Abstract
Purpose:
The purpose of this review is to provide a summary of the rationale for engaging patients in research as well as to review the established and envisioned advantages and strategies for patient-researcher partnerships. The authors of this article, which include a patient and 4 researchers in kidney disease, discuss the expected benefits and opportunities for patient engagement in their respective research programs. The 4 research programs span the spectrum of kidney disease and focus on enhancing bone health, increasing living donor kidney transplants, improving medication adherence, and preventing kidney transplant rejection.
Sources of Information:
The sources of information for this review include published studies on the topics of patient engagement and the 4 research programs of the new investigators.
Key Findings:
(1) Patient, health care provider, and researcher partnerships can contribute useful insights capable of enhancing research in kidney disease. (2) Regardless of the research program, there are various strategies and opportunities for engagement of patients with lived experience across the various stages of research in kidney disease. (3) Envisioned advantages of patient-researcher partnerships include: targeting patient-identified research priorities, integrating patients’ experiential knowledge, improving study design and feasibility through patient-researcher input, facilitating dissemination of research findings to other patients, effectively responding to patient concerns about studies, and inspiring researchers to conduct their research.
Limitations:
The limitations of the current review include the relative scarcity of literature on patient engagement within the field of kidney disease.
Implications:
The findings of the current review suggest that it will be important for future studies to identify optimal strategies for patient engagement in setting research priorities, study design, participant recruitment, execution of research projects, and knowledge dissemination and translation.
Keywords: antibody-mediated rejection, living kidney donation, medication adherence, health literacy, mineral bone disease, patient engagement, patient partnership, patient experiential knowledge, KRESCENT Program
Abrégé
Objet:
Le but de cet article synthèse est de fournir un résumé des raisons justifiant de faire participer les patients à la recherche. On veut également examiner les avantages établis et envisagés, de même que les stratégies de partenariats patients-chercheurs. Les auteurs de cet article, un patient et quatre chercheurs dans le domaine des maladies rénales, discutent des bénéfices espérés et des débouchés attendus de l’implication des patients dans leurs programmes de recherche respectifs. Les quatre programmes de recherche étudiés couvrent un spectre étendu dans le domaine des maladies du rein, et se concentrent sur l’amélioration de la santé osseuse, l’augmentation du nombre de greffes provenant de donneurs vivants, l’amélioration de l’observance à la médication et la prévention du rejet de la greffe.
Sources:
Les sources consultées comprennent les recherches publiées sur le thème de la participation des patients en recherche et sur les quatre programmes de recherche des chercheurs participants (amélioration de la santé osseuse, augmentation du nombre de greffes provenant de donneurs vivants, amélioration de l’observance à la médication et prévention du rejet de greffe).
Principales conclusions:
(1) Les partenariats entre les patients, les professionnels de la santé et les chercheurs peuvent apporter de précieuses informations susceptibles de faire avancer la recherche sur les maladies rénales. (2) Peu importe le programme de recherche, il existe plusieurs stratégies et possibilités pour encourager la participation de patients et le partage de leur expérience lors des différentes étapes de la recherche sur les maladies rénales. (3) On discute des nombreux avantages attendus des partenariats patients-chercheurs, notamment le ciblage des priorités de recherche établies par les patients, l’intégration des connaissances tirées de l’expérience des patients, l’amélioration de la conception et de la faisabilité des études par les apports des patients et des chercheurs, la diffusion facilitée des résultats de la recherche auprès des autres patients, la réponse efficace aux soucis des patients en regard des études, et la source de motivation fournie aux chercheurs pour la poursuite de leurs études.
Limites de l’étude:
Les résultats sont limités par le fait qu’il existe peu de recherches ayant porté sur la participation des patients à la recherche sur les maladies rénales.
Implications:
Les résultats de cette étude suggèrent qu’il sera important pour les études ultérieures de définir les stratégies optimales favorisant la participation des patients lors de l’établissement des priorités de recherche, de la conception de l’étude, du recrutement des participants, de l’exécution des projets de recherche et au moment de la diffusion et du transfert des connaissances.
What was known before
There is an increasing acknowledgment that engaging patients in the research process enhances the relevance of research to patients and the quality of care ultimately delivered.
What this adds
We review the limited literature on patient engagement in kidney research and suggest specific strategies and opportunities for engaging patients in future research in enhancing bone health in chronic kidney disease, increasing living donor kidney transplants, improving medication adherence, and preventing kidney transplant rejection.
Introduction
The Canadian Strategy for Patient-Oriented Research (SPOR)1 and the American Patient-Centered Outcomes Research Institute (PCORI)2 recognize that patient engagement will form an integral part of the future of health research. In Canada, specifically for kidney disease, the Canadians Seeking Solutions and Innovations to Overcome Chronic Kidney Disease (Can-SOLVE CKD) initiative was launched as a nationwide partnership of patients, researchers, health care providers, and key stakeholders. The aim of this initiative is to perform patient-oriented research (which includes engaging patients in research) to ultimately improve the delivery of care for patients with chronic kidney disease (CKD).
