Abstract
Background
Delirium is a common complication after cardiac surgery and is associated with increased morbidity and mortality. However, whether rigorously-assessed postoperative delirium is associated with increased length of stay in the intensive care unit (LOS-ICU), length of stay (LOS), and hospital charges is not clear.
Methods
Patients (n=66) undergoing coronary artery bypass and/or valve surgery were enrolled in a nested cohort study. Rigorous delirium assessments were conducted using the Confusion Assessment Method. LOS-ICU and LOS were obtained from the medical record and hospital charges from administrative data reported to the state. Because of the skewed distribution of outcome variables, outcomes were compared using rank-sum tests, as well as median regression incorporating propensity scores.
Results
Patients who developed delirium (56%) vs. no delirium (43%) had increased median LOS-ICU (75.6 hours [IQR 43.6–136.8] vs. 29.7 hours [IQR 21.7–46.0]; p=0.002), increased median LOS (9 days [IQR 6–16] vs. 7 days [IQR 5–8]; p=0.006), and increased median hospital charges ($51,805 [IQR $44,041-$80,238] vs. $41,576 [IQR $35,748-$43,660]; P=0.002). In propensity score models adjusted for patient and surgical characteristics and complications, the results for LOS-ICU and cost remained highly significant, although the results for LOS were attenuated based on the specific statistical model. Increased severity of delirium was associated with both increased LOS-ICU and charges in a dose-response manner.
Conclusions
Delirium after cardiac surgery is independently associated with both increased length of stay-ICU and higher hospital charges. Since delirium is potentially preventable, targeted delirium-prevention protocols for high-risk patients may represent an important strategy for quality improvement.
Keywords: Delirium, Outcomes, Quality Care (Management)
Delirium is increasingly recognized as a common complication after cardiac surgery. (1) The fact that it is potentially modifiable (2–4) makes it an attractive target for improving quality of care in cardiac surgery patients. The incidence of delirium after cardiac surgery has been estimated to be between 30–50%, (5) and importantly, depends on the methodology of the assessment, with the hypoactive form of delirium often missed in the absence of a rigorous assessment. (6) Although previously thought to be transient with few consequences, recent evidence has demonstrated that delirium after cardiac surgery is associated with increased mortality, (7) functional decline, (8) and cognitive decline (9).
However, the association between delirium after cardiac surgery and length of stay and hospital charges has not been well defined. Prior studies have demonstrated an association between delirium and increased length of stay, (10–12) but these studies have been generally limited in several ways. First, a lack of sensitive methodology in delirium assessment may have underestimated the incidence of delirium, particularly hypoactive delirium, which is common in the cardiac care units, leading to misclassification bias. Second, failure to consider patient baseline characteristics may have resulted in confounding, since vulnerable patients are more likely both to develop delirium and to have increased length of stay. Finally, the economic impact of delirium after cardiac surgery has not been well characterized.
In this study, we tested the hypothesis that rigorously assessed delirium after cardiac surgery would be associated with increased length of stay in the intensive care unit (LOS-ICU), length of stay (LOS), and hospital charges, even after adjusting for important patient characteristics.
Patients and Methods
This was a prospective observational study, nested in an ongoing randomized trial evaluating the association between cerebral blood flow autoregulation (13) and brain injury after cardiac surgery (www.clinicaltrials.gov NCT00981474). The study was approved by the Johns Hopkins Institutional Review Board (Baltimore, MD), and individual written informed consents were obtained. Patients were enrolled between October 2012 and February 2014. Inclusion criteria were coronary artery bypass graft (CABG) and/or valve surgery that required cardiopulmonary bypass and an elevated risk of stroke or encephalopathy, based on a Johns Hopkins risk score composed of history of stroke, presence of carotid bruit, hypertension, diabetes, and age, which generally excluded patients in the lowest quartile of risk. (14) Exclusion criteria were dialysis, non-English speaking, contraindications to MRI, and emergency surgery. As part of the main trial, patients were randomized 1:1 to blood pressure targets during cardiopulmonary bypass based on measures of cerebral autoregulation vs. standard of care targets. During the nested cohort study, 798 patients were screened, of which 453 did not meet enrollment criteria, 151 were not approached for logistical reasons (such as unavailability of staff or emergent procedure), 117 declined participation, and 77 were enrolled. Of the 77 enrolled patients, 5 patients withdrew, 2 patients did not have baseline cognitive assessments and so were excluded, and 4 patients did not have delirium assessments, leaving 66 patients for analysis.
