Abstract
Objective:
To evaluate the current scientific evidence for the applicability, safety and effectiveness of pathways of enhanced recovery after emergency surgery (ERAS).
Methods:
We undertook a search using PubMed and Cochrane databases for ERAS protocols in emergency cases. The search generated 65 titles; after eliminating the papers not meeting search criteria, we selected 4 cohort studies and 1 randomized clinical trial (RCT). Data extracted for analysis consisted of: patient age, type of surgery performed, ERAS elements implemented, surgical outcomes in terms of postoperative complications, mortality, length of stay (LOS) and readmission rate.
Results:
The number of ERAS items applied was good, ranging from 11 to 18 of the 20 recommended by the ERAS Society. The implementation resulted in fewer postoperative complications. LOS for ES patients was shorter when compared to conventional care.
Mortality, specifically reported in three studies, was equal or lower with ERAS. Readmission rates varied widely and were generally higher for the intervention group but without statistical significance.
Conclusions:
The studies reviewed agreed that ERAS in emergency surgery (ES) was feasible and safe with generally better outcomes. Lower compliance with some of the ERAS items shows the need for the protocol to be adapted to ES patients. More evidence is clearly required as to what can improve outcomes and how this can be formulated into an effective care pathway for the heterogeneous ES patient.
Key Words: Enhanced recovery after surgery (ERAS), Enhanced recovery, Emergency, Surgery
Introduction
Emergency Surgery (ES) is a key hospital service, with the highest proportion of cases in General Surgery. Surgical mortality is a major concern, with reports of rates as high 80% of all surgical mortality being as a result of emergency surgical interventions [1]. There are currently strong recommendations that the delivery model of ES needs to be changed in order to improve efficiency and quality of care [2]. Despite the issues being appreciated and discussed, there is uncertainty about how best to proceed. One of the proposed measures to improve outcomes has been the recommendation to implement enhanced recovery programmes (ERAS) [1,3].
ERAS programmes are evidenced-based protocols designed to standardize and optimize perioperative care in order to reduce surgical trauma, perioperative physiological stress and organ dysfunction [4]. Although published initially for colorectal surgery in 2005, they are now well established for many other surgical conditions (http://www.erassociety.org). There is already substantial evidence in the literature demonstrating the effectiveness of adopting ERAS based protocols in elective surgery [5-9], resulting in a change of clinical practice. Intuitively, ERAS could benefit ES patients due to its design to reduce surgical stress and return functional status more efficiently. The aim of our work was to evaluate the current scientific evidence for the applicability, safety and effectiveness of Enhanced Recovery pathways in ES.
Materials and Methods
Protocol and registrationThe review has been registered in PROSPERO (International prospective register of systematic reviews, http://www.crd.york.ac.uk/PROSPERO/searchadvanced.php) with the Registration number: CRD42016049268 and was reported in accordance with PRISMA statements (http://prisma-statement.org).
Eligibility criteria and search We undertook a search using PubMed and Cochrane databases for ERAS protocols in emergency cases. The search was restricted to the last 10 years in order to avoid pre ERAS guideline studies and to allow for greater homogeneity in the studies to be reviewed. No language restrictions were applied.
The following search string was used for PubMed and adapted for Cochrane: (enhanced[All Fields] AND recovery[All Fields] AND ("emergencies"[MeSH Terms] OR "emergencies"[All Fields] OR "emergency"[All Fields]) AND ("surgery"[Subheading] OR "surgery"[All Fields] OR "surgical procedures, operative"[MeSH Terms] OR ("surgical"[All Fields] AND "procedures"[All Fields] AND "operative"[All Fields]) OR "operative surgical procedures"[All Fields] OR "surgery"[All Fields] OR "general surgery"[MeSH Terms] OR ("general"[All Fields] AND "surgery"[All Fields]) OR "general surgery"[All Fields])) AND ("2006/01/01"[PDat]: "2016/10/16"[PDat] AND "humans"[MeSH Terms]).
An additional search using “fast-track” OR “multimodal” AND “emergency”, with no date restrictions, did not produce further relevant studies.
