Abstract
Objectives
To determine if 1) Osteoarthritis (OA)-related pain is associated with the diurnal cortisol pattern and cortisol levels; 2) the diurnal pattern of cortisol varies with severity of OA pain and 3) the association between OA pain and cortisol is mediated by daily experience variables (DEV).
Design
In a community-based study of changes in regional and widespread pain among women with OA, participants (n=31) completed daily diaries and collected three saliva samples daily for 7 days. Severity of OA-related pain was assessed by the validated Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain subscale. Multilevel regression analyses estimated associations between OA pain and diurnal cortisol levels and slopes, controlling for body mass index, medication use, time and day. Mediation analyses examined DEV as potential mediators of the association between OA pain and cortisol.
Results
The mean age was 57 years and average BMI 31kg/m2. Mean WOMAC pain subscale score was 8.8. Women with higher WOMAC pain scores had higher cortisol throughout the day. The estimated association of WOMAC with cortisol [ß 0.083(0.02, 0.15) p =0.009] represents a ~ 9% increase in cortisol for every unit increase in WOMAC pain score. Women with WOMAC pain scores ≥ 9 had higher cortisol levels than those with scores <9. Examination of DEV revealed no significant mediated associations between these relationships at the daily level.
Conclusion
In women with OA, disease-related pain is positively associated with cortisol production, particularly with greater pain severity. Future studies should explore biologic mediating variables between OA pain and cortisol.
Keywords: osteoarthritis, cortisol, pain, female
Introduction
The link between pain (acute or chronic), function of the hypothalamic–pituitary–adrenal (HPA) axis and the subsequent effect on cortisol levels has been well-documented in the literature1. Cortisol has important regulatory functions including glucose production, maintenance of the central nervous system, and anti-inflammatory properties that limit the spread of pain1. Cortisol release follows a diurnal pattern, with peak levels after waking and a steady decline occurring throughout the rest of the day2. The HPA axis is the physiological response of the body to stress with its end role being the release of cortisol from the adrenal glands, the result of a cascade of reactions after the HPA axis is triggered2. Pain is a potential stressor and therefore activator of the HPA axis. Depending on the level of threat that an individual associates with the perception of pain, the physiological response may be exaggerated, resulting in cortisol dysfunction3,4.
Studies of cortisol in people with chronic pain have occurred in a variety of musculoskeletal conditions such as rheumatoid arthritis, low back pain and whiplash5–7. In contrast few have examined the association of pain and cortisol in people with osteoarthritis (OA). Besides pain, cortisol dysfunction and OA share several common factors including links with obesity, metabolic syndrome and inflammation8,9. The experience of pain in OA is known to be intermittent and chronic with the former potentially overlaying the latter10. A consequence of intermittent pain flares is hypercortisolism that may lead to obesity, a common comorbidity of OA11. In a study of the association of cortisol with acute, chronic (radicular or degenerative low back pain) or intermittent pain (headache), Strittmatter et al12 compared serum cortisol levels to healthy controls sampled at 4 separate time points in 24 hours. Results showed that those with intermittent pain had the greatest changes in cortisol levels throughout the day12.
To our knowledge, there has been only one study examining the association between pain and cortisol in people with diagnosed OA. In a study of men with chronic OA pain (defined as OA lasting more than 3 mos and disease process confirmed by Kellgren and Lawrence radiographs) and the association with neuroendocrine function, Khoromi et al13 reported no difference in mean cortisol levels in both blood and urine compared to healthy controls. Sex differences are known to exist in the response of the HPA axis14,15 and in regards to pain, this may be due to differences in sex hormones or psychosocial factors16,17. However, the association of pain and cortisol in women with OA has not been studied.
