Abstract
The aim of this study was to compare the mid-term effects and benefits of ultrasound (US)-guided and fluoroscopy (FL)-guided medial branch blocks (MBBs) for chronic lower lumbar facet joint pain through pain relief, functional improvement, and injection efficiency evaluation.
Patients with chronic lumbar facet joint pain who received US (n = 68) or FL-guided MBBs (n = 78) were included in this retrospective study. All procedures were performed under FL or US guidance. Complication frequency, therapeutic effects, functional improvement, and the injection efficiency of MBBs were compared at 1, 3, and 6 months after the last injection.
Both the Oswestry Disability Index (ODI) and the verbal numeric pain scale (VNS) improved at 1, 3, and 6 months after the last injections in both groups. Statistical differences were not observed in ODI and VNS between the groups (P > .05). The proportion of patients who reported successful treatment outcomes showed no significant differences between the groups at different time points. Logistic regression analysis showed that sex, pain duration, injection methods, number of injections, analgesic use, and age were not independent predictors of a successful outcome. US guidance was associated with a significantly shorter performance time.
US-guided MBBs did not show significant differences in analgesic effect and functional improvement compared with the FL-guided approach. Therefore, by considering our data from this retrospective study, US-guided MBBs warrant consideration in the conservative management of lower lumbar facet joint pain.
Keywords: fluoroscopy, nerve block, ultrasound
1. Introduction
Lumbar facet joints have been implicated in the cause of chronic pain in 15% to 45% of patients with chronic low back pain (LBP),[1–5] which was based upon their responses toward controlled, diagnostic blocks according to the criteria set by the International Association for the Study of Pain.[6]
Treatment effects for facet join pain in 3 types of intervention, including intraarticular injection, medial branch nerve block (MBB), and neurolysis using radiofrequency, have been reported.[5] Appropriate management methods for the facet joint pain are still in argument.[7–12] The long-term therapeutic effects of intra-articular injections for the facet joints have not been satisfactory compared with neurolysis using radiofrequency.[7,13] However, they showed that MBBs can be used as an alternative to neurolysis using radiofrequency.[5,7,13]
MBBs using computed tomography (CT) or fluoroscopy (FL) have been performed for the diagnosis and treatment of clinical facet joint pain.[14] The patients may be exposed to considerable radiation dose to identify the symptomatic joint or to rule out facet joint pain during MBBs using CT or FL. However, an ultrasound (US)-guided approach can be safe and reliable without radiation exposures and special spaces for radiation devices.[15] Recently, US-guided MBBs demonstrated high success rates, cost-effectiveness, and fewer complications than conventional methods.[15–18]
However, in previous studies, only needle location, safety, and the short-term therapeutic effects were observed. Hence, this retrospective study aimed to evaluate mid-term pain, and the functional improvement of the patient facilitated by US-guided MBBs in comparison to FL-guided MMBs. In addition, the incidence of adverse event, US treatment outcomes and efficiency (decreased performance time) were also evaluated as secondary outcomes.
2. Methods
2.1. Study design
This retrospective, comparative study of chart data maintained patient privacy and data confidentiality throughout the research process. Approval from the institutional review board of the corresponding author's affiliated university was obtained. This study did not have direct contact with the study group and all the patient identifiers were discarded from the data set at the time of initial collection, thus we got a waiver of informed consent.
2.2. Subjects
Potential participants were those who received US or FL-guided MBBs at the outpatient clinic of the rehabilitation center from January 2013 to December 2014. Their baseline information was checked by self-assessment questionnaires about pain level and functional status. Electronic clinical records and questionnaire responses were retrospectively reviewed to determine data compliance and inclusion criteria.
We selected patients 18 years of age or older who had received a US or FL-guided MBBs for the chronic lumbar spinal pain treatment. And the pain duration should be at least 3 months over. A local anesthetic block was used to diagnose lumbar facet joint pain.[3,5] Patients who did not respond to conservative care including analgesic medication and physical therapy for at least 4 weeks were included in this study. They all reported at least 5 points on the verbal numeric pain scale (VNS) and experienced pain on most days for 3 months over. Patients with a previous history of spinal stenosis, herniated lumbar disc, psychological problems, any active infectious or inflammatory diseases, rheumatoid disorders, or neurological diseases such as lumbar radiculopathies, Parkinson disease, and stroke were excluded. Patients who had received lumbar-related surgical treatment were also excluded.
2.3. Injection methods
FL or US-guided MBBs used in the treatment of symptomatic lower lumbar facet joint pain was common practice in our service. Before the provision of consent, the patient received detailed information regarding the procedure, expected benefits, and risks.
The procedures were performed by a physician (Y. Park) with more than 7 years of experience with US and FL-guided procedures. All treatments were performed as an outpatient procedure. Accuvix XQ (Samsung Medison, Seoul, Korea) with a linear probe at 6 to 12 MHz was used as US device.
In accordance with the standard practice, we performed a US examination to identify all important structures before skin disinfection wrap the US transducer in a sterile cap. Starting at the sacrum, the sonographic long-axis view begins with the transducer positioned at the midline (Fig. 1A).[19] Once the long-axis midline is obtained, the transducer is gently shifted laterally until a “saw-tooth” hyperechoic line is seen; this bony structure represents the superior and inferior articular process of the lumbar laminae (Fig. 1B).[19] As the probe is moved further in the lateral direction, a hyperechoic dotted line representing the transverse processes (TPs) appears and a hyperechoic soft tissue between them (Fig. 1C). The TPs were counted caudally from the highest mark to the 5th lumbar vertebrae and sacrum.