Commonly cited advantages for seeking patient engagement in research include the need to harness health care practices to study outcomes,3 respond to patient-identified health priorities,4 and increase return on investment in health research.5 It is becoming clear that patients need to be involved in health care research.6 Cukor and colleagues7 recently reported on 7 studies to demonstrate what meaningful patient engagement in clinical research on kidney disease could include. They concluded that by having patients and investigators work together, it is possible to refine the selection of research questions and outcomes to be studied and implement changes identified by research into clinical management.7
The term patient-researcher partnership designates the mode in which patients are engaged in research, which implies that each partner contributes something of equal value to the common enterprise. It encompasses more than having patients engaged as study participants. Furthermore, it is important to make a distinction between patients as research partners and patients as participants in surveys or focus groups. In the latter, patients are participants and their feedback and discussions serve to answer the research question. In patient-research partnerships, patients’ input is sought to direct the various phases (preparatory, execution, and translational) of the research project.8
While identifying the nature of patients’ contribution to research remains a challenge,9 many authors suggest that patient-researcher partnerships have a positive impact on health care and study outcomes because patients’ experiential knowledge about their illness can provide investigators with unique insights into protocol design, implementation, and knowledge translation.1,10-12 Although patient engagement is relatively new, there are ways in which patients can be engaged as partners in health research.12 First, patients can participate in setting research priorities,13 and thus enhance relevance of patient outcomes, an initiative which has already taken place for patients on or nearing dialysis treatment.14 Second, patients can help develop study protocols and consent forms, which are highly comprehensible to patients, and in turn, facilitate recruitment of study participants and improve the efficiency of data collection. Finally, patients can help disseminate information to study participants and efficiently respond to their questions about the trial and its procedures.2 However, these examples have yet to be generalized within the wider health research community.
Although many patients have been engaged in research priority setting initiatives, a recent review determined that 80% of available clinical research does not address the top 10 research priorities established by patients on or nearing dialysis.15 Specifically, 4 of the top 10 research priorities determined through a Canadian survey and initiative were not represented in the journals reviewed, including management of pruritus, increasing access to transplantation, addressing the psychosocial impact of kidney failure, and the effects of dietary restriction.15 The authors suggest that insufficient patient involvement across the various stages of research is likely the main reason for this discrepancy. It should be noted that the review conducted a search of only 15 top nephrology and medical journals, and it is therefore possible that research meeting the above priorities was published in other lower impact journals and those from other disciplines.15 The authors cite several advantages to aligning priorities of researchers with those of patients including the principal of fairness (addressing issues that patients see as problematic since they are the most impacted stakeholders), facilitation of translation of research to clinical practice, and facilitation of funding by the public.15 Although current initiatives such as SPOR1 and PCORI2 are likely to increase the amount of research that targets patient-identified research priorities, it is important to note that there is a place for research outside of patient-identified priorities, which contributes to innovation and important discoveries that may similarly have a real impact on patient quality of life.15 Nonetheless, the results of this systematic review were concerning and suggested that greater involvement of patients across all study stages, especially at the proposal stage, is needed.
Although it is true that patients’ own stories can be powerful sources of inspiration and help remind investigators why and for whom they are conducting research, it is only one part of patients’ contribution to the process. The patient-author of this article, a researcher in the field of education, is currently engaged in four health research projects as a patient expert. Although it is straightforward to carry out operational roles (patient recruitment, focus group facilitation, etc.), he is aware that conveying insights on study priorities and developing relevant questions requires more than a lived experience as a patient. This has little to do with openness on behalf of the researchers and implies analyzing one’s experience so as to focus on broader issues regarding living with the disease. This does not happen quickly without external help and necessitates that strategies for engaging patients in research be developed.
Strategies for Patient Engagement in Research
One such strategy is to “emancipate” patients from such labels such as the “interstitial nephritis patient” or “kidney transplant patient.” These tend to reinforce the idea that patients only contribute their personal experience, which does little to advance the field of work. The “emancipation” therefore hinges upon the development of an analytical perspective toward patients’ own experience and disease,16 which allows for joint development of research priorities and questions.12
Joint training models, such as that of Marlett and colleagues’ study,16 identify four stages for the development of a patient-researcher partnership. The stages are as follows: (1) gaining competence to take on appropriate roles as patients and as researchers (which involves providing joint training to patients and researchers with regard to methodologies and infrastructure such as funding, ethics, career development, etc.); (2) applying the emerging partnership competency to advancing the project; (3) blending the roles of a patient and researcher; and finally (4) “seeding change” by identifying opportunities for future integration of patients’ voice and experience in research.16 In this view, patient engagement implies important changes to research practices, such that patient-oriented research can then represent “collaborative research done by, with, and for patients to inform health care and health research decisions and questions.”16 Future research can develop instruments based on frameworks to evaluate whether patients have been engaged across the research continuum. Such instruments could be completed by patients and researchers who collaborated on the same study to allow for a comparison of the degree of patient engagement from both perspectives. This would help both patients and researchers reflect on the partnership that occurred, any discrepancies bet-ween initial engagement aims and the actual degree of participation, as well as on the barriers to and advantages of patient engagement.
Patient Engagement Within Kidney Research Programs in Canada
In this article, a patient-researcher and four new investigators discuss how patient engagement can be implemented strategically and effectively in their respective research programs. These four research programs address outcomes that cause significant morbidity in patients suffering from kidney disease, including the enhancement of mineral and bone health, increase in living donor kidney transplants, improvement in medication adherence, and the prevention of kidney transplant rejection by optimizing donor-recipient compatibility. Not surprisingly, the outcomes of each of these programs are as important to patients as they are to researchers.
Mineral and Bone Disorders in CKD
Mineral and bone disorders (MBD) in patients with CKD can lead to increased fractures, vascular morbidities, and mortality.17-19 Traditionally, the focus has been on decreasing the parathyroid hormone–induced bone turnover that contributes to bone loss and fragility.20 However, despite optimizing parathyroid hormone levels in patients with CKD, mineral and bone complications still remain highly prevalent in this population.21 Therefore, research aimed at new mechanisms leading to MBD in patients with CKD is needed.
