Abstract
Introduction: There is increasing evidence concerning adverse health consequences of low vitamin D levels. We determined whether there is any surrogate for measuring vitamin D in people older than 70 years and the relation between index of multiple deprivation (IMD) and vitamin D levels. Methods: Blood samples from 241 patients were included in this analysis. Concurrent measurements for 25-hydroxyvitamin D (25(OH)D), parathyroid hormone (PTH), and bone profile are reported. Results: The prevalence of total vitamin D insufficiency/deficiency (defined as total vitamin D <50 nmol/L) was 57.5% overall. Even for patients with vitamin D deficiency, a significant proportion had PTH, normal calcium, phosphate, and alkaline phosphatase levels. For patients with vitamin D <25 nmol/L, 62.7% had a PTH within reference range, 83.1% had normal serum-adjusted calcium, 80.6% had normal phosphate, and 85.1% had a normal serum alkaline phosphatase. With increasing quintiles of IMD, there was a 22% increased risk of vitamin D deficiency/insufficiency from quintiles 1 to 5, in age- and sex-adjusted logistic regression models (odds ratio [OR] = 1.22, 95% confidence interval [1.01, 1.47]; p = .034). Conclusion: No other parameter is currently adequate for screening for vitamin D deficiency in older people. A higher IMD is associated with lower vitamin D levels in older people.
Keywords: vitamin D deficiency, social deprivation, screening, older people
Introduction
Vitamin D insufficiency and deficiency have been related to a range of adverse health outcomes (Pludowski et al., 2013). In the United Kingdom, vitamin D deficiency has generally been defined as 25-hydroxyvitamin D (25(OH)D) <25 nmol/L (Hyppönen & Power, 2007) or <50 nmol/L (Holick et al., 2011), and insufficiency in vitamin D has been defined as 25(OH)D between 25 and either 50 nmol/L (Hyppönen & Power, 2007) or 75 nmol/L (Holick et al., 2011). Nationwide surveys in the United Kingdom showed that >50% of the adult population have insufficient levels of vitamin D and that 16% have severe deficiency during the winter and spring (Hyppönen & Power, 2007; Pearce & Cheetham, 2010).
Vitamin D deficiency is associated with muscle weakness (Schott & Wills, 1976) and is common in older people (Gloth, Gundberg, Hollis, Haddad, & Tobin, 1995). Older people are prone to develop vitamin D deficiency because of various risk factors: decreased dietary intake, diminished sunlight exposure, reduced skin thickness, impaired intestinal absorption, and impaired hydroxylation in the liver and kidneys (Holick, 1995; McKenna, 1992).
In a Health Service England survey of people aged ≥65 years, 57% of women and 49% of men had serum 25(OH)D <50 nmol/L. Only 16% of men and 13% of women aged ≥65 years had serum 25(OH)D levels ≥75 nmol/L. Importantly, there was no discernible improvement in vitamin D status in 2005 compared with 2000 (Hirani & Primatesta, 2010).
Many investigators have estimated vitamin D status by examining the relation between serum 25(OH)D, which is considered to be the best estimate of vitamin D status, and serum parathyroid hormone (PTH; Yan et al., 2000). The concept behind these estimates is that there is a threshold for serum 25(OH)D below which secondary hyperparathyroidism (and bone loss) occurs.
The serum concentration of 25(OH)D below which PTH begins to rise has been estimated to be between 25 and 122 nmol/L (Yan et al., 2000). The wide range of these estimates may be related to the varied ethnicity and ages of the populations studied, variations in calcium intake, the presence of illness that may affect PTH concentrations in the elderly, renal insufficiency, and lack of standardization of assays for 25(OH)D (Fraser & Milan, 2013). Despite these limitations, there has been a progressive increase in the volume of vitamin D requests received by laboratories in the United Kingdom and elsewhere in recent years (Kennel, Drake, & Hurley, 2010; Sattar, Welsh, Panarelli, & Forouhi, 2012; Vogeser, 2010), with a significant number of older people identified as having suboptimal vitamin D status (Holick, 2007).