Pain mechanisms in OA are currently not well understood and a challenge exists in trying to unravel the discordance found between structural findings typical of the disease and patient reported pain18. Given the common factors shared by cortisol dysfunction and OA, along with the greater frequency of the disease in women and concomitant severity of pain19, it is important to understand the role that the HPA axis and cortisol dysfunction may have in the pain experience of women with OA. The purpose of this study was to determine 1) if OA-related pain is associated with the diurnal cortisol pattern and cortisol levels; 2) if the diurnal pattern of cortisol varies with severity of OA pain and 3) if the association between OA pain and cortisol is mediated by daily variations in pain, fatigue, pain catastrophizing, stress and affect. We hypothesized that there would be a significant association of WOMAC pain with cortisol levels. We further hypothesized that cortisol levels may drop in response to greater pain and that this association may be mediated by any of the daily experience variables.
Methods
Sample
This is a secondary analysis of data from a community-based study of changes in regional and widespread pain among women with chronic pain in Arizona, USA. The sample was recruited between May 2002 and March 2004 through physician referral, ads in the local newspaper, and posted fliers. Potential participants were screened by telephone to determine eligibility. Eligibility requirements for this analysis included: 1) female; 2) physician-confirmed diagnosis of osteoarthritis of the hip, knee or spine; and 3) onset of symptoms within the last 5 years or a current pain rating of ≥40 on a 0 to 100 scale in the past month. Exclusion criteria were: 1) autoimmune or other comorbid disorders causing widespread pain, inflammation, and fatigue (e.g., fibromyalgia, ankylosing spondylitis;) 2) pending litigation regarding the pain condition; and 3) use of daily corticosteroids. The study was approved by the Institutional Review Board at Arizona State University. Participants provided written informed consent for all study activities after completion of the initial eligibility screening and before their physicians were contacted for diagnosis confirmation. The current analysis includes data from 31women with OA who were enrolled in the larger study (final n = 257).
Procedures
There were four components to the larger study of women with chronic pain: a daily diary field assessment of symptoms, mood, and cortisol; an in-home assessment of physical and mental health symptoms; laboratory tests of stress reactivity under controlled conditions; and follow-up of illness course after 2 years. Only data from the field and home assessments are used in the current analysis. After initial screening, participants were provided with a laptop computer for completion of daily diary reports before bedtime each evening for up to 7 consecutive days. Reports were time-stamped to verify completion and allowed only one report to be completed per day. Diary questionnaires included measures of pain, pain catastrophizing, fatigue, positive and negative affect, and stress. Over the same period, participants also collected saliva samples three times a day (at 10 AM, 4 PM, and 8 PM), using cotton salivettes (Sarstedt, Newton, North Carolina). To assess compliance, participants were given a 200-mL bottle with a cap that registered all opening times (MEMS TrackCap, Aardex Ltd., Sion and Zug, Switzerland). Participants were instructed that the bottle was to be opened only at the scheduled collection times and to remove only one swab each time. A digital alarm was set to the scheduled times to serve as a reminder. Participants returned saturated swabs to the salivette tubes, recorded the actual collection time on the tube, and at the end of each day, stored the new samples in their home freezer. In-home clinical assessments were conducted between 1 day and 27 days (mean, 7.0 days) after completion of the daily diaries and saliva collection. At this time, participants also completed questionnaires regarding experiences of OA-related pain. The frozen saliva samples were then taken to the laboratory and stored at −20°C until analysis.
Measures
Salivary cortisol
An in-house radioimmunoassay (Department of Reproductive Physiology, University of Liege) was performed in duplicate on 50 μL of saliva, with salivary free cortisol in competition with a high-performance liquid chromatography preparation of cortisol-3CMO coupled with 2-125 iodohistamine as tracer for specific antibodies raised against cortisol-3CMO-BSA20. The lower detection limit of the assay was 0.2 nmol/L. The intra- and interassay coefficients of variation were <5% and <12%, respectively. All samples from an individual were analyzed in the same assay to reduce sources of variability. Two cortisol measures had values above the physiological range (>1585 ng/dL)2 and were excluded from the statistical analysis. Over the 7 days, participants collected a median of 18 valid saliva samples (range, 12–21) with an overall sample of 570 cortisol measures.