Literature discussing the role of patient engagement in the study of MBD in CKD is currently nonexistent. There are, however, multiple opportunities to engage patients in innovative research strategies and improve our understanding of MBD. Moreover, patients can help with knowledge dissemination and translation by informing other CKD patients on risk factors for bone disease. For example, given their own experiences, patients can design educational materials that can best inform other patients on the importance of mineral and bone dysregulation, in particular when CKD progresses from predialysis to end-stage renal disease (ESRD). Patients can present at conferences and highlight gaps in knowledge on the pathophysiology and therapy of bone disease in CKD. This would increase awareness of their disease and its complications in a public forum.
One of the major challenges faced by researchers and health care providers in CKD-MBD is related to the fact that gold standard iliac crest biopsies are considered too invasive by many health care providers.22 One strategy to overcome this barrier is to have interventional radiologists perform the procedure under ultrasound-guidance, thus optimizing the diagnostic yield while minimizing patient complications. Whether this strategy will indeed be acceptable and whether radiology-assisted iliac crest biopsies will enhance CKD-MBD research also need to be studied. Having patients’ input and help in designing and implementing a program of radiology-assisted iliac crest biopsies is clearly an opportunity for patient engagement in CKD-MBD research. More specifically, the opinions on the intervention of patients with an indication for iliac crest biopsy could be sought. Information regarding patients’ perception of the technique’s invasiveness or inconvenience could be gathered once the intervention has been performed. Thereafter, these patients could participate in future research protocols that include iliac crest biopsies to better understand the pathophysiology of CKD-MBD. Eventually, these patients could be part of the nephrologists’ research team for enhancement of their bone health. Finally, when seeking to prevent and treat MBD, patients with CKD may be more influential in educating other patients on the importance of lifestyle choices and adherence to recommended treatments. Patients could adapt research findings on MBD and make them more comprehensible to a wider patient audience. They could help design instructional material for other patients on strategies to control MBD complications (ie, diet restriction, adherence to phosphate chelators, or vitamin D treatments).
These specific strategies could lead to significant improvements in patients’ quality-of-life and in maintaining their daily activities, which are top priorities for ESRD and hemodialysis patients.23 Having patients participate throughout the research program by developing new research protocols will improve the quality and relevance of MBD management in patients with CKD.
Living Kidney Donation
For patients with ESRD, kidney transplantation is associated with improved long-term survival and better quality-of-life compared with the alternative treatment of dialysis.24,25 Compared with kidneys from deceased donors, kidneys from living donors have superior patient and graft survival.26 Unfortunately, the need for an organ transplant has been increasing and many patients with ESRD die while on the deceased donor waiting list. Improving the rates of living donor kidney transplantations is one strategy to help meet this growing demand for available organs.27
Patient involvement in this area of research has been primarily through surveys, interviews, and focus groups to better understand the barriers that potential living kidney donors face throughout the evaluation and donation process.28-34 These studies have provided invaluable information on the experiences of potential living kidney donors with the health care system. They have also generated possible interventions to improve the efficiency and lower the costs of the donor evaluation process. These studies provide insights into how these issues may be personalized to meet the specific barriers faced by various donors of different ages, genders, race/ethnicity, socioeconomic status, and education.33,35,36 Recently, there has been increasing interest in engaging patients in research surrounding living kidney donation. Patients, including living kidney donor candidates and previous living kidney donors and their respective friends and family, can be actively involved in research, not only as study participants, but as scientific partners. Having a donor-centered approach to living kidney donation may help physicians and health care providers better understand donors’ tolerance for risk and motivations for donating.37 Their unique perspective can help guide researchers toward the key issues facing donors, and their involvement can complement and enhance interventional strategies.
Research in understanding the barriers to living kidney donation and safely increasing living donor kidney transplantation rates features prominently in the Can-SOLVE CKD initiative. While the first 2 key areas involve the early diagnosis of CKD and treatment of CKD, the third priority of the Can-SOLVE initiative is to increase living donor kidney transplantation. In all three areas, the goal is to integrate patient engagement throughout the entire process.
Patients and their families have been an integral part of the Can-SOLVE CKD initiative. As members of this collaborative team, their input and suggestions have guided the overall objectives of this research program. Patient input on this initiative has identified 2 main areas for which interventions can be targeted. The first is to develop personalized educational materials to help potential transplant candidates explore the option of living donor kidney transplantation. The second is to improve efficiency in the potential living kidney donor evaluation process. Researchers have incorporated patients into every step of this initiative. In addition to developing research priorities, patients and their families can guide protocol development by providing a unique perspective on feasibility and acceptability. Developing gender-specific as well as racially sensitive and culturally sensitive strategies, including those related to the Canadian Indigenous population, will require patient input and opinion. Patient voice, stemming from lived experience of CKD, is seen as key component to the strategies’ success. Patients can also assist in the recruitment of study participants and even be part of the intervention itself. For example, one potential intervention to improve the living donor evaluation process is the involvement of a donor advocate—someone who is easily accessible to living donor candidates and who can answer important questions about the evaluation and/or donation process. A previous living kidney donor and/or their family member would be an ideal donor advocate for future living donor candidates as they have been through the experience themselves and can provide their unique perspective on how to navigate through the evaluation process most efficiently and what to expect from the donor testing, such as a renal nuclear medicine scan. Improving donor evaluation efficiency and patient satisfaction may lead to an increase in living donor kidney transplants, which is a Canadian research priority. These priorities also align with a recent consensus conference held in 2014 in the United States, which also included patients as members of the committee.38
Medication Adherence and Health Literacy in Kidney Transplant Recipients
Although many risk factors for immunosuppressant nonadherence in kidney transplant recipients have been identified,39-42 several of these are not amenable to interventions. Health literacy, which is defined as one’s ability to access, process, and understand health-related information and services to make good health decisions43 is a modifiable risk factor for which interventional strategies should be developed. In Canada, 60% of individuals lack sufficient health literacy skills.44 Many studies have demonstrated that lower health literacy is a risk factor for worse patient outcomes, including those with kidney disease.45-55 Emerging research seeks to better understand the relationship between a potentially amendable factor, like health literacy, and medication adherence with the eventual goal of developing health literacy-based interventions to improve adherence in kidney transplant recipients.56
Patient engagement in the research of medication adherence in kidney transplant patients is sparse. It is limited to focus groups discussing reasons for nonadherence from patients’ perspective as well as eliciting patient feedback on a mobile medication adherence assessment tool.57,58 Going forward, there are various opportunities for engaging patients along the stages of the development of a health-literacy intervention aimed at improving medication adherence. Usually, the earliest that a patient is exposed to and permitted the opportunity to offer his or her feedback on an intervention is at the pilot study stage. By this point, however, many of the intervention’s characteristics have already been decided upon by the researchers and may be difficult to amend. Many of the questions which interventional pilot studies seek answers for, such as general practicality, feasibility of used measures and intervention, recruitment success rate, and patient acceptance may largely be addressed by the inclusion of a patient as a research collaborator in the design of an interventional study.