Study selection
Titles and abstracts were scrutinized; duplicates and citations were removed and full text articles of studies matching search criteria were included. Papers focused on ES that were other than abdominal were excluded. References of relevant studies were then reviewed for possible additional papers. ERAS guidelines recommend a total of 20 elements (divided into preoperative, intraoperative and postoperative) however not all of these are feasible for emergency patients and no restriction was placed on the number of elements applied as part of the protocol in each study. (Appendix 1 - Guidelines for perioperative care in elective colonic surgery: ERAS Society recommendations). After the search, study selection was independently performed by two authors (MP, LP) and disputes were resolved by discussion or the judgement of a third reviewer (PS) as to which papers should be included if required.
Quality and risk of bias assessment
Two reviewers (MZ, IMC) independently assessed the quality and risk of bias of the papers selected using SIGN levels of evidence and grades of recommendation (http://www.sign.ac.uk/methodology/checklists.html).
Data collection
Data extracted for analysis consisted of: patient age, type of surgery performed, ERAS elements implemented, surgical outcomes in terms of postoperative complications, mortality, length of stay (LOS) and readmission rate.
Results
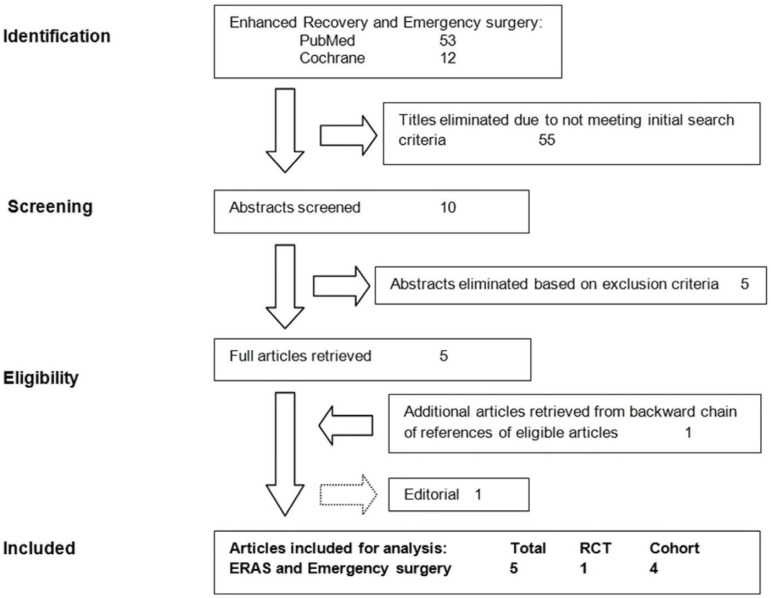
The search on ERAS and ES generated 65 titles. After eliminating the papers not meeting initial search criteria (55 papers), we selected 10 abstracts for screening; of these, 5 were eligible and one additional article was retrieved from backward chain of references. The flow chart in Figure 1 gives a summary of the article selection process.
Fig. 1.
Flow chart of the study
One of the 5 papers initially considered eligible was an Editorial, evaluated as relevant. Although this could not be used for the findings due to not providing outcome data, it was included for the discussion, offering experts’ opinion (Level 4 of evidence) [10].
Quality and risk of bias assessment is given in Table 1. Three out of the five papers were cohort studies rated as acceptable quality (level of evidence 2+), one cohort study as high (2++) and one RCT as poor quality (1-).
Table 1.
Quality and risk of bias assessment (SIGN)
| Study | Type | Overall assessment of the study | Level of evidence |
|---|---|---|---|
| Gonenc [11] | RCT | Low quality | 1- |
| Lohsiriwat [12] | Cohort | High quality | 2++ |
| Wisely [13] | Cohort | Acceptable | 2+ |
| Roulin [14] | Cohort | Acceptable | 2+ |
| Verheijen [15] | Cohort | Acceptable | 2+ |
Study characteristics
Baseline data and results from each study are shown in Table 2. The impact of ERAS on a total number of 311 emergency patients was assessed, in comparison to 605 patients consisting of 235 emergency patients receiving Conventional care (CC) and 370 elective patients receiving ERAS. Outcomes reported were based on 30 day follow up in the majority of studies. Outcomes reported by study are summarized in Table 3.