Perioperative Care
General anesthesia was induced and maintained with midazolam (0.15 mg kg−1), fentanyl (5–20 μg kg−1) and isoflurane. Cardiopulmonary bypass was with a non-occlusive roller pump, a membrane oxygenator, and an arterial line filter ≤40-μm. Non-pulsatile flow was maintained between 2.0–2.4 L/min m−2, using alpha-stat pH management. Patient rewarming targeted nasal temperature <37oC. Postoperative sedation was with propofol 20–75 μg kg−1 min−1. Patients requiring >24 hours of ventilation received fentanyl and/or midazolam.
Delirium assessment
Delirium was assessed using rigorous methodology. For non-intubated patients, delirium was assessed in-person by trained research assistants using the validated Confusion Assessment Method (CAM). (15) The assessment was composed of a structured cognitive exam, which included the Mini-Mental State Examination, Digit Span Forwards and Backwards, and timed Months of the Year Backwards. Additionally, researchers queried the patient, nurses, patient families, and the medical record for evidence of delirium. Similarly, delirium severity was evaluated after each assessment using the Delirium Rating Scale-Revised-98, a validated 16-item scale which assesses the severity of delirium symptoms over the previous 24 hours. (16) CAM-ICU was used for intubated patients. (17) Delirium assessments were conducted daily on the first 4 postoperative days because of evidence that >90% of delirium occurs within this time frame. (18) When research staff were not available for assessments (generally one day each weekend), we conducted a validated chart review (19) and queried nurses and family for events since the prior assessment, thereby obtaining data to support a delirium diagnosis in the interval days. Delirium subtype (hyperactive, hypoactive, mixed) was classified using observations of psychomotor agitation or retardation. (20)
Quality assurance methods for delirium assessment included a formal training protocol for research assistants, co-ratings of patients with author KN approximately every two weeks, and bimonthly meetings of delirium experts and research assistants to discuss assessments. We measured agreement between researchers, and kappa statistics are between 0.7–0.8, which is consistent with substantial agreement.
Outcome Data
LOS-ICU and LOS were obtained from the electronic medical record. Charge data was obtained from an administrative database used by the hospital billing department for reporting to the State of Maryland. This database includes all charges from the date of surgery until the date of hospital discharge. The unique structure of medical reimbursement in Maryland means that payment rates for insurers (both public and private) are determined by a rate-setting commission and are uniform amongst payors, thus avoiding cost-shifting to privately insured patients. There was no adjustment for inflation since all patients were enrolled within a 16-month period.
Statistical Analysis
Statistical analyses were conducted using SAS version 9.2 (SAS Institute Inc, Cary, NC)
Baseline characteristics of the study population were compared using t-tests, rank-sum tests, and chi-square tests. To compare outcomes among patients with and without delirium who were otherwise similar with respect to covariates, a propensity score analysis was performed. (21) We a priori included factors for propensity score analysis based on prior studies or clinical suspicion that these factors might be confounders in the association of delirium and the outcomes. For the propensity score model, we used age, race, gender, Beck Depression Inventory score, Mini-Mental State Examination score, European System for Cardiac Operative Risk Evaluations (euroSCORE), active drug/alcohol use, preoperative hemoglobin, surgical procedure, total cardiopulmonary bypass time, number of units of red blood cells transfused intraoperatively, randomization group, and any postoperative occurrence (prior to delirium diagnosis) of sepsis, stroke, new intra-aortic balloon pump, dialysis, multi-organ system failure, or multiple inotropic drugs for 24 hours. The propensity score was estimated using logistic regression, and the propensity score model was checked for balance, for model fit, and for the ability to differentiate between delirious and non-delirious patients. The propensity score was used to calculate an inverse probability weight, which was then used as a weight in weighted median regression models. Because LOS-ICU, LOS, and charges were non-normally distributed, we used median regression models for the main analyses. As sensitivity analyses based on comments from reviewers, we also incorporated additional comorbidities (stroke, diabetes, atrial fibrillation, congestive heart failure, hypertension, myocardial infarction, peripheral vascular disease, and significant valvular disease) into the propensity score and used the Society for Thoracic Surgeons risk score instead of the EuroSCORE.
Results
Patient Characteristics
The incidence of delirium was 56%(37/66). Delirium was first diagnosed on postoperative day 1 in 26(39.4%) patients, on postoperative day 2 in 8(12.1%) patients, and on postoperative day 3 in the remaining 3(4.6%) delirious patients. Hypoactive delirium was present in 18/37(49%), with the remainder being hyperactive delirium (5/37[14%]) mixed hypoactive and hyperactive (8/37[21.6%]), and difficult to classify (6/37[16.2%]). Characteristics of patients by delirium status are shown in Table 1. Overall median LOS-ICU in the cohort was 45.7 hours (IQR 25.1–86.6), overall median LOS was 7 days (IQR 6–13); and median charges were $45,459 (IQR $36,607–$67,807).
