Abstract
Background:
Prediction of acute kidney transplant rejection remains imperfect despite several known risk factors. There is an increasing appreciation of the potential importance of the vitamin D pathway in immunological disease and transplantation.
Objective:
The purpose of this study was to determine the association of 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D with acute rejection.
Design:
This was a prospective cohort study.
Setting:
Three academic adult kidney transplant programs in Ontario, Canada, were chosen.
Patients:
All consecutive adult patients at the 3 institutions who received a solitary kidney transplant, were able to provide written informed consent, and planned to be followed at the same center post-operatively were included.
Measurements:
Serum concentration of 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D were measured at baseline, 3, and 6 months post-transplantation. Acute rejection was classified using Banff criteria.
Methods:
The co-primary outcome was the association between 25-hydroxyvitamin D or 1,25-dihydroxyvitamin D and time to first occurrence of biopsy-proven acute rejection (BPAR) within the first year after kidney transplantation. Cox proportional hazards models were fitted taking into account the time-varying nature of serum concentrations of 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D.
Results:
From 556 screened patients, data on 327 kidney transplant recipients are included. First BPAR occurred in 54 (16.5%) patients. In adjusted Cox proportional hazards models, the serum concentration of 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D was not associated with acute renal transplant rejection (hazard ratio 1.00; 95% [confidence interval] CI, 0.87-1.14, per 10 nmol/L increase, and hazard ratio 0.97; 95% CI, 0.84-1.12, per 10 pmol/L increase, respectively).
Limitations:
Given the observational design, we cannot rule out the possibility of residual confounding that limited our ability to detect a clinically significant effect of vitamin D metabolites on acute rejection.
Conclusions:
A low serum concentration of 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D is not associated with an increased risk of acute kidney transplant rejection following kidney transplantation.
Keywords: 1,25-dihydroxyvitamin D; 25-hydroxyvitamin D; acute rejection; renal transplant
Abrégé
Contexte:
La prévision du rejet aigu d’une greffe de rein demeure imparfaite en dépit des connaissances au sujet de plusieurs facteurs de risque. On remarque cependant un intérêt croissant en regard de l’importance potentielle que pourrait jouer la voie métabolique de la vitamine D dans la maladie immunologique et la transplantation.
Objectif:
Le but de cette étude était d’établir si les concentrations sériques de 25-dihydroxyvitamine D et de 1,25-hydroxyvitamine D sont associées au phénomène de rejet aigu du greffon.
Modèle d’étude:
Il s’agit d’une étude de cohorte prospective.
Cadre de l’étude:
L’étude s’est tenue au sein de trois programmes universitaires de greffe de rein chez des adultes en Ontario, au Canada.
Patients:
L’étude a inclus tous les patients adultes ayant reçu une greffe solitaire de rein dans les trois établissements concernés, qui pouvaient fournir un consentement écrit et qui avaient prévu être suivis au même centre à la suite de l’intervention.
Mesures:
Les concentrations sériques de 25-hydroxyvitamine D et de 1,25-dihydroxyvitamine D ont été mesurées tout juste après l’intervention et à nouveau après 3 mois et 6 mois. Le rejet aigu a été déterminé en utilisant la classification de Banff.
Méthodologie:
Le principal critère attendu était une corrélation entre un faible taux de 25-hydroxyvitamine D et de 1, 25-dihydroxyvitamine D et le moment où survient le premier cas avéré par biopsie d’un rejet aigu au cours de l’année suivant la transplantation. Pour l’analyse, des modèles de risques proportionnels de Cox ont été adaptés en tenant compte du fait que les concentrations sériques de 25-dihydroxyvitamine D et de 1,25-hydroxyvitamine D varient au fil du temps.
Résultats:
Parmi les 556 patients sélectionnés, on a retenu les données de 327 receveurs d’une transplantation rénale. Les cas avérés par biopsie d’un premier rejet aigu sont survenus chez 54 de ces patients (16,5 %). Dans les modèles de risques proportionnels de Cox adaptés, les concentrations sériques de 25-hydroxyvitamine D et de 1, 25-dihydroxyvitamine D n’ont pas été associées à un rejet aigu de greffe rénale (risque relatif à 1,00 [IC à 95 %: 0,87, 1,14] par augmentation de 10 nmol/L et à 0,97 [IC95: 0,84, 1,12] par augmentation de 10 pmol/L, respectivement).
Limites de l’étude:
Compte tenu du modèle observationnel de l’étude, nous ne pouvons exclure la possibilité que des variables résiduelles confondantes aient limité notre capacité à détecter un effet clinique significatif des métabolites de la vitamine D sur le rejet aigu.
Conclusions:
Une faible concentration sérique de 25-hydroxyvitamine D et 1, 25-dihydroxyvitamine D n’est pas associée à un risque accru de rejet aigu du greffon consécutif à une transplantation rénale.
What was known before
Vitamin D3 is made in the skin from 7-dehydrocholesterol under the influence of ultraviolet light. Vitamin D is metabolized to 25-hydroxyvitamin D and then to the 1,25-dihydroxyvitamin D via 1-alpha-hydroxylase found predominately in the kidney. 1,25-dihydroxyvitamin D is the ligand for the vitamin D receptor that is found on multiple cell types of cells in the immune system. Stimulation of the receptors appears to lead to a more tolerogenic phenotype that might decrease the risk of acute kidney transplant rejection. This hypothesis is supported by studies in animals but the results in humans have been inconclusive.