In recruiting patient research partners, kidney researchers may seek recommendations of health care providers for potentially interested patients or invite patients with an interest in research on medication-non adherence to serve as research collaborators/advisors. Researchers may also wish to make use of online research-focused forums for kidney transplant recipients to access the voice and feedback of a larger group of patients. The aim would be to develop interventions, which are not merely scientifically sound, but which are also feasible, practical, and acceptable to patients.
Providing patients with an introduction to the research project by presenting existing scientific literature on the link between health literacy and medication adherence will help to inform patients’ subsequent provision of feedback on the design of the intervention. It may also aid patient-research collaborators in spreading awareness of the intervention to other patients and at the conclusion of the study motivate the dissemination of findings in a meaningful manner to the greater community of kidney transplant recipients.
Most importantly, however, patients would collaborate with researchers in the choice of measures and the delivery method particulars of the intervention. In this case, patients would be engaged in selecting among various measures of health literacy and medication adherence that are available. Patient preferences may be based on factors (readability, length, resources required, invasiveness), which may otherwise be in the researcher’s blind sight. Patient study partners may help in advising researchers on the number and duration of sessions as well as the delivery mode of intervention (eg, phone, group, individual, electronic, face-to-face) which is most acceptable to them.
Patient engagement at the implementation level would involve piloting the intervention with the patient research partner and further eliciting and incorporating patient feedback into its final design. In summary, the active inclusion of patients’ feedback on the design of an intervention to improve medication adherence is anticipated to improve the feasibility, acceptability, and practicality of an intervention, which would likely facilitate patient recruitment, participation, and dissemination of study findings to kidney transplant peers.
Donor-Recipient Compatibility and Prevention of Antibody-Mediated Rejection
Although kidney transplantation improves survival in comparison with dialysis,24 graft loss is associated with increased mortality,59 decreased quality-of-life, and increased health care expenditures.60 Antibody-mediated rejection (ABMR) is now widely recognized as the leading cause for premature graft loss.61 ABMR occurs when kidney transplant recipients develop donor-specific antibodies (DSA) to human leukocyte antigens (HLA) on transplanted organs.62-68 To prevent ABMR, organ allocation schemes promote transplantation from blood type and HLA-compatible donors, and kidney transplant recipients are prescribed lifelong immunosuppression.
There is a body of evidence showing that kidney transplant recipients have a strong focus on graft survival, an aversion to returning to dialysis, and a willingness to accept side effects and adverse outcomes of immunosuppressants as a necessary part of the treatment.69-71 In a recent pilot study that was set to elicit preferences and acceptable trade-offs in kidney transplant recipients, graft survival was once again identified as the most important outcome. Interestingly, potentially debilitating side effects, such as severe diarrhea and nausea, were given similar weight by patients even though they carry a lower risk of serious adverse outcomes than graft loss.72 Realizing that priorities of patients’ and other stakeholders may differ from those of researchers, a recent initiative, the Standardised Outcomes in Nephrology (SONG),73 has been established to identify research outcomes that are deemed meaningful and relevant to patients, caregivers, clinicians, researchers, policy makers, and other relevant stakeholders.
Because allograft survival can be optimized through prevention of ABMR, and the risk of ABMR can be reduced by improved donor-recipient immune compatibility, future research into kidney transplant candidates’ inclination to accept trade-offs such as prolonged wait times to transplantation to achieve improved donor-recipient matching may be ascertained by surveying/interviewing kidney transplant candidates and recipients. Moreover, in line with the Can-SOLVE CKD initiative, rather than research participants, patient may also wish to be further engaged as research partners and consultants. In this capacity, patients can advise on effective communication with study participants on the involved trade-offs in a way that would be approachable to their peers. Patients can also advise on strategies to evaluate the attitudes of blood type and HLA-compatible living donor and kidney transplant candidate pairs to participate in paired exchange programs at the provincial and national levels in an effort to prevent CKD and early graft loss.
Patient-researchers’ input can also be sought on matters of ABMR surveillance, diagnosis, and therapy. In this aim, patient-researchers can help set priorities and codesign studies on preferable invasive and noninvasive ABMR surveillance strategies (eg, anti-HLA antibody monitoring, genomic and proteomic biomarkers, biopsies, and imaging modalities) and schedules. Moreover, patient-researchers’ voice can provide unique insights into potential barriers to study enrollment and shed light on causes of participant attrition, the Achilles heel of interventional studies.74 This is of particular significance in research involving kidney transplant candidates and recipients where the number of engaged participants is limited to begin with. Insights learned would be of great benefit when embarking on the design and execution of future clinical trials evaluating therapies for clinically evident and subclinical ABMR. Although some of these insights may be obtained through patient surveys, patient-collaborators are more likely to remain actively engaged throughout the life cycle of the research project.
