The acquisition of a novel tool behavior to drink water spreads culturally through families in wild chimpanzees.
Keywords: chimpanzees, Pan troglodytes, tool use, social transmission, kin transmission
Abstract
Current research on animal culture has focused strongly on cataloging the diversity of socially transmitted behaviors and on the social learning mechanisms that sustain their spread. Comparably less is known about the persistence of cultural behavior following innovation in groups of wild animals. We present observational data and a field experiment designed to address this question in a wild chimpanzee community, capitalizing on a novel tool behavior, moss-sponging, which appeared naturally in the community in 2011. We found that, 3 years later, moss-sponging was still present in the individuals that acquired the behavior shortly after its emergence and that it had spread further, to other community members. Our field experiment suggests that this secondary radiation and consolidation of moss-sponging is the result of transmission through matrilines, in contrast to the previously documented association-based spread among the initial cohort. We conclude that the spread of cultural behavior in wild chimpanzees follows a sequential structure of initial proximity-based horizontal transmission followed by kin-based vertical transmission.
INTRODUCTION
In recent years, the notion of animal culture has become widely accepted, largely because of evidence for social transmission of novel behaviors in both captive and field studies (1–5). Several definitions have been proposed for cultural behaviors these definitions differ mainly in their descriptions of the underlying transmission mechanisms (6). For instance, Fragaszy and Perry (7) define tradition, the core component of every definition of animal culture, as “a distinctive behaviour pattern shared by two or more individuals in a social unit, which persists over time and that new practitioners acquire in part through socially aided learning” (p. xiii). We use the same definition here but do not distinguish between traditions and cultural behaviors [for example, see the study of Whiten et al. (8)].
In the wild, animal cultural behavior has been studied mainly with observational methods, particularly the method of exclusion (9–11) [but see the study of Biro et al. (12)], whereas captive studies typically use both observational and experimental approaches (13). The method of exclusion is controversial because it is opaque with regard to the cognitive processes involved in learning and because it is difficult to rule out alternative explanations, such as the influence of genetic and environmental differences (14). In contrast, although experimental approaches can generate more powerful results, they usually lack ecological validity (15).
In a recent study carried out in Budongo Forest, Uganda, Hobaiter et al. (2) reported that a newly invented natural behavior in wild chimpanzees (Pan troglodytes schweinfurthii) of the Sonso community, moss-sponging, spread through the social network via social learning. Despite an intense and continuous behavioral research program, which started in the mid-1990s (16), moss-sponging had never been observed until 2011. The behavior was first displayed by the alpha male in November 2011 upon which it spread rapidly through spatiotemporal association to another seven individuals. However, between 2011 and 2013, the behavior was seldom observed by researchers and long-term field assistants, raising questions about its maintenance in this community consisting of around 70 individuals.
Our study aimed to determine whether moss-sponging was still present and had further propagated throughout the community to become a component of its group-specific behavioral portfolio. We used this opportunity to address an important issue in the animal culture literature: the scarcity of documented evidence for the maintenance of behavioral innovations before they can become cultural traits characteristic of a group (14, 17, 18).
Although there is considerable literature on behavioral innovations (19) and their spread, particularly in captivity (5), there are only few well-documented cases for the persistence of natural behavioral innovations in wild populations, especially in the tool use context, and, even in those cases, the information is often incomplete (20, 21). The textbook case for innovation is the milk bottle foil cap piercing behavior of parids in England in the 1950s, but little is known of the social dynamics underlying this behavior (22). Another classic case is pine cone eating in Israeli black rats (Rattus rattus) (23), a behavior shown to spread through stimulus enhancement, with pups learning by interacting with the partially eaten food left by their mothers (23). However, in these cases, as well as in more recent studies in whales (1) and chimpanzees (24), the major problem is that the initial innovation was not observed, making the precise dynamics of propagation difficult to understand (2). The best evidence for behavioral innovations and subsequent spread comes from long-term research on Japanese macaques (Macaca fuscata), where several feeding and play behavior innovations by single individuals have spread to other group members (25). A well-known example is the transmission of sweet potato washing, invented by a juvenile female “Imo” (26). Here, the behavior initially spread to individuals with close social ties to the initiator (that is, playmates and kin). Thus, the propagation of the behavior occurred between individuals with a strong social affinity and with whom cofeeding was possible, namely, to other females, which have a strong grouping tendency, and to kin (26, 27). The spread within family units went upward, from younger to older matriline members (26, 27). Although the authors were able to meticulously describe the slow propagation process of the behavior, it was not possible to discriminate between the two mechanisms responsible for the spread, spatial proximity and kinship, because mothers and children spend most of their time together and usually cofeed (28). More relevant in this species is stone-handling, a solitary object play behavior consisting of repetitively manipulating stones in various ways, which has been documented in various troops (29). In the best-documented case, the behavior has been studied since 1979 [Arashiyama B troop, near Kyoto, Japan (30)]. The behavior was socially transmitted (31, 32), first horizontally from the innovator, a 3-year-old female, to other similarly aged individuals and then vertically from the females to their offspring (29). Although stone-handling could potentially lead to tool use behavior (33, 34), it is generally considered a nonadaptive, functionless play behavior in Japanese macaques (30, 34).
The goal of the current study was to investigate the mechanisms of maintenance of moss-sponging in a wild chimpanzee community in the years following its initial emergence and its rapid spread among a restricted number of individuals in 2011. At that time, moss-sponging was only seen in eight individuals, when it was used exclusively to absorb mineral-rich water from a single location, a natural clay pit (figs. S1 and S2), despite the fact that the site was visited by most group members (35). Effectively, this led to the coexistence of two cultural subgroups: individuals that relied on the traditional drinking technique seen in all chimpanzee communities, that is, manufacturing of a leaf-sponge to ingest liquids (“clay-pit leaf-spongers”), and individuals that used moss to absorb clay water in addition to the traditional technique (“clay-pit moss-spongers”). Crucially, Hobaiter et al. (2) estimated that 85 to 99% of moss-sponging acquisitions were made through social transmission. Some of this was due to individuals reusing moss-sponges discarded by others, a potential case of stimulus enhancement, which also qualifies as social learning (36). However, for moss-sponging to truly qualify as cultural behavior, we predicted that individuals that socially acquired the behavior in 2011 should continue to moss-sponge in the current study, that is, 3 years later. Furthermore, we predicted that if moss-sponging had spread to other individuals by social learning in the 3-year interval since the initial study, it should have spread preferentially to individuals that were socially close (either by family ties or by spatial proximity) to the original eight moss-spongers.