Table 1.
Patient and Surgical Characteristics
| No Delirium (n=29) | Delirium (n=37) | P-value | |
|---|---|---|---|
| Age (years), mean±SD | 69±8 | 70±7 | 0.49a |
| Male, n(%) | 23(79.3) | 28(75.7) | 0.73b |
| Race, n(%) | 0.75c | ||
| White | 23(79.3) | 31(83.8) | |
| African American | 3(10.3) | 4(10.8) | |
| Other | 3(10.3) | 2(5.4) | |
| Baseline MMSE, median(IQR) | 28(26–29) | 27(26–28) | 0.13d |
| Education (years), median(IQR) | 15 (12–17) | 13 (12–17) | 0.26 |
| Beck Depression Inventory, median(IQR) | 7.5(3.5–12) | 7.5(4–14) | 0.83d |
| Current smoking, n(%) | 3(10.3) | 2(5.4) | 0.65c |
| Current drug or alcohol, n(%) | 13(44.8) | 21(56.8) | 0.34b |
| euroSCORE, median(IQR) | 5(3–6) | 6(4–9) | 0.05c |
| Comorbidities, n(%) | |||
| Stroke | 2(6.9) | 3(8.1) | 1.0c |
| Carotid bruit | 3 (10.3) | 4 (10.8) | 1.0c |
| Atrial fibrillation | 3(10.3) | 5(13.5) | 1.0c |
| Coronary artery disease | 21(72.4) | 34(91.9) | 0.05b |
| Congestive heart failure | 1(3.5) | 4(10.8) | 0.38c |
| Hypertension | 26(89.7) | 35(94.6) | 0.45b |
| Myocardial infarction | 9(31.0) | 13(35.1) | 0.73b |
| Peripheral vascular disease | 2(6.9) | 12(32.4) | 0.02c |
| Diabetes, n(%) | 13(44.8) | 21(56.8) | 0.34 |
| Hemoglobin (mg/dL), mean±SD | 13.3±1.5 | 12.3±1.8 | 0.02a |
| Surgery, n(%) | 0.02c | ||
| CABG | 15(51.7) | 25(67.6) | |
| CABG/Valve | 2(6.8) | 9(24.3) | |
| Valve | 12(41.3) | 3(8.1) | |
| Bypass time (min), mean±SD | 108±58 | 122±69 | 0.37a |
| Cross-clamp time (min), mean±SD | 72±30 | 77±41 | 0.55a |
| Packed red blood cell transfusions intraoperatively, median(IQR) | 0(0–3) | 2(0–7) | 0.07d |
| Midazolam dose intraoperatively (mg), mean±SD | 9±4 | 9±4 | 0.88a |
| Fentanyl dose intraoperatively (mcg), mean±SD | 1386±411 | 1227 ±344 | 0.10a |
| Delirium Rating Scale-Revised-98 median(IQR) | 3(2–4) | 11(8–14) | <0.001 |
| Postoperative | |||
| In-hospital mortality | 0(0) | 3(8.1) | 0.25 c |
| Re-admission to ICU | 1(3.5) | 5(13.5) | 0.22 c |
| Benzodiazepines on postoperative days 1–4, n(%) | 1(3.5) | 2(5.4) | 1.00 c |
| New intra-aortic balloon pump, n(%) | 0(0) | 4(11.1) | 0.12c |
| Cerebrovascular accident, n(%) | 1(3.5) | 3(8.1) | 0.62c |
| Transient Ischemic Attack, n(%) | 0 (0) | 1(2.9) | 1.0c |
| Sepsis, n(%) | 0(0) | 2(5.4) | 0.50c |
| Dialysis, n(%) | 0(0) | 3(8.6) | 0.25c |
| Multi-Organ System Failure | 0(0) | 1(2.9%) | 1.0c |
| Multiple inotropic drugs, n(%) | 0(0) | 3(8.1) | 0.25b |
| 30-day readmission | 0(0) | 2(5.4) | 0.5 c |
Student’s t-test;
Chi-squared test;
Fisher’s exact test; d Wilcoxon rank-sum test
Abbreviations: CABG=Coronary Artery Bypass Graft; IQR=Inter-Quartile Range; SD=Standard Deviation; MMSE= Mini-Mental State Examination; STS= Society of Thoracic Surgeons
LOS-ICU and LOS
Median LOS-ICU was higher in patients with delirium (75.6 hours, IQR 43.6–136.8) compared to patients without delirium (29.7 hours, IQR 21.7–46.0; P=0.002). As shown in Figure 1a, there was a “dose-response” between severity of delirium and LOS-ICU (p=0.012). In adjusted propensity score models, the estimated median LOS-ICU was 37.2 hours longer (95%CI 25.6–48.7 P<0.001) for patients with delirium compared to without delirium. Among patients with purely hypoactive or hyperactive delirium compared to patients with no delirium, median LOS-ICU in adjusted propensity score models was significantly longer in both the hypoactive patients (41.9 hours; 95%CI 17.3–66.5 hours; p=0.001) and the hyperactive patients (47.6 hours; 95%CI 9.4–85.9 hours; p=0.02).