Disease-related pain
Severity of OA-related pain was assessed by the valid and reliable Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain subscale21. The subscale is comprised of 5 items, scaled from 0 (none) to 4 (extreme). Higher scores indicate greater pain with scores ranging from 0–2021.
Daily experience variables
At the end of each day, participants rated their pain, pain catastrophizing, affect, stress, and fatigue. Mean scores for each measure were calculated across the 7 days for each participant. Pain was assessed with the question, “What number describes your average level of arthritis pain today?”. Responses ranges were from 0 (no pain) to 100 (pain as bad as it can be). Pain catastrophizing was assessed using two items from the Coping Strategies Questionnaire22. Ratings were on a Likert scale of 1 (strongly disagree) to 5 (strongly agree). Daily catastrophizing scores were computed by averaging the two items. Cronbach’s α=0.90 for values averaged across the 7 days. Positive affect and negative affect were assessed using the Positive and Negative Affect Schedule- ratings of 20 affect terms on 5-point scales (1 - very slightly or not at all to 5 - extremely)23. The items, averaged across 7 days for each person, yielded a Cronbach’s α coefficient of 0.98 for positive affect and 0.92 for negative affect. Interpersonal stress was obtained by averaging daily responses on the four items “Overall, how stressful were your relations with your (friends, spouse/partner, family, and co-workers,) today?” for each rated on a 4-point scale (1 -not at all to 4 - extremely). Fatigue was assessed with the item “What number best describes your average level of fatigue today?,” with response ranges from 0 (none) to 100 (fatigue as bad as it can be).
Demographics and medication use
Demographic variables collected included age in years, sex, marital status and level of education. Body mass index (BMI) was calculated from self-reported height and weight. Participants also provided information on medication use.
Statistical analyses
Cortisol values were natural-log transformed to correct positive skew in their distribution. Descriptive statistics were examined for each variable. Multilevel models, which take into account the hierarchical clustering of the data24 were used. The model used to estimate effects on cortisol had three levels: data were collected three times a day (measurement level); for 7 consecutive days (day level); clustered within participants (person level). However, the three-level models failed to converge; consequently, the final models did not estimate models separately at the daily level but included day number as a covariate. We used maximum likelihood estimation to test fixed effects, but allowed for random intercepts in the model. To test for potential person level confounders, bivariable multilevel models were built for each of the independent variables (age, BMI, education, marital status, income, exogenous hormones, and medication class (analgesics, nonsteroidal anti-inflammatory drugs, COX-2 inhibitors, antidepressants, anxiolytics, hypnotic sedatives, synthetic thyroid hormones, topical corticosteroids, inhaled steroids, antihypertensives, anticonvulsants)) and the dependent variable of cortisol level. Those reaching a p-value of < 0.20 were retained for the multivariable model. In addition, the final decision to exclude variables with p>0.20 considered whether they were important confounders, in the causal pathway or colliders. To assess the diurnal pattern of cortisol, a model with an interaction term of time × WOMAC pain subscale score was fitted as a function of time. Next the association of cortisol levels with WOMAC pain subscale was assessed without the interaction term. To assess the association of severity of OA pain with cortisol, in the absence of any known cut-point for severity of the WOMAC pain subscale that is applicable to a general OA population, a Student’s t-test using the median split (=9) of WOMAC pain subscale scores was used. For our third objective, mediation analyses were conducted using the product of the co-efficients approach, which calculates the statistical significance of the product of the paths from the predictor to the mediator (the a path) and from the mediator to the outcome (the b path)25. Estimation of mediated effects using the ab product coefficient is preferable to the calculation of the difference of effects of the direct and indirect paths (c′ − c) due to concerns about the requirement of normality in the multivariate distributions of the direct and indirect effects26. Significance of the mediated effect was calculated at an alpha level of 0.05. We employ the term mediation to refer to the statistical analysis, but cannot infer causality from the observed associations. Analyses were conducted using the Statistical Package for the Social Sciences (SPSS, v.22 IBM, Chicago Ill.) and Mplus, v.6.12 (Muthen & Muthen, CA), using the TYPE = COMPLEX command which allows for estimation of lower-level effects while accounting for clustering effects.