Figure 1.
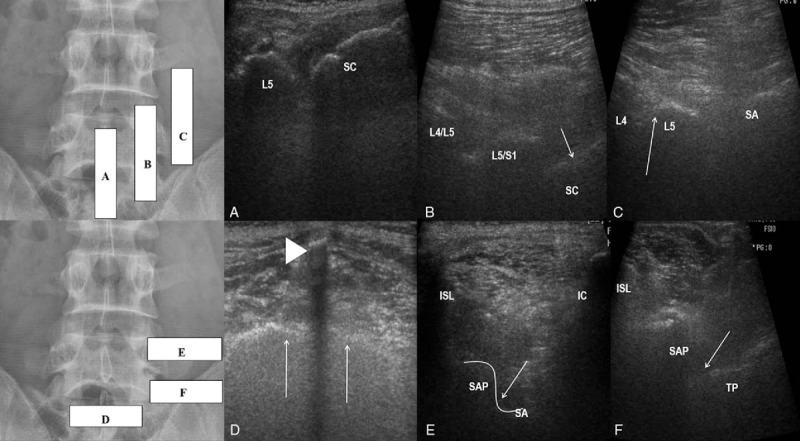
Ultrasound-guided medial branch block. (A) Long-axis view of the lumbar spine showing the L5 spinous process and median S1 crest (SC). (B) Long-axis view of the lumbar spine with L4/L5 and L5/S1 facet joint contours and the S1 (arrow) dorsal foramen. (C) Long-axis view showing the L4 and L5 transverse processes and the sacral ala (SA). Upper edge of the transverse process, or the sacral ala, immediately lateral to the superior articular process is the correct anatomical target (arrow). (D) Short-axis view of the sacrum showing the S1 median crest (arrow head) and the surface of the sacrum (arrow). (E) Short-axis view of L4/L5 segment showing the interspinous ligament (ISL), L5 superior articular process (SAP), and L4 transverse process (TP). Target point on between the L5 superior articular process (SAP) and the L4 transverse process for an approach toward the right-sided L4 medial branch (arrow). (F) Short-axis view of the lumbosacral segment showing the interspinous ligament (ISL), S1 superior articular process (SAP), the sacral ala (SA), and the iliac crest (IC). Target point is between the S1 superior articular process (SAP) and the sacral ala for an approach to the right-sided L5 dorsal ramus (arrow).
After the completion of the long-axis view scan, then the transducer was rotated into a short-axis view to delineate the sacrum as a bony landmark distinguished by the first distinct midline bony protuberance at the level of the S1 median sacral crest (Fig. 1D).[15,19] From the aforementioned landmark, the transducer was then moved cephalad to visualize the junction of the S1 superior articular process (SAP) with sacral ala as the anatomical target for MBBs (L5 dorsal ramus) (Fig. 1E) and the angle between the SAP and TP as the L3/4 MBBs (Fig. 1F).
After sterile skin preparation, a 23-gauge, 9- or 11-cm spinal needle was directed in the US plane at an angle of about 45° to 60° to the skin, advancing laterally and medially until the needle tip reached the target and bony contact (Fig. 2A).[15,16,19] A long-axis paravertebral view was obtained to identify the position of the needle in the cephalad margin of the TP (Fig. 2B).[15,16,18,19] The L5 dorsal ramus block can be technically challenging due to the height of the iliac crest. If the iliac crest covered the field of view, the injection was performed using an out-of-plane (OOP) approach.[19]
Figure 2.
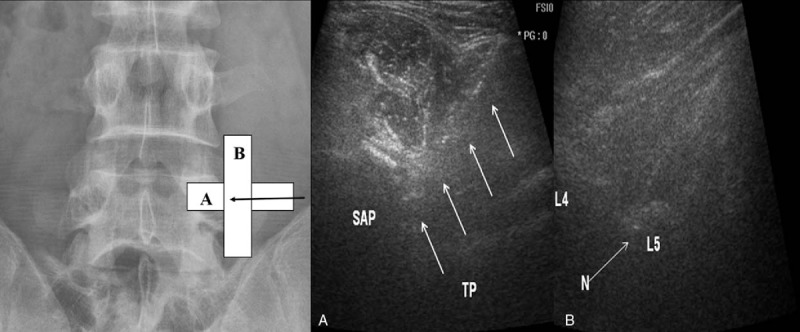
Ultrasound-guided medial branch block by a posterolateral approach with short-axis view and an in-plane free-hand technique. (A) The needle (arrow) is positioned using short axis in-plain approach to the angle between superior articular process (SAP) and the transverse process (TP) for a right-sided L4 medial branch block. (B) Check-up of the needle tip (N) positioned at the upper part of the L5 transverse process (L5) is facilitated using a long-axis and out-of-plane view.