In the present study, we have assessed the relation between 25(OH)D levels and biochemical markers of calcium status in consecutive patient samples for individuals aged >70 years sent to the Salford Royal Hospital Laboratory (Salford, UK).
Our objectives were the following:
To determine whether measuring PTH levels/bone profile is sufficient to identify older patients with vitamin D insufficiency or deficiency, or whether vitamin D should be measured directly.
To assess the impact of social disadvantage on these findings.
Material and Methods
A total of 288 serum specimens from individuals with requests for 25(OH)D, calcium, phosphate, PTH, alkaline phosphatase (ALP), and creatinine analyzed on the same sample at Salford Royal Hospital were identified retrospectively via a search of the laboratory database. This covered the period from November 2011 to November 2012 and represented all the older patients referred for an assay of vitamin D.
Requests from renal wards or renal/endocrinology outpatient locations were excluded, as were patients with an estimated glomerular filtration rate (eGFR) <60 mL/min. Multiple requests from the same patients were excluded, with only the earliest specimen received within the time period reviewed included, leaving 241 individual requests. These consisted of 49 outpatients and 192 general practitioner (GP) surgery attendees being screened for vitamin D deficiency; 94% were of White European ethnicity.
Permission for this study was sought through the Salford information governance committee and Salford ethics committee. This is a laboratory-based study, so we did not have access to GP records, including information about diagnoses and medication.
Details of the subjects included in the present study are given in Table 1. The 25(OH)D was measured via an in-house reverse-phase ultra-performance liquid chromatography–tandem mass spectrometry (UPLC-MS/MS) method following liquid–liquid extraction, which fractionates the D2 and D3 forms, and the combined results formed the total 25(OH)D value. The limit of detection for both forms was 5 nmol/L. LC-MS/MS assay precision (CV) was 13.2% for 25(OH)D2 at 42.1 nmol/L and 9.3% at 93.9 nmol/L. For 25(OH)D3, the coefficient of variation (CV) was 11.0% at 41.7 nmol/L and 9.9% at 95.8 nmol/L. Patients with detectable D2 levels were likely to be those on vitamin D2 replacement treatment, although this may not necessarily be the case. Serum calcium, ALP, phosphate, albumin, creatinine, and PTH were assayed using Roche automated chemistry and immunoassay platforms (Hoffmann-La Roche, Grenzacherstrasse 124, CH-4070 Basel, Switzerland). We have reported serum-adjusted calcium where this was calculated. The assay used for albumin was the bromocresol green dye-binding method, and the formula used to calculate adjusted calcium was adjusted calcium = calcium mmol/L + 0.02 × (40 − albumin g/L).
Table 1.
Sample by Total 25-Hydroxyvitamin D Status According to Mayo Medical Laboratories Classification (Kennel et al., 2010).
| Vitamin D category (nmol/L) |
|||||
|---|---|---|---|---|---|
| <10 Severe deficiency (n = 28) |
10-24.9 Deficiency (n = 39) |
25.0-49.9 Insufficiency (n = 71) |
50-74.9 Adequate (n = 69) |
≥75.0 Optimal (n = 29) |
|
| % normal PTH | 71.4 | 56.4 | 67.6 | 84.1 | 79.3 |
| % normal calcium | 71.4 | 74.4 | 78.9 | 92.8 | 93.1 |
| % normal phosphate | 82.1 | 79.5 | 91.6 | 91.3 | 93.1 |
| % normal alkaline phosphatase | 89.3 | 82.1 | 80.3 | 84.1 | 86.2 |
Note. Reference ranges: PTH: 15 to 65 ng/L, calcium: 2.2 to 2.6 mmol/L, phosphate: 0.8 to 1.5 mmol/L, alkaline phosphatase: 35 to 105 IU/L female, alkaline phosphatase: 40 to 130 IU/L male. PTH = parathyroid hormone.
The costs of the assays are as follows: total 25(OH)D profile (25(OH)D2 and 25(OH)D3), £8.40 (€10.97); bone profile (calcium, phosphate, albumin and ALP), £4.75 (€6.21); and PTH, £2.75 (€3.59).