What this adds
We have shown that low serum concentrations of 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D are not associated with an increased risk of acute rejection following kidney transplantation.
Introduction
Kidney transplantation is the treatment of choice for end-stage renal disease as it prolongs survival,1 improves quality of life, and is less costly when compared with dialysis.2 However, the full potential of this treatment option is not being realized as many kidney transplants fail prematurely. Although there has been a significant reduction in the risk of acute rejection over the last several decades,3 the occurrence and severity of acute rejection remain strong predictors of long-term graft outcome.4 Several risk factors for acute rejection have been identified and include the number of human leukocyte antigen (HLA) mismatches, delayed graft function, deceased donor, repeat transplant, panel reactive antibody level, race, and female gender.5-7 However, predicting acute rejection in the individual patient remains imperfect, which suggests that other, yet undiscovered, factors may be important.
The vitamin D receptor is found in most cell types of the immune system including macrophages, dendritic cells, CD4+ T cells, and CD8+ T cells.8 1,25-Dihydroxyvitamin D has been shown to inhibit antigen stimulated T-cell proliferation as well as inhibit the production of interleukin-2 (IL-2), IL-12, and gamma interferon.9,10 Furthermore, 1,25-dihydroxyvitamin D can direct naïve CD4+ cells toward a Th2 phenotype, which may be important in allograft tolerance.8 Patients with chronic kidney disease are known to be deficient in both 1,25-dihydroxyvitamin D11 and 25-hydroxyvitamin D,12-14 but the impact of these deficiencies on the risk of acute rejection after kidney transplantation remains speculative.
The objective of this study was to determine the association between serum 1,25-dihydroxyvitamin D and serum 25-hydroxyvitamin D concentrations and the risk of biopsy-proven acute rejection (BPAR) following kidney transplantation.
Materials and Methods
Study Population
This was a multi-center, prospective cohort study from 3 academic kidney transplant programs (Ottawa, London, Toronto–University Health Network) in Ontario, Canada, from August 2007 to December 2014. We included all consecutive adult patients at the 3 institutions who received a solitary kidney transplant from August 1, 2007, to December 29, 2012, were able to provide written informed consent, and planned to be followed at the same center post-operatively. The study was approved by the Research Ethics Board at each institution. The study was carried out according to the Declaration of Helsinki and Declaration of Istanbul. Demographic data, past medical history, potential risk factors for acute rejection, and immunosuppression were collected on each individual at the time of transplantation. Study visits occurred at baseline, 3 months, 6 months, and 12 months post-transplantation.
Immunosuppression Protocol
Immunosuppression for low immunological risk patients typically included basiliximab or anti-thymocyte globulin (total dose 3 mg/kg) plus methylprednisolone for induction followed by tacrolimus, mycophenolate mofetil, and prednisone for maintenance therapy. For patients at high risk to develop diabetes mellitus, cyclosporine was used instead of tacrolimus. Basiliximab or low-dose anti-thymocyte globulin was substituted with high-dose anti-thymocyte globulin (total dose of 5-7 mg/kg) for high-risk patients and those with delayed graft function.
Measurement of Vitamin D
Samples for 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D were collected at baseline (immediately pre-transplant), 3 months, and 6 months post-transplantation and stored at −80°C until analyzed. The investigators were unaware of the results. Serum 25-hydroxyvitamin D concentration was measured using an antibody-based radioimmunoassay (DiaSorin Inc, Stillwater, Minnesota, USA). This method detects both 25-hydroxyvitamin D3 (cholecalciferol, produced in vivo) and 25-hydroxyvitamin D2 (ergocalciferol, contained in foods and 25-hydroxyvitamin D supplements) on an equimolar basis, therefore providing an accurate measure of true 25-hydroxyvitamin D status.15 The level of agreement and correlation between the DiaSorin radioimmunoassay and high-performance liquid chromatography methods was excellent.16,17 The coefficient of variation (CV) for the radioimmunoassay was 10% to 12% in the range of 35 to 150 nmol/L. The 1,25-dihydroxyvitamin D analysis was performed by semi-automated enzyme immunoassay (IDS, Immunodiagnostic Systems, United Kingdom) that involved extraction of 1,25-dihydroxyvitamin D from potential cross-reactants by incubation with a solid-phase monoclonal antibody followed by an enzyme-linked immunosorbent assay. Briefly, the immuno-extraction gel was washed and purified; 1,25-dihydroxyvitamin D was eluted off and incubated overnight with a highly specific anti-1,25-dihydroxyvitamin D antibody. 1,25-Dihydroxyvitamin D linked–biotin was added followed by enzyme-labeled (horseradish peroxidase) avidin. The microtiter plate was read using a Perkin Elmer VICTOR3 V (Waltham, Massachusetts) plate reader at 450 nm (reference 650 nm). The CV was less than 13% at all levels. All commonly available vitamin D supplements, including vitamin D3 (cholecalciferol), alfacalcidol, and calcitriol, available in our renal transplant centers are detected with these 2 assays.
Outcomes
The primary outcome was the time from kidney transplantation to the first occurrence of BPAR within the first-year post-transplant. Kidney transplant biopsies were all performed for indication and were classified as per the Banff criteria.18
Statistical Analysis
Based on an anticipated BPAR rate of 12% and a power of 80%, we estimated that 310 participants would be required to detect a relative risk of 1.5 for acute rejection in patients with the lowest levels versus the highest levels of the vitamin D metabolites.
