Abstract
We conducted a retrospective review of 168 patients with invasive fungal infections from January 2000 to December 2011 in 2 neonatal intensive care units. Patients with Candida bloodstream infection (BSI, n = 152) were further analyzed. C albicans was the most common species overall (47%); however, there was an increase in non–albicans sp from 2006 to 2011. Candida BSI clearance rates were lower in extremely low birth weight infants (77% vs 93%, P = .01) and in patients with C albicans infections (77% vs 91%, P = .01). Clearance rates improved from 2000 to 2005 (70% - 90%) to 2006 to 2011 (86% -100%). Combination antifungal use increased during the later years (73% vs 49%, P < .05) and in patients with end-organ dissemination (83% vs 54%, P < .05). We concluded that extremely low birth weight infants and C albicans infection are factors associated with nonclearance of Candida BSI. Successful clearance of Candida BSI improved in 2006 to 2011, perhaps due to increase in non–albicans species and the use of combination antifungals.
Keywords: Candida, NICU, invasive fungal infections, clearance, dissemination
Introduction
Invasive fungal infections (IFIs) are an important cause of nosocomial infection in the neonates, especially the extremely premature infants given the immaturity of the immune system.1 Infection from various fungi including Candida, Aspergillus, Malassezia, and Blastomyces sp have been reported in these patients.2 Candida bloodstream infections (BSIs) remain the most common infection among all IFIs and is associated with significant mortality and adverse neurodevelopmental outcome.2,3 While C albicans remains the most commonly isolated species in most studies, infection with non–albicans species, especially C parapsilosis, is increasingly being reported.4,5 C albicans is thought to be more virulent and has been associated with higher rates of end-organ dissemination and mortality as compared with non–C albicans species.1,3,6
Given the high rates of morbidity and mortality associated with IFIs, appropriate antifungal therapy remains vital in the management of these patients. Amphotericin B and fluconazole are the 2 most widely used antifungals for treatment of IFIs in neonates.7 Over the past decade, fluconazole has been increasingly used as a prophylactic agent to prevent IFI in high-risk premature infants.7,8 However, the impact of antifungal prophylaxis on the epidemiology of IFI in neonates is not well understood. In addition, other clinical variables including gestational age, birth weight, prior surgery, antimicrobial use, and presence of central venous catheters (CVCs) that may also affect the outcome of IFIs have not been studied extensively.9
The objective of our research was to study the epidemiology of IFIs including trends, outcome, clinical and laboratory characteristics, and antifungal utilization in our neonatal intensive care unit (NICU).
Materials and Methods
We conducted a retrospective chart review of all patients diagnosed with IFI during their admission to the NICUs at the Detroit Medical Center. Our NICUs have a unique set-up, with a dual facility within the main hospital campus, one each at Hutzel Women’s Hospital and at Children’s Hospital of Michigan. The duration of study ranged from January 2000 until December 2011. Approval was obtained from the institutional review board at Wayne State University School of Medicine.
Patient Selection
All patients with diagnoses of fungal infections including the terms such as invasive fungal infections, fungal infections, probable fungal infection, possible fungal infection, fungemia, candidiasis, candidemia, aspergillosis, fusarium, blastomycosis, and histomycosis were identified by the diagnoses list at discharge and the records of the hospital’s microbiology laboratory that serves both the NICUs. Patients with Candida isolated from lung, stool, or skin were considered to be colonized and thus were excluded.
Data Collection
Demographic and Clinical Data
Age, gender, race, underlying diagnosis, history of prematurity, predisposing factors such as primary or acquired immunodeficiency, use of immunosuppressive agent, presence and type of CVC, prior surgical procedure, administration of total parenteral nutrition, and antibiotic usage prior to the onset of the fungal infection; type of fungal infection; degree of infection (dissemination); and use of prophylactic antifungal therapy. Results of cerebrospinal fluid analysis, echocardiography, abdominal ultrasound, and dilated eye exams were reviewed to assess for possible end-organ dissemination.