After identification of the needle position following negative aspiration test for blood, a volume of 1 mL of a mixture of 1% lidocaine (0.5 mL) and dexamethasome (5 mg/mL at 0.5 mL) was injected under real-time US guidance with short-axis view. During injection, it was necessary to check for appropriate needle placement by observing for hypoechoic expansion resultant from the injectate via real-time US. A failure to properly identify hypoechoic expansion may indicate improper placement or intravascular injection. Following this, the needle would be repositioned.
All FL-guided MBBs were performed on prone-positioned patients using a posterior approach. At the level L3 to L4, MBBs are done by targeting the junction of the upper border of the TP and SAP.[20,21] The L5 dorsal ramus is blocked in the groove between the ala of the sacrum and the SAP of S1.[20,21] The MBBs were performed on a minimum of 2 nerves to block a single joint.
Spine needle 22-G (Spinocan; BRAUN, Melsungen, Germany) was placed on the anatomical target; 0.2 mL of the nonionic contrast medium Omnipaque 300 (GE Healthcare, Carrigtohill Co., Cork, Ireland) can be injected to test for the incidence of venous uptake. If venous uptake occurred, the needle was readjusted by 1 to 2 mm and the test was repeated.[21] If there was no venous uptake, a volume of 1 mL of a mixture of 1% lidocaine (0.5 mL) and dexamethasone (5 mg/mL at 0.5 mL) was injected into the target nerve (Fig. 3A, B).
Figure 3.
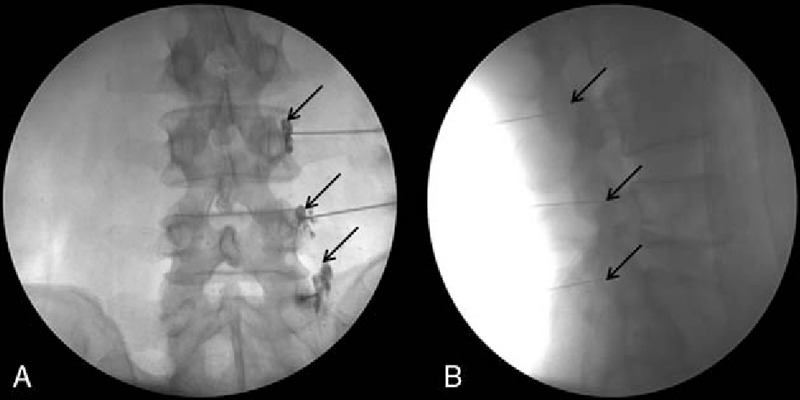
Fluoroscopy-guided medial branch block. (A) Anteroposterior view. The contrast medium filled the L4/L5 superior articular processes for the L3/L4 medial branch block and the groove between the ala of the sacrum and the superior articular process of the sacrum for the L5 dorsal ramus. (B) Lateral view.
Basically, patients received 2 consecutive therapeutic injections at a 2-week interval. The following list indicates the patient satisfaction scores after the therapeutic injections. The patient satisfaction score was measured on the 5th-grade scale after 2 weeks of the first injection (<0, indicating no effect at all; 1, indicating a bad response; 2, indicating a fair response; 3, indicating a good response; and ≥4, indicating an excellent response). Each score, with reference to the patients’ experience of pain alleviation, is indicated thusly: “excellent” meaning that the patient was “satisfied with the treatment result as expected”; “good,” indicating that the pain relief was “not as much as expected but willing to try this treatment next time when pain redevelops”; “fair” indicating that the treatment “had some effect but not enough to choose the same treatment next time when pain re-develops”; and finally, “bad,” indicating that the treatment had the “same effect as the prior treatment or worse.”
However, there were some exceptions to the 2 successive injection protocols. Patients who showed significant improvement for the pain (≥50% improvement on the VNS score) did not get a second injection. A second injection or reevaluation was not considered if the pain worsened, no change, or the patient satisfaction score was less than or equal to the “Fair” grade. If the patient satisfaction score is “Good” despite a VNS score improvement of less than 50%, a second injection was scheduled. As all patients did not show improvement with treatments such as anti-inflammatory drugs and 4-week physical therapy, there were no restrictions on the duration of previous exercise programs, pharmacotherapy, or return to work. Specific physical therapy, occupational therapy, brace, or other specific interventions have not been utilized.
2.4. Review of the clinical date
We used a standardized chart abstraction form to collect demographic data, treatments, pain severity, analgesic use, and functional evaluation. Follow-up interviews were performed by nursing staff who were not involved in the procedure and were performed during the visit at 1, 3, and 6 months postinjection.
