Abstract
Medullary thick ascending limbs (mTAL) regulate Na balance and therefore blood pressure. We previously showed that cell swelling and luminal flow activates the mechano-sensitive channel TRPV4 in mTAL.
Aim
We hypothesized that TRPV4 mediates flow-induced increases in intracellular Ca (Cai) in rat mTALs.
Methods
We performed ratiometric measurements of Cai in perfused mTALs.
Results
Increasing luminal flow from 0 to 20 nL min-1 caused Cai to peak 231±29 nmol L-1 above basal concentrations (n=18). The general TRPV inhibitor ruthenium red at 15 and 50 umol L-1 reduced peak Cai by 41±9 (p<0.01;n=5) and 77±10 % (p<0.02;n=6). The selective TRPV4 inhibitor RN1734 at 10 and 50 umol L-1 reduced peak Cai by 46±11 (p<0.01;n=7) and 76±5% (p<0.02;n=5) respectively. To specifically target TRPV4, mTALs were transduced with adenoviruses expressing TRPV4 small hairpin (sh) RNA. In non-transduced control mTALs, luminal flow generated a peak increase in Cai of 111±21 nmol L-1 (n=8). In TRPV4shRNA-transduced mTALs, the Cai peak was reduced to 56±8 nmol L-1 (p<0.03,n=9). Removing extracellular Ca completely abolished flow-induced increases in Cai. Increasing luminal flow in the presence of hexokinase 20 (U/ml) to scavenge extracellular ATP did not modify significantly the increases in Cai induced by luminal flow. Finally, we studied the effect of the TRPV4 selective agonist GSK1016790A on Cai. In the absence of luminal flow, GSK1016790A (10 nmol L-1) increased Cai from 60±11 nmol L-1 to 262±71 nmol L-1 (p<0.05;n=7).
Conclusion
We conclude that flow-induced increases in Cai are mediated primarily by TRPV4 in the rat mTAL.
Keywords: mechanosensation, TRP channels, nitric oxide
Introduction
The thick ascending limb of the loop of Henle (TAL) plays a critical role in salt homeostasis and blood pressure regulation because it reabsorbs approximately 30 % of the NaCl filtered by the glomerulus (Burg, 1982). This is best evidenced by the fact that pathologies that cause elevated TAL NaCl reabsorption increase blood pressure while those that reduce it cause hypotension (Jung et al., 2011, Hebert, 2003).
Luminal flow through the nephron is not static; rather it constantly changes. Acutely, flow changes due to tubuloglomerular feedback and peristalsis of the renal pelvis (Dwyer and Schmidt-Nielsen, 2003, Holstein-Rathlou and Marsh, 1990). Increases in volume and salt intake also rapidly enhance luminal flow (Mozaffari et al., 1991, Arendshorst and Beierwaltes, 1979). Hypertension and glomerular hyperfiltration in the early stages of diabetes chronically elevate luminal flow (DiBona and Rios, 1978, Pollock et al., 1991). Increases in luminal flow have been shown to augment nitric oxide and superoxide production, and ATP release from TALs (Cabral et al., 2010, Cabral and Garvin, 2011, Cabral et al., 2012). They have also been shown to enhance K secretion and Na reabsorption in the collecting duct (Woda et al., 2003, Woda et al., 2001, Morimoto et al., 2006).
Increasing luminal flow has been shown to elevate intracellular Ca (Cai) in both thick ascending limbs and collecting ducts (Woda et al., 2002, Liu et al., 2003, Jensen et al., 2007). This is thought to be the initiating signal for flow-induced changes in different physiological parameters such as ionic transport in both nephron segments. However, the transporters mediating the flow-induced increase in Cai are not well understood. The transient receptor potential vanilloid 4 (TRPV4) channel, a member of the TRPV family of cation channels, displays mechano-sensitive activation (O’Neil and Heller, 2005, Liedtke, 2007). It transduces cellular stretch and flow-induced shear stress into Ca influx increasing Cai in vascular smooth muscle and endothelial cells (Mendoza et al., 2010, Kohler et al., 2006, Filosa et al., 2013). In the nephron, Berrout et al showed that TRPV4 mediates flow-induced Cai increases in the mouse collecting duct (Berrout et al., 2012). We have previously shown that TRPV4 is expressed in the rat TAL and mediates cell swelling-induced ATP release in this segment (Cabral and Garvin, 2014, Silva and Garvin, 2008); however it is not known whether this channel mediates the increase in Cai caused by luminal flow in this segment. We hypothesized that TRPV4 mediates flow-induced elevation of Cai in rat TALs.
Materials and Methods
Animals
Male Sprague-Dawley rats weighing 100 to 150 g from Charles River Breeding Laboratories, Wilmington, MA, were fed a diet containing 0.22% sodium and 1.1% potassium (Purina, Richmond, IN) for at least 5 days prior to the experiments. All protocols were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of Case Western Reserve University following the National Institutes of Health Guidelines for Use and Care of Experimental Animals.
Solutions and Chemicals
Fura 2-AM and 4-Bromo A-23187 were purchased from Invitrogen (Eugene, OR). Ruthenium red, hexokinase, GSK1016790A and EGTA (Ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid tetrasodium salt) were obtained from Sigma-Aldrich (St. Louis, MO) and RN1734 from Tocris Bioscience (Bristol, United Kingdom). The composition of the physiological saline used to perfuse and bathe the tubules was (in mmol L-1): 130 NaCl, 4 KCl, mix of monobasic and dibasic phosphate, 1.2 MgSO4, 6 L-alanine, 0.1 L-arginine, 1 trisodium citrate, 5.5 glucose, 2 calcium dilactate, and 10 4-(2-hydroxyethyl) piperazine-1-ethanesulfonic acid (HEPES), pH 7.4 at 37°C. The calcium-free solution was (in mmol L-1): 130 NaCl, 4 KCl, 1.2 MgSO4, 6 L-alanine, 0.1 L-arginine, 5.5 glucose, 0.1 ethylene glycol tetraacetic acid (EGTA) and 10 4-(2-hydroxyethyl) piperazine-1-ethanesulfonic acid (HEPES), pH 7.4 at 37°C. Solutions were adjusted to 290 ± 3 mOsm kg-1 H2O with NaCl as necessary as measured by freezing-point depression.