Samples were classified on the basis of total 25(OH)D status as defined by the Mayo Medical Laboratories Reference ranges (Table 1; Kennel et al., 2010). Serum calcium, phosphate, PTH, and ALP results in each vitamin D category were compared with those in the defined optimal vitamin D group (≥75 nmol/L).
Index of Multiple Deprivation (IMD)
The English Indices of Deprivation (Cabinet Office, 2001) combine factors of housing, social, and economic issues to give a single deprivation score for small areas, known as Lower Super Output Areas (LSOA) in England. An overall weighted aggregation IMD is generated based on seven criteria, and each area is ranked from the least to most deprived. The criteria and their associated weightings are as follows: income deprivation, 22.5%; employment deprivation, 22.5%; health deprivation and disability, 13.5%; education, skills, and training deprivation, 13.5%; barriers to housing and services, 9.3%; crime, 9.3%; and living environment deprivation, 9.3%. The indices are a widely used standard measure for comparing areas across the country and can help to identify areas with high levels of overall deprivation or areas with specific concerns, health for example, that may not be recognized from the overall index. The measures of deprivation are collected nationally and published every 3 to 4 years; hence, data behind the 2010 IMD are for the year 2008 and as such, will not reflect any policy changes, economic changes, or regeneration effects since then. In the IMD 2010, the most deprived LSOA in England is given a rank of 1 and the least deprived is ranked 32,482.
Each vitamin D result was aligned with an associated postcode and linked its LSOA using the GeoConvert tool (http://geoconvert.mimas.ac.uk). Subsequently, the 2010 IMD and rank were linked to the LSOA for the specified postcode.
Statistical Analysis
The data were analyzed with the statistical package “Intercooled Stata” version 12.2 (StataCorp, Texas). The normal distribution of continuous variables was examined with Shapiro–Wilk’s test. Geometric means (95% confidence intervals [CIs]) are presented where the underlying data were skewed. Comparison of means was assessed using t test or ANOVA. Chi-square test was used to compare proportions. Correlations between continuous variables used Spearman coefficients.
Logistic regression was used to assess associations. Model numbers varied depending on the variables included, with any missing values causing the participant to be dropped from the model. Not all measurements were obtained on all individuals, accounting for slight differences in the number of individuals included in the models.
Results
Specimens were identified from 241 patients above the age of 70 years. A full data set was available for 236 patients aged >70 years (Table 1), and 71% of the patients referred for vitamin D testing were women. The levels of 25(OH)D3 were higher in women at 40.6 (95% CI [36.6, 45.0]) nmol/L versus 32.0 (95% CI [26.4, 38.9]) nmol/L (F = 3.75, p = .043) in men; 25 (OH)D2 levels (geometric means) were similar in men and women.
The prevalence of total vitamin D insufficiency or deficiency (defined as total vitamin D <50 nmol/L) was 57.5% overall, with men having a higher prevalence than women (60.0% vs. 56.1%).
By vitamin D status, 28 patients (11.9%) had severe vitamin D deficiency (25(OH)D < 10 nmol/L), 39 (16.5%) had vitamin D deficiency (25(OH)D 10-24.9 nmol/L), 71 (30.1%) had vitamin D insufficiency (25(OH)D 25-49.9 nmol/L), 69 (29.2%) had adequate vitamin D status (25(OH)D 50-74.4 nmol/L), and 29 (12.3%) had optimal vitamin status (25(OH)D ≥ 75 nmol/L).
Geometric mean PTH levels (including those age-adjusted) were lower in men at 43.0 (95% CI [38.3, 42.4]) ng/L versus 51.1 (95% CI [46.3, 56.4]) ng/L for women (F = 4.9, p = .01).
Relation Between PTH and 25(OH)D Levels
As expected, PTH levels rose as vitamin D levels fell: 25(OH)D = 50 nmol/L, PTH = 58.0 ng/L; 25(OH)D = 10 nmol/L, PTH = 83.7 ng/L (F = 3.4, p = .01).
