Abstract
In this study, we aimed to compare the effect of desflurane and sevoflurane on postoperative nausea and vomiting and pain in patients receiving opioid-based intravenous patient-controlled analgesia (IV-PCA) after thyroidectomy.
We reviewed the electronic medical records of 1042 patients administered opioid-based IV-PCA after a thyroidectomy at Chung-Ang University Hospital between January 1, 2010 and June 30, 2016. We classified the patients into 2 groups according to the inhalation anesthetic used for anesthesia: desflurane versus sevoflurane (groups D and S, n = 587 and 455, respectively). Then, propensity scoring was used to select 234 matched subjects between both groups based on their confounding factors. A propensity score matching method was used to match patients from the 2 groups in a 1:1 ratio.
Before the propensity score analysis, there was no significant difference between the 2 groups. However, after the propensity score matching, the frequency of complete remission (CR, defined as no nausea and vomiting) was significantly higher in group S than it was in group D. The number of patients administered rescue antiemetics on day 0 in group S was lower than that in group D, although it was not statistically significant.
In patients receiving opioid-based IV-PCA after thyroidectomy, sevoflurane seems to be more beneficial in achieving CR than desflurane was. However, further randomized controlled studies are needed to confirm this conclusion.
Keywords: desflurane, opioid-based patient control analgesics, postoperative nausea and vomiting, sevoflurane, thyroidectomy
1. Introduction
Thyroid cancer is the most common malignancy of the endocrine system.[1] In particular, papillary thyroid cancer is the 5th major malignancy, and most afflicted women have a favorable prognosis.[2] For the treatment of this condition, open thyroidectomy is performed worldwide with low morbidity and mortality.[3] Thyroidectomy is associated with relatively moderate pain; therefore, many patients require pain control and management during the early postoperative phase,[4,5] and the pain can progress to a chronic form. Patient-controlled analgesia (PCA), which allows patients to self-administer pain medications, is known to control postoperative pain efficiently.[5,6] However, opioid-based PCA causes side effects such as postoperative nausea and vomiting (PONV).[7–9]
Numerous factors, such as sex, history of smoking and motion sickness, use of opioid and method for anesthesia, and type of surgery, are related to the induction of PONV. On the basis of these factors, the incidence of PONV is high in patients after thyroidectomy[4,10,11] and is reported to be 44% to 80%.[12,13] Vagal stimulation during surgical manipulation and changes in the level of circulating thyroid hormones play a role in triggering nausea.[10] Further, there is strong evidence that volatile anesthetics are emetogenic.[14]
Desflurane and sevoflurane have a characteristic low blood solubility, which results in a rapid onset of action and emergence from anesthesia.[15,16] However, their use increases PONV in a dose-dependent manner irrespective of the choice of the agent.[17] Numerous studies have compared the effect of desflurane and sevoflurane on PONV with conflicting results. Although a recent meta-analysis by Macario et al[18] showed no difference in PONV frequency between desflurane and sevoflurane use, some studies reported an increased incidence with desflurane compared to that observed with sevoflurane use.[19,20]
Therefore, we compared the effects of sevoflurane and desflurane on PONV in patients receiving opioid-based intravenous patient-controlled analgesia (IV-PCA) after thyroidectomy by using a propensity-score matching analysis. Additionally, we compared the severity of postoperative pain between both treatments.
2. Methods
The Institutional Review Board at Chung-Ang University Hospital approved the present study (IRB no C2016056 [1799]). This was a retrospective cohort study using prospectively collected data of 1042 patients receiving fentanyl-based IV-PCA after thyroidectomies at Chung-Ang University Hospital from January 1, 2010 to June 30, 2016. Since the study involved the evaluation of preexisting de-identified electronic medical records of patients, the requirement for informed consent was waived. The Strengthening the Reporting of Observational Studies in Epidemiology checklist was used for the preparation of this manuscript.[21]
2.1. PCA protocol
In our institution, we used the existing standardized IV-PCA protocol of the Department of Anesthesiology and Pain Medicine: standardized to continuously infuse 1 mL/h and a 1-mL bolus with a 15-min lockout interval. For the thyroidectomy, the 100-mL IV-PCA solution contained 15 μg/kg fentanyl, ketorolac 90 mg or nefopam 60 mg, ramosetron 0.3 mg, and palonosetron 0.25 mg or granisetron 3 mg.