Ethics
The procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (Arizona State Institutional Review Board, Tempe, Arizona) and with the Helsinki Declaration of 1975, as revised in 2000. All subjects gave informed consent to participate in this research.
Results
The mean age was 57 years (range 38–72) and average BMI 31kg/m2 (standard deviation (sd) 9.9). The average length of OA diagnosis in years was 4.4 (sd 3.2). The WOMAC pain subscale mean was 8.8 (sd 3.2), see table 1.
Table 1.
Sample characteristics n=31
| Variable | Mean (sd) |
|---|---|
| Age in years | 56.9 (9.3) |
| BMI | 31.2 (9.9) |
| Duration of OA diagnosis in years | 4.4 (3.2) |
| WOMAC pain subscale score (0–20) | 8.8 (3.1) |
| Daily diary reports | |
| Pain (1–100) | 50.9 (20.5) |
| Stress (1–4) | 1.3 (0.5) |
| Fatigue (0–100) | 43.0 (26.5) |
| Pain catastrophizing (1–5) | 1.9 (0.9) |
| Negative affect (1–5) | 1.4 (0.6) |
| Positive affect (1–5) | 2.7 (1.1) |
| N (%) | |
| Marital status – cohabitation | 15 (48.4) |
| Post secondary education or higher | 25 (80.6) |
| Income > 40,000 | 14 (46.7) |
| Medications | |
| NSAIDS | 10 (32.3) |
| Cox 2 | 4 (12.9) |
| Anxiolytics | 4 (12.9) |
| Antidepressants | 10 (32.3) |
| Analgesics | 9 (29.0) |
| Anticonvulsants | 3 (9.7) |
| Blood pressure | 7 (22.6) |
| Thyroid | 7 (22.6) |
| Hormones | 17 (54.8) |
| Hypnotics | 4 (12.9) |
| Steroids | 3 (9.7) |
Bivariable assocation of cortisol levels and potential confounders
Of the independent variables tested, only BMI, use of anticonvulsants and inhaled steroids had p <0.20 and were therefore retained in all subsequent multilevel analyses.
Association of Diurnal Cortisol Pattern, cortisol levels and WOMAC pain subscale
There was a significant decline in cortisol levels as the day progressed (Unstandardized ß = −0.608, p < .001) indicating a 47% decline throughout the day. A nonsignificant time × WOMAC interaction indicated that WOMAC pain scores did not alter the trajectory of cortisol levels throughout the day [Unstandardized ß 0.009 (−0.04, 0.06) p=0.724]. However, analyses revealed that there was a significant association of WOMAC pain subscale scores with average daily cortisol levels [Unstandardized ß 0.083(0.02, 0.15) p =0.009] (see table 2) representing an 8.7% increase in cortisol per one-unit increase in WOMAC pain score.
Table 2.
Multilevel model of WOMAC pain on Cortisol
| Variable | Unstandardized ß Co-efficient | P value | Interpretation* (Raw Cortisol Levels) |
|---|---|---|---|
| Day | −0.001 (−0.06, 0.06) | 0.971 | NS |
| Time | −0.608 (−0.76, −0.46) | <0.001 | 46% decrease throughout the day |
| WOMAC | 0.083 (0.02, 0.15) | 0.009 | 8.7% increase/unit WOMAC |
| BMI | 0.012 (−0.01, 0.04) | 0.361 | NS |
| Anticonvulsants | 0.824 (0.62, 1.10) | <0.001 | 127% increase if used |
| Steroids | −0.260 (−0.14, 0.70) | 0.197 | NS |
% change in raw cortisol per unit change in predictor [exp (ß)] - 1.
Association of severity of WOMAC pain scores and diurnal pattern
The median value of WOMAC pain subscale scores (=9) was used to determine if the diurnal pattern of cortisol varied with OA pain severity. Analyses revealed an overall significant difference in women with WOMAC pain scores ≥ 9 compared to those lower than 9 [back-transformed mean (95%CI) 66.7 ng/dl (59.5, 74.1) versus 46.0 ng/dl (40.9, 51.7) p<0.001 respectively]. See Figure 1. It is unknown whether the difference in these values is biologically significant.