Table 2.
Baseline characteristics of selected studies
| Study |
Year |
No. of patients Intervention (Comparison) |
Age years (mean) |
Pathology / Type of surgery |
Items of ERAS applieda |
|||
|---|---|---|---|---|---|---|---|---|
| Pre. (7) | Intra. (6) | Postop. (7) | ||||||
| ERAS (CCb)all ES |
Gonenc | 2014 | 21(26) | 18-66 (35±13.2) | Perforated ulcer | 2 | 2 | 4 |
| Lohsiriwat | 2014 | 20(40) | 57.6±13.2 | Colorectal | 1 | 6 | 5 | |
| Wisely | 2014 | 201(169) | 18-95 (68 median) | Abdominal surgery | 4 | 5 | 5 | |
| ESc (Elective)all ERAS |
Roulin | 2014 | 28(63) | 18+ (64±19.5) | Colorectal | 6 | 6 | 6 |
| Verheijen | 2011 | 41 (307) | >18 (not specified) | Colorectal | 4 | 3 | 4 | |
ERAS: Enhanced Recovery after Surgery;
CC: Conventional care;
ES: Emergency surgery
In relation to Appendix 1 - Guidelines for perioperative care in elective colonic surgery: ERAS Society recommendations
Table 3.
Outcomes reported by study
| Study |
Postoperative complications
Intervention (Comparison) |
Mortality (%) Intervention (Comparison) |
Length of Hospital stay (days)
Intervention (Comparison) |
Readmission rate (%) Intervention (Comparison) |
||
|---|---|---|---|---|---|---|
| Overall (%) | Classification % | |||||
| Gonenc | 23.8 (26.9)P=0.8 | Superficial-type SSI Organ/space-type SSI Ileus Pulmonary |
0 (3.84) P=0.37 9.52 (7.69) P=0.67 9.52 (19.23) P=0.76 4.76 (15.38) P=0.48 |
0 (3.8)P=0.36 | 3.8±1.9 (6.9±2.2)(mean)P=0.0001 | 19 (7.6)P=0.47 |
| Lohsiriwat | 25 (48)P = 0.094 | Clavien-Dindo:-Grade II-V | 10 (20) P=0.47 |
0 (0) | 5.5 (7.5)(median)P=0.009 | 0 (0) |
| Wisely | No overall rate given | Major complications Minor complication |
31% overall.Significantlyless with ERAS P=0.002 79 (83) P =0.46 |
10 (10) | 8 (8)(median) | 10(8)P =0.88 |
| Roulin | 64 (51)P=0.26 | Clavien-Dindo:-Grade I-II -Grade IIIa-IVb -Grade V | 36 (38) P=0.4721 (11) P=0.27 (2) |
Not reported | 8 (5)(median)P=0.006 | 3.57 (1.58)P=0.52 |
| Verheijen | 4 (5)anastomotic leaks | - | - | 3% overall | 14 (7)(median) | 10 (10) |
Analysis of findings
Three studies compared ERAS to CC in emergency surgery (1 RCT and 2 cohort studies) [11-13]. All studies showed post-operative complication rate reduction in patients receiving ERAS, with a statistically significant reduction in major complications in one study [13].
LOS was similarly reduced by 2-3 days in 2 studies [11,12], with statistical significance and mortality rates did not increase or were improved upon (0 vs. 3.8%) [11]. In the cohort studies readmission rates were not increased by the implementation of ERAS, however in the RCT an increase from 7.6 to 19% was reported [11] although this was not considered statistically significant.
Two studies [14,15] compared emergency to elective post operative outcomes for colorectal surgery within an ERAS pathway. There was no statistically significant difference in the results between intervention and control groups for complication and readmission rates, although Verheijen et al., [15] only reported anastomotic leaks for complication rates. Mortality was not reported by either study.
Current ERAS Guidelines (Appendix 1) were used as the measure against which the enhanced recovery protocols reported to be applied in each study were evaluated. The total number of items per study ERAS protocol ranged from 8 to 18 (Table 2).