2.2. Data collection
Using the data recorded by a nurse dedicated to the management of patients administered IV-PCA, we noted the demographic and perioperative factors related to PONV. The nurse undertook only the tasks related to IV-PCA and made the rounds at least once a day to investigate associated issues including pain and PONV. She had 5 years of clinical experience and collected the data after being trained in the standardized protocols of pain and PONV investigation. We excluded the data collected during the first 2 years of the PCA rounds of the nurse (2008–2009). The data consisted of 18 demographic characteristics and perioperative factors known to be closely related to PONV. Specifically, we collected data on the type of anesthetic agents used (desflurane vs. sevoflurane), age, sex, height, weight, history of smoking, PONV, use of nitrous oxide (N2O) and remifentanil, use of anticholinergics (e.g., glycopyrrolate) as premedication, use of preintubation and intraoperative opioids, and the use of nefopam, ketorolac, ramosetron, palonosetron, or granisetron in IV-PCA, dosage of fentanyl in IV-PCA, and operation duration.
Additionally, the severity of pain and nausea, number of vomiting episodes, headache, use of rescue antiemetics and analgesics, and complete remission (CR) measured on postoperative day 0 and 1 were analyzed. CR was defined as no nausea and vomiting during postoperative day 0 and 1. Severities of pain and nausea were recorded using a 10-point visual analog scale (VAS) and a numeric rating scale (0 = no nausea, 1 = mild, 2 = moderate, 3 = severe, and 4 = worst imaginable), respectively.
2.3. Inclusion and exclusion criteria
All the patients who underwent a thyroidectomy with inhalation anesthetics were included. The exclusion criteria were age < 16 years, performance of reoperation, no report of the type of inhalation anesthetics used, use of total IV anesthesia, and refusal of PCA.
2.4. Statistical analysis
Both groups (S and D) were compared based on demographic and perioperative characteristics using the chi-squared or Fisher exact test for categorical data and compared t tests were used for continuous variables. Because this was a retrospective cohort study, patients were not randomized before the interventions. Therefore, we used a propensity score matching method to reduce the bias due to confounding.[22] A logistic regression model was created to calculate the propensity score. Patients in each group were allocated a propensity score, which reflected their possibility of going under general anesthesia with desflurane. The following variables were tested to determine the propensity score: age, height, weight, smoking status, history of PONV, type of anesthetic agent (desflurane vs. sevoflurane), premedication, use of N2O and remifentanil, opioid administration before intubation, and PCA constituents including fentanyl, ketorolac, nefopam, ramosetron, palonosetron, and granisetron.[23]
The patients in each group were matched in a 1:1 ratio using a propensity score matching method. A patient in group S was matched with 1 in group D based on the similarity of their propensity scores. Patients whose caliper radius was over 0.001 at the nearest available matching were excluded from this study.
The standardized differences for covariates were tested to estimate the relevance of balance between the matched groups. Standardized difference is the difference in means between both groups in units of standard deviation. If the standardized difference between both groups was <20%, their comparability was considered to be good.[24]
We additionally calculated the simplified Apfel score before and after propensity score matching.[25]
For continuous variables, data distribution was first evaluated for normality using the Shapiro–Wilk test. Normally distributed data were then compared using parametric methods while non-normally distributed data were analyzed using nonparametric methods. Before the matching, an unpaired t test or Mann–Whitney U test was used to compare the continuous variables, and the chi-squared test or Fisher exact test was used to compare the descriptive variables.
After the matching, statistical differences between groups D and S were evaluated using the paired t test, the Wilcoxon signed-rank test, or the McNemar test. The continuous variables were expressed as the mean ± standard deviation and the descriptive variables were expressed as absolute numbers (%). The 95% confidence intervals for the difference were also calculated. P values < .05 were considered statistically significant. All the analyses were conducted using the statistical package for the social sciences software suite (version 23, IBM Corp, Armonk, NY).
3. Results
We reviewed the records of 1042 patients who received opioid-based IV-PCA after thyroidectomy. The number of patients who were administered sevoflurane and desflurane inhalation anesthesia was 455 and 587, respectively. The demographic characteristics and perioperative elements are shown in Table 1. We generated 234 matched pairs using the propensity score matching, which was effectively performed for both groups to counterpoise each preoperative variable.