Figure 1.
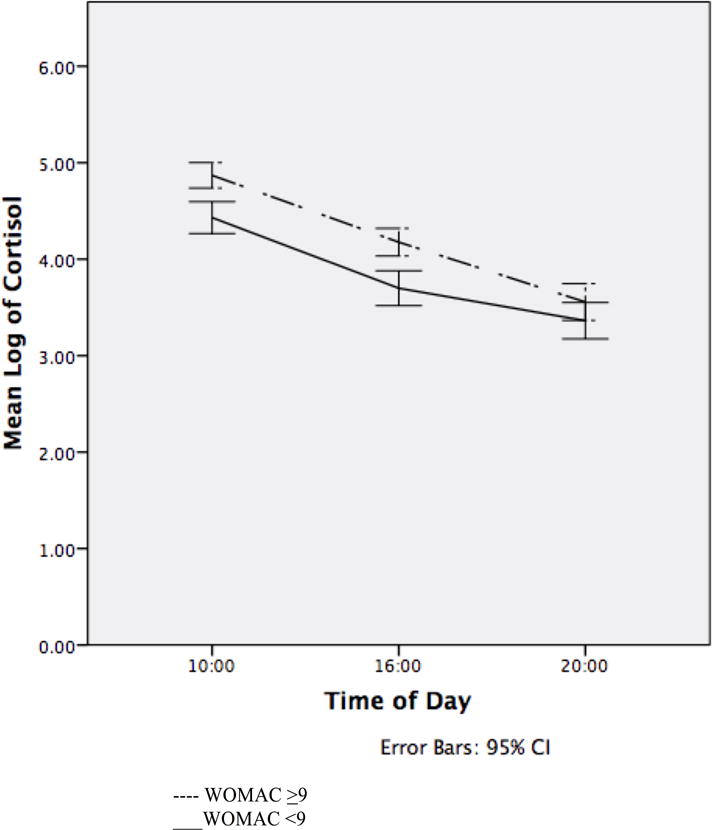
Diurnal Cortisol as a Function of WOMAC pain scores.
Mediation of OA pain and cortisol by daily experience variables
Individual multilevel mediation models were built for each of the daily experience variables, pain, pain catastrophizing, affect, stress, and fatigue. As summarized in table 3, results indicated none of the daily experience variables significantly mediated the association between WOMAC pain scores and cortisol levels.
Table 3.
Multilevel regression estimates of the effects of average daily experience variables on cortisol level in relation to WOMAC pain scores
| Variable | Unstandardized ß Co-efficient | P value |
|---|---|---|
| Pain WOMAC pain |
0.004 0.080 |
0.177 0.016 |
| Stress WOMAC pain |
−0.067 0.084 |
0.515 0.009 |
| Fatigue WOMAC pain |
0.002 0.081 |
0.397 0.015 |
| Pain catastrophizing WOMAC pain |
0.090 0.079 |
0.328 0.011 |
| Negative mood WOMAC pain |
−0.009 0.083 |
0.939 0.010 |
| Positive mood WOMAC pain |
−0.036 0.085 |
0.662 0.009 |
Discussion
Our results indicate that in women with OA, disease-related pain is positively associated with greater mean levels of cortisol production at each time point of cortisol sampling, and that cortisol levels increase when pain severity is greater. However, OA-related pain was not found to predict significantly different trajectories of cortisol levels throughout the day. These results demonstrate that women with OA, having greater pain severity, may demonstrate altered tonic HPA axis function. To our knowledge, there are no other studies of women with OA examining the association of disease-related pain and cortisol. In a review of disparities between the sexes in cortisol responses to pain-related stress, differences were reported to be variable14. However, a more recent study of differences in diurnal cortisol patterns between the sexes in people with chronic pain, found that men had lower concentrations in the afternoon27. In a previous study of chronic pain in men with OA13 no differences were found between those with OA and healthy controls and pain severity was not accounted for. Other differences include the fact that cortisol measurements were taken for a 24-hr period, and a smaller sample (n=16) was used. Given the small number of studies examining the association of OA pain and cortisol and the greater profile of women with the disease, future work is warranted to clarify if differences between the sexes exist in this population.