Grade of recommendation (SIGN)
Summarizing the current evidence in the studies analyzed and using SIGN Revised grading system for recommendations in evidence based guidelines (Appendix 2), grade C of recommendation was givenbased on: 1 Cohort study level 2++ and one Cohort study level 2+, comparing ERAS to CC in ES; and 2 Cohort studies level 2+ comparing ES to Elective surgery with ERAS. The RCT (level 1-) could not be used for establishing the grade of recommendation due to its a high risk of bias.
Discussion
To our knowledge this is the first review that evaluates the evidence with regard to the feasibility and effectiveness of ERAS in ES. The studies we found focusing on ERAS in ES were scarce, with two distinct comparators of either conventional care or elective surgery, different pathologies (abdominal, perforated ulcer and colorectal) and different mean ages.In addition, the heterogeneity of the scales used to report postoperative complications in the studies (Clavien-Dindo [16], major-minor complications, superficial-organ/space type SSI) and the fact that only 3 out of the five studies reported mortality rates, limited the comparative analysis between studies. Despite this, some valuable general observations could be made.
Complication rates were reduced in four out of the five studies and readmission rates were equal or not increased significantly; the exception was Gonenc et al., [11] with a higher readmission rate for the intervention group, but no explanation was offered for the difference. This could be in relation to the low LOS achieved in this group.
LOS was significantly reduced with ERAS in comparison to CC in 2 out of the 3 studies. Advance age is common in patients requiring emergency surgery and the 3 studies with higher mean age had the higher LOS; this observation, however, is not identified by the individual studies. Although LOS was measured by all five studies, it has been argued that it is not a reliable measure when evaluating the effectiveness of ERAS and that the return to functional status is a more valid one [16].
Mortality is a key issue in ESand has been specifically identified as one to be addressed. The three studies that reported mortality outcomes for both intervention and control groups found rates to be the same or better [11] with ERAS application. Co-morbidities are well known contributing factors to mortality. Two studies excluded higher risk patients, as assessed by ASA and POSSUM [12,14] which could have contributed to their low mortality rates.
The quality of the studies analysed in this review is mainly acceptable, with 3 cohort studies assessed as level 2+ and one as 2++. The only RCT was assessed as having a high risk of bias (level of evidence 1-), being non blinded, randomization being made at the end of the surgical procedure [11], employing many exclusion criteria and has been proved to deviate from clinical trial protocol [18]. The results from this study therefore need to be used with caution.
The application rate of ERAS items demonstrated in the studies analysed was between 11 [15] and 18 [14], with the exception of Gonenc et al. applying only 8 elements. The study undertaken by Verheijen et al., [15] was focused on several patient groups (emergency-elective, younger-elderly, open-laparoscopic, benign-malignant) and the reporting of ERAS elements was generic. Current ERAS guidance recommends the implementation of 20 items in order to provide a comprehensive pathway leading to better outcomes. We found no obvious correlation between the number of items applied and improved outcomes in the studies we reviewed.
It has been stated that in ES the implementation of all ERAS preoperative components may not always be feasible [3]. Pre-operative optimisation by cessation of smoking and alcohol consumption four weeks before surgery is clearly not achievable in ES cases. We found that of the seven pre-operative elements (Appendix 1), the range of implementation was from 1 to 6; from the six intra-operative items the implementation ranged from 2 to 6; and from the seven post-operative items the implementation range was from 4 to 6. We also observed that there was variability in the way some elements were applied, most notably for early post operative oral feeding and mobilisation.
Although we did not analyse the possible correlation between specific elements applied in the studies reviewed and their outcomes, the impact of individual elements of an ERAS programme on post-operative results have been undertaken. The ERAS study group[19]identified 2 key elements which had an independent positive impact on post operative outcomes: perioperative intravenous fluid management and preoperative carbohydrate treatment and Brandstrup et al.,[20] has demonstrated the important impact of fluid management on post-operative outcomes; however neither of these studies was specific to ES.The trial undertaken by Gonenc et al.,[11] stated that 3 key elements produced better outcomes in their intervention group: non NGT usage, early oral feeding and use of NSAIDs. More research is still needed in relation to identifying which elements of ERAS might have greater impact and whether individual influence plays a more significant role than the number of elements applied. It is also necessary to consider how these factors might vary in emergency surgery and with different patient groups within it.