Outcome measurement was assessed by the Oswestry Disability Index (ODI), and the VNS. ODI was one of the most commonly used disease-specific measurement tool for patients with LBP.[22] ODI is calculated on the basis of each score, which consists of 10 items. Each of the 10 items is scored from 0 to 5, and the sum is added and multiplied by the factor of 2. Therefore, the ODI ranges from 0 to 100. When using VNS, the patient was asked to rate the pain from 0 to 10, with 0 and 10 representing “no pain” and “worst pain,” respectively. There were a total of 11 integers inclusive of 0 and 10.[23]
Successful outcomes were defined as patients with a VNS score of more than 50% improved and ODI improved by more than 40%. Patients who failed to meet these criteria or who, subsequent to undergoing MBBs, underwent an invasive procedure during a follow-up period were considered to have failed the treatment. Patients with successful treatment outcomes were referred to be responsive and patients without successful treatment outcomes were referred to be nonresponsive. Independent variables such as injection method, number of injections, pain duration, sex, and age were recorded on the medical chart. Predictive variables, such as the classification of the patients’ age, were categorized into 5 age groups: those who were <39 years old, and those between the age brackets of 40 to 49, 50 to 59, 60 to 69, and >70 years old accordingly. Pain duration was also treated as a potential predictor and classified as acute or subacute, chronic for less than 6 months or more than 6 months.[24] The performance time and the number of needle passes were recorded. For the US guidance, performance time was defined as the time interval between the point of contact of the US probe with the patient's skin and the completion of the injectate.[25,26] For FL guidance, performance time was defined as the temporal interval between the first radiographic image and the end of the second injection.[25,26]
We checked if there were any adverse events such as severe back pain just after injection, facial flushing, or vasovagal reaction sign. Each patient handed the questionnaire after the injection and asked them to complete it within 48 hours, and returned it after 2 weeks of follow-up visits.
2.5. Statistics
Age, body mass index (BMI), pain duration, analgesic use, and the number of injections were compared using Pearson Chi-square and Wilcoxon rank-sum test were used. At each time point, VNS and ODI were compared by repeated-measures analysis of variance (ANOVA), and the Bonferroni correction was performed for post-hoc comparison. The Pearson Chi-square was used to test the differences in proportions. Fisher exact test was used wherever the expected value was less than 5. Univariate analysis was performed to evaluate the relationship between the possible outcome predictors and the therapeutic effect by using Pearson Chi-square test. Logistic regression analysis was performed to assess whether the injection method, the number of injections, sex, pain duration, analgesic use, and age were independent predictors of a successful outcome. Statistical analyses were performed with SAS Enterprise Guide 4.1 (SAS Institute Inc. 2006). The level of statistical significance was set as P < .05.
3. Results
Of the 214 MBBs, using either US (n = 94) or FL (n = 120), performed during this study, the inclusion criteria were met for 146 (68.2%) injections. Fifty-four (25.2%) injections were excluded, as the patient did not complete the follow-up survey. Fourteen (6.5%) injections were excluded because patients were withdrawn owing to an incidence of rheumatoid disorders (n = 6) and stroke (n = 8). Finally, 68 patients in the US and 78 patients in the FL group were included in this study (Fig. 4).
Figure 4.
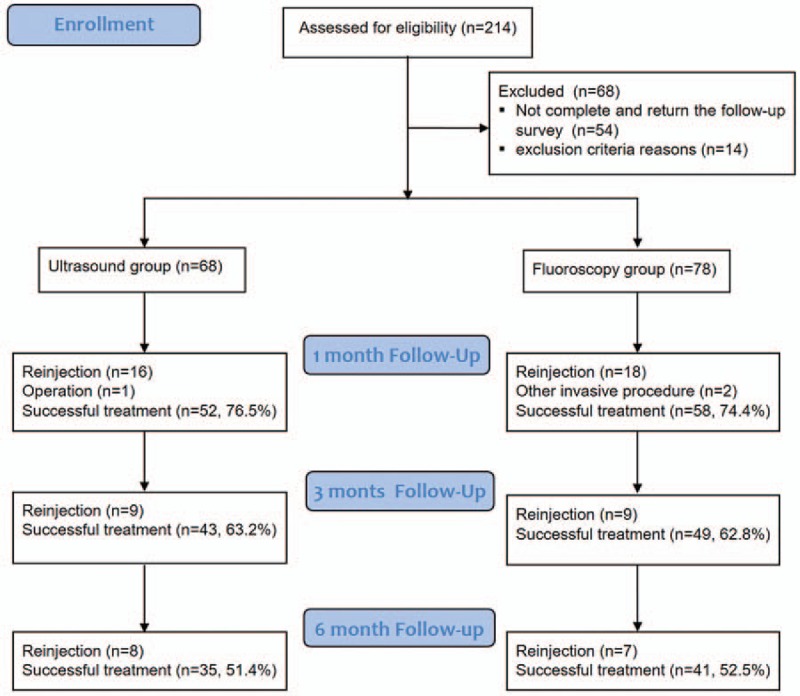
Subjects flow diagram.
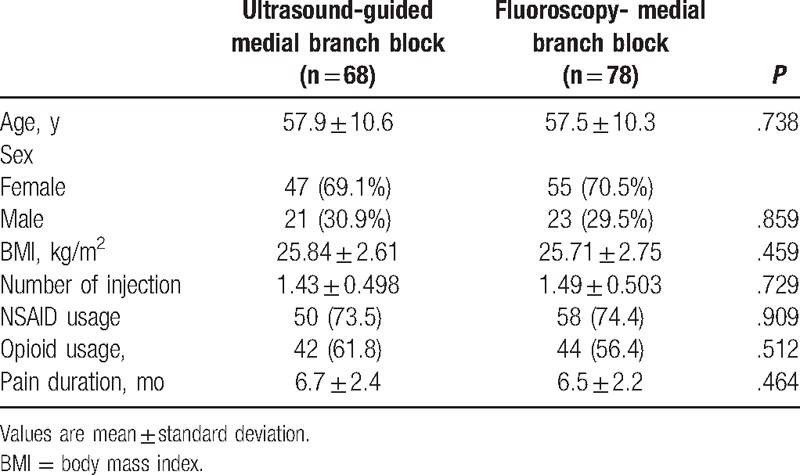
The average age of the patients was 57.5 ± 10.3 years in the FL group and 57.9 ± 10.6 years in the US group, without significant difference. There were no significant differences in general characteristics such as sex, BMI, duration of pain, use of analgesic, and number of injections (Table 1).