To explore these questions, we carried out a field experiment at the same clay pit at which the original innovation took place. During 2 months in 2014, we presented subjects with a choice of two different materials by providing moss next to the naturally available leaves, which allowed us to systematically categorize group members as either moss- or leaf-spongers 3 years after the original innovation. From our long-term database, we could assign each individual to its matriline (that is, mother-offspring units) and could document the association patterns of individuals since the time of innovation in 2011. We then combined long-term observational data from Budongo with our cross-sectional experimental results to describe the spread of moss-sponging over the 3 years following its innovation.
RESULTS
Data were collected in 2014, 3 years after the first appearance of the moss-sponging behavior. At the time of this study, community members still visited the site of innovation, the clay pit filled with mineral-rich suspension.
In our experiment, five of the eight initiators in the study of Hobaiter et al. (2) moss-sponged at least once at the clay pit, one was observed leaf-sponging only, one never visited the site, and, for another one, the sponge material chosen could not be determined. In addition, a total of 48 individuals (~69% of the community) visited the experimental site and drank at the clay pit using either moss- or leaf-sponges. We excluded eight individuals for whom we could not confirm that artificially provided moss was available during the experiment, leaving 40 participating individuals (median number of trials per individual, 3;range, 1 to 17; Table 1). Individual technique preference could not be tested because of the low numbers of data points per individual. Of the 40 participants, 22 (55%) moss-sponged during at least one experimental trial (that is, 5 of the 8 initiators in Hobaiter et al.’s study and 17 new moss-spongers), and 18 exclusively leaf-sponged (45%; Table 1). In comparison, in Hobaiter et al.’s study (2), 8 of 32 individuals (25%) moss-sponged to extract the clay pit mineral suspension, a notable increase. Nevertheless, leaf-sponging remained the predominant technique, with 33 of 40 individuals (83%) leaf-sponging at least once during the experiment, with 18 exclusive leaf-spongers, compared to 22 participants moss-sponging at least once, with 7 exclusive moss-spongers.
Table 1. List of participants sorted by matrilines and their choices during the experiment (number of leaf-sponging event, number of moss-sponging event, total number of sponging events, proportion of leaf-sponging, and proportion of moss-sponging). F, female; M, male.
| ID (N = 40) | Age (years) | Sex | Matriline | No. of leaf-sponging | No. of moss-sponging | Total | Percentage of leaf-sponging | Percentage of moss-sponging |
| AN | 24.3 | F | AN* | 4 | 0 | 4 | 100 | 0 |
| CC | 14.3 | F | CC* | 3 | 0 | 3 | 100 | 0 |
| GL | 38.3 | F | G | 4 | 0 | 4 | 100 | 0 |
| GR | 8.3 | F | G | 8 | 0 | 8 | 100 | 0 |
| HR | 5.0 | F | H | 3 | 4 | 7 | 43 | 57 |
| HT | 36.5 | F | H | 1 | 4 | 5 | 20 | 80 |
| HW | 21.3 | M | H | 0 | 1 | 1 | 0 | 100 |
| HY | 9.1 | F | H | 7 | 5 | 12 | 58 | 42 |
| IN | 15.3 | F | IN* | 2 | 1 | 3 | 67 | 33 |
| JS† | 8.4 | M | J | 0 | 1 | 1 | 0 | 100 |
| KG | 38.5 | F | K1 | 2 | 0 | 2 | 100 | 0 |
| KI | 11.2 | F | K1 | 0 | 3 | 3 | 0 | 100 |
| KP | 6.1 | F | K1 | 2 | 0 | 2 | 100 | 0 |
| KC | 8.2 | M | K2 | 2 | 0 | 2 | 100 | 0 |
| KL | 35.5 | F | K2 | 3 | 0 | 3 | 100 | 0 |
| KH | 6.3 | F | K3 | 3 | 2 | 5 | 60 | 40 |
| KS | 12.2 | M | K3 | 1 | 1 | 2 | 50 | 50 |
| KU | 35.4 | F | K3 | 0 | 1 | 1 | 0 | 100 |
| KB† | 7.8 | F | K4 | 1 | 2 | 3 | 33 | 67 |
| KR† | 13.0 | F | K4 | 0 | 4 | 4 | 0 | 100 |
| KW† | 33.4 | F | K4 | 0 | 1 | 1 | 0 | 100 |
| KZ | 19.8 | M | K4 | 1 | 0 | 1 | 100 | 0 |
| KA | 15.8 | F | KA* | 1 | 0 | 1 | 100 | 0 |
| MB | 5.9 | M | M1 | 1 | 0 | 1 | 100 | 0 |
| ML | 39.5 | F | M1 | 1 | 0 | 1 | 100 | 0 |
| MI | 7.0 | F | M2 | 4 | 1 | 5 | 80 | 20 |
| MK | 34.4 | F | M2 | 3 | 2 | 5 | 60 | 40 |
| MS | 23.3 | M | N | 3 | 0 | 3 | 100 | 0 |
| NB† | 52.3 | F | N | 3 | 1 | 4 | 75 | 25 |
| NT | 11.6 | F | N | 6 | 0 | 6 | 100 | 0 |
| OK | 18.5 | F | OK* | 2 | 0 | 2 | 100 | 0 |
| PS | 16.3 | M | PS* | 6 | 1 | 7 | 86 | 14 |
| RF | 7.3 | F | R | 1 | 1 | 2 | 50 | 50 |
| RH | 49.5 | F | R | 0 | 4 | 4 | 0 | 100 |
| RM | 12.1 | F | R | 2 | 0 | 2 | 100 | 0 |
| RS | 17.0 | F | R | 2 | 1 | 3 | 67 | 33 |
| TM | 10.5 | F | TM* | 1 | 1 | 2 | 50 | 50 |
| UP | 15.3 | F | UP* | 11 | 6 | 17 | 65 | 35 |
| ZD | 13.4 | M | Z | 1 | 0 | 1 | 100 | 0 |
| ZG | 17.4 | M | ZG* | 1 | 0 | 1 | 100 | 0 |
| Median | 2 | 1 | 3 | 78 | 22.5 | |||
| Total | 96 | 48 | 144 |
*Individuals without a known mother (they were assigned their own matriline).