Microbiological Data
Culture results from blood, urine, and body fluids as appropriate were reviewed.
Treatment Data
Antifungal prophylaxis, empiric and definitive therapy with single or combination antifungal drugs were noted.
Outcome Measures
Primary outcome in the form of microbiologic clearance of infection or candidemia was recorded. Clearance was defined as sustained negative blood cultures for fungal species for 2 consecutive weeks with clinical improvement after therapy was initiated.
Statistical Analysis
Descriptive statistics (mean, standard deviation, rate, ratio, and proportion) were used to characterize the study sample, including demographic factors (age, gender, race, age of diagnosis), types of infection, and utilization of antifungal medication. The incidence of IFIs before and after introduction of routine antifungal treatment were compared using χ2 test with P < .05 (2-sided). Time trend of IFIs was assessed by tabulating and plotting the total number of patients with IFIs per year over the study period from 2000 to 2011. Observed time trends were further assessed using Cochran-Armitage trend test.
Results
A total of 226 patients were identified as having a possible fungal infection during the study period. Nine of these patients were excluded as their medical records were unavailable for further review. Of the remaining 217 patients, 49 patients were excluded after a detailed review of their medical records suggested fungal colonization rather than a true infection. One hundred and sixty-eight patients were eventually included in the study (Figure 1).
Figure 1.
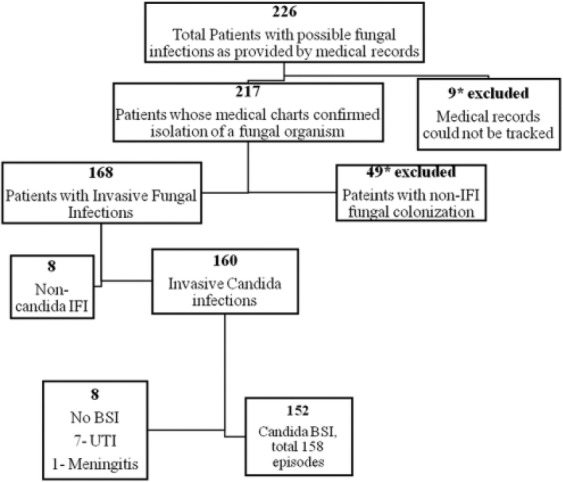
Flowchart demonstrating selection of study patients and the types of invasive fungal infections (IFIs) seen.
Non-Candida IFI: Malassezia sp (5), Aspergillus sp (2), and Rhodotorula sp (1). BSI, bloodstream infections.
The overall incidence of IFIs in our NICUs was 9.8 per 1000 NICU admissions per year (range 2.7-20.2). The predominant IFI seen in our study cohort was invasive candidiasis present in 160 patients (95.2%) of which majority (152/160) had Candida bloodstream infection (BSI). Other pathogens isolated were Malassezia sp (5), Aspergillus sp (2), and Rhodotorula sp (1). The demographic characteristics and the predominant clinical manifestations seen with different types of IFIs (non–Candida BSI) are shown in Table 1.
Table 1.
Characteristics of IFIs (Non–Candida BSI) in the study populations.