Figure 1 shows the relation between mean PTH and categorized degree of vitamin D sufficiency/deficiency, derived by ANOVA of PTH across the categories of vitamin D status. This showed that while there was a clear relationship, there was significant variability in PTH levels in each group. Furthermore, use of vitamin D levels as the dependent variable in multivariate linear regression showed that higher circulating PTH levels were associated with lower vitamin D (normalized β = −0.14, p = .02), independent of age (β = −0.3, p = .6); male sex (β = −0.11, p = .003), adjusted calcium (β = 0.21, p = .002), and phosphate levels (β = −0.19, p = .006). The relationship was insufficiently strong to allow prediction of vitamin D levels from the PTH result.
Figure 1.
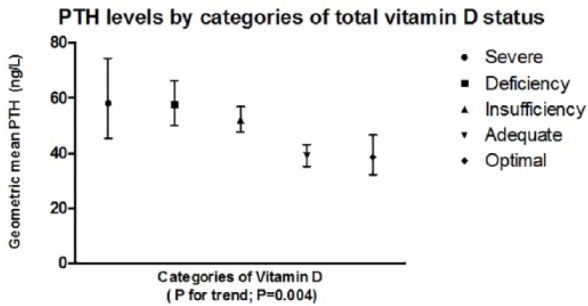
Plot demonstrating the increase in PTH levels by categories of decreasing total 25-hydroxyvitamin D status.
Note. Plot shows means with interquartile ranges in men and women aged ≥70 years. PTH = parathyroid hormone.
Relation Between Calcium, Phosphate and ALP, and 25(OH)D Levels
There was no overall trend in mean serum-adjusted calcium across categories of 25(OH)D status, derived by ANOVA of PTH across the categories of vitamin D status (Figure 2). Similarly, for phosphate, there was no trend in mean serum values across categories of 25(OH)D status, nor was there any trend for ALP (data not shown).
Figure 2.
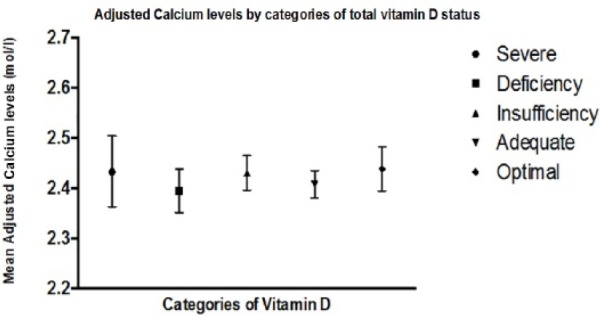
Plot to demonstrate no difference in calcium levels by categories of total 25-hydroxyvitamin D status in men and women aged ≥70 years.
Note. Plot shows means with interquartile ranges.
Proportion of Individuals With Severe Vitamin D Deficiency With Other Bone Profile Parameters Within the Reference Range
Even for patients aged ≥70 years with 25(OH)D deficiency, a significant proportion of patients had PTH, calcium, phosphate, and ALP levels within the laboratory reference range. Notably, for patients with 25(OH)D <25 nmol/L, 62.7% had a normal PTH, 83.1% had normal serum calcium, 80.6% had normal phosphate, and 85.1% had a normal serum ALP (Table 1).
Analysis by IMD
Based on LSOA, the geographical area from which the IMD is derived, derived from seven criteria, a higher IMD by quintile (higher quintiles associated with greater disadvantage; Cabinet Office, 2001) was associated with a greater likelihood of having a low level of 25(OH)D (<25 nmol/L; Figure 3). There was a 22% increased likelihood of 25(OH)D deficiency/insufficiency for each increase in quintile of IMD, in age- and sex-adjusted logistic regression models (odds ratio [OR] = 1.22, 95% CI [1.01, 1.47]; p = .034).
Figure 3.
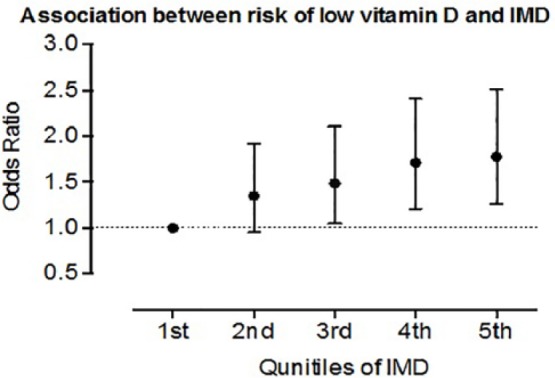
Relation between IMD and risk of vitamin D deficiency.