Table 1.
Patient characteristics in total and matched cohorts.
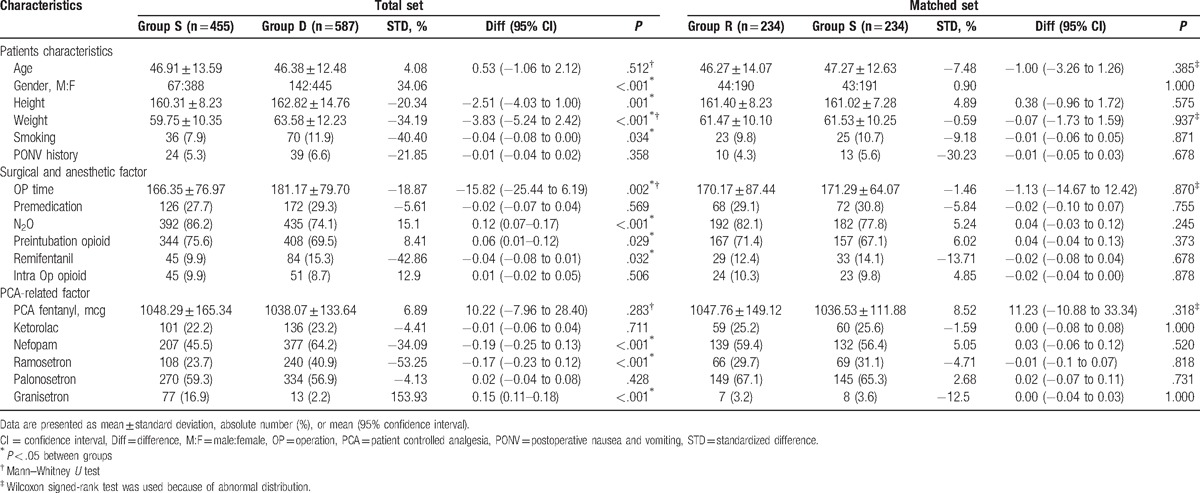
Before but not after the propensity score matching, the sex, height, weight, operation time, and N2O, preintubation opioid, remifentanil, nefopam, ramosetron, and granisetron use in PCA were significantly different between both groups (Table 1). Simplified Apfel score was significantly higher in group S than that in group D before propensity score matching (2.83 ± 0.55 vs. 2.71 ± 0.70, P = .002), but this difference disappeared after propensity score matching (2.76 ± 0.60 vs. 2.77 ± 0.65, P = .883).
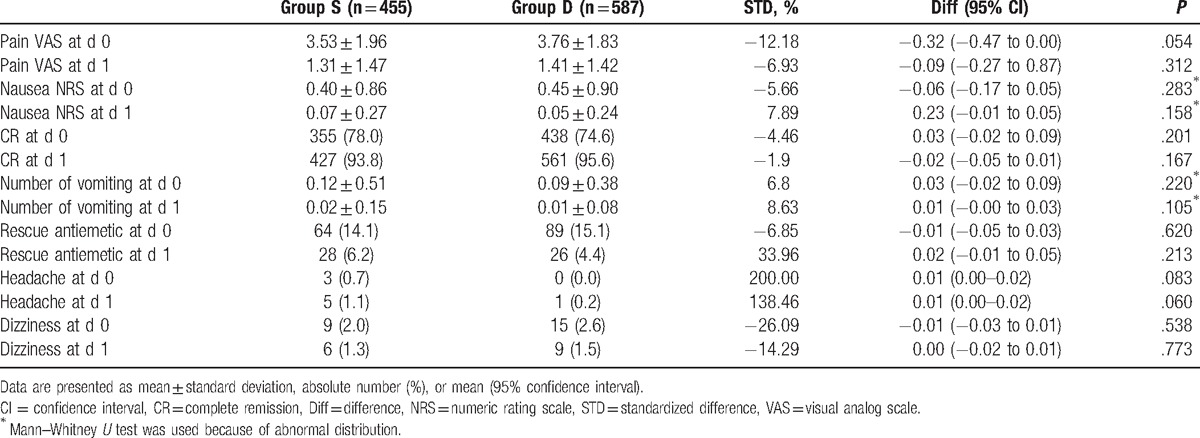
There was no significant difference between both groups before the propensity score matching (Table 2).