†Initial moss-spongers in the study of Hobaiter et al. (2).
Given that the number of moss-spongers had increased substantially over the 3 years following innovation, we were interested in the transmission patterns within the group. We tested two hypotheses, that is, that transmission occurred vertically (within matrilines) or horizontally (across the community, following association patterns). Using a generalized linear mixed model with binomial error structure, we estimated the probability of manufacturing a moss-sponge by individuals in a given experimental trial, taking into account all potential demonstrators from which the subject may have learned the technique. Crucially, the full model included two terms corresponding to the two potential transmission modes. For the vertical transmission hypothesis, we tested whether having a moss-sponger in the matriline affected the probability of using moss in the experiment. For the horizontal transmission hypothesis, we tested the interaction term between the association strength between the subject and the potential demonstrator (as a proxy for the possibility to learn socially) and the sponging technique of the potential demonstrator. Specifically, if horizontal transmission was at work, we expected to see a positive relationship between the association index and the probability to moss-sponge, but only with potential demonstrators that were moss-spongers themselves.
We found significant support for the vertical transmission hypothesis [β = 1.35 ± 0.11, z = 12.80; likelihood ratio test (LRT): χ2(1) = 184.22, P = 0.000; Fig. 1 and table S1] but no support for horizontal transmission [β = −0.03 ± 0.09, z =−0.290; LRT: χ2(1) = 0.682, P = 0.877; Fig. 1 and table S1]. In particular, subjects with a moss-sponger in the matriline were more likely to use a moss-sponge during the experiment than subjects without a moss-sponger in the matriline (Fig. 2), whereas moss-spongers were not more strongly associated with other community members displaying moss-sponging from which they could have learned the behavior.
Fig. 1. Social transmission of cultural behavior in wild chimpanzees.
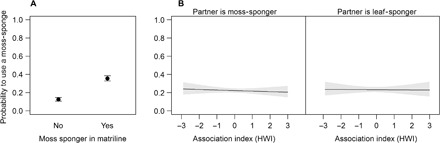
(A) Probability of moss-sponging by subjects depending on whether they have at least one moss-sponger in the matriline: parameter estimates with SEs. (B) Probability of moss-sponging by subjects depending on their association history with other moss- or leaf-spongers in the group, calculated using the half-weight index (HWI): model estimates with SEs (shaded areas). The numerical results are provided in table S1.
Fig. 2. Proportion of trials with subjects using moss to manufacture a sponge.
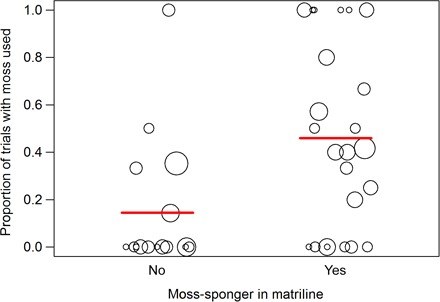
The size of the circles corresponds to the total number of trials observed (that is, the larger the circle is, the more trials the subject had). The red lines represent the means. Note that the figure represents raw data. Results of the generalized linear mixed model are provided in fig. S1 and table S1.
In a complementary analysis, we investigated the distribution of techniques across individuals (as opposed to the technique used in a given experimental trial). We devised a permutation procedure in which we randomly assigned techniques to subjects and estimated the effects of having a moss-sponger in the matriline and a subject’s average association index with other moss-spongers on the probability that a subject was a moss-sponger. We then compared the observed effects of these two factors to the expected effects resulting from our permutation procedure. We found that the observed effect of having a moss-sponger in the matriline was significantly larger than we would expect (10,000 permutations, P = 0.048; fig. S3). This result suggests again that having a moss-sponger in the matriline increases the likelihood of belonging to the moss-sponging subgroup. We found no such effect with regard to the average association with other moss-spongers (10,000 permutations, P = 0.494; fig. S4).
DISCUSSION
Here, we used natural and experimental observations to assess the propagation pattern and the maintenance of a novel tool technique, moss-sponging, first observed in 2011. Although we cannot exclude the possibility that some individuals developed the moss-sponging behavior at the time of our experiment, our cross-sectional design aimed to uncover the present state of knowledge of the chimpanzees visiting the clay pit and to analyze whether the moss-sponging behavior had spread in the community following its original innovation. Our results showed that, over a period of 3 years, the behavior spread from a small number of founder individuals (8) to 17 additional group members. Considering that moss-sponging is transmitted socially and that only a subset of the community members uses this technique (without any compelling genetic or environmental alternative explanations at hand), we consider this behavior a subculture within the Sonso community. Nevertheless, most moss-spongers did not abandon their default drinking technique, leaf-sponging, and manufactured both leaf- and moss-sponges in parallel (Table 1).
Another major finding was that moss-sponging mainly increased in prevalence within matrilines (Fig. 3), and not through either an association network or by individual learning. Because kin usually associate together, kin-based transmission and association-based transmission are therefore often confounded and hard to discriminate (37, 38). However, in our study, we were able to disentangle the two transmission mechanisms of close spatial association and close social links; our results suggest that both mechanisms are at work, albeit at different stages of the cultural transmission process. Specifically, we found that individuals from matrilines with moss-sponging individuals were more likely to be moss-spongers themselves compared to individuals from matrilines that did not have a moss-sponger. In contrast, moss-spongers did not associate more with each other per se, unlike other studies on cultural behavior [for example, see the study of Reynolds et al. (39)]. Mere spatiotemporal associations with other moss-spongers alone could not explain the distribution, in contrast to the initial spread reported by Hobaiter et al. (2). This suggests that, in chimpanzees, cultural behaviors become established by a scaffolding of two distinct mechanisms. Although the initial spread of cultural behavior appears to propagate through spatial associations, subsequent expansion proliferates mainly through matrilines (Fig. 2). This pattern is reminiscent of findings in Japanese macaques, where various behaviors also spread throughout the group in possibly similar ways. Compared to the macaque cases, all possible acquisition pathways (adults to adults, youngsters to youngsters, and adults to youngsters or reverse) were observed (2). Additionally, during this propagation phase, the transmission was not strictly vertical; otherwise, only the five matrilines of the eight initiators would have used this technique after 3 years, which was not the case. Rather, the behavior propagated within a network of close associates, possibly kin-bonded and allies, leading to the coexistence of a subculture, the moss-spongers, within the community. Other factors, such as opportunities for social learning or the identity of the model, which we could not analyze at this stage of the spread of moss-sponging, may subsequently explain why a given behavior becomes dominant during the consolidation phase.