Note. Higher IMD is associated with a higher likelihood of vitamin D deficiency (25-hydroxyvitamin D of <25 nmol/L). Plot shows hazard ratio with interquartile ranges. IMD = index of multiple deprivation.
Discussion
The majority of patients aged ≥70 years whom we studied did not show any aberration of bone profile or PTH on the basis of established reference ranges in the face of low circulating 25(OH) levels (Table 1).
The relation between higher IMD and lower vitamin D levels is an important finding and replicates that of Webster (2013) in a multiethnic population in Birmingham, United Kingdom. In that study, statistically significant correlations were found between (a) median 25(OH)D and percentage of non-White population, (b) percentage of non-White population and IMD, and (c) percentage of population classified as severely deficient and IMD. The reasons for this phenomenon are complex and relate to an interaction between environmental, dietary, and demographic factors (Brennan et al., 2009; Grimes, 2011).
We have demonstrated in this older persons group that no surrogate bone marker is adequate in terms of identifying those individuals who have vitamin D deficiency. We previously reported similar findings in a general adult population sample (Heald et al., 2015); 31% of the older patients in this survey were included in the analysis for the previous study.
The additional cost of measuring the serum vitamin D must be weighed against the potential health economic benefits of normalizing vitamin D status in those individuals found to have suboptimal levels. The number of women tested for vitamin D insufficiency was higher for all categories than for men. This probably relates to the higher prevalence of osteoporosis in women and particularly older women, for which vitamin D estimation is a commonly requested investigation. Increased testing in females has been reported elsewhere (LeBlanc, Chou, Zakher, Daeges, & Pappas, 2014). In the United Kingdom, as in other countries with publicly funded health care systems, screening for Vitamin D status tends to happen more often in women than in men.
It is known that vitamin D insufficiency or deficiency is common in older people. Of 824 people aged >70 years from 11 European countries, 36% of men and 47% of women had wintertime serum 25(OH)D3 concentration <30 nmol/L (van der Wielen et al., 1995). In relation to this landmark article by van der Wielen et al. (1995), we have provided further evidence that free-living older Europeans, regardless of geographical location, are at substantial risk of inadequate vitamin D status. The corollary is that dietary enrichment or supplementation with vitamin D should be seriously considered in this group given that in our study, we found 57.5% of people overall to have low Vitamin D levels.
Vitamin D deficiency has been reported to affect predominantly the weight-bearing antigravity muscles of the lower limb, which are necessary for postural balance and walking (Glerup et al., 2000; Schott & Wills, 1976), and a significant correlation between serum 25(OH)D3 concentration and the occurrence of falls in elderly people has been reported (Stein et al., 1999). Furthermore, supplementation for 8 weeks with vitamin D and calcium in 148 elderly women with a serum 25(OH)D3 concentration <50 nmol/L resulted in a 9% decrease in body sway and fewer falls per subject over 1 year of follow-up as compared with calcium monotherapy (Pfeifer et al., 2000). Thus, measurement of vitamin D status in older people, with vitamin D supplementation if required, should be an integral part of their ongoing health management in primary care. In the United Kingdom, vitamin D replacement is most simply given with Fultium-D3 800 IU capsules at the dose of 1 to 2 capsules daily.
The degree of overlap between 95% CIs for the mean values observed for all markers in all vitamin D categories and the numbers of people with normal bone parameters with 25(OH)D levels <25 nmol/L or even <10 nmol/L (Table 1) reinforces the view that none of the less expensive markers (bone profile: calcium, phosphate, and ALP, cost £4.75 [€6.21]; and PTH, cost £2.75 [€3.59]) can be used as a surrogate measure of vitamin D status (£8.40 [€10.97]). Thus, other markers of bone metabolism are insufficient surrogates for measurement of vitamin D when screening for vitamin D insufficiency/deficiency.