Table 2.
Postoperative variables in total unmatched cohorts.
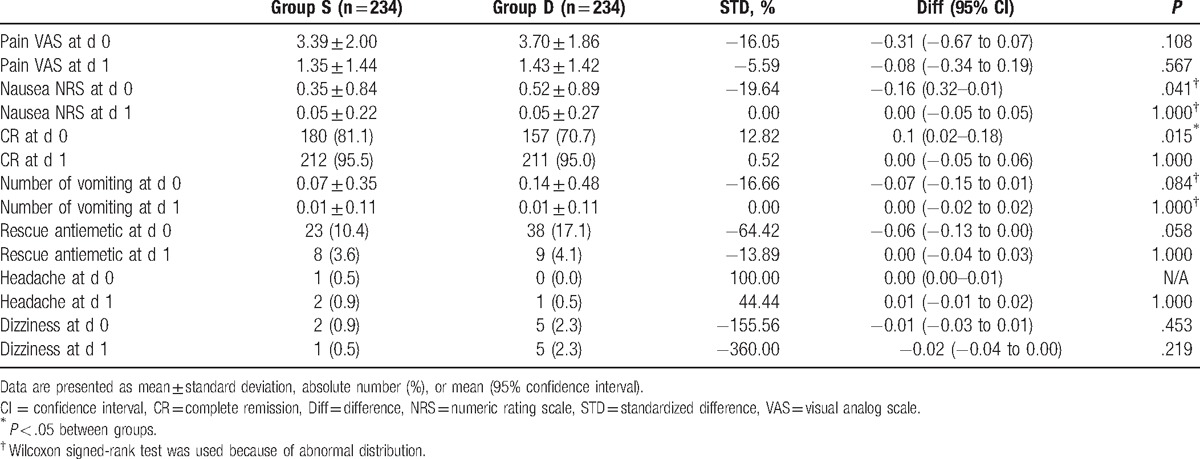
The propensity-matched set showed a significant difference in CR on day 0 between the 180 and 157 patients in groups S and D (81.1% vs. 70.7%, P = .015). The number of patients who required rescue antiemetic was not significantly different between group S and group D (10.4% vs. 17.1%, P = .058) (Table 3).
Table 3.
Postoperative variables in matched cohorts.
4. Discussion
In this study, there were no significant differences in the incidence of PONV between the 2 groups before the propensity score matching. However, sevoflurane was more beneficial than desflurane in achieving CR on day 0 after the propensity score matching (180 [81.1%] vs. 157 [70.7%], P = .015). Further, although it was not statistically significant (P = .058), the number of patients administered rescue antiemetics on postoperative day 0 was lower in group S than that in group D (10.4% vs. 17.1%).
Thyroidectomy is associated with relatively moderate pain, and many patients require pain control medication during the early postoperative phase[4,5]; this pain can progress to the chronic form. Opioid-based PCA, which allows patients to self-administer pain medications, is widely used in both major and minor surgeries and provides a safe and efficient strategy for controlling postoperative pain.[5,6] However, opioid-based PCA causes side effects such as PONV.[7–9]
PONV is one of the complications that occur after anesthesia and surgery.[7,14] Many patients find it harder to tolerate PONV than postoperative pain. Despite recent drug advances, the incidence of PONV is approximately 20% to 30%.[17,26] PONV may result in patient discomfort, dissatisfaction, aspiration of gastric contents, and suture dehiscence. PONV development is associated with various factors including female sex, history of PONV or motion sickness, smoking status, younger age, duration of volatile anesthetic administration, and use of postoperative opioids.[17] Additionally, the characteristics of the patients, surgery, and type of anesthesia influence PONV development.[26–28]
In this study, all the patients had at least 1 risk factor for the Apfel score (opioid-based PCA), and nearly 80% of the patients were women. Further, the incidence of PONV is known to increase in thyroidectomy, particularly when volatile anesthetics are used. Patients undergoing thyroid surgery are at high risk for PONV development. Sonner et al[10] hypothesized that vagal stimulation during surgical manipulation of the neck may be responsible. Additionally, surgical manipulation contributes to the changes in circulating thyroid hormone levels, which play a role in triggering nausea.[10]
Apfel et al[7] reported that volatile anesthetics were the single greatest factor affecting the incidence of emesis in the first 2 h after an operation and their use increased PONV in a dose-dependent manner irrespective of the choice of the agent.[11] Moreover, volatile anesthetics are strongly responsible for the induction of PONV, and their avoidance reduces the incidence of PONV by 19%.[29]
Both sevoflurane and desflurane are inhalation anesthetics with low solubility. They are widely used for general anesthesia because of their various advantages including rapid induction and emergence.[15,16] In this study, there was a significant difference in CR on day 0 between 180 and 157 patients in groups S and D (81.1% and 70.7%, respectively, P = .015). This result was consistent with that of another study, which reported that compared with desflurane, sevoflurane decreased the incidence of PONV.[19] Specifically, a lower incidence of PONV was observed after sevoflurane than after desflurane anesthesia (36% vs. 67%, respectively) after breast surgery.