Fig. 3. Model of the two-dimensional transmission pattern.
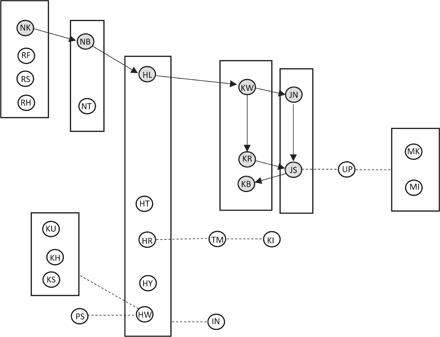
Circles represent all individuals seen moss-sponging at least once between 2011 and 2014. The eight original moss-spongers from Hobaiter et al.’s study (2) are represented in gray. The arrows represent the social transmission found by Hobaiter et al. (2). The rectangles represent matrilines in which vertical social learning took place, the main transmission mechanism involved in the spread. The dashed lines represent the possible learning path of the cases (n = 7) in which individuals acquired the behavior in the absence of vertical transmission because individuals did not have moss-sponging kin, suggesting learning from individuals that they were highly associated with (linked with the individual with the highest association index).
We can think of several explanations for this observed change in the acquisition pattern, from horizontally to vertically governed. First, the change of transmission could be a by-product of special circumstances. The behavior arose at a time when the clay pit contained a suspension with high levels of minerals (35), which appeared to be an exceptionally valuable resource (35, 39). Over time, the mineral content of the suspension may have decreased, because of intense exploitation by chimpanzees and guereza monkeys (Colobus guereza; N.L., personal observation). Similarly, individuals may have started to exploit additional mineral sources (35), which could have led to a decrease in competition at the clay pit, with fewer opportunities for horizontal transfer. High levels of competition have been proposed as the main cause of innovation of moss-sponging behavior in 2011 (2). During this study, the average number of individuals simultaneously present at the pit was 2.2 (data extracted from camera trap clips), suggesting a possible decrease in competition between 2011 and 2014. Transmission dynamics of cultural behavior may thus have been influenced by variation in both ecological factors and social competition.
Another hypothesis is that the acquisition of cultural behavior generally follows a two-stage process in chimpanzees, in that innovations first spread via proximity-based social networks, whereas subsequent stabilization within the larger group happens within relevant social units, which, for chimpanzees, are the matrilines (40). However, this may also depend on the type of transmitted behavior: Different patterns of transmission may apply to different types of behaviors [for example, sexual behavior may not be learned by immature individuals; see the discussion by Huffman and Hirata (25)]. In the case of tool use behavior, previous research has shown that chimpanzee mothers play an important role (41–45). Transmission through family members may be magnified by the chimpanzees’ fusion-fission social system (40), mainly because young individuals do not have regular contact with group members other than members of their matriline (45), although this does not appear to be a necessary precondition [for example, Japanese macaques (31)]. Moreover, chimpanzees are especially tolerant toward family members, which may preferentially facilitate transmission of novel behavior, a model proposed by Matsuzawa et al. (46) as “education by master-apprenticeship.” Possibly driven by a desire to act like others (that is, de Waal’s Bonding- and Identification-based Observational Learning model) (47), an individual may also have a strong tendency to copy matrilineal members. Our analyses show that within-matriline transmission is not solely restricted to unidirectional mother-offspring dyads but also probably includes cases of transmission from offspring to the mother (H matriline; see Fig. 3) or between offspring (R or H matrilines; see Fig. 3), as seen in Japanese macaque sweet potato washing (26). According to this hypothesis, the fostering ground for cultural transmission in chimpanzees and macaques is the entire matriline (that is, mother and offspring and the offspring of offspring), in line with what has been proposed for human cultural transmission (48).
Nevertheless, we cannot completely rule out alternative explanations of our results. First, on the basis of Hobaiter et al.’s findings (2), we assumed that moss-sponging was generally socially learned between 2011 and the time of this study. It is possible, of course, that by artificially providing moss at the clay pit to sample group members, we increased the affordance of moss and thereby made individual learning possible. However, we do not find individual learning–based explanations very plausible, for the following reasons. On the one hand, during the initial propagation phase in 2011, social learning–based models were overwhelmingly more supported than individual learning–based models, and there is no obvious reason why chimpanzees would suddenly switch from social to individual learning in subsequent years. Of course, each moss-sponger has to individually learn the behavior, but in all likelihood, this was facilitated by the social influence exerted by other group members that acted as models. On the other hand, the existence of a continuous social influence is supported by the fact that the distribution of moss-spongers in the group in our study was biased toward kin and not randomly, as we would expect if the transmission mechanism had been asocial. A random distribution would indeed be expected if individual learning was at work, as was found for the second behavior studied by Hobaiter et al. (2), leaf-sponge reuse, in the same context.
Second, it could be argued that moss-sponging matrilines were genetically more predisposed to this type of tool use than other matrilines. For example, moss-sponging family members may generally show more traits essential for acquiring new behaviors, such as curiosity, inventiveness, or boldness. According to this second explanation, the recent cohort of moss-spongers was more likely to acquire moss-sponging because of their genetic predisposition, albeit by individual learning. However, similarly, we do not find the family-specific genetic hypothesis very plausible. If we accept that genetic predispositions are the underlying process at play in the propagation phase investigated here, the same reasoning should apply to the initial spread of the behavior described in Hobaiter et al.’s study (2). Following this reasoning, the behavior should have appeared multiple times in response to the environmental stimulus, rather than follow a pattern based on observational learning only. Additionally, some kind of genetic relatedness between individuals should be observed in both the original and new cohorts of moss-spongers. Because of chimpanzees’ philopatric system, females tend to have a more diverse genetic background compared to males (49), but offspring will be sired by a limited number of resident males in the community. If moss-sponging innovation results from genetic predispositions, we should thus expect that offspring from the same father, which are genetically more related, display the same propensity to innovate the new technique. In the Sonso community, of seven fathers with at least two documented offspring, five had offspring using different sponging technique at the clay pit (table S3), making it unlikely that a genetic predisposition triggered moss-sponging innovation.