Preparation of isolated and perfused medullary thick ascending limbs
Medullary TALs were isolated and perfused as previously described. Briefly, rats were anesthetized with ketamine (100 mg kg-1 body wt i.p.) and xylazine (20 mg kg-1 body wt i.p.). The abdominal cavity was opened and the left kidney superfused with ice-cold 150 mmol L-1 NaCl , then removed and placed in physiological saline (4°C). Coronal slices were cut and TALs isolated from the outer medulla under a stereomicroscope at 4 to 10°C. TALs ranging from 0.7 to 1.0 mm were transferred to a temperature-regulated chamber and perfused using concentric glass pipettes at 37 ± 1°C. Luminal perfusion rates were 0 or 20 nL min-1 and basolateral flow rate was 1 mL min-1.
Measurements of Cai
Isolated TALs were loaded with 1 umol L-1 FURA 2-AM for 30 min and washed for 30 min using dye-free physiological saline at 37 ± 1°C. The TRPV4 antagonists (ruthenium red and RN1734) and the ATP scavenger hexokinase were present in both luminal and basolateral sides throughout the 30 min-washing period. FURA 2 was excited at 340 nm and 380 nm. The ratio between the emitted fluorescence of both wavelengths is proportional to Cai. Fluorescence was imaged digitally with an image intensifier and a charge-coupled device camera. Fluorescence images were taken every two seconds in the absence and presence of luminal flow. Data were recorded using Metafluor version 7 imaging software (Universal Imaging, Downington, PA). Cai was calibrated at the end of each experiment with 5 mmol L-1 EGTA and 10 umol L-1 4-Br-A23187. Cai was calculated using the following equation as previously described:
Where Kd is the Ca dissociation constant of FURA 2 (224 nmol L-1); R is the ratio between 340 nm and 380 nm of each sample; Fmin is the fluorescence intensity of 380nm during EGTA; Fmax is the fluorescence intensity of 380 nm during 4-Br-A23187; Rmin is the minimum ratio between 340 nm and 380 nm during EGTA and Rmax is the maximum ratio between 340 nm and 380 nm during 4-Br-A23187.
For each experiment where luminal flow was tested, we calculated and analyzed the following variables: peak increase of Cai (nmol L-1); rate of rise (nmol L-1 s-1); half-life of the decay (seconds); and the plateau of the decay (nmol L-1). In the set of experiments where the TRPV4 agonist GSK1016790A was tested in the absence of luminal flow, the peak increase of Cai and the rate of rise were analyzed.
In vivo adenoviral transduction of medullary thick ascending limbs
Small hairpin TRPV4-RNA (shRNA) expressing adenoviruses under the control of the H1 mouse RNA polymerase promoter were delivered to mTAL as previously described (Silva and Garvin, 2008, Ortiz et al., 2003). Briefly, rats were anesthetized with ketamine (60 mg kg-1 body wt i.p.) and xylazine (20 mg kg-1 body wt i.p.). After anesthesia, the left kidney was exposed through a flanked incision and the renal artery and vein clamped. Then 4 infusions of 20 μL of adenovirus at a final concentration of 1.2 × 10 -12 particles ml-1 were injected at the cortico-medullary boundary at a flow rate of 20 uL min-1 using a 30 gauge needle and syringe pump. The needle was removed from the injection site 30 s after each infusion was completed to avoid bleeding and leakage of the virus. After a total time of 8 min the vascular clamps were removed, the kidney returned to the abdomen and the wound sutured. After surgery, animals were kept warm and under direct observation until they recovered from the anesthesia and appeared alert. Then they were returned to their cages, where food and water was available ad libitum. Experiments were performed 72 hs after surgery.
Statistical Analysis
Results are presented as mean ± standard error. An unpaired Student’s t test was used to calculate the difference between no flow and flow conditions in separated experimental groups. A p value of < 0.05 was taken as significant. A one-way ANOVA test followed by a Bonferroni correction for multiple comparisons was used to analyze the groups in the set of experiments where higher concentrations of the two different TRPV4 antagonists were used.
Results
To start testing our hypothesis, we first studied the effect of luminal flow on Cai. Figure 1 shows a representative experiment in which increasing luminal flow elevated Cai. In the group of control experiments, basal Cai was 59±6 nmol L-1. Luminal flow caused Cai to increase at a rate of 25±4 nmol L-1 s-1 resulting in a peak Cai of 290±31 nmol L-1 (p<0.0001, n=18, compared to basal Cai). Cai then declined in a single exponential decay with a half-time of 44±10 s reaching a plateau value of 85±10 nmol L-1. Mean data for control experiments are shown in Table 1.
Figure 1.
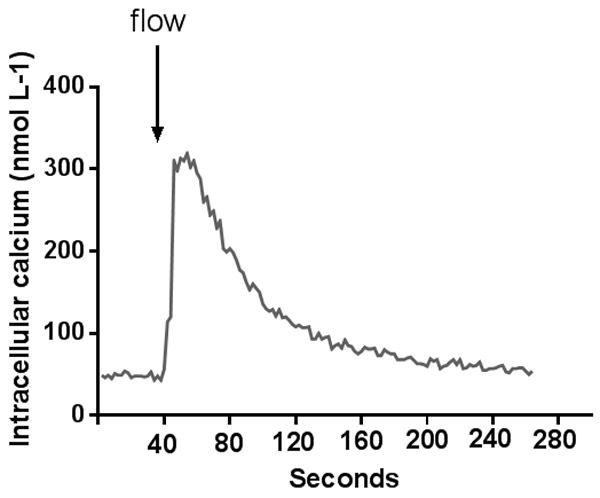
Representative trace showing the behavior of intracellular calcium after luminal flow is increased in isolated mTALs.
Table 1.