Figure 1.
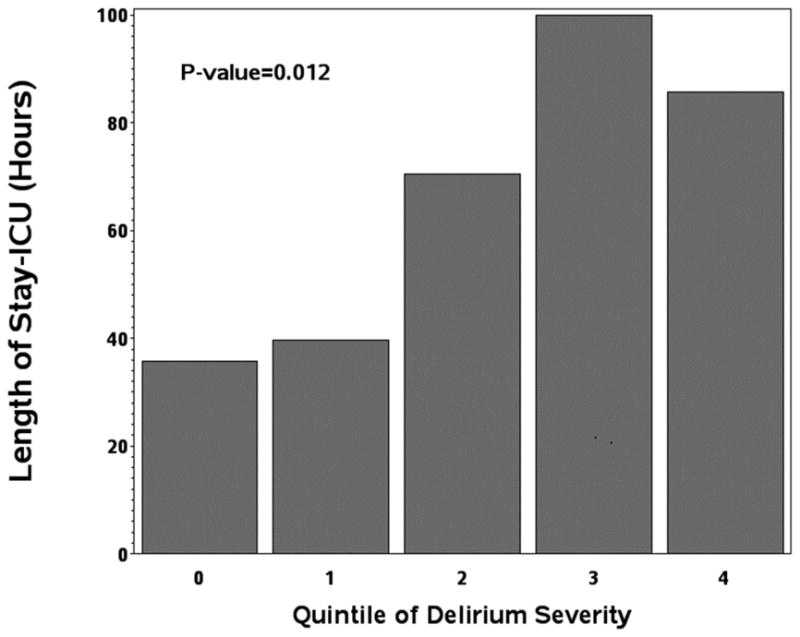
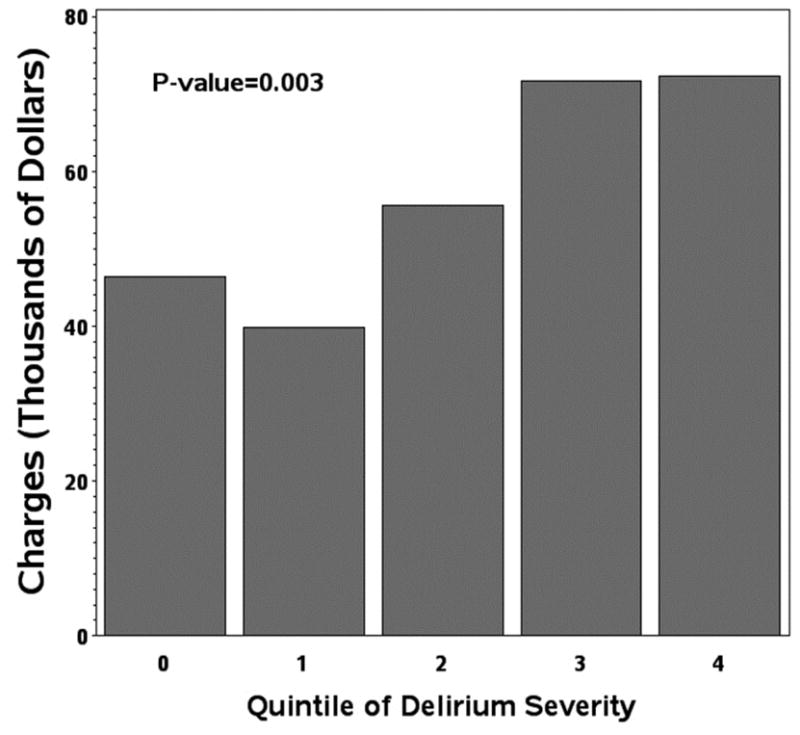
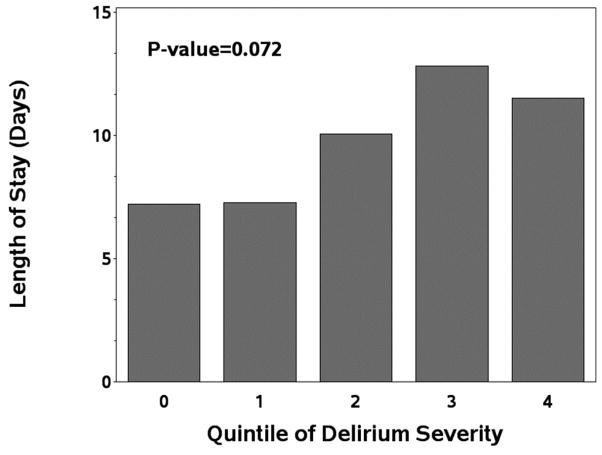
Figure 1A: Length of stay-ICU
Figure 1B: length of hospital stay
Figure 1C: hospital charges in relation to quintile of delirium severity.