In conclusion, our experiment allowed us to decipher how a new tool-assisted drinking behavior persisted in a subgroup of the Sonso community and how it became part of their cultural repertoire. We also showed that although chimpanzees had the same opportunity to individually learn the moss-sponging behavior by taking the provided moss or the discarded moss-sponges at the clay pit, only a subset of them incorporated this behavior in their repertoire, by learning from members of their matriline. To our knowledge, this is the first study to disentangle the influence of proximity-based association networks and matrilines on the propagation and maintenance of a tool use invention, by showing how the two transmission patterns operate both during the emergence and during the consolidation of a novel behavior within a group. Ultimately, because the propagation phase is not strictly vertical, it is likely that novel cultural behavior will be able to reach the “habitual” or “customary” level in a population over time following this model (9).
Our findings have some implications for understanding cultural transmission in modern humans, where cultural behavior also tends to spread through close family units, although not exclusively so (50). Considering that early human groups lived in fission-fusion social systems, likely similar to chimpanzees, and had cultural repertoires similar to them (51), it is possible that early human cultural transmission followed the pattern found here, that is, an initial founding event (potentially triggered by ecological necessity and opportunity) followed by early spread to close associates and subsequent consolidation within the close family units. However, human evolution is also characterized by increasingly more complex social cognition and a social life in large and highly cooperative groups with cooperative breeding and teaching, suggesting that the transmission of culture has become more efficient and widespread, extending beyond the original family core.
MATERIALS AND METHODS
Study site and subjects
The study was conducted in the Budongo Forest Reserve in Western Uganda. The reserve consists mainly of moist, semideciduous tropical forest at a mean altitude of 1100 m. The Sonso community uses a core home range of approximately 7 km2 (52), and community members have been habituated to the presence of human observers since the mid-1990s (16). At the beginning of the study period in 2014, the community consisted of 68 individuals, 21 adult females, 12 adult males, 9 subadult females, 3 subadult males, 13 juvenile females, 3 juvenile males, 3 infant females, and 4 infant males, following Reynolds’ classification (16). By the end of the study, 3 new infant males had been born and 1 adult female had immigrated, resulting in a community size of 72 individuals.
Experimental design
The aim of the experiment was to investigate the choice of sponge material at a clay pit (N1 43.107; E31 32.473) 3 years after the emergence of the novel moss-sponging behavior within the community. At the time of the study, the clay pit site, where the moss-sponging behavior started in 2011 (2), consisted of two ground water holes at the bottom of two trees (figs. S1 and S2). The cavities were filled with rainwater enriched with minerals dissolved from a high concentration of clay in the soil. Clumps of moss [Orthostichella welwitschii (Duby)], collected in swamp areas within the chimpanzees’ natural home range and naturally hanging from tree branches, were hung in trees around the clay pit. No leaves were provided because the clay pit is located in a tree-dense area, with a large choice of leafy trees regularly used by the chimpanzees to manufacture leaf-sponges. We set up a “Bushnell HD Trophy Cam” motion-sensitive video camera during the first sampling period (11 September to 18 November 2014) and added two more cameras during the second sampling period (30 November to 8 December 2014) to monitor the area from three different angles. The cameras started recording videos as soon as a movement at the clay pit was detected and were set to 1-min recording clips with a 1-s interval between triggers. Although this setup was ideal with regard to the possible precluding disturbance of the presence of the researchers on less-habituated individuals during the experiment and the systematic recording of any interaction with the clay pit at any time of the day, it did not allow data collection of the audience and possible direct observation of moss-sponging behavior by other group members during the trials outside of the camera range.
Statistical analysis
Experimental data collected for the analyses were as follows: (i) the technique (leaf-sponging or moss-sponging) used by the subjects during tool-assisted drinking bouts, (ii) the age and sex of the subjects, (iii) whether subjects had at least one moss-sponger in their matriline, and (iv) the subject–potential demonstrator dyad association index. “Leaf-sponging” was defined as using a wad of crumpled or folded leaves to absorb and consume liquid; “moss-sponging” was defined as using a clump of moss or mixture of moss and leaves for the same purpose. A tool-assisted drinking bout (that is, a “trial”) started when an individual was seen (i) manufacturing a sponge, (ii) reusing a discarded sponge, or (iii) adding material to an existing sponge. It ended when the individual (i) discarded the manufactured sponge without using it, (ii) stopped sponging to start with another activity, (iii) altered the structure of the sponge by adding material (leaf or moss), or (iv) went out of sight. A matriline consists of the mother and her offspring. We did not consider fathers because only 24 of the 40 participants had a known father: 14 of 24 died before the emergence of moss-sponging, and 5 did not participate in the experiment. As a consequence, the father’s sponging technique was only documented for 5 participants but unknown for 19. Furthermore, several studies (42–44, 53) have shown the crucial role of the mother in the youngsters’ acquisition of behaviors, mainly because of their constant association during the first years of life (54), which is not found with the father (55). Nulliparous immigrant females each got assigned their own matriline ID (Table 1). A potential demonstrator was an individual whose preferences for moss or leaves were known. A potential demonstrator was defined as (i) a moss-sponger if it had been observed moss-sponging at least once between 2011 and 2014 [that is, the individuals that moss-sponged during the experiment at the clay pit, the eight initiators, and one additional individual (NT) that was observed using a moss-sponge during an unrelated experiment that took place 10 months before our experiment at the clay pit] or (ii) a leaf-sponger, if it has been observed exclusively leaf-sponging. Note that NT’s classification as a potential moss- or leaf-sponger demonstrator had no effect on our conclusions (tables S1 and S2).
The association index between the subjects and the potential demonstrators was calculated using the half-weight index (56, 57) based on party compositions collected from November 2011 to December 2014 by experienced field assistants. We calculated the association index for all dyads that comprised individuals that were at least 3 years old during the study period (November 2011 to December 2014; n = 67), resulting in n = 2211 dyads (= 67 × 66/2). For 10 of 2211 dyads, association indices were not calculated because the two individuals were not coresident in the community (that is, n = 4 dyads between a female that emigrated during the study period and an infant that turned 3 years old after the female emigrated, n = 3 dyads between a female that emigrated during the study period and a female that immigrated after the emigrant left the group, n = 2 dyads between an individual that died during the study period and a female that immigrated in the group after the death of the individual, and n = 1 dyad between an infant that turned 3 years old and a female that died before the infant turned 3 years old).