Baseline characteristics of the study participants were summarized for the cohort. For 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D, the mean, standard deviation, median, and interquartile range were reported. In addition, the proportion of participants who had vitamin D deficiency (25-hydroxyvitamin D level <50 nmol/L) and vitamin D insufficiency (25-hydroxyvitamin D level 50-75 nmol/L) were determined according to the Endocrine Society guidelines.19
Extended Kaplan-Meier plots for time to first BPAR were created incorporating the time-varying nature of the serum concentrations of 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D (by tertiles).20 Comparisons of the survival functions between the groups were made using the log-rank test. Cox proportional hazards models were used to analyze time to first BPAR accounting for the time-varying nature of the serum concentration of 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D (ie, the vitamin D measurement used in the model was always the value preceding the acute rejection). In these time-dependent Cox models, vitamin D status was handled in the following ways: (1) as a time-dependent continuous variable and (2) by tertiles of 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D. Other covariates that were considered included age, sex, body mass index, race, pre-emptive transplantation, time on dialysis pre-transplant, donor type (living or deceased), repeat transplantation, delayed graft function, immunosuppressive medications, panel reactive antibody, and the number of HLA mismatches. Univariable analyses are presented first followed by a multivariable model adjusted for variables that have been associated with both acute rejection and vitamin D status (age, sex, body mass index, race, and time on dialysis). As serum vitamin D “sufficiency” status has not been defined from an immunological perspective, the univariable and multivariable models were repeated with the serum 25-hydroxyvitamin D level less than 25 nmol/L versus a level higher than or equal to 25 nmol/L. Missing 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D concentrations were excluded from the analysis, and no imputation was used. A 2-sided P value less than .05 was considered statistically significant. All analyses were performed using SAS statistical software, Version 9.3.
Results
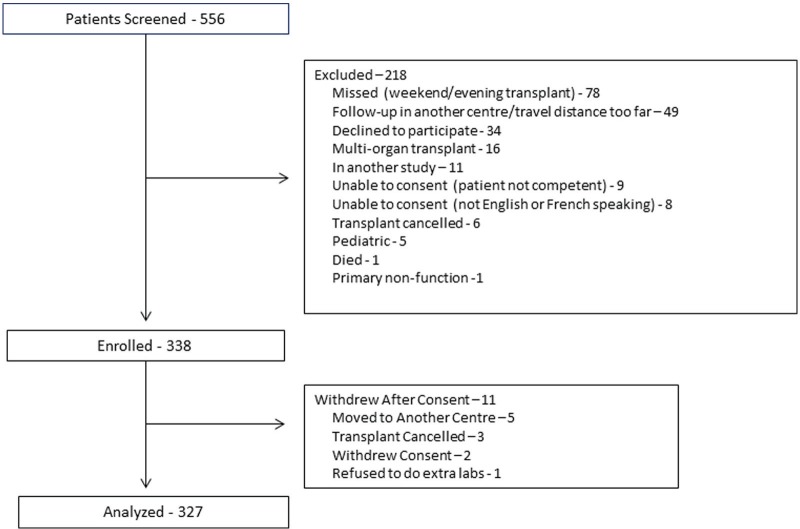
From 556 screened patients, 338 patients were enrolled, and 327 patients were included in the analytic cohort (Figure 1). The mean (SD) age was 51 (14) years. Participants were mostly male (67%) and white (84.9%; Table 1). The most common causes of end-stage renal disease were glomerulonephritis, other and diabetes mellitus. The majority of patients were first-time transplants (88%). At the time of transplant, approximately 60% of patients were taking some type of vitamin D supplement. Although these medications were not continued immediately post-transplant, 27, 3, and 9 patients were taking vitamin D3, alfacalcidol, or calcitriol, respectively, in follow-up (Table 1). Most patients (86.9%) received induction immunosuppression, with similar proportions receiving basiliximab and anti-thymocyte globulin (Table 1). The most common maintenance immunosuppression regimen included tacrolimus, mycophenolate, and prednisone. During follow-up, 4 patients were switched from tacrolimus to cyclosporine and 5 patients were switched from mycophenolate to azathioprine. Five patients were treated with sirolimus, 2 with leflunomide, and 1 patient received a combination of cyclophosphamide, rituximab, and eculizumab.
Figure 1.
Flow diagram of patients in the study.
Table 1.
Baseline Characteristics of the Study Population.