A separate issue to the application of ERAS items is whether patients are able to comply with individual element application. Only one study in our review looked specifically at patient compliance to elements of ERAS; Roulin et al., [14] reported an overall patient compliance of 57% in ES. This was compared to 77% in elective ERAS patients. Difficulties identified were: pre-operative carbohydrate loading, NGT early removal/non routine use, postoperative fluid management, nutrition and early mobilization. However, the difference was no longer evident from the first postoperative day and functional recovery was similar in both ES and elective patients following an ERAS pathway [14]. Wisely et al.,[13] identified the ERAS elements considered to be appropriate for ES patients. Based on their study findings, most elements were considered appropriate in varying degrees. Laparoscopic surgery, avoiding resection‐site drain and general anaesthetic ± epidural anaesthesia was identified as appropriate only for some ES patients [13]. These findings could provide a baseline for further investigation for ES patients.The main limitation of this review is the heterogeneity and quality of the studies evaluated. This is due to the fact that there is little information on ERAS programmes in ES and therefore we did not eliminate any of the studies which met our inclusion criteria. Quiney et al., [10] attributed the small number of studies evaluating the impact of ERAS in ES partially to the difficulty to apply many of the ERAS principles. However this difficulty does not prohibit the use of evidence-based practice, on which ERAS is based.
In conclusion, the studies reviewed agreed that ERAS in ES was feasible and safe with generally better outcomes, but needs to be adapted for this patient group as compliance with all ERAS elements can be difficult to achieve. A tailored ERAS pathway would better serve this population along with a multidisciplinary team approach. The limited number of trials and studies focusing on ERAS in ES clearly indicates that this is still a new area to explore. More evidence is required as to what can improve outcomes and how this can be formulated into an effective care pathway for the heterogeneous ES patient.
Acknowledgments
Melanie Radcliff, BA, for assisting with the English translation.
Appendix I
Guidelines for perioperative care in elective colonic surgery: Enhanced Recovery After Surgery (ERAS®) Society recommendations.
| Item | Recommendation | Evidence level | Recommendation grade |
|---|---|---|---|
| Preoperative information, education and counselling | Patients should routinely receive dedicated preoperative counselling. | Low | Strong |
| Preoperative optimisation | Preoperative medical optimisation is necessary before surgery. | Alcohol: Low | Strong |
| Smoking and alcohol consumption (alcohol abusers) should be stopped four weeks before surgery. | Smoking: High | ||
| Preoperative bowel preparation | Mechanical bowel preparation should not be used routinely in colonic surgery. | High | Strong |
| Preoperative fasting and carbohydrate treatment | Clear fluids should be allowed up to 2 h and solids up to 6 h prior to induction of anaesthesia. | Solids and fluids: Moderate | Fasting guidelines: Strong |
| Preoperative oral carbohydrate treatment should be used routinely. In diabetic patients carbohydrate treatment can be given along with the diabetic medication. | Carbohydrate loading, overall: Low | Preoperative carbohydrate drinks: Strong | |
| Carbohydrate loading, diabetic patients: Very low | Preoperative carbohydrate drinks, diabetic patients: Weak | ||
| Preanaesthetic medication | Patients should not routinely receive long- or short-acting sedative medication before surgery because it delays immediate postoperative recovery. | High | Strong |
| Prophylaxis against thromboembolism | Patients should wear well-fitting compression stockings, have intermittent pneumatic compression, and receive pharmacological prophylaxis with LMWH. Extended prophylaxis for 28 days should be given to patients with colorectal cancer. | High | Strong |