Intracellular calcium parameters of control experiments before and after luminal flow is increased in isolated mTALs (SEM=standard error of the mean).
| Basal | Peak-increase* | Δ peak increase-basal | Rate of rise | Decay half-life | Plateau | |
|---|---|---|---|---|---|---|
| Mean | 59 | 290 | 231 | 25 | 44 | 85 |
| SEM | 6 | 31 | 29 | 4 | 10 | 10 |
Units: Basal (nmol L-1); Peak-increase (nmol L-1); Δ peak increase-basal (nmol L-1); Rate of rise (nmol L-1 s-1); Decay half-life (s) and Plateau in (nmol L-1).
p<0.0001, n=18, compared to basal Cai.
To investigate whether flow-induced increases in Cai were due to TRPV4, we next tested the ability of two TRPV4 inhibitors ruthenium red and RN1734 to blunt flow-induced increase in Cai . In the presence of 15 and 50 umol L-1 ruthenium red peak Cai induced by luminal flow was reduced by 41±9 (p<0.01, n=5) and 77±10 % (p<0.02, n=6; Figure 2). The rate of rise, decay half-life and plateau were not significantly different from control experiments (Figure 4 A-C). We then examined the effects of the specific TRPV4 inhibitor RN1734 (Vincent et al., 2009). In the presence of 10 and 50 umol L-1 RN1734 the peak increase in Cai induced by luminal flow was reduced by 46±11 (p<0.01, n=7) and 76±5 % (p<0.02, n=5; Figure 3). The rate of rise was also significantly reduced with RN1734 50 umol L-1 (p<0.01). The decay half-life and the plateau were not significantly different from control experiments (Figure 4 A-C).
Figure 2.
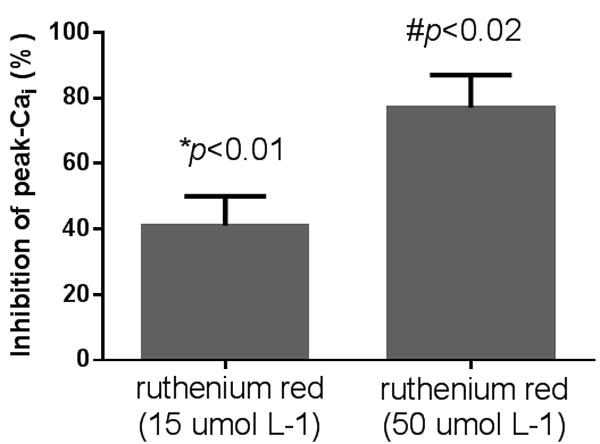
Effect of theTRPV blocker ruthenium red on flow-induced Cai increases by isolated mTALs. Ruthenium red at 15 and 50 umol L-1 reduced peak Cai by 41±9 (*p<0.01;n=5 compared to control) and 77±10 % (#p<0.02,n=6 compared to control) respectively.
Figure 4.
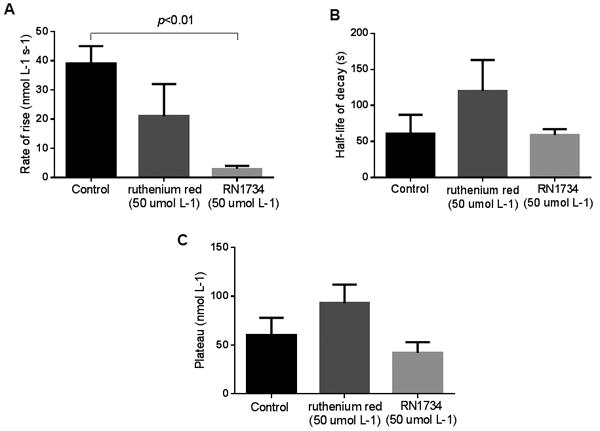
Effect of increasing luminal flow on Cai in the absence (n=6) or presence of the TRPV blocker ruthenium red (50 umol L-1) (n=6) and the TRPV4 blocker RN 1734 (50 umol L-1) (n=5) by isolated mTALs. A) effect of increasing luminal flow on rate of rise of Cai (p < 0.01, only RN1734 compared to control); B) half-life of decay after the peak-increase in Cai, C) plateau of the decay.
Figure 3.
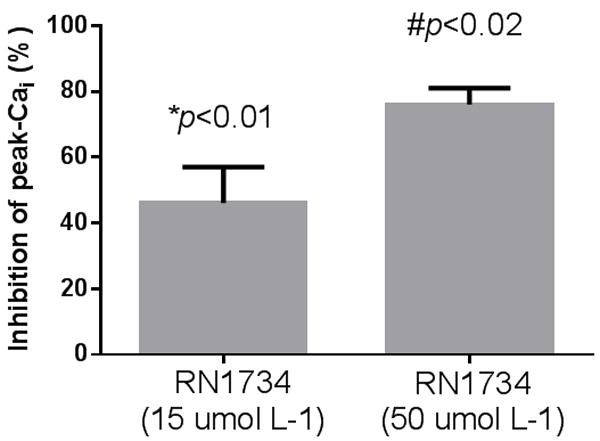
Effect of theTRPV4 blocker RN1734 on flow-induced Cai increases by isolated mTALs. RN1734 at 10 and 50 umol L-1 reduced peak Cai by 46±11 (*p<0.01;n=7 compared to control) and 76±5 % (p<0.02;n=5 compared to control) respectively.
To avoid any confounding effects caused by pharmacological compounds, we studied the role of TRPV4 by reducing its expression using TRPV4-shRNA. We have previously shown that total TRPV4 expression can be reduced by 50-70 % by transducing mTALs with a TRPV4-shRNA expressing adenoviral vector (Silva and Garvin, 2008, Cabral and Garvin, 2014). In non-transduced control mTALs, luminal flow generated a peak-increase in Cai of 111 ± 21 nmol L-1 (n=8). The rate of rise was 10 ± 4 nmol L-1 s-1, the decay half-life was 43 ± 6 s and the plateau was 32 ± 3 nmol L-1. In mTALs transduced with a TRPV4-shRNA, the peak increase in Cai was reduced to 56 ± 8 nmol L-1 (p<0.03, n=9). The rate of rise and a decay half-life of 69 ± 20 s were not significantly different from non-transduced control mTALs. The plateau at 19 ± 3 nmol L-1 was significantly lower than non-transduced control mTALs (p<0.01) (Figure 5A-D).