Table 1.
General characteristics of the patients.
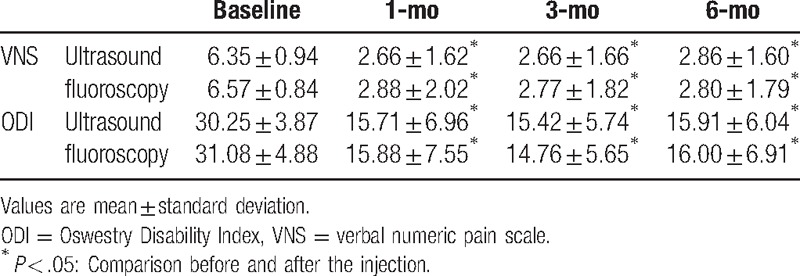
ODI and VNS showed significant improvement at 1, 3, and 6 months after the last injection in both groups. No significant difference in ODI and VNS between the 2 groups was present at baseline, or at 1, 3, and 6 months after the last injections (Table 2). For the period, 16 patients were reinjected and a single patient underwent surgery at the US-guided group for 1 month. Fifty-two patients (76.5%) were successfully treated. Meanwhile, 18 patients were reinjected and 2 patients underwent an invasive procedure at 1 month in the FL-guided group. Fifty-eight (74.4%) patients showed successful treatment outcome. At 3 months, 9 patients were reinjected and 43 (63.2%) patients showed successful outcomes in the US-guided group. In addition, 9 patients were reinjected and 49 (62.8%) patients showed successful outcomes in FL-guided group. At 6 months, 8 patients were reinjected and 35 (51.4%) patients showed successful treatment outcome in the US-guided group. Furthermore, 7 patients were reinjected and 1 patient underwent invasive procedure, with a total of 41 (52.5%) patients showing successful treatment outcome in the FL-guided group (Fig. 4). There was no significant difference in treatment success rates between the 2 groups at each evaluation period.
Table 2.
Comparison of verbal numeric pain scale (VNS) and Oswestry Disability Index (ODI) from baseline to 1, 3, and 6 months after the last injection.
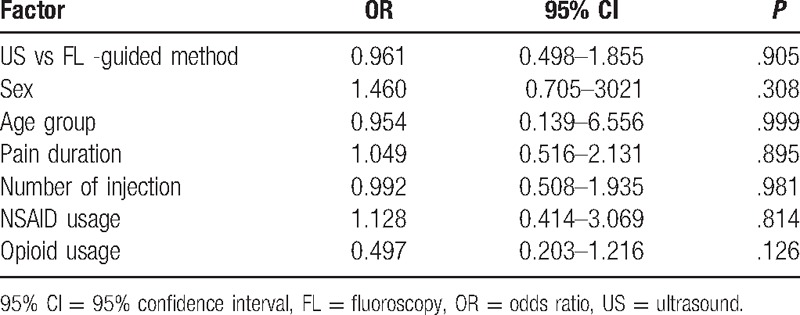
Injection method, sex, age, duration of pain, analgesic use, and number of injection were not independent predictors of the MBBs efficacy as indicated using univariate and multiple logistic regression analyses (P > .05) (Tables 3 and 4).
Table 3.
Univariate analysis for the possible outcome predictors for injection effectiveness at follow-up.
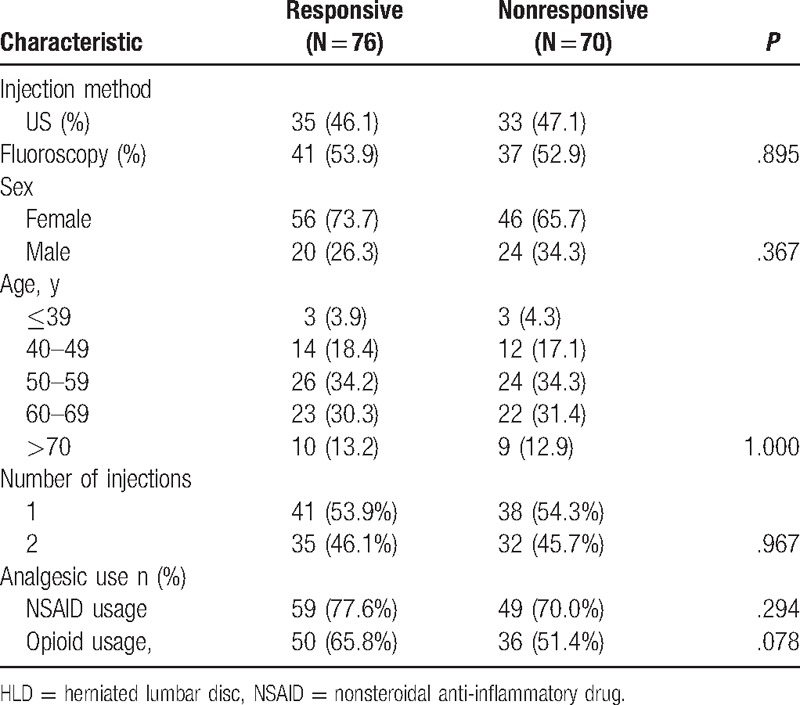
Table 4.
