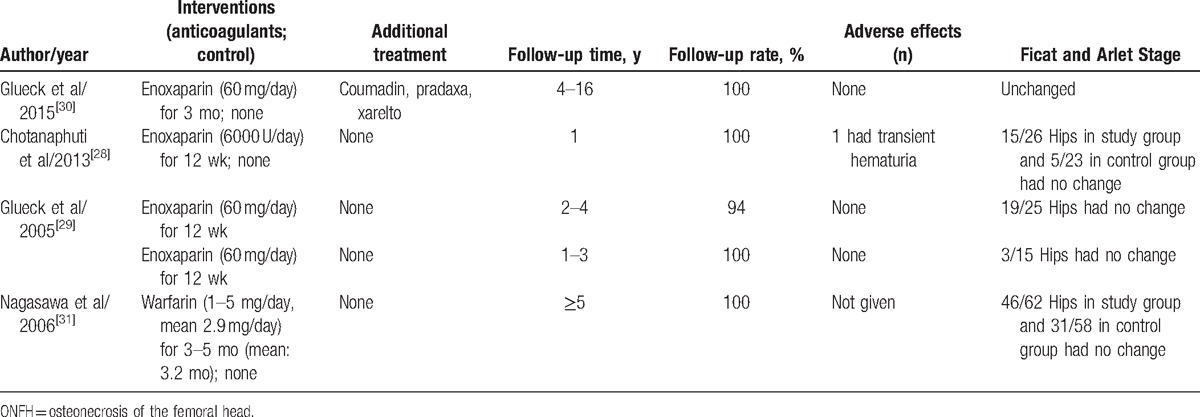
3.1. Primary ONFH outcomes analysis
Glueck et al[30] studied 6 patients (four men and two women (9 hips, 8 Ficat stage II, 1 stage I) with familial thrombophilia (5 Factor V Leiden heterozygotes, 1 with resistance to activated protein C [RAPC]). They used enoxaparin (60 mg/day) for 3 months for every subjects, In 4 patients, coumadin was used in long term with the international normalized ratio targeted between 2 and 3, and 1 patient was given pradaxa 150 mg twice per day after 90 days of enoxaparin, whereas in 1 patient, coumadin was later supplanted by xarelto, 20 mg/day. They found that after the anticoagulants therapy, 9 hips in the 6 patients (8 originally Ficat II and 1 Ficat I) remained unchanged, contrasted to untreated ONFH, Ficat stage II, wherein 50% to 80% of hips progress to collapse (Ficat stages III-IV) within 2 years after diagnosis.[32] Within 3, 3, 3, 9, and 16 months after starting anticoagulation, 5 patients became pain-free and remained asymptomatic throughout follow-up, the rest 1 patient required prcocet for pain. There were no significant bleeding episodes. These authors confirmed that long-term anticoagulation started before segmental collapse of the head of the femur (Ficat stages III), in patients with thrombophilic Factor V Leiden or RAPC, would be expected to stop the progression of idiopathic osteonecrosis and relieve symptoms, thus preventing the need for total hip replacement.
Another study with primary ONFH was conducted by Chotanaphuti et al.[28] Thirty-six patients (25 men and 11 women) diagnosed of idiopathic osteonecrosis of the hips with 49 hips been in the precollapsed stage were randomized into 2 groups averagely; the study group consisting of 26 hips had been administered with 6000 U of enoxaparin daily for 12 weeks, whereas 23 hips in the control group had not received additional treatment. All patients had been radiographically evaluated with x-ray of the studied hips at 3, 6, 12, 18, and 24 months. At 24 months’ follow-up, 15 hips (57.7%) from the study group remained in precollapse stage, whereas only 5 hips (21.7%) in the control group remained in precollapse stage (P = .042). In the study group, 10 of 11 hips (90.9%) that were in the Ficat & Arlet stage 0 or I at the time of enrollment have not progressed beyond stage II, whereas only 5 of 15 hips (33.3%) that were initially in stage II had remained unchanged, and only 1 patient from the study group developed transient hematuria, which spontaneously subsided. They concluded that enoxaparin administration for the idiopathic osteonecrosis of the hip in precollapse stage can significantly prevent the progression of the disease in 24 months of follow-up.