| Antimicrobial prophylaxis and skin preparation | Routine prophylaxis using intravenous antibiotics should be given 30–60 min before initiating surgery. Additional doses should be given during prolonged operations according to half-life of the drug used. Preparation with chlorhexidine-alcohol should be used. |
High | Strong |
| Standard anaesthetic protocol | A standard anaesthetic protocol allowing rapid awakening should be given. | Rapid awakening: Low | Strong |
| The anaesthetist should control fluid therapy, analgesia and haemodynamic changes to reduce the metabolic stress response. | Reduce stress response: Moderate | ||
| Open surgery: mid-thoracic epidural blocks using local anaesthetics and low-dose opioids. | Open surgery: High | ||
| Laparoscopic surgery: spinal analgesia or morphine PCA is an alternative to epidural anesthesia. | Laparoscopic surgery: Moderate | ||
| PONV | A multimodal approach to PONV prophylaxis should be adopted in all patients with ≥2 risk factors undergoing major colorectal surgery. If PONV is present, treatment should be given using a multimodal approach. |
Low | Strong |
| Laparoscopy and modifications of surgical access | Laparoscopic surgery for colonic resections is recommended if the expertise is available. | Oncology: HighMorbidity: LowRecovery/LOSH: Moderate | Strong |
| Nasogastric intubation | Postoperative nasogastric tubes should not be used routinely.Nasogastric tubes inserted during surgery should be removed before reversal of anaesthesia. | High | Strong |
| Preventing intraoperative hypothermia | Intraoperative maintenance of normothermia with a suitable warming device and warmed intravenous fluids should be used routinely to keep body temperature >36 °C. | High | Strong |
| Perioperative fluid management | Patients should receive intraoperative fluids (colloids and crystalloids) guided by flow measurements to optimise cardiac output. | Balanced crystalloids: High | Strong |
| Vasopressors should be considered for intra- and postoperative management of epidural-induced hypotension provided the patient is normovolaemic. | Flow measurement in open surgery: High | ||
| The enteral route for fluid postoperatively should be used as early as possible, and intravenous fluids should be discontinued as soon as is practicable. | Flow measurement in other patients: Moderate | ||
| Vasopressors: High Early enteral route: High | |||
| Drainage of peritoneal cavity after colonic anastomosis | Routine drainage is discouraged because it is an unsupported intervention that is likely to impair mobilisation. | High | Strong |
| Urinary drainage | Routine transurethral bladder drainage for 1–2) days is recommended. | Low | Routine bladder drainage: Strong |
| The bladder catheter can be removed regardless of the usage or duration of thoracic epidural analgesia. | Early removal if epidural used: Weak | ||
| Prevention of postoperative ileus | Mid-thoracic epidural analgesia and laparoscopic surgery should be utilised in colonic surgery if possible. | Thoracic epidural, laparoscopy: High | Thoracic epidural, fluid overload, nasogastric decompression, chewing gum and alvimopan: Strong |
| Fluid overload and nasogastric decompression should be avoided. | Chewing gum: Moderate | Oral magnesium: Weak |
|
| Chewing gum can be recommended, whereas oral magnesium and alvimopan may be included. | Oral magnesium, alvimopan: Low | ||
| Postoperative analgesia | Open surgery: TEA using low-dose local anaesthetic and opioids.Laparoscopic surgery: an alternative to TEA is a carefully administered spinal analgesia with a low-dose, long-acting opioid. | TEA, open surgery: HighLocal anaesthetic and opioid: ModerateTEA not mandatory in laparoscopic surgery: Moderate | Strong |
| Perioperative nutritional care | Patients should be screened for nutritional status and if at risk of under-nutrition given active nutritional support. | Postoperative early enteral feeding, safety: High | Postoperative early feeding and perioperative ONS: Strong |