[30] We assumed that the higher frequency of PONV in group D might be due to higher irritation and shorter emergence time of desflurane compared with those of sevoflurane. More airway irritation of desflurane may increase PONV. Shorter emergence time of desflurane than sevoflurane might make the patients in group D request more PCA bolus than those in group S. And more frequent analgesic request (especially opioid) leads to higher incidence of PONV. Additionally, higher degree of drowsiness in patients anesthetized with sevoflurane in the early recovery period may shield the patients from experiencing early PONV in group S.[30]
However, the results of our study differed from those of a previous meta-analysis[18] that showed no difference in PONV frequency between desflurane and sevoflurane. These differences may be because our study included high-risk patients (opioid-based PCA, a high proportion of women, nonsmokers, and thyroid surgery), and the meta-analysis included heterogeneous types of surgeries and patients.
There were no statistically significant differences in severity of pain between the 2 groups in the present study. These results are consistent with those of previous studies, which reported that there was no statistically significant difference in the postoperative pain associated with sevoflurane and desflurane.[31,32] However, these results are different from those of Iannuzzi et al[33] who reported that patients receiving desflurane exhibited a faster recovery from anesthesia but an earlier and more intense perception of pain after surgery than those who received sevoflurane. This observation might have been due to the differences in the types of surgery, the intensity of pain, and the strategy of analgesic use.
Remifentanil and N2O are commonly used as adjuvant anesthetic to clinical practices. Use of N2O itself is also a well-known risk factor for PONV.[17] As remifentanil can induce acute tolerance and hyperalgesia,[34] use of remifentanil during surgery can increase the postoperative analgesic use. Thus, remifentanil can also induce higher PONV theoretically. However, the incidence and severity of PONV was lower in the remifentanil group than those in the N2O group in patients receiving fentanyl-based IV-PCA after thyroidectomy.[35] Thus, the differences in use of remifentanil and N2O can lead to differences in severity and incidence of PONV. In our study, although uses of remifentanil and N2O were significantly different before matching, these differences were disappeared after matching. Thus, these differences may not affect the degree of PONV.
The present study has several limitations. First, the nurse evaluated the patients’ postoperative symptoms only once a day during the visit to patients who received opioid-based PCA. Patients were asked to rate their symptoms using the numerical rating scale for nausea, the number of vomiting episodes, headache, dizziness, and the VAS of pain. This might have caused recall bias, and the incidence of PONV might be underestimated. Second, as this was a retrospective study, the relationship between the cause and effect could not be proven. Because there might be selection bias and unmeasured confounding factors, we did our best to analogize the best correlation from our observation study using propensity score matching.
The propensity score matching was based on the type of inhalation anesthetic. Therefore, we were able to minimize the bias and confounding. Third, this was a single-center study conducted in Korea. A multicenter study would be able to provide more accurate and reliable results.
Despite these limitations, this study can be considered to provide a meaningful comparison of the effect of sevoflurane with that of desflurane on PONV, especially in a high-risk group of patients administered inhalation anesthetics during thyroidectomy and opioid-based PCA after thyroidectomy.