Figure 5.
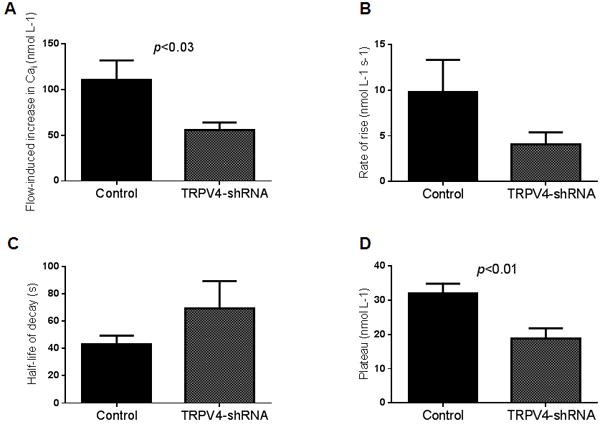
Effect of increasing luminal flow on Cai by isolated mTALs from non-transduced control kidneys (n=8) and TRPV4shRNA-transduced kidneys (n=9). A) effect of increasing luminal flow on peak-increase in Cai (p < 0.03 compared to control); B) effect of increasing luminal flow on rate of rise of Cai; C) half-life of decay after the peak-increase in Cai, D) plateau of the decay (p < 0.01 compared to control).
We then tested the effects of luminal flow on Cai increases in the absence of extracellular calcium. During no flow conditions, Cai was 52±14 nmol L-1 and the peak Cai was 55±11 nmol L-1 after luminal flow was increased (n=9) (Figure 6). These data indicate that removing extracellular Ca completely abolished flow-induced increases in Cai.
Figure 6.
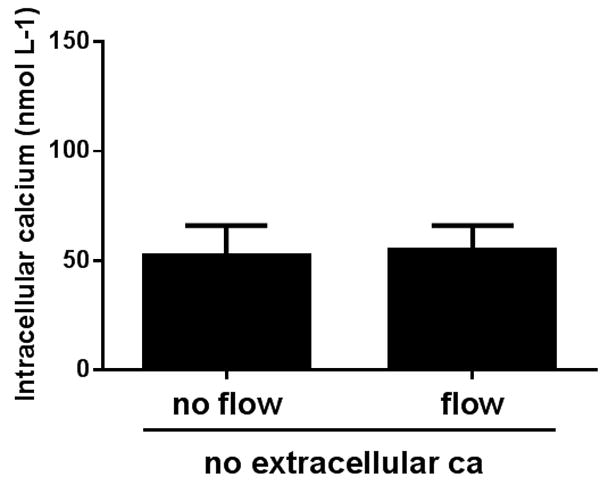
Effect of increasing luminal flow on Cai in the absence of extracellular calcium by isolated mTALs (n=5).
We have previously shown that increasing luminal flow stimulated the release of ATP to the extracellular space (Cabral et al., 2012). Therefore, in order to study whether ATP is involved in flow-induced increases in Cai, we performed a series of experiments in which the effects of luminal flow on Cai were tested in control mTALs and in mTALs that were previously treated with hexokinase (20 U/ml) to degrade extracellular ATP. In control mTALs, luminal flow generated a peak-increase in Cai of 110 ± 39 nmol L-1 (p<0.02, n=7, compared to no flow conditions). The rate of rise was 19 ± 9 nmol L-1 s-1, the decay half-life was 22 ± 3 s and the plateau was 59 ± 11 nmol L-1. In the presence of hexokinase, the peak increase in Cai was 78 ± 22 nmol L-1 (p<0.005, n=8, compared to no flow conditions). The rate of rise was 11 ± 2 nmol L-1 s-1, the decay half-life was 15 ± 4, and the plateau was 65 ± 16 nmol L-1. None of these parameters were significantly different among the two groups (Figure 7 A-D).
Figure 7.
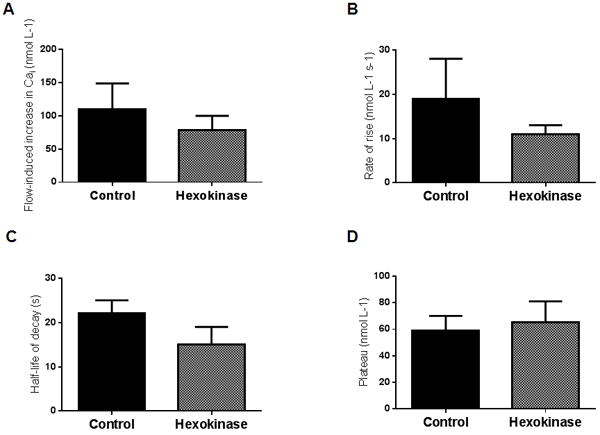
Effect of increasing luminal flow on Cai in the absence (n=7) or presence of the ATP scavenger hexokinase (U mL-1) by isolated mTALs. A) effect of increasing luminal flow on peak-increase in Cai; B) effect of increasing luminal flow on rate of rise of Cai; C) half-life of decay after the peak-increase in Cai; D) plateau of the decay.
Finally, to show that activation of TRPV4 is not only necessary but sufficient to generate increases in Cai in mTALs, we tested the TRPV4 selective agonist GSK1016790A (Thorneloe et al., 2008) without increasing luminal flow. In the absence of luminal flow, Cai concentration was 60 ± 11 nmol L-1. Exposure of mTALs to GSK1016790A (10 nmol L-1) increased the Cai concentration up to 262 ± 71 nmol L-1 (p<0.05, n=7). The rate of rise was 2 ± 1 nmol L-1 s-1 (Figure 8). This set of experiments suggests that stimulation of TRPV4 channels can mimic the response of mTALs to increased luminal flow.
Figure 8.