Multiple logistic regression analysis for the possible outcome predictors for injection effectiveness at follow-up.
The performance time was significantly lower with US than with FL (at 323 vs 430 seconds; both P < .001). There was no clinically significant decrease in the use of analgesics (nonsteroidal anti-inflammatory drug, NSAID, and opioid) between the 2 groups at 6 months after injection. Immediately after the procedure, a vasovagal reaction was present in 4 patients in the US group and 6 patients in the FL group. Two patients in the US group and 3 in the FL group showed a transient headache (P > .05). Overall, 3 in the US group and 5 in the FL group reported temporary pain aggravation (back or the lower extremity) 48 hours after injection during 2-week follow-up. None of the patients reported headache suggesting postlumbar puncture syndrome, decompensated heart disease, or diabetes. No case of infection or hematoma was reported for 2 weeks after the procedure. Blood aspiration before injection was reported in 7% of the FL group and 0% of the US group. Intravascular contrast spread was observed in 6% of the FL group.
4. Discussion
This retrospective study showed clinically meaningful and significant improvements in all parameters at the end of a mid-term period in both FL and US group.
Traditionally, MBBs have been performed with FL or CT guidance. However, these methods require an exposure to radiation, have a higher cost relative to other methods, and involve bulky devices.[18] In contrast, US provides an imaging form that is unrelated to radiation exposure and with radiation exposure and identifies soft tissue targets.[18] Greher et al[15] have reported that 50 bilateral US-guided approaches to the lumbar medial branch were performed in 5 embalmed cadavers, in which 45 of the 50 needle tips were located at the correct target point. Shim et al[18] reported that in 20 patients diagnosed with lumbar facet joint mediated pain, 101 needles were positioned by the US toward the lumbar segment under FL control, in which 96 needles were positioned correctly and 2 injections had an intravenous distribution of the contrast agent. The mean pain score on the VNS decreased from 52 to 16 after the block. Greher et al[16] performed 28 US-guided lumbar MBBs in 5 patients. They reported a high success rate, no complications, no sign of a nerve root block, and no incidence of other neurologic symptoms. Two of the five patients had no pain during the evaluation period after 30 minutes and 3 had a 50% reduction in pain scores.
Compared to the FL-guided procedure, the US-guided procedure had several limitations. The resolution of US is limited in the deeper layers due to the physical characteristics of the waves. It could be difficult to check if the needle tip is located at the target point. There are several techniques to resolve this problem. First, Marhofer and Chan[27] described the trichotomous technique of alignment, rotation, and tilting movements of the US transducer while scanning to allow for the improved placement of the needle tip and shaft for better visualization. For the second method, correct injection into the target lesion was similarly identifiable by high shadow filling conferred by US contrast agents.[28] With regard to the third method, hydrolocalization was the term attributed by Bloc et al.[29] This maneuver was generally performed by observing the movement of the surrounding tissue with moving the inserted needle.
The second limitation is that US-guided MBBs cannot clearly detect intravascular injections or inadvertent foraminal spreads. FL-guided MBBs at the lumbar level of the spine are, on average, likely to be intravascular at an incidence of 3.7% of the procedures performed.[30] Therefore, severe complications can occur if arterial corticosteroid injection is performed. Although the location of small vessels can be visualized by color Doppler, it is difficult to detect small and deep vessels in obese people. Even if we did not visualize a small critical vessel through US, we could not necessarily exclude its presence. In the case of adding corticosteroid, nonparticular corticosteroid is recommended for the safety. In the case of infiltration of particular agent into microvessel cannot be identified with US alone, it can cause neurological damage.
The L5 dorsal ramus block can be technically challenging due to the elevation of the iliac crest.[19] If the iliac crest was obscuring the view of the US, the injection would be performed using a short-axis OOP approach.[19] The OOP approach has difficulties with accurately targeting the site of interest, as the on-screen visualization of the hyperechoic dot may ambiguously represent either the needle tip or the needle shaft.[31] In this study, both the short-axis and long-axis views were used to determine the position of the target lesion to ensure for appropriate needle tip placement. When the L5 dorsal ramus block was performed in the OOP approach, the transducer was located at the L5/S1 level in the short-axis view.[19] The angle between SAP S1 and the sacral ala is centered on the image. The needle was inserted directly caudal to the midpoint of the transducer toward the caudocephalad direction until the tip of the needle contacted the bone. Then, the transducer was rotated to obtain the long-axis view, followed by positioning at the sacral ala within the plane of the TP. Hypoechoic expansion generated by the injectate from the needle tip was checked via real-time US. If there was a failure to identify this phenomenon, the needle was guided again to ensure for appropriate needle placement, as to avoid unintentional intravascular injection.
In this study, the performance time was significantly quicker with US than with FL. This may have occurred as a result of 2 reasons. Firstly, fluoroscopic imaging requires anteroposterior (AP), lateral, and oblique views for the appropriate placement of the needle, which is a critical step for safe needle placement and for the correct identification of the target lesion. This can be time-consuming. Furthermore, as the procedure involved intermittent FL, an ample amount of time was required, in addition to the requirement of lengthier performance times to allow for the injection of the contrast media.