Other studies have demonstrated increased fracture risk at 25(OH)D levels <50 nmol/L (Buchebner et al., 2014) and increased PTH levels at 25(OH)D levels <75 nmol/L (Yan et al., 2000). Vitamin D insufficiency/deficiency has been associated with numerous major disorders including cardiovascular disease, type 2 diabetes, and some forms of cancer. Consequently, we have seen a huge increase in the demand for measurement of serum 25(OH)D levels in the United Kingdom. For example, a sixfold increase in the workload was seen in hospitals in London, United Kingdom, over a period of 4 years (Sattar et al., 2012).
The strengths of this study include the significant number of consecutive older people assessed and the use of a single assay platform for measuring vitamin D status. Weaknesses are that this is not a systematic population study, with blood samples taken over a wide time frame, so no inferences can be drawn regarding prevalence of vitamin D deficiency in the healthy normal older individuals from North West England. Furthermore, we do not know if some of these individuals were taking over-the-counter, self-prescribed vitamin D. Finally, we have no information for the majority of patients about action taken in the light of the results.
In conclusion, the results presented in this report indicate that social disadvantage is associated with a higher likelihood of vitamin D deficiency in older people and that none of the other available parameters is currently adequate for screening for vitamin D deficiency. Treating vitamin D deficiency, once identified, will improve health outcomes for older people because of the beneficial effects of Vitamin D on musculoskeletal and cardiovascular health.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- Brennan S. L., Henry M. J., Wluka A. E., Nicholson G. C., Kotowicz M. A., Williams J. W., Pasco J. A. (2009). BMD in population-based adult women is associated with socioeconomic status. Journal of Bone and Mineral Research, 24, 809-815. [DOI] [PubMed] [Google Scholar]
- Buchebner D., McGuigan F. E., Gerdhem P., Malm J., Ridderstrale M., Akesson K. (2014). Vitamin D insufficiency over 5 years is associated with increased fracture risk—An observational cohort study of elderly women. Osteoporosis International, 25, 2767-2775. [DOI] [PubMed] [Google Scholar]
- Cabinet Office. (2001). A new commitment to neighbourhood renewal: National strategy action plan (Report by the Social Exclusion Unit). London, England: Author. [Google Scholar]
- Department of Health. (1998). Nutrition and bone health: With particular reference to calcium and vitamin D. Report on health and social subjects 49. London, England: The Stationery Office. [Google Scholar]
- Fraser W. D., Milan A. M. (2013). Vitamin D assays: Past and present debates, difficulties, and developments. Calcified Tissue International, 92, 118-127. [DOI] [PubMed] [Google Scholar]
- Glerup H., Mikkelsen K., Poulsen L., Hass E., Overbeck S., Andersen H., . . . Eriksen E. F. (2000). Hypovitaminosis D myopathy without biochemical signs of osteomalacic bone involvement. Calcified Tissue International, 66, 419-424. [DOI] [PubMed] [Google Scholar]
- Gloth F. M., III, Gundberg C. M., Hollis B. W., Haddad J. G., Tobin J. D. (1995). Vitamin D deficiency in homebound elderly persons. Journal of the American Medical Association, 274, 1683-1686. [DOI] [PubMed] [Google Scholar]
- Grimes D. S. (2011). Vitamin D and the social aspects of disease. Quarterly Journal of Medicine, 104, 1065-1074. [DOI] [PubMed] [Google Scholar]
- Heald A. H., Anderson S. G., Scargill J. J., Short A. D., Holland D., Livingston M., . . . Fryer A. A. (2015). Measuring vitamin D levels: Surrogates are insufficient. International Journal of Clinical Practice, 69, 131-135. [DOI] [PubMed] [Google Scholar]
- Hirani V., Primatesta P. (2010). Urgent action needed to improve vitamin D status among older people in England! Age and Ageing, 39, 62-68. [DOI] [PubMed] [Google Scholar]