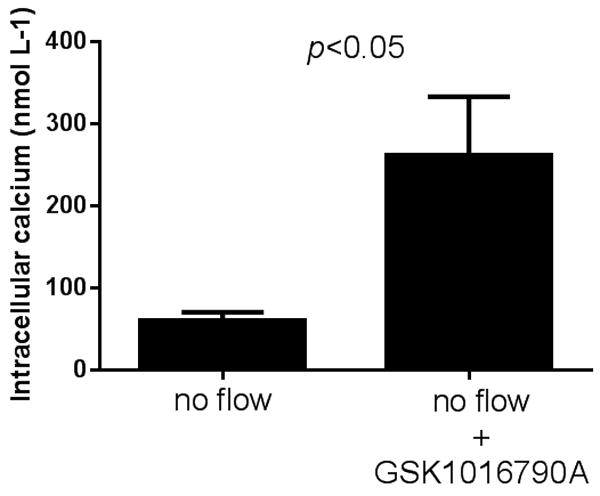
Effect of the TRPV4 selective agonist GSK1016790A (10 nmol L-1) on Cai by isolated thick ascending limbs in the absence of luminal flow (p < 0.05 compared to no flow conditions; n = 7).
Discussion
Luminal flow has been shown to elevate Cai in several cells including the thick ascending limb (Jensen et al., 2007, Rohatgi et al., 2008, Liu et al., 2003). We hypothesized that this is due to activation of TRPV4. We found that: 1) increasing luminal flow elevated Cai; 2) antagonizing TRPV4 channels with two pharmacological compounds blocked this effect; 3) TRPV4-specific targeting with adenoviruses expressing shRNA blunted the increase in Cai generated by luminal flow and 4) activating TRPV4 in the absence of luminal flow stimulated an increase in Cai. Altogether, these data suggest a pivotal role of TRPV4 in mediating increases of Cai concentrations in response to luminal flow.
Raising luminal flow from 0 to 20 nL min-1 in perfused thick ascending limbs elevated Cai. We found that the rate of rise was 25 ± 4 nmol L-1 s-1 leading to a peak of 231 ± 29 nmol L-1. The peak was transient and decayed with a single exponential half-life of 44 ± 10 s and plateaued at a concentration of 85 ± 10 nmol L-1. The fact that the decay of the peak fit a single exponential provides evidence that it is due to activation of a single process.
To study whether flow-stimulated Cai was due to TRPV4, we targeted TRPV4 using three different approaches. First, ruthenium red was used to block TRPV4 activation. We found that ruthenium red concentration dependently diminished the peak flow-induced Cai. The decay half-life was not significantly different before and after ruthenium red. This would be expected if only a single type of transporter was responsible for the decay.
Ruthenium red does not specifically block TRPV4. It blocks other transient receptor potential channels as well (Vennekens et al., 2008). Thus we next used RN 1734 a commercial selective TRPV4 blocker (Vincent et al., 2009). We found that RN1734 also concentration-dependently diminished the peak flow-induced Cai. The decay half-life was not significantly different before and after RN. This would be expected if only a single type of transporter was responsible for the decay. Unlike ruthenium red, RN1734 significantly decreased the rate of rise.
The IC50 of RN 1734 for TRPV4 is 2.3 umol L-1 while the IC50 of RN 1734 for other members of the TRP family is between > 30 umol L-1 to 100 umol L-1 (Vincent et al., 2009). Therefore, the concentrations used in our experiments exceeded the IC50 for TRPV4 channels by 4 and 20-fold while approximating the lower limit of the IC50 for other members of the TRP family. Based on these data, one can presume that the concentrations we used selectively target TRPV4 channels. However, to our knowledge RN1734 has only been tested among a few other members of the transient receptor potential super family of channels. Thus we specifically targeted TRPV4 with adenoviruses expressing shRNA. The participation of TRPV4 on flow-induced Cai increase could also be studied by transducing mTALs with a TRPV4shRNA. This approach has the advantage of reducing the expression of proteins in a transient manner decreasing the likelihood of activation of compensatory mechanisms. Nonetheless, transducing mTALs with a TRPV4shRNA only reduced flow-induced Cai increase by approximately 50 ± 7%. This is likely due to a partial reduction in TRPV4 expression levels as we have previously shown (Silva and Garvin, 2008, Cabral and Garvin, 2014). It is well known that this approach not always lead to a total suppression of protein expression. In this regard, we have previously shown that transducing mTALs with TRPV4shRNA decreased TRPV4 protein expression by approximately 50-70 %. Thus, the increase of Cai that cannot be prevented, might be mediated by the remaining protein that could not be reduced with TRPV4shRNA. Also, there is a possibility that reducing the expression of TRPV4 could affect different pools of the protein such as the intracellular pool or the membrane targeted fraction of the protein. In a previous publication we found that the effect of the TRPV4 agonist GSK1016790A on NO production was reduced in TRPV4shRNA-transduced mTALs suggesting that this procedure can affect the membrane-targeted fraction of TRPV4 (Cabral and Garvin, 2014).
Finally, we targeted TRPV4 using the selective agonist GSK1016790A. We saw that pharmacological activation of TRPV4 was a sufficient stimulus to elicit an increase in Cai in the absence of mechanical stimulation exerted by luminal flow. Together our findings indicating that TRPV4 could serve as a transducer of luminal flow in the rat mTAL are novel.
Elevating luminal flow increases shear stress, cellular stretch and pressure. TRPV4 activation has been suggested to be able to transduce each of these mechanical stimuli in several other cell types (O’Neil and Heller, 2005, Mochizuki et al., 2009, Hartmannsgruber et al., 2007, Wu et al., 2007). Perhaps the most studied mechanical stimuli leading to activation of these channels in different cell types is cellular stretch. We have previously shown that hypotonicity-induced ATP release in the mTAL is mediated by TRPV4 activation (Silva and Garvin, 2008). Given that hypotonicity causes cell swelling, the TRPV4-mediated ATP release was likely mediated by cellular stretch. This effect could be blunted by removing extracellular calcium. Additionally, cellular stretch has been shown to generate increases in Cai in the collecting duct although it is not clear what channel mediates this (Liu et al., 2003).