| Baseline characteristics | n (%) |
|---|---|
| Age, y; mean (SD) | 50.5 (13.8) |
| Female gender | 108 (33%) |
| Body mass index; mean (SD) | 27 (5) |
| Race | |
| Caucasian | 275 (84.9) |
| Black | 14 (4.3) |
| Other | 13 (4.0) |
| Aboriginal | 11 (3.4) |
| Asian | 10 (3.1) |
| Latin American | 1 (0.3) |
| Primary etiology of renal disease | |
| Glomerulonephritis | 69 (21.1) |
| Other | 69 (21.1) |
| Diabetes mellitus | 68 (20.8) |
| Polycystic kidney disease | 46 (14.1) |
| Hypertension | 27 (8.3) |
| Unknown | 15 (4.6) |
| Primary transplant | 288 (88.3) |
| Ever receive renal replacement therapy | 269 (82.8) |
| Dialysis vintage; median (IQR), days | 829 (391-1450) |
| PRA Class I | |
| 0 | 178 |
| 1-20 | 113 |
| >20 | 36 |
| PRA Class II | |
| 0 | 156 |
| 1-20 | 104 |
| >20 | 67 |
| HLA mismatch | |
| 4-6 | 197 (61.2) |
| 1-3 | 96 (29.8) |
| 0 | 29 (.0) |
| Vitamin D (immediately pre-transplant) | 194 (59.5) |
| Calcitriol | 108 (33.0) |
| Alfacalcidol | 66 (20.2) |
| Cholecalciferol | 28 (8.6) |
| Other | 17 (5.2) |
| Doxercalciferol | 1 (0.3) |
| Ergocalciferol | 1 (0.3) |
| Living donor | 151 (46.1) |
| Delayed graft function | 17 (5.2) |
| Immunosuppressant medications (initial) | |
| Intravenous methylprednisolone | 325 (99.0) |
| Prednisone | 309 (94.8) |
| Induction therapy | |
| Basiliximab | 136 (41.6) |
| Anti-thymocyte globulin | 148 (45.3) |
| Neither | 43 (13.2) |
| Calcineurin inhibitor | |
| Tacrolimus | 282 (86.2) |
| Cyclosporine | 40 (12.2) |
| Neither | 5 (1.5) |
| Anti-metabolite | |
| Mycophenolate mofetil | 302 (92.4) |
| Mycophenolate sodium | 21 (6.4) |
| Neither | 4 (1.2) |
| IVIG | 31 (9.5) |
| Pre-transplant plasmapheresis | 12 (3.7) |
| Other | 10 (3.1) |
Note. SD = standard deviation; IQR = interquartile range; PRA = panel reactive antibody; HLA = human leukocyte antigen; IVIG = intravenous immunoglobulin.
The mean (SD) serum concentration of 25-hydroxyvitamin D at the time of transplant was 64.3 (30.4) nmol/L; 36% of patients were classified as deficient and 30% insufficient (Table 2). The concentration of 25-hydroxyvitamin D remained stable over time (Figure 2). The mean (SD) serum concentration of 1,25-dihydroxyvitamin D at the time of transplant was 22.5 (22.1) pmol/L and increased approximately 3.5-fold over the first 6 months post-transplant (Table 2, Figure 3).
Table 2.
25-Hydroxyvitamin D and 1,25-Dihydroxyvitamin D Serum Concentration Over Time.
| Baseline | 3 months | 6 months | |
|---|---|---|---|
| 25-hydroxyvitamin D (nmol/L) | |||
| N | 307 | 301 | 293 |
| Mean (SD) | 64.3 (30.4) | 63.5 (25.9) | 67.0 (27.6) |
| Median (IQR) | 59 (40-85) | 60 (47-77) | 63 (49-87) |
|
| |||
| 25-hydroxyvitamin D (nmol/L) | N (%) | N (%) | N (%) |
| Deficient | 111 (36) | 91 (30) | 79 (27) |
| Insufficient | 91 (30) | 127 (42) | 112 (38) |
| Sufficient | 105 (34) | 83 (28) | 102 (35) |
|
| |||
| 1,25-dihydroxyvitamin D (pmol/L) | |||
| N | 298 | 272 | 262 |
| Mean (SD) | 22.5 (22.1) | 74.5 (35.3) | 85.6 (41.3) |
| Median (IQR) | 14 (8-29) | 73 (50-95) | 82 (54-105) |
Note. SD = standard deviation; IQR = interquartile range.
Figure 2.
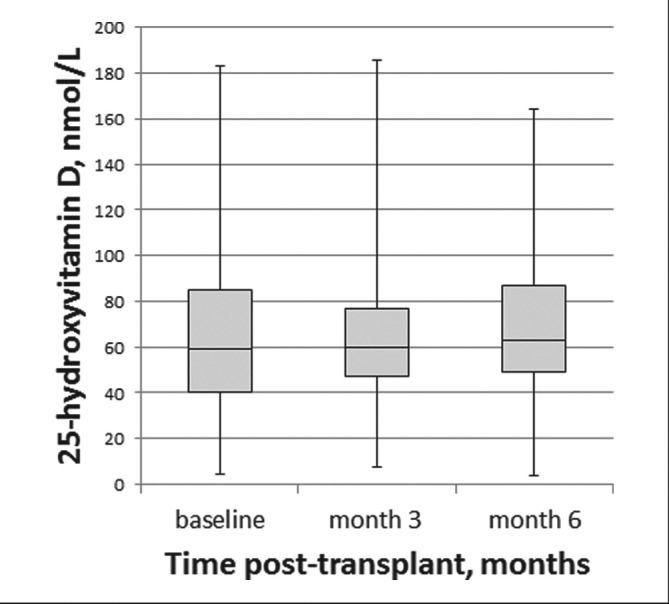
Box and Whisker plots of the change in serum concentration of 25-hydroxyvitamin D post-transplant.
Figure 3.
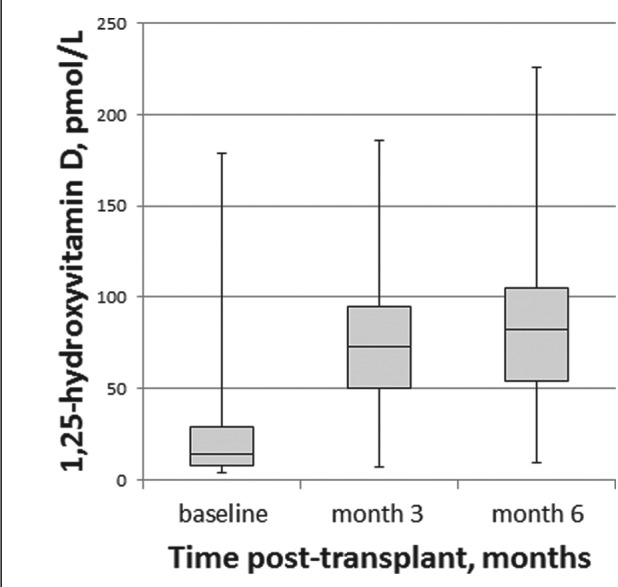
Box and Whisker plots of the change in serum concentration of 1,25-dihydroxyvitamin D post-transplant.