Abbreviations: ICU= Intensive Care Unit
NOTES
Article Type = Original Article
Similarly, median LOS was higher in patients with delirium (9 days, IQR 6–16) compared to without delirium (7 days, IQR 5–8; P=0.006, unadjusted). As shown in Figure 1b, there was a similar trend towards a “dose-response” between severity of delirium and LOS (p=0.07). In the primary adjusted propensity score model, the estimated median length of stay was 4 days longer, (IQR 2.65–4.35, p<0.001) for patients with delirium compared to without delirium. Among patients with purely hypoactive or hyperactive delirium compared to patients with no delirium, median LOS in adjusted propensity score models was significantly increased only in the hyperactive patients (6 days longer; 95%CI 1.4–10.6 days; p=0.01).
Charges
Similar to LOS-ICU and LOS, median total charges were greater among patients with delirium ($51,805, IQR $44,041-$80,238) compared to without delirium ($41,576, IQR $35,748-$43,660; P=0.002). As shown in Table 2, all types of charges (with the exception of supplies) were significantly higher among patients with delirium compared to without delirium. Figure 1c demonstrates a “dose response”, between severity of delirium and hospital charges (P=0.003). In adjusted propensity score models, patients with delirium had median hospital charges that were greater by $10,339 (95%CI $1,969-$18,709, P=0.02) compared to patients without delirium. There was no difference in costs between patients with purely hypoactive or hyperactive delirium compared to patients with no delirium in adjusted propensity score models.
Table 2.
Categories of hospital charge data for patients with and without delirium
| No Delirium (n=29) | Delirium (n=37) | P-valuea | |||
|---|---|---|---|---|---|
|
|
|||||
| Median | IQR | Median | IQR | ||
| Pharmacyb | $931 | $724–1090 | $1431 | $1045–2247 | <0.001 |
| Laboratoryc | $2703 | $2275–3466 | $5319 | $3361–8513 | <0.001 |
| Operating | $7911 | $7561–8556 | $9680 | $8094–11,208 | 0.002 |
| Roomd | |||||
| Radiology | $555 | $341–1041 | $1224 | $684–3485 | 0.002 |
| Servicese | |||||
| Suppliesf | $8853 | $6858–12,903 | $8473 | $6487–13,476 | 0.67 |
| Therapyg | $1473 | $1092–2180 | $2685 | $1574–7069 | <0.001 |
| Routineh | $13,515 | $9700–$16,555 | $17,441 | $13,310–32,955 | 0.002 |
| Otheri | $3112 | $2401–4590 | $4367 | $3167–7078 | 0.003 |
P-value was calculated using Wilcoxon rank sum test and is unadjusted.
Pharmacy charges generally included all drugs (intra- and post-operative)
Laboratory charges generally included all laboratory tests and blood charges
Operating room charges generally included operating and recovery room charges
Radiology services generally included postoperative radiologic scans
Supplies charges generally included surgery and anesthesia intraoperative and postoperative supplies
Therapy charges generally included occupational, physical, respiratory, and speech therapy.
Routine charges generally included daily hospital care charges
Other charges generally included anesthesia care
Abbreviations: IQR= Inter-Quartile Range
Sensitivity Analyses
We conducted several sensitivity analyses to further examine these findings. First, we examined how the timing of postoperative delirium affected LOS-ICU, because we were concerned that patients with long LOS-ICU might be prone to develop delirium (reverse causation). We compared the subset of patients who developed “early” delirium (on postoperative days 0–1 only) compared to non-delirious patients and found similar results to the main model—increased LOS-ICU in the “early” delirium group (44.8 hours longer; 95%CI 16.4–73.2 hours; p=0.003). Second, we incorporated additional covariates into the propensity score model as described in the methods, and we substituted Society for Thoracic Surgeons risk score for the EuroSCORE. In each of these models, we found a consistent independent association of delirium with both increased LOS-ICU and increased cost. However, the association between delirium and increased LOS was attenuated in the model with additional covariates (p=0.1) and in the model using the Society for Thoracic Surgeons risk score (p=0.27).