| Variable | Candida UTI Without BSI (N = 7) | Candida Meningitis (N = 1) | Malassezia (N = 5) | Aspergillus (N = 2) | Rhodotorula (N = 1) |
|---|---|---|---|---|---|
| Age (days), median (range) | 50 (27-150) | 10 | 90 (60-120) | 86 (22-150) | 50 |
| Gender | Male: 7 | Female | Males: 3; females: 2 | Male: 2 | Female |
| Ethnicity | African American = 3 | African American | African American: 4 | African American: 1 | African American |
| Caucasian = 2 | Caucasian: 1 | Caucasian: 1 | |||
| Others = 2 | |||||
| Site of infection | Urinary tract infection | CNS | Bloodstream infection | Pulmonary | Bloodstream infection |
| Gestational age (weeks) | <28 weeks: 5 | 25 weeks | <28 weeks: 4 | <28 weeks:1 | 29 weeks |
| 28-32 weeks: 1 | 28-32 weeks: 1 | >32 weeks: 1 | |||
| >32 weeks: 1 | |||||
| Birth weight (g) | <1000: 4 | 730 | <1000: 4 | 1000-1500: 1 | 1085 |
| 1000-1500: 1 | 1000-1500: 1 | >1500: 1 | |||
| >1500: 2 | |||||
| Risk factors | Urinary catheter = 7 | CVC, TPN, ABx | TPN: 5 | Immunodeficiency (1), ABx (1) | TPN, ABx |
| Antifungals used | C-AMB = 2 | C-AMB | C-AMB: 2 | L-AMB: 2 | L-AMB |
| L-AMB = 3 | L-AMB: 2 | ||||
| Fluconazole = 2 | Fluconazole: 1 | ||||
| Cure | 5 (71%) | 1 (100%) | 5 (100%) | 1 (50%) | 1 (100%) |
Abbreviations: IFI, invasive fungal infection; BSI, bloodstream infection; UTI, urinary tract infection; CVC, central venous catheter; TPN, total parenteral nutrition; C-AMB, conventional amphotericin B deoxycholate; L-AMB, liposomal amphotericin B.
Patients With Candida Invasive Fungal Infections
Of 160 patients, 8 patients had Candida IFIs without evidence of a Candida BSI. Of these, 7 patients had urinary tract infection (UTI) and one had meningitis due to C albicans (Figure 1). Of the 152 patients with Candida BSI, recurrent BSIs were seen in 6 patients, leading to a total of 158 episodes during the study period. As majority of IFIs were due to Candida BSI in our study group, we further studied the characteristics of this group of patients (Table 2).
Table 2.
Characteristics of Patients With Candida BSI (Candidemia) IFIs.
| Patient Characteristics (N = 152) | Value |
|---|---|
| Age (days), median (range) | 26 (4-390) |
| Male gender (%) | 54 |
| Race | |
| African American (%) | 74 |
| Caucasian (%) | 16 |
| Gestational agea, median (range) | 28 (22-41) |
| ≤28 weeks | 70 |
| 29-32 weeks | 18 |
| >32 weeks | 12 |
| Birth weight (g)a, median (range) | 820 (400-5600) |
| <1000 g | 61 |
| 1001-1500 | 20 |
| ≥1500 g | 19 |
Abbreviations: BSI, bloodstream infection; IFI, invasive fungal infection.
Data available for 151 episodes.
Patients With Candida BSI
The median age at diagnosis was 26 days (range: 4-390 days). The majority of these patients (74%) were African American, and the male-to-female ratio was 1.08:1. The median gestational age was 28 weeks (range: 22-41 weeks) with 70% of patients born at ≤28 weeks of gestation. The median birth weight of the study population was 820 g (range: 400-5600 g), with 61% patients categorized as extremely low birth weight (birth weight less than 1000 g). Most patients had one or more risk factors for IFI, with the most common factor being prior administration of antibiotics, which was seen in 95% patients (Figure 2). C albicans accounted for 48% of the total isolates followed by C parapsilosis (38%; Figure 3). Overall, non–albicans species accounted for 52% of Candida BSI.
Figure 2.
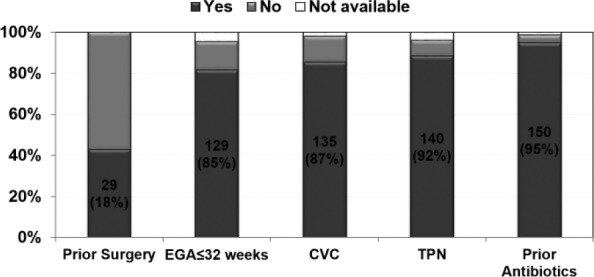
Associated risk factors for Candida BSI.