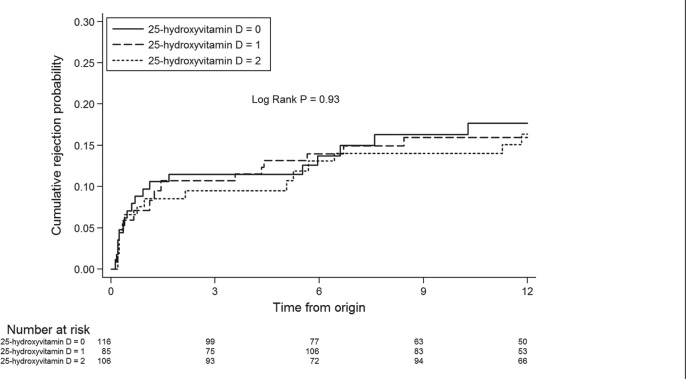
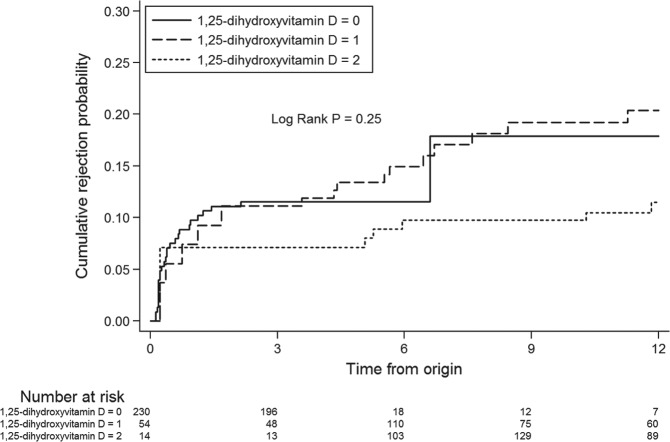
First BPAR occurred in 54 (16.5%) patients. By extended Kaplan-Meier analysis, time to first BPAR was not significantly different across tertiles of serum 25-hydroxyvitamin D (log-rank P = .93; Figure 4) or 1,25-dihydroxyvitamin D (log-rank P = .25; Figure 5). In univariate analysis, a lack of induction therapy was associated with an increased risk of acute rejection (Table 3). Lower levels of HLA mismatch were associated with a decreased risk of acute rejection. The serum concentration of 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D were not associated with acute rejection either as continuous variables or divided into tertiles (Table 3).
Figure 4.
Extended Kaplan-Meier curve of the association between tertiles of 25-hydroxyvitamin D serum concentration and time to acute rejection.
Note. 25-hydroxyvitamin D: 0, lowest tertile; 1, middle tertile; 2, highest tertile.
Figure 5.
Extended Kaplan-Meier curve of the association between tertiles of 1,25-dihydroxyvitamin D serum concentration and time to acute rejection.
Note. 1,25-dihydroxyvitamin D: 0, lowest tertile; 1, middle tertile; 2, highest tertile.
Table 3.
Univariate Risk Factors for Time to First Episode of Acute Rejection.
| Potential risk factors | HR (95% CI) | P value |
|---|---|---|
| Age | 0.99 (0.97-1.01) | .31 |
| Sex (male vs female—REF) | 1.42 (0.77-2.60) | .26 |
| Body mass index | 1.04 (0.98-1.10) | .17 |
| Race (white vs other—REF) | 1.22 (0.55-2.69) | .63 |
| Pre-emptive transplantation (yes vs no—REF) | 1.41 (0.64-3.12) | .40 |
| Time on dialysis pre-transplant (per month) | 0.99 (0.99-1.01) | .81 |
| Donor type (deceased vs living—REF) | 1.07 (0.63-1.84) | .79 |
| Repeat transplantation (yes vs no—REF) | 0.79 (0.37-1.66) | .53 |
| Delayed graft function (yes vs no—REF) | 0.79 (0.11-5.69) | .81 |
| Induction | ||
| Basiliximab | REF | REF |
| Anti-thymocyte globulin | 1.50 (0.82-2.77) | .19 |
| Neither | 2.24 (1.05-4.78) | .04 |
| Calcineurin inhibitor | ||
| Tacrolimus | REF | REF |
| Cyclosporine | 0.54 (0.20-1.51) | .24 |
| Anti-metabolite | ||
| Mycophenolate mofetil | REF | REF |
| Mycophenolate sodium | 0.80 (0.25-2.56) | .71 |
| IVIG (yes vs no) | 1.02 (0.41-2.56) | .97 |
| Pre-transplant plasmapheresis (yes vs no) | 2.50 (0.90-6.92) | .08 |
| PRA Class I | ||
| 0 | REF | REF |
| 1-20 | 0.76 (0.41-1.41) | .38 |
| >20 | 1.55 (0.74-3.26) | .25 |
| PRA Class II | ||
| 0 | REF | REF |
| 1-20 | 0.91 (0.49-1.69) | .76 |
| >20 | 1.32 (0.54-3.20) | .55 |
| HLA mismatch | ||
| 4-6 | REF | REF |
| 1-3 | 0.49 (0.24-0.97) | .04 |
| 0 | 0.49 (0.15-1.58) | .23 |
| 25-hydroxyvitamin D (per 10 nmol/L) | 1.01 (0.92-1.12) | .79 |
| 25-hydroxyvitamin D (by tertile) | ||
| Highest (74-186 nmol/L) | REF | REF |
| Middle (51-73 nmol/L) | 1.04 (0.51-2.11) | .92 |
| Lowest (4-50 nmol/L) | 1.13 (0.58-2.23) | .72 |
| 25-hydroxyvitamin D | ||
| ≥25nmol/L | REF | 0.51 |
| <25nmol/L | 1.48 (0.46-4.78) | |
| 1,25-dihydroxyvitamin D (per 10 pmol/L) | 0.95 (0.86-1.06) | .38 |
| 1,25-dihydroxyvitamin D (by tertile) | ||
| Highest (77-226 pmol/L) | REF | REF |
| Middle (31-76 pmol/L) | 2.24 (0.85-5.94) | .10 |
| Lowest (4-30 pmol/L) | 2.12 (0.70-6.44) | .19 |
Note. HR = hazard ratio; CI = confidence interval; REF = reference group; IVIG = intravenous immunoglobulin; PRA = panel reactive antibody; HLA = human leukocyte antigen.