| Perioperative fasting should be minimised. Postoperatively patients should be encouraged to take normal food as soon as lucid after surgery. | Improved recovery and reduction of morbidity: Low | IN could be considered in open colonic resections: Weak | |
| ONS may be used to supplement total intake. | Perioperative ONS (well-fed patient): Low Perioperative ONS (malnourished patient): Low IN: Low |
||
| Hyperglycaemia is a risk factor for complications and should therefore be avoided. | Using stress reducing elements of ERAS to minimise hyperglycaemia: Low | Using stress reducing elements of ERAS to minimise hyperglycaemia: Strong | |
| Postoperative glucose control | Several interventions in the ERAS protocol affect insulin action/resistance, thereby improving glycaemic control with no risk of causing hypoglycemia. | Insulin treatment in the ICU: Moderate | Insulin treatment in the ICU (severe hyperglycaemia): Strong |
| For ward-based patients, insulin should be used judiciously to maintain blood glucose as low as feasible with the available resources. | Glycaemic control in the ward setting: Low | Insulin treatment in ICU (mild hyperglycaemia): Weak | |
| Insulin treatment in the ward setting: Weak | |||
| Early mobilisation | Prolonged immobilisation increases the risk of pneumonia, insulin resistance and muscle weakness. Patients should therefore be mobilised. | Low | Strong |
U. O. Gustafsson, M. J. Scott, W. Schwenk, N. Demartines, D. Roulin, N. Francis, et al. - Guidelines for Perioperative Care in Elective Colonic Surgery: Enhanced Recovery After Surgery (ERAS) Society Recommendations, World J Surg (2013) 37:259–284
Appendix II
SIGN Levels of Evidence and Grades of Recommendation
Levels of evidence
1++
High quality meta-analyses, systematic reviews of RCTs, or RCTs with a very low risk of bias
1+
Well conducted meta-analyses, systematic reviews of RCTs, or RCTs with a low risk of bias
1−
Meta-analyses, systematic reviews or RCTs, or RCTs with a high risk of bias
2++
High quality systematic reviews of case-control or cohort studies or
High quality case-control or cohort studies with a very low risk of confounding, bias, or chance and a high probability that the relationship is causal
2+
Well conducted case-control or cohort studies with a low risk of confounding, bias, or chance and a moderate probability that the relationship is causal
2−
Case-control or cohort studies with a high risk of confounding, bias, or chance and a significant risk that the relationship is not causal
3
Non-analytic studies, eg case reports, case series
4
Expert opinion
Grades of recommendations
A
At least one meta-analysis, systematic review, or RCT rated as 1++ and directly applicable to the target population or
A systematic review of RCTs or a body of evidence consisting principally of studies rated as 1+ directly applicable to the target population and demonstrating overall consistency of results
B
A body of evidence including studies rated as 2++ directly applicable to the target population and demonstrating overall consistency of results or
Extrapolated evidence from studies rated as 1++ or 1+
C
A body of evidence including studies rated as 2+ directly applicable to the target population and demonstrating overall consistency of results or
Extrapolated evidence from studies rated as 2++
D
Evidence level 3 or 4 or
Extrapolated evidence from studies rated as 2+
http://www.sign.ac.uk/guidelines/fulltext/50/annexoldb.html.
Conflicts of Interest:
None declared.
References
- 1.Advancing Surgical Standards (ASS) Emergency surgery policy briefing. The Royal College of Surgeons UK: [(September, 2014)]. Available from: https://www.rcseng.ac.uk/-/media/files/.../rcs-emergency-surgery-policy-briefing.pdf. [Google Scholar]
- 2.Association of Coloproctology of Great Britain and Ireland (AUGIS); Association of Upper Gastro-intestinal Surgeons & Association of Surgeons of Great Britain and Ireland. The future of Emergency general surgery: A joint document. 2015. Available from: http://www.augis.org/wp-content/uploads/2014/05/Future-of-EGS-joint-document_Iain-Anderson_140915.pdf.