Glueck et al[29] also performed a study including patients with primary and secondary ONFH. In their study, 16 patients (age 45 ± 8 years, 5 women and 11 men) had primary osteonecrosis (25 hips: 13 Stage I and 12 Stage II); they were treated with enoxaparin (60 mg/day in a preloaded syringe) administered for 12 weeks, with serial hip radiographs taken every 36 weeks to ≥108 weeks. During the study, 1 patient was lost to follow-up at 36 weeks, and 1 patient, on worsening of left hip osteonecrosis, stopped follow-up at 36 weeks despite no change in his right hip, and another patient progressed to Ficat Stage III and IV osteonecrosis and received THA at 36 weeks. At ≥108 weeks or more of follow-up (mean, 161 weeks; range, 108–216 weeks) in 13 patients (20 hips) with primary osteonecrosis, 19 hips (95%) had no change from Ficat Stages I and II osteonecrosis. At ≥108 weeks’ follow-up in patients with primary osteonecrosis, preservation of 95% of hips, compared favorably with untreated historic controls of approximately 20% with 2 years hip preservation.[32–34]
3.2. Secondary ONFH outcomes analysis
As mentioned above, in the study published in 2005 by Glueck et al,[29] secondary ONFH patients were also included. There were 12 patients (age, 36 ± 9 years; 5 women and 7 men) who had osteonecrosis secondary to long-term and high-dose corticosteroid use (15 hips: 5 Stage I and 10 Stage II). Of which 12 patients continued using corticosteroids during the study, 2 patients had systemic lupus erythematosus (SLE). After enoxaparin treatment (60 mg/day for 3 months) and with ≥108 weeks of follow-up, in 12 patients with secondary osteonecrosis, only 3 of 15 hips (20%) had osteonecrosis that remained at Ficat Stages I and II, whereas 12 (80%) had osteonecrosis that had progressed to Ficat Stages III and IV. This 20% hip preservation were the same as untreated historic controls after 2 years.[32–34] When comparing the outcomes between the primary and secondary ONFH, the percent of hips remaining at Stages I and II in patients with primary osteonecrosis (95%) was much greater (P < .001) than in patients with secondary osteonecrosis (20%). So they concluded that for osteonecrosis secondary to corticosteroid use, enoxaparin did not alter progression to Ficat stages III or IV.
Another study searching secondary ONFH was conducted by Nagasawa et al.[31] They conducted a prospective clinical trial for the prevention of steroid-associated ONFH in SLE patients using warfarin. In their study, 60 patients consisting of 5 males and 55 females ranging from 16 to 58 years of age were selected; all of them were requested to be newly diagnosed as having SLE and to require high-dose (≥40 mg/day) prednisolone as the initial treatment including pulse therapy with 1000 mg/day of methylprednisolone for 3 days. Plain radiography and MRI were used first at 3 months after the beginning of steroid treatment and subsequently every year for >5 years to make a diagnosis of ONFH. Patients were alternately assigned to either of 2 groups. In one group, patients received warfarin (1–5 mg/day, mean 2.9 mg/day) starting together with steroid therapy for 3 to 5 months (mean, 3.2 months). Patients in the other group were observed without warfarin (control). Silent ONFH developed in 19 of 58 hips (33%) in the control group, compared to 13 of 62 hips (21%) in the warfarin group, without significant difference (P = .13). However, symptomatic ONFH developed in 8 of 58 hips (14%) in the control group. In comparison, it appeared in only 3 of 62 hips (4.8%) in the warfarin group, with only a trend toward significance (P = .08). The authors concluded that there was no statistically significant effect of anticoagulant therapy on the prevention of ONFH induced by corticosteroid. Besides, warfarin tended to prevent only symptomatic ONFH other than silent ONFH induced by corticosteroid.
3.3. Adverse events analysis
Except 1 patient from the study group developed transient hematuria after 12 weeks of enoxaparin injection according to Chotanaphuti et al.[28] None of the studies noted serious adverse effects related to anticoagulants administration.
4. Discussion
The overall goal of our review was to make a summary conclusion of whether anticoagulants could prevent or treat ONFH before its collapse stage by synthesizing evidence from previous studies. In this review, we searched 3 databases, but only found 4 clinical trial articles published to determine the effect of anticoagulants on preventing and treating the ONFH. Two of them reported the result of enoxaparin for primary ONFH, and one article was related to prevention of ONFH using warfarin, whereas the last one included primary and secondary ONFH with the treatment of enoxaparin.
Generally speaking, various limitations presented in our review should not be ignored. First, the numbers of the included studies and the subjects in each of the study were not so sufficient, the smallest study only consisted of 6 patients, and 2 of the 4 articles used observational noncontrolled methods. Second, there was not a unified standard in dose and time of anticoagulants use either, and the follow-up time was different among the studies, so some clinical heterogeneity was very likely to exist.