Figure 3.
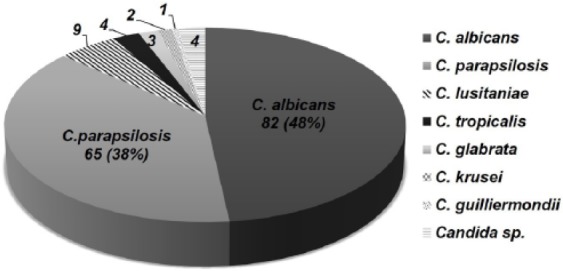
Candida species isolated during the study period.
Data on dissemination of candidemia to various organ systems was available in 126 patients. Of these, a third of patients (48/126) had one or more organ involvement as a result of Candida BSI. Kidney involvement based on sonographic demonstration of the presence of fungal balls was seen in 30 patients followed by meningitis in 12 patients, endocarditis in 8 patients, and various forms of eye involvement in 7 patients (endophthalmitis = 3, vitritis = 2, chorioretinitis = 2). Dissemination was significantly more common in patients with gestational age less ≤32 weeks (P = .04; Table 3).
Table 3.
Factors Associated With Dissemination of Candida BSI (Dissemination Data Were Available in 126 Patients).
| Variable | Dissemination = 48, n (%) | No Dissemination = 78, n (%) | χ2 | P Value |
|---|---|---|---|---|
| Age ≤28 days | 31 (65) | 38 (49) | 3.019 | .08 |
| Male gender | 24 (50) | 42 (54) | 0.176 | .68 |
| EGA ≤32 weeksa | 45 (96) | 59 (82) | —b | .04 |
| Birth weight ≤1000 ga | 28 (61) | 45 (62) | 0.005 | .92 |
| Early removal of CVC | 35 (72) | 57 (73) | 0.000 | .98 |
| Candida albicans species | 25 (52) | 32 (41) | 1.467 | .22 |
| Clearance of infection | 41 (85) | 65 (83) | 0.097 | .75 |
Median duration of antifungal treatment prior to documented clearance of candidemia was 5.5 days (range: 1-61 days). Table 4 presents the patient characteristics and factors associated with clearance and nonclearance of candidemia. Clearance of candidemia was documented in 132/158 episodes (84%). Significant risk factors associated with decreased clearance of candidemia were birth weight ≤1000 g (P = .01) and infection with C albicans species compared to non–albicans species (P = .004). We also observed a trend toward increased rate of clearance in patients with early removal of CVC within 3 days after diagnosis of candidemia, although this was not statistically significant (P = .07). Of the 26 patients in whom nonclearance was documented, mortality was 100% within 5 days of the last positive culture for Candida.
Table 4.
Patient Characteristics and Factors Associated With Clearance and Nonclearance of Candida BSI.
| Variable | Clearance = 132, n (%) | Nonclearance = 26, n (%) | χ2 | P Value |
|---|---|---|---|---|
| Age ≤28 days | 70 (53) | 13 (50) | 0.080 | .77 |
| Male gender | 72 (55) | 13 (26) | 0.181 | .67 |
| EGA ≤32 weeksc | 111 (87) | 22 (92) | —b | .55 |
| Birth weight ≤1000c | 72 (57) | 20 (83) | —b | .01 |
| Early removal (within 3 days of diagnosis) of CVC | 37 (41) | 2 (14) | —b | .07 |
| Candida albicans species | 56 (42) | 19 (73) | 8.185 | .004 |
| Age ≤28 days | 70 (53) | 13 (50) | 0.080 | .77 |
Abbreviations: BSI, bloodstream infection; EGA, estimated gestational age; CVC, central venous catheter.
Data not available for 3 patients.
Fisher exact test.
Data not available for 7 episodes.