- Holick M. F. (1995). Environmental factors that influence the cutaneous production of vitamin D. American Journal of Clinical Nutrition, 61(Suppl.), 638S-645S. [DOI] [PubMed] [Google Scholar]
- Holick M. F. (2007). Vitamin D deficiency. The New England Journal of Medicine, 357, 266-281. [DOI] [PubMed] [Google Scholar]
- Holick M. F., Binkley N. C., Bischoff-Ferrari H. A., Gordon C. M., Hanley D. A., Heaney R. P. . . . Endocrine Society. (2011). Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. Journal of Clinical Endocrinology & Metabolism, 96, 1911-1930. [DOI] [PubMed] [Google Scholar]
- Hyppönen E., Power C. (2007). Hypovitaminosis D in British adults at age 45 y: Nationwide cohort study of dietary and lifestyle predictors. American Journal of Clinical Nutrition, 85, 860-868. [DOI] [PubMed] [Google Scholar]
- Kennel K. A., Drake M. T., Hurley D. L. (2010). Vitamin D deficiency in adults: When to test and how to treat. Mayo Clinic Proceedings, 85, 752-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc E., Chou R., Zakher B., Daeges M., Pappas M. (2014). Screening for vitamin D deficiency: Systematic review for the U.S. preventive services task force recommendation (Evidence Synthesis No. 119, AHRQ Publication No. 13-05183-EF-1). Rockville, MD: Agency for Healthcare Research and Quality. [PubMed] [Google Scholar]
- McKenna M. J. (1992). Differences in vitamin D status between countries in young adults and the elderly. The American Journal of Medicine, 93, 69-77. [DOI] [PubMed] [Google Scholar]
- Pearce S. H. A., Cheetham T. D. (2010). Diagnosis and management of vitamin D deficiency. British Medical Journal, 340, Article b5664. [DOI] [PubMed] [Google Scholar]
- Pfeifer M., Begerow B., Minne H. W., Abrams C., Nachtigall D., Hansen C. (2000). Effects of a short-term vitamin D and calcium supplementation on body sway and secondary hyperparathyroidism in elderly women. Journal of Bone and Mineral Research, 15, 1113-1118. [DOI] [PubMed] [Google Scholar]
- Pludowski P., Holick M. F., Pilz S., Wagner C. L., Hollis B. W., Grant W. B., . . . Soni M. (2013). Vitamin D effects on musculoskeletal health, immunity, autoimmunity, cardiovascular disease, cancer, fertility, pregnancy, dementia and mortality-a review of recent evidence. Autoimmunity Reviews, 12, 976-989. [DOI] [PubMed] [Google Scholar]
- Sattar N., Welsh P., Panarelli M., Forouhi N. G. (2012). Increasing requests for vitamin D measurement: Costly, confusing, and without credibility. The Lancet, 379, 95-96. [DOI] [PubMed] [Google Scholar]
- Schott G. D., Wills M. R. (1976). Muscle weakness in osteomalacia. The Lancet, 1, 626-629. [DOI] [PubMed] [Google Scholar]
- Stein M. S., Wark J. D., Scherer S. C., Walton S. L., Chick P., Di Carlantonio M., . . . Flicker L. (1999). Falls relate to vitamin D and parathyroid hormone in an Australian nursing home and hostel. Journal of the American Geriatrics Society, 47, 1195-1201. [DOI] [PubMed] [Google Scholar]
- van der Wielen R. P., Löwik M. R., van den Berg H., de Groot L. C., Haller J., Moreiras O., van Staveren W. A. (1995). Serum vitamin D concentrations among elderly people in Europe. The Lancet, 346, 201-210. [DOI] [PubMed] [Google Scholar]
- Vogeser M. (2010). Quantification of circulating 25-hydroxyvitamin D by liquid chromatography-tandem mass spectrometry. The Journal of Steroid Biochemistry & Molecular Biology, 121, 565-573. [DOI] [PubMed] [Google Scholar]
- Webster C. (2013). Relationship of total 25-OH vitamin D concentrations to indices of multiple deprivation: Geoanalysis of laboratory results. Annals of Clinical Biochemistry, 50, 31-38. [DOI] [PubMed] [Google Scholar]
- Yan L., Prentice A., Zhang H., Wang X., Stirling D. M., Golden M. M. (2000). Vitamin D status and parathyroid hormone concentrations in Chinese women and men from north-east of the People’s Republic of China. European Journal of Clinical Nutrition, 54, 68-72. [DOI] [PubMed] [Google Scholar]