Footnotes
Abbreviations: CR = complete remission, IV-PCA = intravenous patient-controlled analgesia, N2O = nitrous oxide, NRS = numerical rating scale, OP = operation, PCA = patient-controlled analgesia, PONV = postoperative nausea and vomiting, VAS = visual analog scale.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Balta AZ, Filiz AI, Kurt Y, et al. Prognostic value of oncoprotein expressions in thyroid papillary carcinoma. Med Oncol 2012;29:734–41. [DOI] [PubMed] [Google Scholar]
- [2].Hung W, Sarlis NJ. Current controversies in the management of pediatric patients with well-differentiated nonmedullary thyroid cancer: a review. Thyroid 2002;12:683–702. [DOI] [PubMed] [Google Scholar]
- [3].Bae DS, Koo do H, Choi JY, et al. Current status of robotic thyroid surgery in South Korea: a web-based survey. World J Surg 2014;38:2632–9. [DOI] [PubMed] [Google Scholar]
- [4].Aunac S, Carlier M, Singelyn F, et al. The analgesic efficacy of bilateral combined superficial and deep cervical plexus block administered before thyroid surgery under general anesthesia. Anesth Analg 2002;95:746–50. [DOI] [PubMed] [Google Scholar]
- [5].Gozal Y, Shapira SC, Gozal D, et al. Bupivacaine wound infiltration in thyroid surgery reduces postoperative pain and opioid demand. Acta Anaesthesiol Scand 1994;38:813–5. [DOI] [PubMed] [Google Scholar]
- [6].Hwang BY, Kwon JY, Kim E, et al. Oxycodone vs. fentanyl patient-controlled analgesia after laparoscopic cholecystectomy. Int J Med Sci 2014;11:658–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Apfel CC, Kranke P, Katz MH, et al. Volatile anaesthetics may be the main cause of early but not delayed postoperative vomiting: a randomized controlled trial of factorial design. Br J Anaesth 2002;88:659–68. [DOI] [PubMed] [Google Scholar]
- [8].Koivuranta M, Laara E, Snare L, et al. A survey of postoperative nausea and vomiting. Anaesthesia 1997;52:443–9. [DOI] [PubMed] [Google Scholar]
- [9].Song JW, Park EY, Lee JG, et al. The effect of combining dexamethasone with ondansetron for nausea and vomiting associated with fentanyl-based intravenous patient-controlled analgesia. Anaesthesia 2011;66:263–7. [DOI] [PubMed] [Google Scholar]
- [10].Sonner JM, Hynson JM, Clark O, et al. Nausea and vomiting following thyroid and parathyroid surgery. J Clin Anesth 1997;9:398–402. [DOI] [PubMed] [Google Scholar]
- [11].Apfel CC, Greim CA, Haubitz I, et al. The discriminating power of a risk score for postoperative vomiting in adults undergoing various types of surgery. Acta Anaesthesiol Scand 1998;42:502–9. [DOI] [PubMed] [Google Scholar]
- [12].Ewalenko P, Janny S, Dejonckheere M, et al. Antiemetic effect of subhypnotic doses of propofol after thyroidectomy. Br J Anaesth 1996;77:463–7. [DOI] [PubMed] [Google Scholar]
- [13].Li B, Wang H. Dexamethasone reduces nausea and vomiting but not pain after thyroid surgery: a meta-analysis of randomized controlled trials. Med Sci Monit 2014;20:2837–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Apfel CC, Stoecklein K, Lipfert P. PONV: a problem of inhalational anaesthesia? Best Pract Res Clin Anaesthesiol 2005;19:485–500. [DOI] [PubMed] [Google Scholar]
- [15].Choi GJ, Baek CW, Kang H, et al. Emergence agitation after orthognathic surgery: a randomised controlled comparison between sevoflurane and desflurane. Acta Anaesthesiol Scand 2015;59:224–31. [DOI] [PubMed] [Google Scholar]
- [16].Wallenborn J, Rudolph C, Gelbrich G, et al. The impact of isoflurane, desflurane, or sevoflurane on the frequency and severity of postoperative nausea and vomiting after lumbar disc surgery. J Clin Anesth 2007;19:180–5. [DOI] [PubMed] [Google Scholar]
- [17].Apfel CC, Heidrich FM, Jukar-Rao S, et al. Evidence-based analysis of risk factors for postoperative nausea and vomiting. Br J Anaesth 2012;109:742–53. [DOI] [PubMed] [Google Scholar]