The mean duration of antifungal treatment in patients with candida BSI was 23 ± 14 days (range: 1 day to 92 days). Among 58 patients treated with various amphotericin B formulations, 25 patients received conventional amphotericin B (amphotericin B deoxycholate), 30 patients received amphotericin B lipid complex (lipid formulation in hospital drug formulary), and 3 patients received liposomal amphotericin B. Over half of the patients received a combination of 2 or more antifungal agents (90/158, 57%). Amphotericin B and fluconazole were the most common combination antifungal therapy used (45/158, 28%). The rates of combination therapy were higher in those patients with organ dissemination (83%) versus those without (54%; P = .004).
Time Trends of Candida Bloodstream Infection
There was a decreasing trend in the incidence of Candida BSI from 2003 to 2011 (Figure 4). Incidence data prior to 2003 were not available. A change in species distribution was also noted with decrease in C albicans infection over the last 5 years of the study (except in 2010); however, this trend did not achieve statistical significance (P = >.05; Figure 5). The clearance rates for Candida BSI improved from about 70% during the early part of the study to almost 100% over the last 5 years (P = <.01; Figure 6). We also noted a trend toward increasing use of combination therapy from 38% in the first half of the study period to 70% or more in the latter part of the study (P = <.05; Figure 7).
Figure 4.
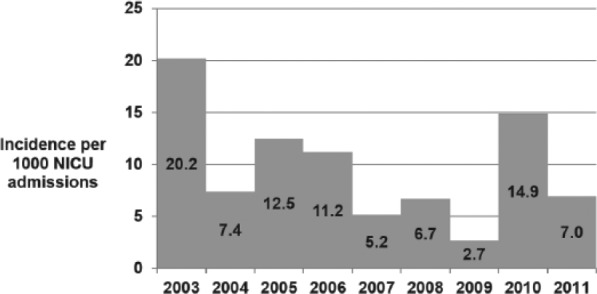
Incidence of Candida BSI from 2003 to 2011.
A negative trend (0.9/1000 annual reduction), P = .218.
Figure 5.
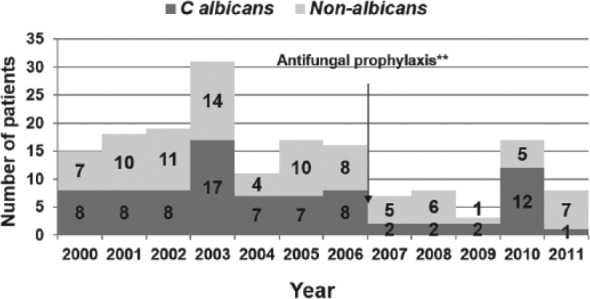
Trend of Candida species over study period.
Cochran-Armitage trend test: Z = 0.34, P > .05.
One patient had 3 episodes of C albicans with prolonged NICU stay in 2010.
Antifungal prophylaxis started in 2007; fluconazole prophylaxis given in selected high-risk patients <1500 g.
Figure 6.
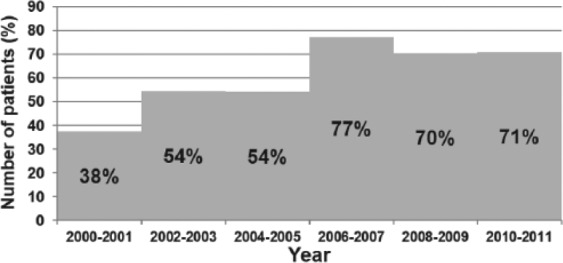
Trend of Candida BSI clearance over study period.
Cochran-Armitage trend test: Z = 3.017, P < .01.
Figure 7.
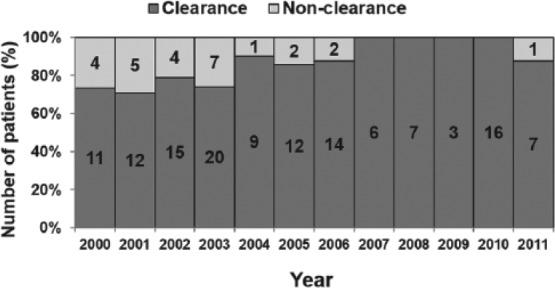
Combination antifungal use during the study period.