In a multivariable model, adjusting for variables that have been previously associated with both acute kidney transplant rejection and vitamin D status (age, sex, body mass index, and time on dialysis) did not alter the results (Table 4).
Table 4.
Multivariable Model Adjusting for Variables Associated With Acute Rejection and Vitamin D Status.
| HR (95% CI) | P value | |
|---|---|---|
| 25-hydroxyvitamin D (per 10 nmol/L) | 0.996 (0.87-1.14) | .96 |
| 25-hydroxyvitamin D (by tertile) | ||
| Highest (74-186 nmol/L) | REF | REF |
| Middle (51-73 nmol/L) | 1.33 (0.49-3.59) | .57 |
| Lowest (4-50 nmol/L) | 1.25 (0.49-3.17) | .64 |
| 25-hydroxyvitamin D | ||
| ≥25nmol/L | REF | .51 |
| <25nmol/L | 1.64 (0.37-7.18) | |
| 1,25-dihydroxyvitamin D (per 10 pmol/L) | 0.97 (0.84-1.12) | .64 |
| 1,25-dihydroxyvitamin D (by tertile) | ||
| Highest (77-226 pmol/L) | REF | REF |
| Middle (31-76 pmol/L) | 1.44 (0.39-5.34) | .58 |
| Lowest (4-30 pmol/L) | 2.25 (0.51-9.84) | .28 |
Note. HR = hazard ratio; CI = confidence interval; REF = reference group.
Discussion
In this large prospective cohort study of kidney transplant recipients, the serum concentrations of 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D at baseline were 64.3 (30.4) nmol/L and 22.5 (22.1) pmol/L, respectively, such that the majority of patients were not vitamin D sufficient as defined for optimal bone health. In the 6 months after kidney transplant, the serum concentration of 25-hydroxyvitamin D remained unchanged but the serum concentration of 1,25-dihydroxyvitamin D increased approximately 3.5 fold from baseline. In the analysis where time-varying concentrations of the vitamin D metabolites were accounted for, 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D were not associated with a decreased risk of BPAR in the first year after kidney transplantation.
Vitamin D levels fall with the declining glomerular filtration rate.21 Mild to moderate renal functional impairment and elevated fibroblast growth factor–23 lead to a reduction in 1-alpha-hydroxylase activity, which is responsible for the conversion of 25-hydroxyvitamin D to 1,25-dihydroxyvitamin D.22 After kidney transplantation, avoidance of sun exposure and immunosuppressive medications may lead to further reductions in vitamin D.21 In our study, greater than 50% of patients had less than sufficient 25-hydroxyvitamin D serum levels as defined by Endocrine Society guidelines. However, this 25-hydroxyvitamin D concentration was higher than that reported in several other studies of kidney transplant recipients.23-25 The reasons for this are unclear as a minority of patients were taking cholecalciferol supplements. The serum concentration of 1,25-dihydroxyvitamin D at baseline was also low despite greater than 50% of patients being treated pre-transplant with the active vitamin D supplement calcitriol or alfacalcidol. These latter results are consistent with similar studies reported in the literature.25,26-28
Vitamin D has important immunomodulatory functions that may affect the risk of acute rejection post kidney transplant through diverse effects on T-cell and dendritic cell function.22 However, the effect of vitamin D on the risk of acute kidney transplant rejection reported in the literature is conflicting likely secondary to the different patient populations, sample sizes, different vitamin D assays, assessment time frames, and definition of outcomes. In the current study, the serum concentration of 25-hydroxyvitamin D analyzed as a time-varying continuous variable was not associated with time to first acute kidney transplant rejection. Furthermore, analysis by population tertiles did not alter the conclusions. Our results are similar to those of Bienaime et al, in which the 25-hydroxyvitamin D level at 3 months was not associated with the risk of acute kidney transplant rejection.23 Our results are different from those of Kim et al in which the 25-hydroxyvitamin D level less than 25 nmol/L measured pre-transplant was associated with a greater risk of acute rejection.25 Their patient population differed from ours in many important ways but it is unclear what the effect of differences in “case-mix” might have on immune function.