- [18].Macario A, Dexter F, Lubarsky D. Meta-analysis of trials comparing postoperative recovery after anesthesia with sevoflurane or desflurane. Am J Health Syst Pharm 2005;62:63–8. [DOI] [PubMed] [Google Scholar]
- [19].Choi JB, Shim YH, Lee YW, et al. Incidence and risk factors of postoperative nausea and vomiting in patients with fentanyl-based intravenous patient-controlled analgesia and single antiemetic prophylaxis. Yonsei Med J 2014;55:1430–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Hough MB, Sweeney B. Postoperative nausea and vomiting in arthroscopic day-case surgery: a comparison between desflurane and isoflurane. Anaesthesia 1998;53:910–4. [DOI] [PubMed] [Google Scholar]
- [21].von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 2008;61:344–9. [DOI] [PubMed] [Google Scholar]
- [22].Perkins SM, Tu W, Underhill MG, et al. The use of propensity scores in pharmacoepidemiologic research. Pharmacoepidemiol Drug Safety 2000;9:93–101. [DOI] [PubMed] [Google Scholar]
- [23].Brookhart MA, Schneeweiss S, Rothman KJ, et al. Variable selection for propensity score models. Am J Epidemiol 2006;163:1149–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Austin PC. A critical appraisal of propensity-score matching in the medical literature between 1996 and 2003. Stat Med 2008;27:2037–49. [DOI] [PubMed] [Google Scholar]
- [25].Apfel CC, Laara E, Koivuranta M, et al. A simplified risk score for predicting postoperative nausea and vomiting: conclusions from cross-validations between two centers. Anesthesiology 1999;91:693–700. [DOI] [PubMed] [Google Scholar]
- [26].Watcha MF, White PF. Postoperative nausea and vomiting. Its etiology, treatment, and prevention. Anesthesiology 1992;77:162–84. [DOI] [PubMed] [Google Scholar]
- [27].Lerman J. Surgical and patient factors involved in postoperative nausea and vomiting. Br J Anaesth 1992;69:24s–32s. [DOI] [PubMed] [Google Scholar]
- [28].Rabey PG, Smith G. Anaesthetic factors contributing to postoperative nausea and vomiting. Br J Anaesth 1992;69:40s–5s. [DOI] [PubMed] [Google Scholar]
- [29].Apfel CC, Korttila K, Abdalla M, et al. A factorial trial of six interventions for the prevention of postoperative nausea and vomiting. N Engl J Med 2004;350:2441–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Karlsen KL, Persson E, Wennberg E, et al. Anaesthesia, recovery and postoperative nausea and vomiting after breast surgery. A comparison between desflurane, sevoflurane and isoflurane anaesthesia. Acta Anaesthesiol Scand 2000;44:489–93. [DOI] [PubMed] [Google Scholar]
- [31].Ortiz J, Chang LC, Tolpin DA, et al. Randomized, controlled trial comparing the effects of anesthesia with propofol, isoflurane, desflurane and sevoflurane on pain after laparoscopic cholecystectomy. Braz J Anesthesiol 2014;64:145–51. [DOI] [PubMed] [Google Scholar]
- [32].Ergonenc J, Ergonenc T, Idin K, et al. The recovery time of sevoflurane and desflurane and the effects of anesthesia on mental and psychomotor functions and pain. Anesth Essays Res 2014;8:367–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Iannuzzi E, Iannuzzi M, Viola G, et al. Desflurane and sevoflurane in elderly patients during general anesthesia: a double blind comparison. Minerva Anestesiol 2005;71:147–55. [PubMed] [Google Scholar]
- [34].Angst MS, Clark JD. Opioid-induced hyperalgesia: a qualitative systematic review. Anesthesiology 2006;104:570–87. [DOI] [PubMed] [Google Scholar]
- [35].Kim MK, Yi MS, Kang H, et al. Effects of remifentanil versus nitrous oxide on postoperative nausea, vomiting, and pain in patients receiving thyroidectomy: propensity score matching analysis. Medicine 2016;95:e5135. [DOI] [PMC free article] [PubMed] [Google Scholar]