In practice, patient-researchers can be recruited at transplant clinics and in the inpatient setting. To tap an even wider population of transplant recipients and candidates, the Canadian Transplant Association can be approached.11,75 To foster mutual respect and active patient participation, adequate time needs to be spent to build reciprocal relationships between investigators and patient-researchers. Expectations and deliverables by both patient-researchers and investigators should be outlined in study protocols in an effort to ensure and effective collaboration.76 It is anticipated that active participation of kidney transplant candidates and recipients in research will help promote patients’ active participation in and adherence to their care plans, thus improving patient experience as well as kidney transplant outcomes.
Conclusion
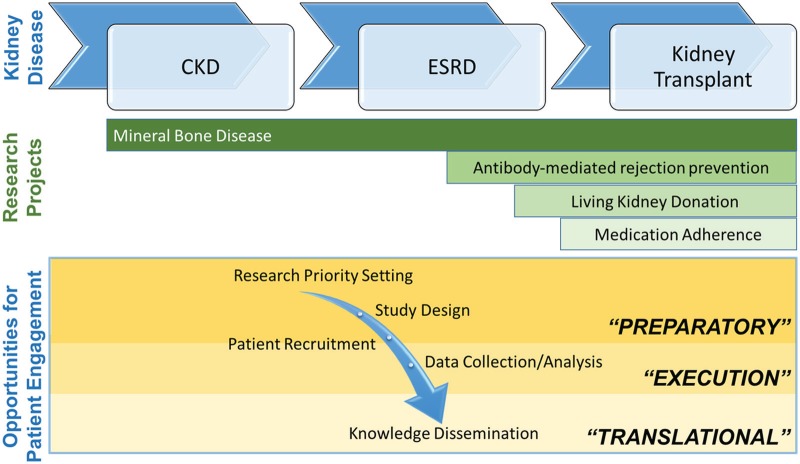
The four research programs presented in this article provide insights into the ways that patient engagement can be incorporated into kidney research. The examples demonstrate that beyond involvement as study participants, patient engagement can be envisioned at a logistical level (patient recruitment, data collection, knowledge dissemination, etc.) and at a conceptual level (research priority setting, protocol design, data analysis, etc.). These three levels emphasize the notion that patient engagement is bound to evolve as research programs progress and is dependent on patients’ wishes and capabilities (Figure 1).
Figure 1.
Opportunities for patient engagement in kidney research along the research continuum (preparatory, execution, and translational stages8) in 4 research projects across the spectrum of kidney disease.
Note. The first research program discusses engagement of patients to improve the quality and relevance of mineral and bone disease management in patients with CKD. The second featured research program discusses patient-researcher partnerships aimed at increasing living donor kidney transplants. The third research program discusses the relevance of patient engagement for the development of an intervention to improve medication adherence in kidney transplant recipients. The final research study discusses opportunities for involving patients in research aimed at preventing antibody-mediated rejection in transplant recipients. CKD, chronic kidney disease; ESRD, end-stage renal disease.
Current funding opportunities have facilitated patient engagement in health research and the accessibility of patients’ experiential knowledge to academic, health, and government authorities. Specifically, SPOR is aimed at bridging the gap between health research and health care outcomes by enhancing the interaction between health-researchers and patients, the end users of study outcomes. Hence, a central initiative of the Can-SOLVE CKD network is to support the development of a consensual language and the necessary skills for investigators and patients to collaborate on CKD research. This is bound to be an iterative collective learning process that improves at each cycle, as researchers and patients learn through repeated experiences of finding solutions together. Beyond an openness to varying opinions and mutual respect, the firm commitment to a common goal—better health outcomes for all—will provide the glue that binds patients and health-researchers together.
Footnotes
List of Abbreviations: ABMR, antibody-mediated rejection; Can-SOLVE CKD, Canadians Seeking Solutions and Innovations to Overcome Chronic Kidney Disease; CKD, chronic kidney disease; DSA, donor-specific antibodies; ESRD, end-stage renal disease; HLA, human leukocyte antigens; HLQ, Health Literacy Questionnaire; MBD, mineral and bone disorders; PCOR, Patient-Centered Outcomes Research; SONG, Standardised Outcomes in Nephrology SPOR, Strategy for Patient-Oriented Research.
Ethics Approval and Consent to Participate: Given that this is a narratve review, ethics approval and consent for participation were not required nor applicable.
Consent for Publication: All authors consented to the publication of this manuscript.
Availability of Data and Materials: Not applicable.
Authors’ Note: N.F. is a kidney transplant recipient who has collaborated with health care professionals to integrate patient perspectives into research aimed at improving clinical outcomes. M.N.D. is pursuing her PhD in Clinical Psychology. N.N.L., F.M.W., and R.S.P. are assistant professors in the Division of Nephrology at their respective institutions. M.N.D., N.N.L., F.M.W., and R.S.P. are awardees of the 2015 KRESCENT awards. M.N.D. received the KRESCENT Allied Health Doctoral Award, and N.N.L., F.M.W., and R.S.P. received New Investigator Awards to support their ongoing research. F.M.W. is also supported by le Fonds de Recherche du Québec en Santé (FRQS). The opinions and conclusions reported in this article are those of the authors and are independent of the funding sources.
Author Contributions: All authors participated in the drafting of the final manuscript. All authors read and approved the final version of the manuscript.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: M.N.D., N.N.L., F.M.W., R.S.P. were supported by the Kidney Research Scientist Core Education and National Training Program (KRESCENT). M.N.D. received the KRESCENT Allied Health Doctoral Award, and N.N.L., F.M.W., and R.S.P. received New Investigator Awards.