Cochran-Armitage trend test: Z = 2.92, P < .05.
Discussion
IFIs are important infections in our NICUs, with Candida BSI being the most common IFI. In our population, more than 80% of the Candida BSIs were seen in neonates with birth weight of <1500 g, which is consistent with published literature.3,10,11 Apart from birth weight, there are several other risk factors associated with invasive IFI in neonates. The National Mycosis Survey study group enrolled 2847 infants from 6 Level 3 NICUs and highlighted various factors that independently predicted higher risk for invasive candidiasis after adjusting for birth weight.11 Several of these risk factors were also noted in our study including prior antibiotic use, presence of CVC, parenteral nutrition, gestational age <32 weeks, and prior abdominal surgery. The incidence of IFI, in general, as well as of Candida infections, varies across different NICUs. Incidence has been reported to be much higher in studies from outside the United States.12,13 The higher incidence of IFIs in our study of 9.8 per 1000 NICU admissions per year is likely reflective of the tertiary care nature of our ICU, which often receives sick infants from other centers, and hence may have overestimated the total incidence of IFIs in our neonatal population.
Candida albicans was the most common isolate in our NICUs followed closely by C parapsilosis. Most studies conducted in the neonatal population have reported C albicans to be the predominant species causing IFI.4,6,14 Although C parapsilosis was the second most common IFI in our patients, it has been reported to be the leading Candida species in other NICU settings.15 In our study, we observed that during the 12-year study period, the proportion of IFIs due to C parapsilosis increased compared to those with C albicans, a pattern also noted by other investigators.14,16 This change in epidemiological trend has been associated with the use of fluconazole prophylaxis in the high-risk NICU babies. In our NICUs, routine fluconazole prophylaxis in high-risk premature infants was implemented in 2006, which likely contributed to the decreasing temporal trend of Candida IFI noted in our study, with a notable decline in C albicans.
Dissemination of Candida infection to end organs has been associated with poorer outcome.17 Dissemination is more common among infants with birth weight <1500 g, a factor seen in almost 80% of our study population, leading to a relatively high dissemination rate of 38% in our study compared to other studies of up to 17%.1,17 In our study, we also noted that infants with estimated gestational age ≤32 weeks were more likely to have end-organ dissemination than those born closer to term. The most common organs where dissemination occurred were the kidneys and central nervous system, as was also noted by other investigators.1,2,5,6,17 The rate of clearance of Candida BSI in our study was comparable in patients with and without dissemination to end organs, although combination antifungals were more likely to be used in patients with end-organ dissemination. This could be related to the perceived severity of infection in patients with end-organ dissemination leading to more aggressive use of antifungal agents and eventually achieving a similar rate of clearance as those without end-organ dissemination. The choice of antifungal treatment in our population was largely dependent on the clinician’s discretion, with susceptibility testing done only in a minority of cases. Routine antifungal susceptibility testing may contribute to judicious use of antifungal agents and possibly achieve higher rates of clearance.
Given the lack of randomized controlled trials on antifungal use in neonates, treatment of fungal infections in this population is mostly extrapolated from studies done in adults. In a survey conducted among members of Pediatric Infectious Disease Society and neonatologists, amphotericin B formulations were used for treatment of neonatal candidemia by the majority (80%) of respondents.18 It was also the most widely used agent in our study. With the availability of multiple antifungal agents with diverse mechanism of actions, there is potential for use of combination therapy that can broaden the overall antifungal coverage and potentially have synergistic effects. In our study, we noted a steady rise in the use of combination antifungals throughout the study period. It was interesting to note that this trend corresponded to the change in the epidemiological pattern of higher proportion of non–C albicans infection as well as with associated higher clearance. Given the retrospective nature of the study, it may be difficult to unravel the exact correlation between these observations.