In contrast, US allowed for the visualization of the contours of the root of the SAP; these were immediately identifiable in short- or long-axis view and were less affected by the patient's position. In addition, the procedure was quicker because the injection was performed under real-time US showing the needle.
There are some limitations to the current study. First, this study was a retrospective designed study. We selected patients with extensive inclusion and exclusion criteria outlined in the Methods section, but were still able to include patients’ heterogeneity in this study. In addition, we could not entirely exclude the patient participation in other treatments such as medication or physical therapy during follow-up periods from our study. Second, both procedures were performed by 1 physician, and thus, this study only reflected the operator experience of one 1, thereby limiting the generalizability of the study results. Third, we could not exclude a placebo effect for the analgesic treatment effect from lack of control group. Finally, whether or not the injectate was properly injected into the targeted area in US-guided MMBs was not checked by FL. This way may have affected the result. Lastly, the BMI of the patients included in this study was relatively low, and US may not have provided good images of these obese patients. Greher et al[15] reported that the quality of the US image was still adequate even in patients who had a BMI of 36 kg/m2; however, recently, Rauch et al[32] controversially provided strong support that MBBs cannot be performed via US guidance in obese patients. As this study lacked obese participants whose BMI were equal to, or greater than, 30 kg/m2, in addition to there being a lack of significant differences observed in the BMI of patients between groups, the results may not have been affected.
In conclusion, the US-guided procedure did not show significant difference in treatment outcomes for pain reduction and functional improvements compared with the FL-guided procedure, but lacked the associated risks of radiation exposure. Therefore, by considering our data from this retrospective study, US-guided MBBs are deserving of consideration for the conservative management of lower lumbar facet joint pain.
Footnotes
Abbreviations: ANOVA = analysis of variance, BMI = body mass index, FL = fluoroscopy, MBBs = medial branch blocks, ODI = oswestry disability index, OOP = out of plane, US = ultrasound, VNS = verbal numeric pain scale.
SHH and KDP contributed equally to this work as first authors.
Financial disclosure statements have been obtained, and no conflicts of interest have been reported by the authors or by any individuals in control of the content of this article.
We certify that no party having a direct interest in the results of the research supporting this article has or will confer a benefit on us or on any organization with which we are associated.
The authors report no conflicts of interest.
References
- [1].Manchikanti L, Pampati VS, Fellows B, et al. The diagnostic validity and therapeutic value of lumbar facet joint nerve blocks with or without adjuvant agents. Curr Rev Pain 2000;4:337–44. [DOI] [PubMed] [Google Scholar]
- [2].Manchikanti L, Singh V, Pampati V, et al. Evaluation of the relative contributions of various structures in chronic low back pain. Pain Physician 2001;4:308–16. [PubMed] [Google Scholar]
- [3].Manchikanti L, Manchukonda R, Pampati V, et al. Prevalence of facet joint pain in chronic low back pain in postsurgical patients by controlled comparative local anesthetic blocks. Arch Phys Med Rehabil 2007;88:449–55. [DOI] [PubMed] [Google Scholar]
- [4].Manchikanti L, Boswell MV, Singh V, et al. Prevalence of facet joint pain in chronic spinal pain of cervical, thoracic, and lumbar regions. BMC Musculoskelet Disord 2004;5:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Manchikanti L, Singh V, Falco FJ, et al. Lumbar facet joint nerve blocks in managing chronic facet joint pain: one-year follow-up of a randomized, double-blind controlled trial: clinical Trial NCT00355914. Pain Physician 2008;11:121–32. [PubMed] [Google Scholar]
- [6].Merskey H, Bogduk N. Classification of chronic pain. In: Merskey H, Bogduk N, editor. Descriptions of Chronic Pain Syndromes and Definition of Pain Terms. 2nd ed. Seattle, WA: IASP Press; 1994. pp. 180–1. [Google Scholar]
- [7].Boswell MV1, Trescot AM, Datta S, et al. American Society of Interventional Pain Physicians. Interventional Techniques: Evidence Based Practice Guidelines in the Management of Chronic Spinal Pain. Pain Physician 2007;10:7–111. [PubMed] [Google Scholar]
- [8].Boswell MV, Colson JD, Sehgal N, et al. A systematic review of therapeutic facet joint interventions in chronic spinal pain. Pain Physician 2007;10:229–54. [PubMed] [Google Scholar]
- [9].Geurts JW, van Wijk RM, Stolker RJ, et al. Efficacy of radiofrequency procedures for the treatment of spinal pain: a systematic review of randomized clinical trials. Reg Anesth Pain Med 2001;26:394–400. [DOI] [PubMed] [Google Scholar]