References
- 1. Canadian Institute for Health Information. Canada’s Strategy for Patient-Oriented Research: Improving Health Outcomes Through Evidence-Informed Care. Ottawa, Ontario: Canadian Institute for Health Information; 2011. [Google Scholar]
- 2. Frank L, Forsythe L, Ellis L, et al. Conceptual and practical foundations of patient engagement in research at the patient-centered outcomes research institute. Qual. Life Res. 2015;24:1033-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Green LA, Fryer GEJ, Yawn BP, Lanier D, Dovey SM. The ecology of medical care revisited. N Engl J Med. 2001;344:2021-2025. [DOI] [PubMed] [Google Scholar]
- 4. Weber C, Fischer S. What the future holds: in response to global health care challenges, innovation has become the new “way of life.” IEEE Pulse. 2014;5:14-17. [DOI] [PubMed] [Google Scholar]
- 5. Schoen C, Osborn R, Doty MM, Squires D, Peugh J, Applebaum S. A survey of primary care physicians in eleven countries, 2009: perspectives on care, costs, and experiences. Health Aff (Millwood). 2009;28:w1171-w1183. [DOI] [PubMed] [Google Scholar]
- 6. Karazivan P, Dumez V, Flora L, et al. The patient-as-partner approach in health care: a conceptual framework for a necessary transition. Acad Med. 2015;90:437-441. [DOI] [PubMed] [Google Scholar]
- 7. Cukor D, Cohen LM, Cope EL, et al. Patient and other stakeholder engagement in patient-centered outcomes research institute funded studies of patients with kidney diseases. Clin J Am Soc Nephrol. 2016;11:1703-1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shippee ND, Domecq Garces JP, Prutsky Lopez GJ, et al. Patient and service user engagement in research: a systematic review and synthesized framework. Health Expect. 2015;18(5):1151-1166. doi: 10.1111/hex.12090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Domecq JP, Prutsky G, Elraiyah T, et al. Patient engagement in research: a systematic review. BMC Health Serv Res. 2014;14:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tattersall RL. The expert patient: a new approach to chronic disease management for the twenty-first century. Clin Med (Lond). 2:227-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pomey M-P, Ghadiri DP, Karazivan P, Fernandez N, Clavel N. Patients as partners: a qualitative study of patients’ engagement in their health care. PLoS ONE. 2015;10:e0122499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Supple D, Roberts A, Hudson V, et al. Erratum to: from tokenism to meaningful engagement: best practices in patient involvement in an EU project. Res Involv Engagem. 2015;1:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Boivin A, Lehoux P, Lacombe R, Burgers J, Grol R. Involving patients in setting priorities for healthcare improvement: a cluster randomized trial. Implement Sci. 2014;9:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Manns B, Hemmelgarn B, Lillie E, et al. Setting research priorities for patients on or nearing dialysis. Clin J Am Soc Nephrol. 2014;9:1813-1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jun M, Manns B, Laupacis A, et al. Assessing the extent to which current clinical research is consistent with patient priorities: a scoping review using a case study in patients on or nearing dialysis. Can J Kidney Health Dis. 2015;2:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Marlett N, Shklarov S, Marshall D, Santana MJ, Wasylak T. Building new roles and relationships in research: a model of patient engagement research. Qual Life Res. 2015;24:1057-1067. [DOI] [PubMed] [Google Scholar]
- 17. Kidney Disease: Improving Global Outcomes CKD-MBD Work Group. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl. 2009;113:S1-S130. [DOI] [PubMed] [Google Scholar]
- 18. Ott SM. Bone disease in CKD. Curr Opin Nephrol Hypertens. 2012;21:376-381. [DOI] [PubMed] [Google Scholar]
- 19. Cozzolino M, Ureña-Torres P, Vervloet MG, et al. Is chronic kidney disease-mineral bone disorder (CKD-MBD) really a syndrome? Nephrol Dial Transplant. 2014;29:1815-1820. [DOI] [PubMed] [Google Scholar]
- 20. Drüeke TB, Massy ZA. Changing bone patterns with progression of chronic kidney disease. Kidney Int. 2016;89:289-302. [DOI] [PubMed] [Google Scholar]
- 21. Garrett G, Sardiwal S, Lamb EJ, Goldsmith DJA. PTH—a particularly tricky hormone: why measure it at all in kidney patients? Clin J Am Soc Nephrol. 2013;8:299-312. [DOI] [PubMed] [Google Scholar]
- 22. Babayev R, Nickolas TL. Bone disorders in chronic kidney disease: an update in diagnosis and management. Semin Dial. 2015;28:645-653. [DOI] [PubMed] [Google Scholar]
- 23. Urquhart-Secord R, Craig JC, Hemmelgarn B, et al. Patient and caregiver priorities for outcomes in hemodialysis: an international nominal group technique study. Am J Kidney Dis. 2016;68:444-454. [DOI] [PubMed] [Google Scholar]
- 24. Wolfe RA, Ashby VB, Milford EL, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999;341:1725-1730. [DOI] [PubMed] [Google Scholar]
- 25. Rabbat CG, Thorpe KE, Russell JD, Churchill DN. Comparison of mortality risk for dialysis patients and cadaveric first renal transplant recipients in Ontario, Canada. J Am Soc Nephrol. 2000;11:917-922. [DOI] [PubMed] [Google Scholar]