From a total of 12.4 hours of video recordings, we identified n = 157 tool-assisted drinking bouts (that is, trials). We excluded n = 13 trials for which the choice of material could not be determined unambiguously from the recordings. Trials for which the subjects were among the original cohort that started using moss-sponges in 2011 (n = 13) were also excluded because we were interested in testing the transmission happening after the original spread in 2011. This resulted in a final data set of n = 131 trials by 35 subjects.
For each trial, we assigned the subject to all potential demonstrators (n = 43). This resulted in 5633 combinations of subjects and potential demonstrators across the 131 trials (5633 = 131 × 43). However, for three trials, the subject, an adult female, immigrated in the group after one potential demonstrator (HL) had emigrated already; they were therefore never coresident, and HL could not have been a possible demonstrator for this female. Thus, our overall data set comprised 3 trials with 41 potential demonstrators + 128 trials with 42 potential demonstrators = 5499 cases, that is, the combination of the subject in a given trial and one potential demonstrator. For each such case (represented as one line in our data table), we assigned the dyad’s association index, the subject’s choice in the trial, whether the potential demonstrator used moss, whether the subject had a moss-sponger in the matriline, and the subject’s age and sex.
We had two major predictor variables in our model. First, for vertical transmission, we included whether subjects had at least one matriline member that had acquired the moss-sponging technique. Second, for horizontal transmission, we included the two-way interaction between the association index (between the subject and the potential demonstrator) and whether moss-sponging was observed in the potential demonstrator at any time between 2011 and 2014. We reasoned that, with potential demonstrators that were moss-spongers themselves, stronger associations should positively correlate with the probability of a subject moss-sponging in our experiment. In contrast, with potential demonstrators that were not moss-spongers, association strength should not affect the subjects’ probability to use moss. In other words, if moss-sponging followed a horizontal transmission, then this should manifest itself as a significant interaction term in the model. We additionally included subject age and sex as control predictors. To account for nonindependence of repeated trials per subject and the way we set up our data set, we included random intercepts in our model for dyad identity, subject ID nested in dyad, and potential demonstrator ID nested in dyad. Both age and association indices were z-transformed (58). Model fitting was conducted in R (v. 3.2.0) (59), using the lme4 package (v. 1.1-7) (60).
Finally, we wanted to test whether the distribution of the two different techniques within the group was random and, therefore, whether moss-sponging was acquired by individual learning. To this end, we first assigned a technique to the 35 subjects that participated to the clay pit experiment by labeling individuals that had been observed moss-sponging at least once at the clay pit as “moss-spongers,” and as “leaf-spongers” otherwise. Using the same technique as the association index in the previous model (see above), we calculated an average association with other moss-spongers in the community [individuals that had been seen moss-sponging at least once at the clay pit and the eight initiators in Hobaiter et al.’s study (2)]. We then ran a generalized linear model on the 35 subjects and tested two main factors (having a moss-sponger in the matriline and the average association with other moss-spongers) along with two control factors (age and sex). To answer the question of whether moss-sponging was randomly distributed among the individuals with respect to the two main factors tested, we reran the model more than 10,000 times and extracted the parameter estimates. In each of the 10,000 models, the assignment of technique to a given individual was randomized. Our randomization kept the proportion of moss-spongers in the group constant; that is, there always were 18 leaf-spongers and 17 moss-spongers. Finally, we compared the original estimates, that is, the effect we actually observed, to the distribution of parameter estimates from our 10,000 models.
Supplementary Material
Acknowledgments
We are grateful to S. Adue for his invaluable help in the field and to all the field assistants for contributing to the long-term data collection. We thank the Uganda National Council for Science and Technology, the Uganda Wildlife Authority, and the President’s office for permission to conduct our research in the Budongo Forest Reserve and the Royal Zoological Society of Scotland for supporting the Budongo Conservation Field Station. We thank C. Ackermann for database management. We are grateful to M. Price for moss species identification and to C. Asiimwe for her expertise and advice regarding chimpanzee health and experimental design. We also thank W. C. McGrew and an anonymous reviewer for helpful comments on earlier versions of this manuscipt. Funding: This work was funded by the European Research Council (ERC) under the European Union’s Seventh Framework Programme (FP7/2007-2013)/ERC grant agreement no. 283871 and the Swiss National Science Foundation (SNSF; project 310030_143359 to K.Z.). T.G. was supported by an Interdisciplinary Project grant (CR13I1_162720/1) from the SNSF. Author contributions: Data collection: N.L.; statistical analysis: C.N. and N.L.; experimental design: N.L., T.G., and K.Z.; manuscript writing: N.L., T.G., C.N., and K.Z.; funding: K.Z. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/3/4/e1602750/DC1
table S1. Results of the generalized linear mixed model in the field experiment, with individual NT included as a potential demonstrator for moss-sponging.
table S2. Results of the generalized linear mixed model in the field experiment, with individual NT included as a potential demonstrator for leaf-sponging (that is, if her moss-sponging event during an unrelated experiment is not taken into account).
table S3. Individuals with a known sponging technique and their affiliation.
fig. S1. General view of the clay pit located between the exposed roots of two adjacent trees (Cynometra alexandri and Mimusops bagshawei) consisting of two cavities.
fig. S2. Closeup view of the two cavities.
fig. S3. Histogram of parameter estimates for the factor moss-sponger in matriline.
fig. S4. Histogram of parameter estimates for the factor average association with moss-spongers.
movie S1. Manufacture of a moss-sponge.
movie S2. Manufacture of a moss-sponge and a leaf-sponge.