In a patient population more similar to ours, Lee at al reported an association between the 25-hydroxyvitamin D level less than 50 nmol/L within 30 days post-transplant and the risk of acute rejection at 1 year.24 However, over half of the 25-hydroxyvitamin D–deficient patients were started on vitamin D supplements within 90 days of transplantation. Only those patients who were supplemented with 1,25-dihydroxyvitamin D rather than 25-hydroxyvitamin D experienced a reduction in the risk of acute rejection. It is possible that 1,25-dihydroxyvitamin D is the important immune modulator and that ongoing impairment of the conversion of 25-hydroxyvitamin D to 1,25-dihydroxyvitamin D after kidney transplant negates the usefulness of 25-hydroxyvitamin, especially for patients with reduced kidney transplant function. Vitamin D levels were not measured again during that study such that the hypothesis remains speculative and is not supported by our study. We were unable to show an association between the serum concentration of 1,25-dihydroxyvitamin D and risk of BPAR. Although the patients in the lowest tertile of serum 1,25-dihydroxyvitmain D appear to be at increased risk of acute rejection, these results were not statistically significant. Furthermore, in a multivariable model where we controlled for variables that have been associated with both acute rejection and vitamin D status, the conclusions were unchanged. Our results are consistent with two smaller studies. Higher serum calcitriol levels in one study and calcitriol supplementation in the other study were not associated with a decreased risk of acute rejection.28,29
The strengths of our study include the prospective design and inclusion of patients from 3 different academic kidney transplant programs in Canada. Only biopsy-proven acute kidney transplant rejection episodes were included in the analysis thereby excluding other potential causes of an increase in serum creatinine. The assays for the determination of 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D were consistent for all patients, each done in only 1 laboratory. Our analysis also took into account changes in serum vitamin D concentration over time such that the vitamin D concentrations prior to the episode of acute rejection were used in the analysis to address the time dependency of vitamin D levels over follow-up.
Limitations should be noted. First, we did have a small percentage of patients with missing lab samples. Second, multivariable models were used to improve the validity of the inferences made from the data but residual confounding cannot be completely excluded. Third, it is possible that we missed a potentially clinically important association between vitamin D metabolites and the risk of BPAR due to our sample size that would have affected our statistical power. Finally, the study represents the experience of 3 kidney transplant centers in Ontario, Canada, and may thus not be fully generalizable to other settings.
In summary, a low serum concentration of 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D was not associated with an increased risk of acute rejection following kidney transplantation. A larger prospective cohort study and/or clinical trial may be necessary to elucidate a small but potentially meaningful relationship between vitamin D and the risk of BPAR in kidney transplant recipients.
Acknowledgments
Drs Zimmerman and Knoll receive salary support from the Department of Medicine at the Ottawa Hospital. Both investigators also receive clinical trials support from the Kidney Research Centre of the Ottawa Hospital Research Institute. The study would not have been possible without patient and research coordinator participation at the three hospitals.
Footnotes
Ethics Approval and Consent to Participate: We received ethics approval from each institution (Ottawa Health Science Network Research Ethics Board, 2006787-01H; Western University Health Sciences Research Ethics Board, 5069; and University Health Network Research Ethics Board, 09-0798-A). All patients provided written informed consent.
Consent for Publication: Consent to publish was obtained from all authors.
Availability of Data and Materials: The data for this study will not be shared at this time as there are several secondary studies that will be reported. Data are available from The Ottawa Hospital Research Ethics Board (privacy@ottawahospital.on.ca) for researchers who meet the criteria for access to confidential data. Anonymized data are available on request by emailing the principal investigator (dzimmerman@toh.on.ca).
Author Contributions: Author contributions include the following: (1) study design, DZ, AAH, SJK, RAB, TR, and GK; (2) data acquisition, DZ, AAH, and SJK; (3) data analysis and interpretation, TZ, SJK, DZ, AAH, SJK, and GK; (4) manuscript preparation, DZ, AAH, SJK, GK, and RAB; and (5) approval of submitted manuscript, all authors. DZ accepts accountability for the overall work.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study received funding support from Physicians’ Services Incorporated Foundation and the Canadian Institutes of Health Research.