Comment
The results of this study demonstrate that postoperative delirium is independently associated with both increased LOS-ICU and hospital charges among patients undergoing cardiac surgery. While not significant in all models, there also was an association between delirium and increased LOS. These results in cardiac surgery complement other studies which have only included non-cardiac surgery patients, (22,23) or intubated ICU patients (24) in whom post-ICU delirium may have been undetected. In addition, prior reports in cardiac surgery have not accounted for potentially important confounding variables (25) or used restricted definitions of delirium, such as CAM-ICU, (12) which has reduced sensitivity in non-intubated patients. These results also support the importance of delirium severity and suggest that further categorizing delirium may have prognostic implications. (26) Importantly, almost half of patients with delirium had the hypoactive subtype, which is difficult to recognize clinically but has been associated with poor outcomes. (12) Indeed, our results show that purely hypoactive delirium was independently associated with increased costs. Finally, this study is unique in quantifying the economic impact of delirium after cardiac surgery. In other populations, including ventilated medical ICU patients, delirium incidence, severity, and duration have been associated with higher costs (11). Prior to this study, however, it was unclear whether results would be similar in cardiac surgery patients due to high prevalence of delirium, different subtypes, and patient baseline comorbidities and functional status.
Although the results of this study do not prove causality, they do highlight the potential importance of targeting high-risk patients with either prevention or rehabilitation strategies. To identify high-risk patients, several risk factors for postoperative delirium have been described, including age, cognition, stroke, alcohol use, activity, laboratory values, and surgery. However, even after identifying high-risk patients, delirium prevention is not easy. Several recent trials in cardiac surgery testing the efficacy of prophylactic steroids (27) and acetylcholinesterase inhibitors (28) have been negative. The strongest evidence for interventions to prevent delirium, at least in non-cardiac surgery patients, are often multi-faceted with examples including the Hospital Elderly Life Program (2) or a formal geriatrics consults (3). Core aspects of these programs include increased mobility, reduction of inappropriate medications, sleep-promotion, and optimization of hydration and electrolytes. However, these programs may be costly to implement and involve additional resources, such as nurse practitioners and therapists, or the use of costly medications, such as dexmedetomidine. (29) The return on these investments may be warranted if delirium-prevention could improve LOS, the need for 1:1 sitters, or functional outcomes, but these tradeoffs need to be considered and evaluated in cardiac surgery populations, as they have been in other populations. (30)
There are multiple reasons why postoperative delirium might lead to increased length of stay-ICU and charges. First, delirious patients are often restrained (either physically or with medications), (12) and this limits mobility, which is increasingly recognized as essential to recovery. (31) Second, delirious patients may not participate in their care because of cognitive or functional limitations. (32) Third, delirious patients may be prone to iatrogenic complications from additional monitoring such as central lines and prolonged use of urinary catheters—both of which increase the risk for iatrogenic infections.
An alternative explanation for our data is that delirious patients have more co-morbid conditions that would be expected to increase length of ICU stay and hospital charges. In order to account for this potential confounding, we rigorously measured patient and surgical characteristics and complications, and adjusted our statistical models using propensity-score methodology. Even with the incorporation of potentially confounding variables, the adjusted models for length of stay in the ICU and overall charges remained significant. However, we cannot rule out the possibility that our results may be due to unmeasured variables, residual confounding, or reverse causation. Indeed, the association between delirium and length of stay was attenuated in models which incorporated additional covariates or additional risk scores.
Strengths of this study include rigorous delirium assessments and adjustment for underlying comorbidities, including cognition, a potentially important confounding variable. Our study has several limitations that should be considered. First, we relied on hospital charge data as a surrogate for cost. As mentioned, unique to the State of Maryland, the Maryland Heath Services Cost Review Commission determines payment rates for insurers—both public and private. The rate for charge payments at the authors’ institutions has historically been cost plus 1–3%, and thus charges approximate cost. (33) Second, the sample size of this study is relatively small, and thus subject to potential confounding by outlying variables and unique events. A larger sample size would allow further examination of the consistency of the results and other confounding or modifying factors. Additionally, there may be residual unmeasured confounding. An average of 9 mg of midazolam were used intraoperatively, and since midazolam may be associated with postoperative delirium, the incidence of delirium may be higher than in practices which routinely use less midazolam, thus limiting generalizability of these results. Finally, a large number of eligible patients were not approached or refused consent, thus introducing the risk of selection bias and limiting generalizability of the results.
In conclusion, delirium is associated with increased length of stay-ICU and increased hospital charges after cardiac surgery, and thus represents an important target for quality improvement measures.