- 26. Medin C, Elinder CG, Hylander B, Blom B, Wilczek H. Survival of patients who have been on a waiting list for renal transplantation. Nephrol Dial Transplant. 2000;15:701-704. [DOI] [PubMed] [Google Scholar]
- 27. Peters TG, Fisher JS, Gish RG, Howard RJ. Views of US voters on compensating living kidney donors. JAMA Surg. 2016;151(8):710-716. [DOI] [PubMed] [Google Scholar]
- 28. Agerskov H, Ludvigsen MS, Bistrup C, Pedersen BD. Relieved or disappointed—experiences of accepted and rejected living kidney donors: a prospective qualitative study. J Clin Nurs. 2015;24:3519-3527. [DOI] [PubMed] [Google Scholar]
- 29. Clemens K, Boudville N, Dew MA, et al. The long-term quality of life of living kidney donors: a multicenter cohort study. Am J Transplant. 2011;11:463-469. [DOI] [PubMed] [Google Scholar]
- 30. Jowsey SG, Jacobs C, Gross CR, et al. Emotional well-being of living kidney donors: findings from the RELIVE Study. Am J Transplant. 2014;14:2535-2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mjøen G, Stavem K, Westlie L, et al. Quality of life in kidney donors. Am J Transplant. 2011;11:1315-1319. [DOI] [PubMed] [Google Scholar]
- 32. Owen M, Lorgelly P, Serpell M. Chronic pain following donor nephrectomy—a study of the incidence, nature and impact of chronic post-nephrectomy pain. Eur J Pain. 2010;14:732-734. [DOI] [PubMed] [Google Scholar]
- 33. Lunsford SL, Shilling LM, Chavin KD, et al. Racial differences in the living kidney donation experience and implications for education. Prog Transplant. 2007;17:234-240. [DOI] [PubMed] [Google Scholar]
- 34. Tong A, Ralph A, Chapman JR, et al. Public attitudes and beliefs about living kidney donation: focus group study. Transplantation. 2014;97:977-985. [DOI] [PubMed] [Google Scholar]
- 35. Adams-Leander S. The experiences of African-American living kidney donors. Nephrol Nurs J. 2011;38:499-508; quiz 509. [PubMed] [Google Scholar]
- 36. Gordon EJ, Feinglass J, Carney P, et al. A culturally targeted website for Hispanics/Latinos about living kidney donation and transplantation: a randomized controlled trial of increased knowledge. Transplantation. 2016;100:1149-1160. [DOI] [PubMed] [Google Scholar]
- 37. Thiessen C, Gordon EJ, Reese PP, Kulkarni S. Development of a donor-centered approach to risk assessment: rebalancing nonmaleficence and autonomy. Am J Transplant. 2015;15:2314-2323. [DOI] [PubMed] [Google Scholar]
- 38. LaPointe Rudow D, Hays R, Baliga P, et al. Consensus conference on best practices in live kidney donation: recommendations to optimize education, access, and care. Am J Transplant 2015;15:914-922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Achille MA, Ouellette A, Fournier S, Vachon M, Hébert M-J. Impact of stress, distress and feelings of indebtedness on adherence to immunosuppressants following kidney transplantation. Clin Transplant. 2006;20:301-306. [DOI] [PubMed] [Google Scholar]
- 40. Denhaerynck K, Dobbels F, Cleemput I, et al. Prevalence, consequences, and determinants of nonadherence in adult renal transplant patients: a literature review. Transpl Int. 2005;18:1121-1133. [DOI] [PubMed] [Google Scholar]
- 41. Gelb SR, Shapiro RJ, Thornton WJL. Predicting medication adherence and employment status following kidney transplant: the relative utility of traditional and everyday cognitive approaches. Neuropsychology. 2010;24:514-526. [DOI] [PubMed] [Google Scholar]
- 42. Goldfarb-Rumyantzev AS, Wright S, Ragasa R, et al. Factors associated with nonadherence to medication in kidney transplant recipients. Nephron Clin Pract. 2011;117:c33-c39. [DOI] [PubMed] [Google Scholar]
- 43. National Institute of Medicine. Health Literacy: A Prescription to End Confusion. Washington, DC; The National Academies Press; 2004. [PubMed] [Google Scholar]
- 44. Canadian Council on Learning. Health Literacy in Canada: A Healthy Understanding. Ottawa, Ontario, Canada: Canadian Council on Learning; 2008. [Google Scholar]
- 45. Estrada CA, Martin-Hryniewicz M, Peek BT, Collins C, Byrd JC. Literacy and numeracy skills and anticoagulation control. Am J Med Sci. 2004;328:88-93. [DOI] [PubMed] [Google Scholar]
- 46. Omachi TA, Sarkar U, Yelin EH, Blanc PD, Katz PP. Lower health literacy is associated with poorer health status and outcomes in chronic obstructive pulmonary disease. J Gen Intern Med. 2013;28:74-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Schillinger D, Grumbach K, Piette J, et al. Association of health literacy with diabetes outcomes. JAMA. 2002;288:475-482. [DOI] [PubMed] [Google Scholar]
- 48. Devraj R, Borrego M, Vilay AM, Gordon EJ, Pailden J, Horowitz B. Relationship between health literacy and kidney function. Nephrology (Carlton). 2015;20:360-367. [DOI] [PubMed] [Google Scholar]
- 49. Cavanaugh KL, Wingard RL, Hakim RM, et al. Low health literacy associates with increased mortality in ESRD. J Am Soc Nephrol. 2010;21:1979-1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gordon EJ, Wolf MS. Health literacy skills of kidney transplant recipients. Prog Transplant. 2009;19:25-34. [DOI] [PubMed] [Google Scholar]
- 51. Green JA, Mor MK, Shields AM, et al. Prevalence and demographic and clinical associations of health literacy in patients on maintenance hemodialysis. Clin J Am Soc Nephrol. 2011;6:1354-1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Escobedo W, Weismuller P. Assessing health literacy in renal failure and kidney transplant patients. Prog Transplant. 2013;23:47-54. [DOI] [PubMed] [Google Scholar]