REFERENCES AND NOTES
- 1.Allen J., Weinrich M., Hoppitt W., Rendell L., Network-based diffusion analysis reveals cultural transmission of lobtail feeding in humpback whales. Science 340, 485–488 (2013). [DOI] [PubMed] [Google Scholar]
- 2.Hobaiter C., Poisot T., Zuberbühler K., Hoppitt W., Gruber T., Social network analysis shows direct evidence for social transmission of tool use in wild chimpanzees. PLOS Biol. 12, e1001960 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aplin L. M., Farine D. R., Morand-Ferron J., Cockburn A., Thornton A., Sheldon B. C., Experimentally induced innovations lead to persistent culture via conformity in wild birds. Nature 518, 538–541 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whiten A., Spiteri A., Horner V., Bonnie K. E., Lambeth S. P., Schapiro S. J., de Waal F. B. M., Transmission of multiple traditions within and between chimpanzee groups. Curr. Biol. 17, 1038–1043 (2007). [DOI] [PubMed] [Google Scholar]
- 5.Whiten A., Mesoudi A., Establishing an experimental science of culture: Animal social diffusion experiments. Philos. Trans. R. Soc. London Ser. B 363, 3477–3488 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whiten A., McGuigan N., Marshall-Pescini S., Hopper L. M., Emulation, imitation, over-imitation and the scope of culture for child and chimpanzee. Philos. Trans. R. Soc. London Ser. B 364, 2417–2428 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D. M. Fragaszy, S. Perry, in The Biology of Traditions: Models and Evidence (Cambridge Univ. Press, 2003), pp. 1–32. [Google Scholar]
- 8.Whiten A., Horner V., Marshall-Pescini S., Cultural panthropology. Evol. Anthropol. 12, 92–105 (2003). [Google Scholar]
- 9.Whiten A., Goodall J., McGrew W. C., Nishida T., Reynolds V., Sugiyama Y., Tutin C. E. G., Wrangham R. W., Boesch C., Cultures in chimpanzees. Nature 399, 682–685 (1999). [DOI] [PubMed] [Google Scholar]
- 10.Rendell L., Whitehead H., Culture in whales and dolphins. Behav. Brain Sci. 24, 309–324 (2001). [DOI] [PubMed] [Google Scholar]
- 11.Perry S., Baker M., Fedigan L., Gros-Louis J., Jack K., MacKinnon K. C., Manson J. H., Panger M., Pyle K., Rose L., Social conventions in wild white-faced capuchin monkeys: Evidence for traditions in a neotropical primate. Curr. Anthropol. 44, 241–268 (2003). [Google Scholar]
- 12.Biro D., Inoue-Nakamura N., Tonooka R., Yamakoshi G., Sousa C., Matsuzawa T., Cultural innovation and transmission of tool use in wild chimpanzees: Evidence from field experiments. Anim. Cogn. 6, 213–223 (2003). [DOI] [PubMed] [Google Scholar]
- 13.Whiten A., Horner V., de Waal F. B. M., Conformity to cultural norms of tool use in chimpanzees. Nature 437, 737–740 (2005). [DOI] [PubMed] [Google Scholar]
- 14.Laland K. N., Janik V. M., The animal cultures debate. Trends Ecol. Evol. 21, 542–547 (2006). [DOI] [PubMed] [Google Scholar]
- 15.Boesch C., What makes us human (Homo sapiens)? The challenge of cognitive cross-species comparison. J. Comp. Psychol. 121, 227–240 (2007). [DOI] [PubMed] [Google Scholar]
- 16.V. Reynolds, The Chimpanzees of the Budongo Forest: Ecology, Behaviour and Conservation (Oxford Univ. Press, 2005). [Google Scholar]
- 17.Laland K. N., Hoppitt W., Do animals have culture?. Evol. Anthropol. 12, 150–159 (2003). [Google Scholar]
- 18.K. N. Laland, B. G. Galef, The Question of Animal Culture (Harvard Univ. Press, 2009). [Google Scholar]
- 19.S. M. Reader, K. N. Laland, Animal Innovation (Oxford Univ. Press, 2003). [Google Scholar]
- 20.Nishida T., Matsusaka T., McGrew W. C., Emergence, propagation or disappearance of novel behavioral patterns in the habituated chimpanzees of Mahale: A review. Primates 50, 23–36 (2009). [DOI] [PubMed] [Google Scholar]
- 21.Kummer H., Goodall J., Conditions of innovative behaviour in primates. Philos. Trans. R. Soc. London Ser. B 308, 203–214 (1985). [Google Scholar]
- 22.Fisher J., Hinde R. A., The opening of milk bottles by birds. Brit. Birds 42, 347–357 (1949). [Google Scholar]
- 23.J. Terkel, in Social Learning in Animals: The Roots of Culture (Academic Press, 1996), pp. 17–47. [Google Scholar]
- 24.O’Malley R. C., Wallauer W., Murray C. M., Goodall J., The appearance and spread of ant fishing among the Kasekela chimpanzees of Gombe: A possible case of intercommunity cultural transmission. Curr. Anthropol. 53, 650–663 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.M. A. Huffman, S. Hirata, in The Biology of Traditions: Models and Evidence (Cambridge Univ. Press, 2003), pp. 267–296. [Google Scholar]
- 26.Kawai M., Newly-acquired pre-cultural behavior of the natural troop of Japanese monkeys on Koshima islet. Primates 6, 1–30 (1965). [Google Scholar]
- 27.S. Hirata, K. Watanabe, M. Kawai, in Primate Origins of Human Cognition and Behavior, T. Matsuzawa, Ed. (Springer, 2001), pp. 487–508. [Google Scholar]
- 28.Kawai M., On the rank system in a natural group of Japanese monkey (I). Primates 1, 111–130 (1958). [Google Scholar]
- 29.M. A. Huffman, J.-B. Leca, C. A. D. Nahallage, in The Japanese Macaques, N. Nakagawa, M. Nakamichi, H. Sugiura, Eds. (Springer, 2010), pp. 191–219. [Google Scholar]
- 30.Huffman M. A., Stone-play of Macaca fuscata in Arashiyama B troop: Transmission of a non-adaptive behavior. J. Hum. Evol. 13, 725–735 (1984). [Google Scholar]
- 31.Nahallage C. A. D., Huffman M. A., Acquisition and development of stone handling behavior in infant Japanese macaques. Behaviour 144, 1193–1215 (2007). [Google Scholar]
- 32.Leca J.-B., Gunst N., Huffman M. A., Indirect social influence in the maintenance of the stone-handling tradition in Japanese macaques, Macaca fuscata. Anim. Behav. 79, 117–126 (2010). [Google Scholar]
- 33.J.-B. Leca, N. Gunst, M. A. Huffman, Thirty years of stone handling tradition in Arashiyama-Kyoto macaques: Implications for cumulative culture and tool use in non-human primates, in The Monkeys of Stormy Mountain: 60 Years of Primatological Research on the Japanese Macaques of Arashiyama, J.-B. Leca, M. A. Huffman, P. L. Vasey Eds. (Cambridge Univ. Press, 2012), pp. 223–257. [Google Scholar]