The prognosis of infants with invasive Candida infections remains poor, with reported mortality rates of 40% to 50%.19-21 In our study, the primary outcome was microbiologic clearance of infection or clearance of Candida BSI; however, we did not follow the course of the infants once clearance was achieved. Nevertheless, mortality within 5 day of the last positive blood culture for Candida was found in all 26 of 158 (16%) infants who did not have documented clearance of Candida infection in their bloodstream. This maybe an overestimate of Candida-related mortality since death may have been due to other causes and may have occurred before the actual documented clearance of Candida BSI. Nonetheless, clearance rates of Candida BSI improved significantly in the latter part of the study period, and corresponded to the use of combination antifungals as well as increased infection with non–albicans sp (C parapsilosis). Further work is necessary to establish if use of combination therapy is indeed associated with better outcome.
Strengths of our study include a large study population from a single medical center. The study also encompassed 12 years of data. However, our study has several limitations including its retrospective nature; results may not be representative of other NICUs across the country. Furthermore, treatment of IFIs is often individualized and can vary between treating neonatologists in consultation with infectious diseases experts. Treatment regimen may not always adhere to established protocols and treatment guidelines. Generalization of the treatment regimen is thus met with these limitations. Moreover, we did not study other factors such as the pharmacokinetics of the antifungal drugs and the associated comorbidities, which may have confounded the clearance of Candida infections.
In summary, Candida BSI was the most common form of fungal infection seen in our NICUs. End-organ dissemination was more common among infants born ≤32 weeks of gestation. Clearance of Candida BSI was lower in extremely low birth weight infants and among those with C albicans infection. There is a trend toward an overall decline in the incidence of Candida infections in our NICU, with an increase in proportions of infection caused by non–C albicans species. This, in addition to the increased use of combination antifungal therapies, may have contributed to an improvement in the clearance of Candida BSI in the recent years. Continued surveillance with multicenter studies and larger number of patients and long-term systematic follow-up is needed to better understand the optimal management of IFIs during the neonatal period.
Author Contributions
RRA: Contributed to conception and design; contributed to acquisition, analysis, or interpretation of data; drafted manuscript; critically revised manuscript; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy.
RLA: Contributed to conception and design; contributed to acquisition, analysis, or interpretation of data; drafted manuscript; critically revised manuscript; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy.
XC: Contributed to conception and design; contributed to analysis, or interpretation of data; critically revised manuscript; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy.
JLL: Contributed to conception and design; contributed to interpretation of data; critically revised manuscript; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy.
JYA: Contributed to conception and design; contributed to interpretation of data; drafted manuscript; critically revised manuscript; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by funding from Astellas Scientific and Medical Affairs, Inc.
References
- 1. Brian Smith P, Steinbach WJ, Benjamin DK., Jr. Invasive Candida infections in the neonate. Drug Resist Updat. 2005;8:147-162. [DOI] [PubMed] [Google Scholar]
- 2. Hundalani S, Pammi M. Invasive fungal infections in newborns and current management strategies. Expert Rev Anti Infect Ther. 2013;11:709-721. [DOI] [PubMed] [Google Scholar]
- 3. Stoll BJ, Hansen N, Fanaroff AA, et al. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics. 2002;110:285-291. [DOI] [PubMed] [Google Scholar]