- 53. Chisholm MA, Fair J, Spivey CA. Health literacy and transplant patients and practitioners. Public Health. 2007;121:800-803. [DOI] [PubMed] [Google Scholar]
- 54. Kazley AS, Hund JJ, Simpson KN, Chavin K, Baliga P. Health literacy and kidney transplant outcomes. Prog Transplant. 2015;25:85-90. [DOI] [PubMed] [Google Scholar]
- 55. Grubbs V, Gregorich SE, Perez-Stable EJ, Hsu C-Y. Health literacy and access to kidney transplantation. Clin J Am Soc Nephrol. 2009;4:195-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Demian MN, Shapiro RJ, Thornton WL. An observational study of health literacy and medication adherence in adult kidney transplant recipients. Clin Kidney J. 2016;9:858-865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Orr A, Orr D, Willis S, Holmes M, Britton P. Patient perceptions of factors influencing adherence to medication following kidney transplant. Psychol Health Med. 2007;12:509-517. [DOI] [PubMed] [Google Scholar]
- 58. Brath H, Morak J, Kästenbauer T, et al. Mobile health (mHealth) based medication adherence measurement—a pilot trial using electronic blisters in diabetes patients. Br J Clin Pharmacol. 2013;76(suppl 1):47-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kaplan B, Meier-Kriesche H-U. Death after graft loss: an important late study endpoint in kidney transplantation. Am J Transplant. 2002;2:970-974. [DOI] [PubMed] [Google Scholar]
- 60. Laupacis A, Keown P, Pus N, et al. A study of the quality of life and cost-utility of renal transplantation. Kidney Int. 1996;50:235-242. [DOI] [PubMed] [Google Scholar]
- 61. Sellarés J, de Freitas DG, Mengel M, et al. Understanding the causes of kidney transplant failure: the dominant role of antibody-mediated rejection and nonadherence. Am J Transplant. 2012;12:388-399. [DOI] [PubMed] [Google Scholar]
- 62. Duquesnoy RJ, Kamoun M, Baxter-Lowe LA, et al. Should HLA mismatch acceptability for sensitized transplant candidates be determined at the high-resolution rather than the antigen level? Am J Transplant. 2015;15:923-930. [DOI] [PubMed] [Google Scholar]
- 63. Duquesnoy RJ. A structurally based approach to determine HLA compatibility at the humoral immune level. Hum Immunol. 2006;67:847-862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Duquesnoy RJ, Askar M. HLAMatchmaker: a molecularly based algorithm for histocompatibility determination. V. Eplet matching for HLA-DR, HLA-DQ, and HLA-DP. Hum Immunol. 2007;68:12-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Claas FHJ, Dankers MK, Oudshoorn M, et al. Differential immunogenicity of HLA mismatches in clinical transplantation. Transpl Immunol. 2005;14:187-191. [DOI] [PubMed] [Google Scholar]
- 66. Duquesnoy RJ, Awadalla Y, Lomago J, et al. Retransplant candidates have donor-specific antibodies that react with structurally defined HLA-DR,DQ,DP epitopes. Transpl Immunol. 2008;18:352-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Marrari M, Duquesnoy RJ. Correlations between Terasaki’s HLA class II epitopes and HLAMatchmaker-defined eplets on HLA-DR and -DQ antigens. Tissue Antigens. 2009;74:134-146. [DOI] [PubMed] [Google Scholar]
- 68. Duquesnoy RJ, Marrari M. HLAMatchmaker-based definition of structural human leukocyte antigen epitopes detected by alloantibodies. Curr Opin Organ Transplant. 2009;14:403-409. [DOI] [PubMed] [Google Scholar]
- 69. Howell M, Tong A, Wong G, Craig JC, Howard K. Important outcomes for kidney transplant recipients: a nominal group and qualitative study. Am J Kidney Dis. 2012;60:186-196. [DOI] [PubMed] [Google Scholar]
- 70. Goldade K, Sidhwani S, Patel S, et al. Kidney transplant patients’ perceptions, beliefs, and barriers related to regular nephrology outpatient visits. Am J Kidney Dis. 2011;57:11-20. [DOI] [PubMed] [Google Scholar]
- 71. Buldukoglu K, Kulakac O, Kececioglu N, Alkan S, Yilmaz M, Yucetin L. Recipients’ perceptions of their transplanted kidneys. Transplantation. 2005;80:471-476. [DOI] [PubMed] [Google Scholar]
- 72. Howell M, Wong G, Rose J, Tong A, Craig JC, Howard K. Eliciting patient preferences, priorities and trade-offs for outcomes following kidney transplantation: a pilot best-worst scaling survey. BMJ Open. 2016;6:e008163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Standardized Outcomes in Nephrology. SONG-TX. http://songinitiative.org/projects/song-tx/. Accessed January 4, 2017.
- 74. Brennan T V, Fuller TF, Vincenti F, et al. Living donor kidney transplant recipients and clinical trials: participation profiles and impact on post-transplant care. Am J Transplant. 2006;6:2429-2435. [DOI] [PubMed] [Google Scholar]
- 75. Concannon TW, Fuster M, Saunders T, et al. A systematic review of stakeholder engagement in comparative effectiveness and patient-centered outcomes research. J Gen Intern Med. 2014;29:1692-1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Carman KL, Dardess P, Maurer M, et al. Patient and family engagement: a framework for understanding the elements and developing interventions and policies. Health Aff (Millwood). 2013;32:223-231. [DOI] [PubMed] [Google Scholar]