Acknowledgments
This work was supported by NIH-RO3AG042331, Jahnigen Career Development Award, Johns Hopkins Pepper Older Americans Independence Center, NIA-P30AG021334, International Anesthesia Research Society, Johns Hopkins Clinician Scientist Award (CB); and NIH-RO1HL092259 (CH).
The authors wish to acknowledge Peter Rabins, Joseph Bienvenue, and Gregory Hobelmann for input on delirium consensus panels.
Footnotes
Set conflict box: Dr Neufeld discloses a financial relationship with Ornim Medical; Dr Hogue with Ornim Medical and Covidien, Inc.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rudolph JL, Jones RN, Levkoff SE, et al. Derivation and validation of a preoperative prediction rule for delirium after cardiac surgery. Circulation. 2009;119(2):229–36. doi: 10.1161/CIRCULATIONAHA.108.795260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Inouye S, Bogardus S, Charpentier P, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Eng J Med. 1999;340:669–676. doi: 10.1056/NEJM199903043400901. [DOI] [PubMed] [Google Scholar]
- 3.Marcantonio E, Flacker J. Reducing delirium after hip fracture: a randomized trial. J Am Geriatr Soc. 2001;49:516–521. doi: 10.1046/j.1532-5415.2001.49108.x. [DOI] [PubMed] [Google Scholar]
- 4.Inouye S, Westendorp R, Sazynski J. Delirium in elderly people. Lancet. 2014;383:911–922. doi: 10.1016/S0140-6736(13)60688-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown C. Delirium in the cardiac surgical ICU. Curr Opin Anesthesiol. 2014;27:117–122. doi: 10.1097/ACO.0000000000000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neufeld KJ, Nelliot A, Inouye SK, et al. Delirium diagnosis methodology used in research: a survey-based study. Am J Geriatr Psychiatry. 2014;14:1–9. doi: 10.1016/j.jagp.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gottesman R, Grega M, Bailey M, et al. Delirium after coronary artery bypass graft surgery and late mortality. Ann Neurol. 2010;67:338–344. doi: 10.1002/ana.21899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rudolph J, Inouye S, Jones R, et al. Delirium: an independent predictor of functional decline after cardiac surgery. J Am Geriatr Soc. 2010;58:643–649. doi: 10.1111/j.1532-5415.2010.02762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saczynski JS, Marcantonio ER, Quach L, et al. Cognitive trajectories after postoperative delirium. N Engl J Med. 2012;367(1):30–39. doi: 10.1056/NEJMoa1112923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Whitlock EL, Torres BA, Lin N, et al. Postoperative delirium in a substudy of cardiothoracic surgical patients in the BAG-RECALL clinical trial. Anesth Analg. 2014;118:809–817. doi: 10.1213/ANE.0000000000000028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Franco K, Litaker D, Locala J, Bronson D. The cost of delirium in the surgical patient. Psychosomatics. 2001;42(1):68–73. doi: 10.1176/appi.psy.42.1.68. [DOI] [PubMed] [Google Scholar]
- 12.McPherson JA, Wagner CE, Boehm LM, et al. Delirium in the cardiovascular ICU. Crit Care Med. 2013;41(2):405–13. doi: 10.1097/CCM.0b013e31826ab49b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ono M, Brady K, Easley R, et al. Duration and magnitude of blood pressure below cerebral autoregulation threshold during cardiopulmonary bypass is associated with major morbidity and operative mortality. J Thorac Cardiovasc Surg. 2014;147(1):483–9. doi: 10.1016/j.jtcvs.2013.07.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McKhann GM, Grega MA, Borowicz LM, et al. Encephalopathy and stroke after coronary artery bypass grafting: incidence, consequences, and prediction. Arch Neurol. 2002 Sep;59(9):1422–8. doi: 10.1001/archneur.59.9.1422. [DOI] [PubMed] [Google Scholar]
- 15.Inouye S, van Dyck C, Alessi C, Balkin S, Siegal A, Horwitz R. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113(12):941–8. doi: 10.7326/0003-4819-113-12-941. [DOI] [PubMed] [Google Scholar]
- 16.Trzepacz P, Mittal D, Torres R, Kanary K, Norton J, Jimerson N. Validation of the delirium rating scale-revised-98: comparison with the delirium rating scale and the cognitive test for delirium. J Neuropsychiatry Clin Neurosci. 2001;13(2):229–242. doi: 10.1176/jnp.13.2.229. [DOI] [PubMed] [Google Scholar]