Shear stress has also been shown to activate TRPV4 channels in endothelial as well as in epithelial cells (Hartmannsgruber et al., 2007, Wu et al., 2007, Taniguchi et al., 2006, Mendoza et al., 2010). Particularly, in mTALs, we have previously reported that shear stress rather than cellular stretch or pressure is the mechanical component involved in flow-induced NO production (Cabral et al., 2010). Additionally, we have recently shown that luminal flow stimulates NO production via activation of TRPV4 channels and that removing extracellular calcium could prevent flow-induced NO production (Cabral and Garvin, 2014). Although here we have not studied in detail the different mechanical components of luminal flow that generate an increase in Cai, it is likely that both shear stress and cellular stretch mediate this phenomenon.
Flow-induced Cai is not an exclusive phenomenon of the rat mTAL. Jensen et al showed that increasing perfusion pressure in isolated TALs from mice generated an increase in Cai (Jensen et al., 2007). In this report, the authors found that flow-induced release of nucleotides that further activate purinergic P2 receptors is the mechanism involved in this phenomenon. They also showed that this event was conserved in the absence of extracellular calcium implicating a P2 receptor-mediated signaling as the only mechanism. In agreement with these findings, we showed before that luminal flow stimulated the release of ATP but did not study the relationship between ATP release and Cai (Cabral et al., 2012). However, in the current report we are showing that 1) flow-induced increases in Cai are mediated primarily by TRPV4 in the rat mTAL; 2) removing extracellular calcium completely abrogated flow-induced Cai increases and 3) scavenging extracellular ATP did not significantly affect this phenomenon. There are several explanations for these discrepancies. First, it is possible that ATP release comes after TRPV4 activation as previously seen in rat mTALs (Silva and Garvin, 2008) as well as in other cells (Shahidullah et al., 2012, Mihara et al., 2011, Mochizuki et al., 2009). Second, it is possible that ATP-degradation products such as ADP are responsible for part of the effect and that the solely elimination of ATP is not enough to block purinergic signaling. Third, luminal flow might trigger different responses in mice and rats. Similar to our findings, Berrout et al reported that flow-induced increase in Cai in the mouse collecting duct depends on TRPV4 activation (Berrout et al., 2012). Even though other members of the transient receptor potential family of channels have been shown to be present in this segment, this report also shows that flow-induced Cai increase is completely blunted in the presence of the TRPV4 blocker ruthenium red and in TRPV4 knockout mice (Berrout et al., 2012).
The mechanisms by which Cai concentrations vary include calcium uptake and released from the endoplasmic reticulum; calcium entry through different membrane-targeted channels; calcium exchange through transporters, among others. More importantly, the kinetics of intracellular calcium from its increase to its decay clearly involved more than one mechanism. In our experiments, antagonizing TRPV4 channels always reduced the peak-increase in Cai. However, the plateau of the decay was only reduced by TRPV4-shRNA and the rate of rise was only reduced by RN1734 (50 umol L-1). The fact that these parameters were only modified in certain experimental conditions might be due to their variability within the group making it difficult to observe statistical significance on subtle variations. The decay half-life was never affected throughout the different experimental conditions. This was not surprising since the decay is likely due to a process that is different from that causing the peak.
Our results suggest that most of the calcium involved in the intracellular increase comes from the extracellular space being TRPV4 the dominant player. However, this might not be the only explanation since the primary cilia present in epithelial cells have been also shown to play an important role in sensing mechanical stimuli (Praetorius and Spring, 2001, Praetorius et al., 2003, Nauli et al., 2003). One of the best known proteins expressed in this structure is the transient receptor potential polycystin 2 (TRPP2) which also allows calcium entry upon mechanical stimulation (Anyatonwu and Ehrlich, 2004, Gonzalez-Perrett et al., 2001, Nauli et al., 2003). Interestingly, it has been demonstrated that TRPP2 and TRPV4 form a heteromeric mechanosensitive channel (Kottgen et al., 2008). We found that TRPV4 antagonists greatly reduced flow-induced Cai increases and that removing extracellular Ca abolished flow-induced Cai increases. However, we cannot ascertain that TRPV4 is the only channel responsible for this phenomenon. Although interesting, the potential interactions of TRPV4 with other channels or even the possibility that calcium influx from the extracellular space might generate calcium release from intracellular stores is beyond the scope of this work.
Acknowledgments
This work was supported in part by grants to J.L.G. from the National Institutes of Health (HL70985) and to P.D.C from the American Heart Association (11POST7490010 and 13POST16280001)
Footnotes
Disclosures
None.