- [10].Niemisto L, Kalso E, Malmivaara A, et al. Cochrane Collaboration Back Review Group. Radiofrequency denervation for neck and back pain: a systematic review within the framework of the Cochrane Collaboration Back Review Group. Spine 2003;28:1877–88. [DOI] [PubMed] [Google Scholar]
- [11].Nelemans P, de Bie R, de Vet HC, et al. WITHDRAWN: injection therapy for subacute and chronic benign lowback pain. Cochrane Database Syst Rev 2007;3:CD001824. [DOI] [PubMed] [Google Scholar]
- [12].Manchikanti L, Singh V, Vilims B, et al. Medial branch neurotomy in management of chronic spinal pain: systematic review of the evidence. Pain Physician 2002;5:405–18. [PubMed] [Google Scholar]
- [13].Boswell MV, Colson JD, Spillane WF. Therapeutic facet joint interventions in chronic spinal pain: a systematic review of effectiveness and complications. Pain Physician 2005;8:101–14. [PubMed] [Google Scholar]
- [14].Kennedy DJ, Shokat M, Visco CJ. Sacroiliac joint and lumbar zygapophysial joint corticosteroid injections. Phys Med Rehabil Clin N Am 2010;21:835–42. [DOI] [PubMed] [Google Scholar]
- [15].Greher M, Kirchmair L, Enna B, et al. Ultrasound-guided lumbar facet nerve block: accuracy of a new technique confirmed by computed tomography. Anesthesiology 2004;101:1195–200. [DOI] [PubMed] [Google Scholar]
- [16].Greher M, Scharbert G, Kamolz LP, et al. Ultrasound-guided lumbar facet nerve block: a sonoanatomic study of a new methodologic approach. Anesthesiology 2004;100:1242–8. [DOI] [PubMed] [Google Scholar]
- [17].Ha DH, Shim DM, Kim TK, et al. Comparison of ultrasonography- and fluoroscopy-guided facet joint block in the lumbar spine. Asian Spine J 2010;4:15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Shim JK, Moon JC, Yoon KB, et al. Ultrasound-guided lumbar medial-branch block: a clinical study with fluoroscopy control. Reg Anesth Pain Med 2006;31:451–4. [DOI] [PubMed] [Google Scholar]
- [19].Siegenthaler A, Eichenberger U. Ultrasound-guided lumbar zygopophysial (facet) nerve blocks. In: Narouze SN, editor. Atlas of Ultrasound-Guided Procedures in Interventional Pain Management. New York: Springer; 2011. 149–60. [Google Scholar]
- [20].Bogduk N. Evidence-informed management of chronic low back pain with facet injections and radiofrequency neurotomy. Spine J 2008;8:56–64. [DOI] [PubMed] [Google Scholar]
- [21].Bogduk N. International Spinal Intervention Society. Lumbar medial branch blocks. Practice guidelines for spinal diagnostic and treatment procedures 1st ed.San Francisco, CA: International Spinal Intervention Society; 2004. 47–65. [Google Scholar]
- [22].Fairbank JC, Pynsent PB. The Oswestry Disability Index. Spine (Phila Pa 1976) 2000;25:2940–52. [DOI] [PubMed] [Google Scholar]
- [23].Hartrick CT, Kovan JP, Shapiro S. The numeric rating scale for clinical pain measurement: a ratio measure? Pain Pract 2003;3:310–6. [DOI] [PubMed] [Google Scholar]
- [24].Sontag MJ. Andrew J, Stanley A. A theoretical overview of the diagnosis and management of low back pain. The Low Back Pain Handbook: a Guide for the Practicing Clinician 1st ed.Philadelphia, PA: Hanley & Belfus; 1993. 39–48. [Google Scholar]
- [25].Finlayson RJ, Etheridge JP, Tiyaprasertkul W, et al. A prospective validation of biplanar ultrasound imaging for C5-C6 cervical medial branch blocks. Reg Anesth Pain Med 2014;39:160–3. [DOI] [PubMed] [Google Scholar]
- [26].Finlayson RJ, Etheridge JP, Vieira L, et al. A randomized comparison between ultrasound- and fluoroscopy-guided third occipital nerve block. Reg Anesth Pain Med 2013;38:212–7. [DOI] [PubMed] [Google Scholar]
- [27].Marhofer P, Chan VW. Ultrasound-guided regional anesthesia: current concepts and future trends. Anesth Analg 2007;104:1265–9. [DOI] [PubMed] [Google Scholar]
- [28].Luchs JS, Sofka CM, Adler RS. Sonographic contrast effect of combined steroid and anesthetic injections: in vitro analysis. J Ultrasound Med 2007;26:227–31. [DOI] [PubMed] [Google Scholar]
- [29].Bloc S, Ecoffey C, Dhonneur G. Controlling needle tip progression during ultrasound-guided regional anesthesia using the hydrolocalization technique. Reg Anesth Pain Med 2008;33:382–3. [DOI] [PubMed] [Google Scholar]
- [30].Verrills] P, Mitchell B, Vivian D, et al. The incidence of intravascular penetration in medial branch blocks: cervical, thoracic, and lumbar spines. Spine (Phila Pa 1976) 2008;15:174–7. 33. [DOI] [PubMed] [Google Scholar]
- [31].Souzdalnitski D, Lerman I, Halaszynski TM. Narouze SN. Atlas of ultrasound-guided procedures in interventional pain management. How to Improve Needle Visibility. New York: Springer; 2011. 35–75. [Google Scholar]
- [32].Rauch S, Kasuya Y, Turan A, et al. Ultrasound-guided lumbar medial branch block in obese patients: a fluoroscopically confirmed clinical feasibility study. Reg Anesth Pain Med 2009;34:340–2. [DOI] [PubMed] [Google Scholar]