Despite of the shortcomings mentioned above, the result of our review offered some useful information about the positive efficacy toward primary ONFH and the unprotected efficacy toward secondary ONFH with the use of anticoagulants. In addition, there were no severe adverse effects associated with anticoagulants treatment reported during follow-up in the included studies. Moreover, to arrive at a convincing conclusion, further studies should be conducted to demonstrate the following aspects: the detailed indication of patient-specific factors should be further unified, including age, mean morbidity time, and life expectancy; the strategy of intervention should be optimized, including timing of treatment initiation and anticoagulants therapy dose and duration; the trial design should be randomized and double-blind when grouping, treating, and examining efficacy.
5. Conclusions
This review not only showed a positive effect on primary ONFH with the prevention and treatment of anticoagulants, but also suggested that for secondary ONFH, anticoagulants cannot play a protective role. So if we can use the anticoagulants to treat primary ONFH before collapse (Ficat stages I or II) or before the pathology of necrosis, this disease will be possibly reversed. Nevertheless, the lack of large-scale randomized, and double-blind studies should be noted; confirmation from further meta-analysis with large-scale, well-designed randomized control trials is required.
Acknowledgments
The authors thank the members of the Department of Epidemiology and Bio-statistics, School of Public Health, Peking University for help with the statistical analysis, and Fan Meng (experienced nurse) for helpful discussions.
Footnotes
Abbreviations: MRI = magnetic resonance imaging, ONFH = osteonecrosis of the femoral head, PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses, RAPC = activated protein C, SLE = systemic lupus erythematosus, THA = total hip arthroplasty.
PG, FG, YW, and ZZ are Joint first authors.
This study was supported by the National Natural Science Foundation of China (81372013, 81672236), Beijing Natural Science Foundation (7174346), and the Research Fund of China-Japan Friendship Hospital (2014–4-QN-29), China-Japan Friendship Hospital Youth Science and Technology Excellence Project (2014-QNYC-A-06).
The authors report no conflicts of interest.
References
- [1].Glueck CJ, Freiberg RA, Wang P. Koo KH, Mont M, Jones L. Treatment of osteonecrosis of the hip and knee with enoxaparin. Osteonecrosis. Berlin, Germany: Springer-Verlag; 2014. 241–7. [Google Scholar]
- [2].Zalavras C, Dailiana Z, Elisaf M, et al. Potential aetiological factors concerning the development of osteonecrosis of the femoral head. Eur J Clin Invest 2000;30:215–21. [DOI] [PubMed] [Google Scholar]
- [3].Korompilias AV, Ortel TL, Urbaniak JR. Coagulation abnormalities in patients with hip osteonecrosis. Orthop Clin North Am 2004;35:265–71. [DOI] [PubMed] [Google Scholar]
- [4].Korompilias AV, Gilkeson GS, Ortel TL, et al. Anticardiolipin antibodies and osteonecrosis of the femoral head. Clin Orthop Relat Res 1997;345:174–80. [PubMed] [Google Scholar]
- [5].Glueck CJ, Freiberg RA, Wang P. Heritable thrombophilia-hypofibrinolysis and osteonecrosis of the femoral head. Clin Orthop Relat Res 2008;466:1034–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Glueck CJ, Freiberg RA, Boppana S, et al. Thrombophilia, hypofibrinolysis, the eNOS T-786C polymorphism, and multifocal osteonecrosis. J Bone Joint Surg Am 2008;90:2220–9. [DOI] [PubMed] [Google Scholar]
- [7].Glueck CJ, Freiberg R, Tracy T, et al. Thrombophilia and hypofibrinolysis: pathophysiologies of osteonecrosis. Clin Orthop Relat Res 1997;334:43–56. [PubMed] [Google Scholar]
- [8].Glueck CJ, Freiberg RA, Fontaine RN, et al. Hypofibrinolysis, thrombophilia, osteonecrosis. Clin Orthop Relat Res 2001;386:19–33. [DOI] [PubMed] [Google Scholar]
- [9].Abeles M, Urman JD, Rothfield NF. Aseptic necrosis of bone in systemic lupus erythematosus. Relationship to corticosteroid therapy. Arch Intern Med 1978;138:750–4. [PubMed] [Google Scholar]
- [10].Boss JH, Misselevich I. Osteonecrosis of the femoral head of laboratory animals: the lessons learned from a comparative study of osteonecrosis in man and experimental animals. Vet Pathol 2003;40:345–54. [DOI] [PubMed] [Google Scholar]