References
- Anyatonwu GI, Ehrlich BE. Calcium signaling and polycystin-2. Biochem Biophys Res Commun. 2004;322:1364–1373. doi: 10.1016/j.bbrc.2004.08.043. [DOI] [PubMed] [Google Scholar]
- Arendshorst WJ, Beierwaltes WH. Renal tubular reabsorption in spontaneously hypertensive rats. Am J Physiol. 1979;237:F38–47. doi: 10.1152/ajprenal.1979.237.1.F38. [DOI] [PubMed] [Google Scholar]
- Berrout J, Jin M, Mamenko M, Zaika O, Pochynyuk O, O’Neil RG. Function of TRPV4 as a mechanical transducer in flow-sensitive segments of the renal collecting duct system. J Biol Chem. 2012 doi: 10.1074/jbc.M111.308411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg MB. Thick ascending limb of Henle’s loop. [Review] [72 refs] Kidney International. 1982;22:454–464. doi: 10.1038/ki.1982.198. [DOI] [PubMed] [Google Scholar]
- Cabral PD, Garvin JL. Luminal flow regulates NO and O2(-) along the nephron. Am J Physiol Renal Physiol. 2011;300:F1047–53. doi: 10.1152/ajprenal.00724.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabral PD, Garvin JL. TRPV4 activation mediates flow-induced nitric oxide production in the rat thick ascending limb. Am J Physiol Renal Physiol. 2014;307:F666–72. doi: 10.1152/ajprenal.00619.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabral PD, Hong NJ, Garvin JL. Shear stress increases nitric oxide production in thick ascending limbs. Am J Physiol Renal Physiol. 2010;299:F1185–92. doi: 10.1152/ajprenal.00112.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabral PD, Hong NJ, Garvin JL. ATP mediates flow-induced NO production in thick ascending limbs. Am J Physiol Renal Physiol. 2012;303:F194–200. doi: 10.1152/ajprenal.00504.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiBona GF, Rios LL. Mechanism of exaggerated diuresis in spontaneously hypertensive rats. Am J Physiol. 1978;235:409–16. doi: 10.1152/ajprenal.1978.235.5.F409. [DOI] [PubMed] [Google Scholar]
- Dwyer TM, Schmidt-Nielsen B. The renal pelvis: machinery that concentrates urine in the papilla. News Physiol Sci. 2003;18:1–6. doi: 10.1152/nips.1416.2002. [DOI] [PubMed] [Google Scholar]
- Filosa JA, Yao X, Rath G. TRPV4 and the regulation of vascular tone. J Cardiovasc Pharmacol. 2013;61:113–9. doi: 10.1097/FJC.0b013e318279ba42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Perrett S, Kim K, Ibarra C, Damiano AE, Zotta E, Batelli M, Harris PC, Reisin IL, Arnaout MA, Cantiello HF. Polycystin-2, the protein mutated in autosomal dominant polycystic kidney disease (ADPKD), is a Ca2+-permeable nonselective cation channel. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:1182–1187. doi: 10.1073/pnas.98.3.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmannsgruber V, Heyken WT, Kacik M, Kaistha A, Grgic I, Harteneck C, Liedtke W, Hoyer J, Kohler R. Arterial response to shear stress critically depends on endothelial TRPV4 expression. PLoS One. 2007;2:e827. doi: 10.1371/journal.pone.0000827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert SC. Bartter syndrome. Curr Opin Nephrol Hypertens. 2003;12:527–532. doi: 10.1097/00041552-200309000-00008. [DOI] [PubMed] [Google Scholar]
- Holstein-Rathlou NH, Marsh DJ. A dynamic model of the tubuloglomerular feedback mechanism. Am J Physiol Renal Physiol. 1990;258:F1448–F1459. doi: 10.1152/ajprenal.1990.258.5.F1448. [DOI] [PubMed] [Google Scholar]
- Jensen ME, Odgaard E, Christensen MH, Praetorius HA, Leipziger J. Flow-induced [Ca2+]i increase depends on nucleotide release and subsequent purinergic signaling in the intact nephron. J Am Soc Nephrol. 2007;18:2062–2070. doi: 10.1681/ASN.2006070700. [DOI] [PubMed] [Google Scholar]
- Jung J, Basile DP, Pratt JH. Sodium reabsorption in the thick ascending limb in relation to blood pressure: a clinical perspective. Hypertension. 2011;57:873–879. doi: 10.1161/HYPERTENSIONAHA.108.120246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler R, Heyken WT, Heinau P, Schubert R, Si H, Kacik M, Busch C, Grgic I, Maier T, Hoyer J. Evidence for a functional role of endothelial transient receptor potential V4 in shear stress-induced vasodilatation. Arterioscler Thromb Vasc Biol. 2006;26:1495–1502. doi: 10.1161/01.ATV.0000225698.36212.6a. [DOI] [PubMed] [Google Scholar]
- Kottgen M, Buchholz B, Garcia-Gonzalez MA, Kotsis F, Fu X, Doerken M, Boehlke C, Steffl D, Tauber R, Wegierski T, Nitschke R, Suzuki M, Kramer-Zucker A, Germino GG, Watnick T, Prenen J, et al. TRPP2 and TRPV4 form a polymodal sensory channel complex. J Cell Biol. 2008;182:437–47. doi: 10.1083/jcb.200805124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liedtke W. TRPV channels’ role in osmotransduction and mechanotransduction. In: HEIDELBERG S-VB, editor. Handb Exp Pharmacol. 2007. [DOI] [PubMed] [Google Scholar]
- Liu W, Xu S, Woda C, Kim P, Weinbaum S, Satlin LM. Effect of flow and stretch on the [Ca2+]i response of principal and intercalated cells in cortical collecting duct. Am J Physiol Renal Physiol. 2003;285:F998–F1012. doi: 10.1152/ajprenal.00067.2003. [DOI] [PubMed] [Google Scholar]