References
- 1. Wolfe RA, Ashby VB, Milford EL, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999;341(23):1725-1730. [DOI] [PubMed] [Google Scholar]
- 2. Laupacis A, Keown P, Pus N, et al. A study of the quality of life and cost-utility of renal transplantation. Kidney Int. 1996;50(1):235-242. [DOI] [PubMed] [Google Scholar]
- 3. Hariharan S, Johnson CP, Bresnahan BA, et al. Improved graft survival after renal transplantation in the United States, 1988 to 1996. N Engl J Med. 2000;342:605-612. [DOI] [PubMed] [Google Scholar]
- 4. Pallardó Mateu L, Sancho Calabuig A, Capdevila Plaza L, et al. Acute rejection and late renal transplant failure: risk factors and prognosis. Nephrol Dial Transplant. 2004;19:38-42. [DOI] [PubMed] [Google Scholar]
- 5. Nair MP, Nampoory MR, Said T, et al. Early acute rejection episodes in renal transplantation in relation to immunosuppression protocols: an audit of 100 cases. Transplant Proc. 2005;37(7):3029-3030. [DOI] [PubMed] [Google Scholar]
- 6. Woodle ES, Alloway RR, Buell JF, et al. Multivariate analysis of risk factors for acute rejection in early corticosteroid cessation regimens under modern immunosuppression. Am J Transplant. 2005;5(11):2740-2744. [DOI] [PubMed] [Google Scholar]
- 7. Heilman RL, Reddy KS, Mazur MJ, et al. Acute rejection risk in kidney transplant recipients on steroid-avoidance immunosuppression receiving induction with either antithymocyte globulin or basiliximab. Transplant Proc. 2006;38(5):1307-1313. [DOI] [PubMed] [Google Scholar]
- 8. Mathieu C, Adorini L. The coming of age of 1,25-dihydroxyvitamin D3 analogs as immunomodulatory agents. Trends Mol Med. 2002;8(4):174-179. [DOI] [PubMed] [Google Scholar]
- 9. Bhalla AK, Amento EP, Serog B, et al. 1,25-Dihydroxyvitamin D3 inhibits antigen-induced T cell activation. J Immunol. 1984;133:1748-1754. [PubMed] [Google Scholar]
- 10. Seibert E, Levin NW, Kuhlmann MK. Immunomodulatory effects of vitamin D in hemodialysis patients. Hemodial Int. 2005;9:S25-S29. [DOI] [PubMed] [Google Scholar]
- 11. National Kidney Foundation. K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis. 2003;42(suppl 3):S1-S201. [PubMed] [Google Scholar]
- 12. Ghazali A, Fardellone P, Puna A, et al. Is low plasma 25-(OH)vitamin D a major risk factor for hyperparathyroidism and Looser’s zones independent of calcitriol? Kidney Int. 1999;55:2169-2177. [DOI] [PubMed] [Google Scholar]
- 13. Gonzalez EW, Sachdeva A, Oliver DA, et al. Vitamin D insufficiency and deficiency in chronic kidney disease. Am J Nephrol. 2004;24:503-510. [DOI] [PubMed] [Google Scholar]
- 14. Mucsi I, Almasi C, Deak G, et al. Serum 25(OH)-vitamin D levels and bone metabolism in patients on maintenance hemodialysis. Clin Nephrol. 2005;64(4):288-294. [DOI] [PubMed] [Google Scholar]
- 15. Hollis BW. Comparison of commercially available (125)I-based RIA methods for the determination of circulating 25-hydroxyvitamin D. Clin Chem. 2000;46:1657-1661. [PubMed] [Google Scholar]
- 16. Hollis BW. Editorial: the determination of circulating 25-hydroxyvitamin D: no easy task. J Clin Endocrinol Metab. 2004;89:3149-3151. [DOI] [PubMed] [Google Scholar]
- 17. Binkley N, Krueger D, Cowgill CS, et al. Assay variation confounds the diagnosis of hypovitaminosis D: a call for standardization. J Clin Endocrinol Metab. 2004;89:3152-3157. [DOI] [PubMed] [Google Scholar]
- 18. Haas M, Sis B, Racusen LC, et al. Banff 2013 meeting report: inclusion of c4d-negative antibody-mediated rejection and antibody-associated arterial lesions. Am J Transplant. 2014;14(2):272-283. [DOI] [PubMed] [Google Scholar]
- 19. Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911-1930. [DOI] [PubMed] [Google Scholar]
- 20. Snapinn SM, Jiang Q, Iglewicz B. Illustrating the impact of a time-varying covariate with an extended Kaplan-Meier estimator. Am Stat. 2005;59(4):301-307. [Google Scholar]
- 21. Levin A, Bakris GL, Molitch M, et al. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney Int. 2007;71:31-38. [DOI] [PubMed] [Google Scholar]
- 22. McGregor R, Li G, Penny H, Lombardi G, Afzali B, Goldsmith D. Vitamin D in renal transplantation—from biological mechanisms to clinical benefits. Am J Transplant. 2014;14:1259-1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bienaime F, Girard D, Anglicheau D, et al. Vitamin D status and outcomes after renal transplantation. J Am Soc Nephrol. 2013;24:831-841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lee JR, Dadhania D, August P, Lee JB, Suthanthiran M, Muthukumar T. Circulating levels of 25-hydroxyvitamin D and acute cellular rejection in kidney allograft recipients. Transplantation. 2014;98(3):292-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kim H, Kang SW, You TH, et al. The impact of pretransplant 25-hydroxy vitamin D deficiency on subsequent graft function: an observational study. BMC Nephrol. 2012;13:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Querings K, Girndt M, Geisel J, et al. 25-hydroxyvitamin D deficiency in renal transplant recipients. J Clin Endocrinol Metab. 2005;91(2):526-529. [DOI] [PubMed] [Google Scholar]
- 27. de Sevaux RG, Hoitsma AJ, van Hoof HJ, Corstens FJ, Wetzels JF. Abnormal vitamin D metabolism and loss of bone mass after renal transplantation. Nephron Clin Pract. 2003;93(1):c21-c28. [DOI] [PubMed] [Google Scholar]
- 28. Falkiewicz K, Boratynska M, Speichert-Bidzinska B, et al. 1,25 Dihydroxyvitamin D deficiency post transplant predicts poorer outcome after renal transplantation. Transplant Proc. 2009;41:3002-3005. [DOI] [PubMed] [Google Scholar]
- 29. O’Herrin JK, Hullett DA, Heisey DM, Sollinger HW, Becker BN. A retrospective evaluation of 1,25-dihydroxyvitamin D3 and its potential effects on renal allograft function. Am J Nephrol. 2002;22:515-520. [DOI] [PubMed] [Google Scholar]