- 34.Huffman M. A., Quiatt D., Stone handling by Japanese macaques (Macaca fuscata): Implications for tool use of stone. Primates 27, 413–423 (1986). [Google Scholar]
- 35.Reynolds V., Lloyd A. W., English C. J., Lyons P., Dodd H., Hobaiter C., Newton-Fisher N., Mullins C., Lamon N., Schel A. M., Fallon B., Mineral acquisition from clay by Budongo forest chimpanzees. PLOS ONE 10, e0134075 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spence K. W., Experimental studies of learning and the higher mental processes in infra-human primates. Psychol. Bull. 34, 806–850 (1937). [Google Scholar]
- 37.C. P. van Schaik, in The Biology of Traditions: Models and Evidence (Cambridge Univ. Press, 2003), pp. 297–328. [Google Scholar]
- 38.Mann J., Stanton M. A., Patterson E. M., Bienenstock E. J., Singh L. O., Social networks reveal cultural behaviour in tool-using dolphins. Nat. Commun. 3, 980 (2012). [DOI] [PubMed] [Google Scholar]
- 39.Reynolds V., Lloyd A., English C., Adaptation by Budongo Forest chimpanzees (Pan troglodytes schweinfurthii) to loss of a primary source of dietary sodium. Afr. Primates 7, 156–162 (2012). [Google Scholar]
- 40.T. Nishida, Chimpanzees of the Lakeshore: Natural History and Culture at Mahale (Cambridge Univ. Press, 2011). [Google Scholar]
- 41.Inoue-Nakamura N., Matsuzawa T., Development of stone tool use by wild chimpanzees (Pan troglodytes). J. Comp. Psychol. 111, 159–173 (1997). [DOI] [PubMed] [Google Scholar]
- 42.Lind J., Lindenfors P., The number of cultural traits is correlated with female group size but not with male group size in chimpanzee communities. PLOS ONE 5, e9241 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lonsdorf E. V., What is the role of mothers in the acquisition of termite-fishing behaviors in wild chimpanzees (Pan troglodytes schweinfurthii)?. Anim. Cogn. 9, 36–46 (2006). [DOI] [PubMed] [Google Scholar]
- 44.Hirata S., Celli M. L., Role of mothers in the acquisition of tool-use behaviours by captive infant chimpanzees. Anim. Cogn. 6, 235–244 (2003). [DOI] [PubMed] [Google Scholar]
- 45.Humle T., Snowdon C. T., Matsuzawa T., Social influences on ant-dipping acquisition in the wild chimpanzees (Pan troglodytes verus) of Bossou, Guinea, West Africa. Anim. Cogn. 12, 37–48 (2009). [DOI] [PubMed] [Google Scholar]
- 46.Matsuzawa T., Biro D., Humle T., Inoue N., Tonooka R., Yamakoshi G., Emergence of culture in wild chimpanzees: Education by master-apprenticeship. Primate Orig. Hum. Cogn. Behav. 2001, 557–574 (2001). [Google Scholar]
- 47.F. B. M. de Waal, The Ape and the Sushi Master: Cultural Reflections by a Primatologist (Basic Books, 2001). [Google Scholar]
- 48.Enquist M., Strimling P., Eriksson K., Laland K. N., Sjostrand J., One cultural parent makes no culture. Anim. Behav. 79, 1353–1362 (2010). [Google Scholar]
- 49.Gruber T., Clay Z., A comparison between bonobos and chimpanzees: A review and update. Evol. Anthropol. 25, 239–252 (2016). [DOI] [PubMed] [Google Scholar]
- 50.P. J. Richerson, R. Boyd, Not by Genes Alone: How Culture Transformed Human Evolution (University of Chicago Press, 2008). [Google Scholar]
- 51.Wynn T., McGrew W. C., An ape’s view of the Oldowan. Man 24, 383–398 (1989). [Google Scholar]
- 52.Newton-Fisher N. E., The home range of the Sonso community of chimpanzees from the Budongo Forest, Uganda. Afr. J. Ecol. 41, 150–156 (2003). [Google Scholar]
- 53.Schneider C., Call J., Liebal K., What role do mothers play in the gestural acquisition of bonobos (Pan paniscus) and chimpanzees (Pan troglodytes)?. Int. J. Primatol. 33, 246–262 (2012). [Google Scholar]
- 54.Pusey A. E., Mother-offspring relationships in chimpanzees after weaning. Anim. Behav. 31, 363–377 (1983). [Google Scholar]
- 55.Lehmann J., Fickenscher G., Boesch C., Kin biased investment in wild chimpanzees. Behaviour 143, 931–955 (2006). [Google Scholar]
- 56.Bejder L., Fletcher D., Bräger S., A method for testing association patterns of social animals. Anim. Behav. 56, 719–725 (1998). [DOI] [PubMed] [Google Scholar]
- 57.Cairns S. J., Schwager S. J., A comparison of association indices. Anim. Behav. 35, 1454–1469 (1987). [Google Scholar]
- 58.Schielzeth H., Simple means to improve the interpretability of regression coefficients. Methods Ecol. Evol. 1, 103–113 (2010). [Google Scholar]
- 59.R Development Core Team, “R: A language and environment for statistical computing” (R Foundation for Statistical Computing, 2015); www.r-project.org/.
- 60.D. Bates, M. Maechler, B. Bolker, S. Walker, “lme4: Linear mixed-effects models using ‘eigen’ and S4,” R package version 1.1-7 (2014); https://cran.r-project.org/web/packages/lme4/index.html.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/3/4/e1602750/DC1
table S1. Results of the generalized linear mixed model in the field experiment, with individual NT included as a potential demonstrator for moss-sponging.
table S2. Results of the generalized linear mixed model in the field experiment, with individual NT included as a potential demonstrator for leaf-sponging (that is, if her moss-sponging event during an unrelated experiment is not taken into account).
table S3. Individuals with a known sponging technique and their affiliation.
fig. S1. General view of the clay pit located between the exposed roots of two adjacent trees (Cynometra alexandri and Mimusops bagshawei) consisting of two cavities.
fig. S2. Closeup view of the two cavities.
fig. S3. Histogram of parameter estimates for the factor moss-sponger in matriline.
fig. S4. Histogram of parameter estimates for the factor average association with moss-spongers.
movie S1. Manufacture of a moss-sponge.
movie S2. Manufacture of a moss-sponge and a leaf-sponge.