- 17.Ely EW, Margolin R, Francis J, et al. Evaluation of delirium in critically ill patients: validation of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) Crit Care Med. 2001;29(7):1370–9. doi: 10.1097/00003246-200107000-00012. [DOI] [PubMed] [Google Scholar]
- 18.Robinson TN, Raeburn CD, Tran ZV, Angles EM, Brenner LA, Moss M. Postoperative delirium in the elderly. Ann Surg. 2009;249(1):173–8. doi: 10.1097/SLA.0b013e31818e4776. [DOI] [PubMed] [Google Scholar]
- 19.Inouye SK, Leo-Summers L, Zhang Y, Bogardus ST, Leslie DL, Agostini JV. A chart-based method for identification of delirium: validation compared with interviewer ratings using the confusion assessment method. J Am Geriatr Soc. 2005;53(2):312–8. doi: 10.1111/j.1532-5415.2005.53120.x. [DOI] [PubMed] [Google Scholar]
- 20.Jones RN, Fong TG, Metzger E, et al. Aging, brain disease, and reserve: implications for delirium. Am J Geriatr Psychiatry. 2010;18(2):117–27. doi: 10.1097/JGP.0b013e3181b972e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399–424. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Markar SR, Smith IA, Karthikesalingam A, Low DE. The clinical and economic costs of delirium after surgical resection for esophageal malignancy. Ann Surg. 2013;258(1):77–81. doi: 10.1097/SLA.0b013e31828545c1. [DOI] [PubMed] [Google Scholar]
- 23.Zheng Z, Lefei P, Ni H. Impact of delirium on clinical outcome in critically ill patients: a meta-analysis. Gen Hosp Psychiatry. 2013;35(2):105–11. doi: 10.1016/j.genhosppsych.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 24.Milbrandt EB, Deppen S, Harrison PL, et al. Costs associated with delirium in mechanically ventilated patients. Crit Care Med. 2004;32(4):955–62. doi: 10.1097/01.ccm.0000119429.16055.92. [DOI] [PubMed] [Google Scholar]
- 25.Stransky M, Schmidt C, Ganslmeier P, et al. Hypoactive delirium After cardiac surgery as an independent risk factor for prolonged mechanical ventilation. J Cardiothorac Vasc Anesth. 2011;25(6):968–74. doi: 10.1053/j.jvca.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 26.Patel SB, Poston JT, Pohlman A, Hall JB, Kress JP. Rapidly reversible, sedation-related delirium versus persistent delirium in the intensive care unit. Am J Respir Crit Care Med. 2014;189(6):658–65. doi: 10.1164/rccm.201310-1815OC. [DOI] [PubMed] [Google Scholar]
- 27.Sauër A-MC, Slooter AJ, Veldhuijzen DS, van Eijk MM, Devlin JW, van Dijk D. Intraoperative dexamethasone and delirium after cardiac surgery. Anesth Analg. 2014;119(5):1046–52. doi: 10.1213/ANE.0000000000000248. [DOI] [PubMed] [Google Scholar]
- 28.Gamberini M, Bolliger D, Lurati Buse GA, et al. Rivastigmine for the prevention of postoperative delirium in elderly patients undergoing elective cardiac surgery—A randomized controlled trial. Crit Care Med. 2009;37(5):1762–8. doi: 10.1097/CCM.0b013e31819da780. [DOI] [PubMed] [Google Scholar]
- 29.Maldonado J, Wysong A, Van der Starre PJA, Block T, Miller C, Reitz B. Dexmedetomidine and the Reduction of Postoperative Delirium after Cardiac Surgery. Psychosomatics. 2009;50:206–217. doi: 10.1176/appi.psy.50.3.206. [DOI] [PubMed] [Google Scholar]
- 30.Rubin FH, Williams JT, Lescisin DA, Mook WJ, Hassan S, Inouye SK. Replicating the hospital elder life program in a community hospital and demonstrating effectiveness using quality improvement methodology. J Am Geriatr Soc. 54(6):969–74. doi: 10.1111/j.1532-5415.2006.00744.x. [DOI] [PubMed] [Google Scholar]
- 31.Schweickert WD, Pohlman MC, Pohlman AS, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet. 2009;373:1874–82. doi: 10.1016/S0140-6736(09)60658-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marcantonio ER, Simon SE, Bergmann MA, Jones RN, Murphy KM, Morris JN. Delirium symptoms in post-acute care: prevalent, persistent, and associated with poor functional recovery. J Am Geriatr Soc. 2003;51(1):4–9. doi: 10.1034/j.1601-5215.2002.51002.x. [DOI] [PubMed] [Google Scholar]
- 33.Arnaoutakis GJ, Allen JG, Merlo CA, et al. Impact of the lung allocation score on resource utilization after lung transplantation in the United States. J Heart Lung Transplant. 2011;30(1):14–21. doi: 10.1016/j.healun.2010.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]