- [11].Moya-Angeler J, Gianakos AL, Villa JC, et al. Current concepts on osteonecrosis of the femoral head. World J Orthop 2015;6:590–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Seamon J, Keller T, Saleh J, et al. The pathogenesis of nontraumatic osteonecrosis. Arthritis 2012;2012:601763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Weinstein RS, Nicholas RW, Manolagas SC. Apoptosis of osteocytes in glucocorticoid-induced osteonecrosis of the hip. J Clin Endocrinol Metab 2000;85:2907–12. [DOI] [PubMed] [Google Scholar]
- [14].Arbab D, Konig DP. Atraumatic femoral head necrosis in adults. Dtsch Arztebl Int 2016;113:31–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kubo T, Ueshima K, Saito M, et al. Clinical and basic research on steroid-induced osteonecrosis of the femoral head in Japan. J Orthop Sci 2016;21:407–13. [DOI] [PubMed] [Google Scholar]
- [16].Yu L, Zhang CH, Guo T, et al. [Middle and long-term results of total hip arthroplasties for secondary post-traumatic arthritis and femoral head necrosis after acetabular fractures]. Zhongguo Gu Shang 2016;29:109–13. [PubMed] [Google Scholar]
- [17].Bejar J, Peled E, Boss JH. Vasculature deprivation--induced osteonecrosis of the rat femoral head as a model for therapeutic trials. Theor Biol Med Model 2005;2:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Norman D, Miller Y, Sabo E, et al. The effects of enoxaparin on the reparative processes in experimental osteonecrosis of the femoral head of the rat. Apmis 2002;110:221–8. [DOI] [PubMed] [Google Scholar]
- [19].Wada M, Kumagai K, Murata MY, et al. Warfarin reduces the incidence of osteonecrosis of the femoral head in spontaneously hypertensive rats. J Orthop Sci 2004;9:585–90. [DOI] [PubMed] [Google Scholar]
- [20].Laroche M. Intraosseous circulation from physiology to disease. Joint Bone Spine 2002;69:262–9. [DOI] [PubMed] [Google Scholar]
- [21].Rajpura A, Wright AC, Board TN. Medical management of osteonecrosis of the hip: a review. Hip Int 2011;21:385–92. [DOI] [PubMed] [Google Scholar]
- [22].Schmitt-Sody M, Kirchhoff C, Mayer W, et al. Avascular necrosis of the femoral head: inter- and intraobserver variations of Ficat and ARCO classifications. Int Orthop 2008;32:283–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Arlet J, Ficat P. [Non-traumatic avascular femur head necrosis. New methods of examination and new concepts]. Chir Narzadow Ruchu Ortop Pol 1977;42:269–76. [PubMed] [Google Scholar]
- [24].Sen RK. Management of avascular necrosis of femoral head at pre-collapse stage. Indian J Orthop 2009;43:6–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Lai KA, Shen WJ, Yang CY, et al. The use of alendronate to prevent early collapse of the femoral head in patients with nontraumatic osteonecrosis. A randomized clinical study. J Bone Joint Surg Am 2005;87:2155–9. [DOI] [PubMed] [Google Scholar]
- [26].Loke C, Ali SS, Johari V. Pharmacology of anticoagulants. Dis Mon 2012;58:424–30. [DOI] [PubMed] [Google Scholar]
- [27].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009;151:264–9. [DOI] [PubMed] [Google Scholar]
- [28].Chotanaphuti T, Thongprasert S, Laoruengthana A. Low molecular weight heparin prevents the progression of precollapse osteonecrosis of the hip. J Med Assoc Thai 2013;96:1326–30. [PubMed] [Google Scholar]
- [29].Glueck CJ, Freiberg RA, Sieve L, et al. Enoxaparin prevents progression of stages I and II osteonecrosis of the hip. Clin Orthop Relat Res 2005;435:164–70. [DOI] [PubMed] [Google Scholar]
- [30].Glueck CJ, Freiberg RA, Wissman R, et al. Long term anticoagulation (4-16 years) stops progression of idiopathic hip osteonecrosis associated with familial thrombophilia. Adv Orthop 2015;2015:138382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Nagasawa K, Tada Y, Koarada S, et al. Prevention of steroid-induced osteonecrosis of femoral head in systemic lupus erythematosus by anti-coagulant. Lupus 2006;15:354–7. [DOI] [PubMed] [Google Scholar]
- [32].Hofmann S, Mazieres B. [Osteonecrosis: natural course and conservative therapy]. Orthopade 2000;29:403–10. [DOI] [PubMed] [Google Scholar]
- [33].Koo KH, Kim R, Ko GH, et al. Preventing collapse in early osteonecrosis of the femoral head. A randomised clinical trial of core decompression. J Bone Joint Surg Br 1995;77:870–4. [PubMed] [Google Scholar]
- [34].Stulberg BN, Davis AW, Bauer TW, et al. Osteonecrosis of the femoral head. A prospective randomized treatment protocol. Clin Orthop Relat Res 1991;268:140–51. [PubMed] [Google Scholar]