- 4. Kaufman D, Fairchild KD. Clinical microbiology of bacterial and fungal sepsis in very-low-birth-weight infants. Clin Microbiol Rev. 2004;17:638-680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Makhoul IR, Kassis I, Smolkin T, Tamir A, Sujov P. Review of 49 neonates with acquired fungal sepsis: further characterization. Pediatrics. 2001;107:61-66. [DOI] [PubMed] [Google Scholar]
- 6. Roilides E, Farmaki E, Evdoridou J, et al. Neonatal candidiasis: analysis of epidemiology, drug susceptibility, and molecular typing of causative isolates. Eur J Clin Microbiol Infect Dis. 2004;23:745-750. [DOI] [PubMed] [Google Scholar]
- 7. Chapman RL. Prevention and treatment of Candida infections in neonates. Semin Perinatol. 2007;31:39-46. [DOI] [PubMed] [Google Scholar]
- 8. Long SS, Stevenson DK. Reducing Candida infections during neonatal intensive care: management choices, infection control, and fluconazole prophylaxis. J Pediatr. 2005;147:135-141. [DOI] [PubMed] [Google Scholar]
- 9. Karlowicz MG, Hashimoto LN, Kelly RE, Jr, Buescher ES. Should central venous catheters be removed as soon as candidemia is detected in neonates? Pediatrics. 2000;106:e63. [DOI] [PubMed] [Google Scholar]
- 10. Fridkin SK, Kaufman D, Edwards JR, Shetty S, Horan T. Changing incidence of Candida bloodstream infections among NICU patients in the United States: 1995-2004. Pediatrics. 2006;117:1680-1687. [DOI] [PubMed] [Google Scholar]
- 11. Saiman L, Ludington E, Pfaller M, et al. Risk factors for candidemia in neonatal intensive care unit patients. The National Epidemiology of Mycosis Survey study group. Pediatr Infect Dis J. 2000;19:319-324. [DOI] [PubMed] [Google Scholar]
- 12. Xia H, Wu H, Xia S, et al. Invasive candidiasis in preterm neonates in China: a retrospective study from 11 NICUS during 2009-2011. Pediatr Infect Dis J. 2014;33:106-109. [DOI] [PubMed] [Google Scholar]
- 13. Barton M, O’Brien K, Robinson JL, et al. Invasive candidiasis in low birth weight preterm infants: risk factors, clinical course and outcome in a prospective multicenter study of cases and their matched controls. BMC Infect Dis. 2014;14:327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kossoff EH, Buescher ES, Karlowicz MG. Candidemia in a neonatal intensive care unit: trends during fifteen years and clinical features of 111 cases. Pediatr Infect Dis J. 1998;17:504-508. [DOI] [PubMed] [Google Scholar]
- 15. Benjamin DK, DeLong E, Cotten CM, Garges HP, Steinbach WJ, Clark RH. Mortality following blood culture in premature infants: increased with Gram-negative bacteremia and candidemia, but not Gram-positive bacteremia. J Perinatol. 2004;24:175-180. [DOI] [PubMed] [Google Scholar]
- 16. Aliaga S, Clark RH, Laughon M, et al. Changes in the incidence of candidiasis in neonatal intensive care units. Pediatrics. 2014;133:236-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zaoutis TE, Greves HM, Lautenbach E, Bilker WB, Coffin SE. Risk factors for disseminated candidiasis in children with candidemia. Pediatr Infect Dis J. 2004;23:635-641. [DOI] [PubMed] [Google Scholar]
- 18. Rowen JL, Tate JM. Management of neonatal candidiasis. Neonatal Candidiasis Study Group. Pediatr Infect Dis J. 1998;17:1007-1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lee BE, Cheung PY, Robinson JL, Evanochko C, Robertson CM. Comparative study of mortality and morbidity in premature infants (birth weight, < 1,250 g) with candidemia or candidal meningitis. Clin Infect Dis. 1998;27:559-565. [DOI] [PubMed] [Google Scholar]
- 20. Faix RG, Kovarik SM, Shaw TR, Johnson RV. Mucocutaneous and invasive candidiasis among very low birth weight (less than 1,500 grams) infants in intensive care nurseries: a prospective study. Pediatrics. 1989;83:101-107. [PubMed] [Google Scholar]
- 21. Adams-Chapman I, Bann CM, Das A, et al. ; Eunice Kennedy Shriver National Institutes of Child Health and Human Development Neonatal Research Network. Neurodevelopmental outcome of extremely low birth weight infants with Candida infection. J Pediatr. 2013;163:961-967. [DOI] [PMC free article] [PubMed] [Google Scholar]