- Mendoza SA, Fang J, Gutterman DD, Wilcox DA, Bubolz AH, Li R, Suzuki M, Zhang DX. TRPV4-mediated endothelial Ca2+ influx and vasodilation in response to shear stress. Am J Physiol Heart Circ Physiol. 2010;298:H466–H476. doi: 10.1152/ajpheart.00854.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihara H, Boudaka A, Sugiyama T, Moriyama Y, Tominaga M. Transient receptor potential vanilloid 4 (TRPV4)-dependent calcium influx and ATP release in mouse oesophageal keratinocytes. J Physiol. 2011;589:3471–82. doi: 10.1113/jphysiol.2011.207829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki T, Sokabe T, Araki I, Fujishita K, Shibasaki K, Uchida K, Naruse K, Koizumi S, Takeda M, Tominaga M. The TRPV4 cation channel mediates stretch-evoked Ca2+ influx and ATP release in primary urothelial cell cultures. J Biol Chem. 2009;284:21257–21264. doi: 10.1074/jbc.M109.020206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto T, Liu W, Woda C, Carattino MD, Wei Y, Hughey RP, Apodaca G, Satlin LM, Kleyman TR. Mechanism underlying flow stimulation of sodium absorption in the mammalian collecting duct. Am J Physiol Renal Physiol. 2006;291:F663–F669. doi: 10.1152/ajprenal.00514.2005. [DOI] [PubMed] [Google Scholar]
- Mozaffari MS, Jirakulsomchok S, Shao ZH, Wyss JM. High-NaCl diets increase natriuretic and diuretic responses in salt-resistant but not salt-sensitive SHR. Am J Physiol. 1991;260:F890–F897. doi: 10.1152/ajprenal.1991.260.6.F890. [DOI] [PubMed] [Google Scholar]
- Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, Li X, Elia AE, Lu W, Brown EM, Quinn SJ, Ingber DE, Zhou J. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet. 2003;33:129–137. doi: 10.1038/ng1076. [DOI] [PubMed] [Google Scholar]
- O’Neil RG, Heller S. The mechanosensitive nature of TRPV channels. Pflugers Arch. 2005;451:193–203. doi: 10.1007/s00424-005-1424-4. [DOI] [PubMed] [Google Scholar]
- Ortiz PA, Hong NJ, Plato CF, Varela M, Garvin JL. An in vivo method for adenovirus-mediated transduction of thick ascending limbs. Kidney Int. 2003;63:1141–9. doi: 10.1046/j.1523-1755.2003.00827.x. [DOI] [PubMed] [Google Scholar]
- Pollock CA, Lawrence JR, Field MJ. Tubular sodium handling and tubuloglomerular feedback in experimental diabetes mellitus. Am J Physiol. 1991;260:F946–F952. doi: 10.1152/ajprenal.1991.260.6.F946. [DOI] [PubMed] [Google Scholar]
- Praetorius HA, Frokiaer J, Nielsen S, Spring KR. Bending the primary cilium opens Ca2+-sensitive intermediate-conductance K+ channels in MDCK cells. J Membr Biol. 2003;191:193–200. doi: 10.1007/s00232-002-1055-z. [DOI] [PubMed] [Google Scholar]
- Praetorius HA, Spring KR. Bending the MDCK cell primary cilium increases intracellular calcium. J Membr Biol. 2001;184:71–79. doi: 10.1007/s00232-001-0075-4. [DOI] [PubMed] [Google Scholar]
- Rohatgi R, Battini L, Kim P, Israeli S, Wilson PD, Gusella GL, Satlin LM. Mechanoregulation of intracellular Ca2+ in human autosomal recessive polycystic kidney disease cyst-lining renal epithelial cells. Am J Physiol Renal Physiol. 2008;294:F890–F899. doi: 10.1152/ajprenal.00341.2007. [DOI] [PubMed] [Google Scholar]
- Shahidullah M, Mandal A, Delamere NA. TRPV4 in porcine lens epithelium regulates hemichannel-mediated ATP release and Na-K-ATPase activity. Am J Physiol Cell Physiol. 2012;302:C1751–61. doi: 10.1152/ajpcell.00010.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva GB, Garvin JL. TRPV4 mediates hypotonicity-induced ATP release by the thick ascending limb. Am J Physiol Renal Physiol. 2008;295:F1090–5. doi: 10.1152/ajprenal.90365.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi J, Tsuruoka S, Mizuno A, Sato JI, Fujimura A, Suzuki M. TRPV4 as a flow sensor in flow-dependent K+ secretion from the cortical collecting duct. Am J Physiol Renal Physiol. 2006 doi: 10.1152/ajprenal.00458.2005. [DOI] [PubMed] [Google Scholar]
- Thorneloe KS, Sulpizio AC, Lin Z, Figueroa DJ, Clouse AK, McCafferty GP, Chendrimada TP, Lashinger ES, Gordon E, Evans L, Misajet BA, Demarini DJ, Nation JH, Casillas LN, Marquis RW, Votta BJ, et al. N-((1S)-1-{[4-((2S)-2-{[(2,4-dichlorophenyl)sulfonyl]amino}-3-hydroxypropanoyl)-1 -piperazinyl]carbonyl}-3-methylbutyl)-1-benzothiophene-2-carboxamide (GSK1016790A), a novel and potent transient receptor potential vanilloid 4 channel agonist induces urinary bladder contraction and hyperactivity: Part I. J Pharmacol Exp Ther. 2008;326:432–442. doi: 10.1124/jpet.108.139295. [DOI] [PubMed] [Google Scholar]
- Vennekens R, Owsianik G, Nilius B. Vanilloid transient receptor potential cation channels: an overview. Curr Pharm Des. 2008;14:18–31. doi: 10.2174/138161208783330763. [DOI] [PubMed] [Google Scholar]
- Vincent F, Acevedo A, Nguyen MT, Dourado M, DeFalco J, Gustafson A, Spiro P, Emerling DE, Kelly MG, Duncton MA. Identification and characterization of novel TRPV4 modulators. Biochem Biophys Res Commun. 2009;389:490–494. doi: 10.1016/j.bbrc.2009.09.007. [DOI] [PubMed] [Google Scholar]
- Woda CB, Bragin A, Kleyman TR, Satlin LM. Flow-dependent K+ secretion in the cortical collecting duct is mediated by a maxi-K channel. American Journal of Physiology - Renal Fluid & Electrolyte Physiology. 2001;280:F786–F793. doi: 10.1152/ajprenal.2001.280.5.F786. [DOI] [PubMed] [Google Scholar]
- Woda CB, Leite M, Jr, Rohatgi R, Satlin LM. Effects of luminal flow and nucleotides on [Ca(2+)](i) in rabbit cortical collecting duct. Am J Physiol Renal Physiol. 2002;283:F437–F446. doi: 10.1152/ajprenal.00316.2001. [DOI] [PubMed] [Google Scholar]
- Woda CB, Miyawaki N, Ramalakshmi S, Ramkumar M, Rojas R, Zavilowitz B, Kleyman TR, Satlin LM. Ontogeny of flow-stimulated potassium secretion in rabbit cortical collecting duct: functional and molecular aspects. Am J Physiol Renal Physiol. 2003;285:F629–39. doi: 10.1152/ajprenal.00191.2003. [DOI] [PubMed] [Google Scholar]
- Wu L, Gao X, Brown RC, Heller S, O’Neil RG. Dual role of the TRPV4 channel as a sensor of flow and osmolality in renal epithelial cells. Am J Physiol Renal Physiol. 2007;293:F1699–F1713. doi: 10.1152/ajprenal.00462.2006. [DOI] [PubMed] [Google Scholar]