- 3.Khan S, Gatt M, Horgan A, Anderson I, MacFie J. Guidelines for implementation of enhanced recovery protocols. Issues in Professional Practice. 2009 [Google Scholar]
- 4.Kehlet H, Wilmore DW. Fast-track surgery. Br J Surg. 2005;92(1):3–4. doi: 10.1002/bjs.4841. [DOI] [PubMed] [Google Scholar]
- 5.Varadhan KK, Neal KR, Dejong CH, Fearon KC, Ljungqvist O, Lobo DN. The enhanced recovery after surgery (ERAS) pathway for patients undergoing major elective open colorectal surgery: a meta-analysis of randomized controlled trials. Clin Nutr. 2010;29(4):434–40. doi: 10.1016/j.clnu.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 6.Adamina M, Kehlet H, Tomlinson GA, Senagore AJ, Delaney CP. Enhanced recovery pathways optimize health outcomes and resource utilization: a meta-analysis of randomized controlled trials in colorectal surgery. Surgery. 2011;149(6):830–40. doi: 10.1016/j.surg.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 7.Wind J, Polle SW, Fung Kon Jin PH, Dejong CH, von Meyenfeldt MF, Ubbink DT, et al. Systematic review of enhanced recovery programmes in colonic surgery. Br J Surg. 2006;93(7):800–9. doi: 10.1002/bjs.5384. [DOI] [PubMed] [Google Scholar]
- 8.Zhuang CL, Ye XZ, Zhang XD, Chen BC, Yu Z. Enhanced recovery after surgery programs versus traditional care for colorectal surgery: a meta-analysis of randomized controlled trials. Dis Colon Rectum. 2013;56(5):667–78. doi: 10.1097/DCR.0b013e3182812842. [DOI] [PubMed] [Google Scholar]
- 9.Gouvas N, Tan E, Windsor A, Xynos E, Tekkis PP. Fast-track vs standard care in colorectal surgery: a meta-analysis update. International journal of colorectal disease. 2009;24(10):1119–31. doi: 10.1007/s00384-009-0703-5. [DOI] [PubMed] [Google Scholar]
- 10.Quiney N, Aggarwal G, Scott M, Dickinson M. Survival After Emergency General Surgery: What can We Learn from Enhanced Recovery Programmes? World J Surg. 2016;40(6):1283–7. doi: 10.1007/s00268-016-3418-0. [DOI] [PubMed] [Google Scholar]
- 11.Gonenc M, Dural AC, Celik F, Akarsu C, Kocatas A, Kalayci MU, et al. Enhanced postoperative recovery pathways in emergency surgery: a randomised controlled clinical trial. Am J Surg. 2014;207(6):807–14. doi: 10.1016/j.amjsurg.2013.07.025. [DOI] [PubMed] [Google Scholar]
- 12.Lohsiriwat V. Enhanced recovery after surgery vs conventional care in emergency colorectal surgery. World J Gastroenterol. 2014;20(38):13950–5. doi: 10.3748/wjg.v20.i38.13950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wisely JC, Barclay KL. Effects of an Enhanced Recovery After Surgery programme on emergency surgical patients. ANZ J Surg. 2016;86(11):883–8. doi: 10.1111/ans.13465. [DOI] [PubMed] [Google Scholar]
- 14.Roulin D, Blanc C, Muradbegovic M, Hahnloser D, Demartines N, Hubner M. Enhanced recovery pathway for urgent colectomy. World J Surg. 2014;38(8):2153–9. doi: 10.1007/s00268-014-2518-y. [DOI] [PubMed] [Google Scholar]
- 15.Verheijen PM, Vd Ven AW, Davids PH, Vd Wall BJ, Pronk A. Feasibility of enhanced recovery programme in various patient groups. Int J Colorectal Dis. 2012;27(4):507–11. doi: 10.1007/s00384-011-1336-z. [DOI] [PubMed] [Google Scholar]
- 16.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–13. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maessen JM, Dejong CH, Kessels AG, von Meyenfeldt MF. Length of stay: an inappropriate readout of the success of enhanced recovery programs. World J Surg. 2008;32(6):971–5. doi: 10.1007/s00268-007-9404-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soreide K. Enhanced recovery in emergency surgery: validity and generalizability of a randomized trial. Am J Surg. 2015;210(3):598–9. doi: 10.1016/j.amjsurg.2014.11.020. [DOI] [PubMed] [Google Scholar]
- 19.Gustafsson UO, Hausel J, Thorell A, Ljungqvist O, Soop M, Nygren J. Adherence to the enhanced recovery after surgery protocol and outcomes after colorectal cancer surgery. Arch Surg. 2011;146(5):571–7. doi: 10.1001/archsurg.2010.309. [DOI] [PubMed] [Google Scholar]
- 20.Brandstrup B, Tonnesen H, Beier-Holgersen R, Hjortso E, Ording H, Lindorff-Larsen K, et al. Effects of intravenous fluid restriction on postoperative complications: comparison of two perioperative fluid regimens: a randomized assessor-blinded multicenter trial. Ann Surg. 2003;238(5):641–8. doi: 10.1097/01.sla.0000094387.50865.23. [DOI] [PMC free article] [PubMed] [Google Scholar]