We were surprised by the absence of mediated associations of the various daily experience variables we investigated particularly given the literature reporting the association of these factors with pain and with OA. This is particularly true for pain catastrophizing and negative affect28,29, the latter encompassing fear or anxiety, sadness or depression, guilt, and hostility30. Pain catastrophizing, depression and fatigue all have reported associations with OA pain and function31,32 but their actual physiological effects on cortisol in this population have been undetermined. Although not specifically measured in this analysis, depression is known to elevate cortisol levels in people with chronic pain,33 however a previous study in people with chronic musculoskeletal pain syndromes reported that depression did not mediate the association between pain and cortisol levels34. However, laboratory studies have demonstrated significant associations between pain catastrophizing and altered morning cortisol levels in people with chronic temporomandibular disorder35 and in people with chronic low back pain, higher stress reactivity was associated with reduced connectivity in parts of the brain known to process pain and these parts in turn mediated the association between cortisol levels and pain36. The lack of association found in our mediation analyses calls into question models that focus particularly on the negative affective consequences of pain as one of the mechanisms of the connection between pain and ill health, particularly in the OA population. It is possible that the lack of significant mediated associations is explainable as a function of the measurement of disease-related pain, as the WOMAC scores were measured only once rather than on a daily basis or multiple times per day.
An important aspect of OA pain is its intermittent nature that can occur mutually with chronic pain typical of the disease process10. Although we did not measure intermittent pain directly, it may be argued that the measurement of average daily pain at the same time as the salivary samples may account for it since multiple pain measures per day are highly correlated with average end of day pain ratings37. Strittmatter12 reported that in people with chronic (low back) or intermittent (headache) pain, compared to healthy controls, significant differences were found in cortisol levels using a cut score of 30 on the McGill pain questionnaire; however this association was only found in the sample taken at 10:00 p.m. and not at any other time (8:00 a.m., 12:00 p.m or 4:00 p.m.). Our sample was chronic in nature, having an average disease duration of more than 4 years. Our findings are in opposition to Strittmatter reporting differences at 10:00 a.m. and 4:00 p.m. but not the later time of 8:00 p.m. We are unable to explain what may account for these contrary findings but propose that differences in the association of pain and cortisol may exist between people with OA compared to those with low back or headache pain. Specifically OA is known to be a disease of low-grade inflammation with frequently observed metabolic changes9 and there are reports of the association of metabolic syndrome with increased OA pain38.
Courties et al39 has suggested that the HPA axis may play a critical role in the development of OA pain and the subsequent vicious cycle that develops between obesity, pain and disability. Cortisol has a role in regulating glucose stores, which when present in excess amounts, has detrimental effects on cartilage and leads to further inflammation9, thus HPA dysfunction may hasten the progress of OA. Cortisol also helps to suppress pro-inflammatory cytokines, such as IL-1β, Il-6, IL-17 and TNFα, known to be mediators of pain in OA by directly acting on the nociceptive system40. Given the lack of mediation by the daily experience variables analyzed, there is an opportunity for future studies to examine alternate explanatory variables, such as pro-inflammatory cytokines or glucose in determining the pathway between OA pain and cortisol. Further, there are reports of the association of psychological factors with inflammatory cytokines41,42 and their co-occurrence could be another avenue for exploration in determining OA-related pain.
There are several strengths to this study to consider. First, as OA pain is complex, more pronounced and more prevalent in women and women appear to have a different experience of pain compared to men, these initial results could help inform our understanding of the complexity of pain mechanisms in women with OA. Daily diaries and collection of multiple salivary cortisol samples for 1 week has provided robust data compared to commonly seen 24-hour periods. Use of multilevel modeling accounts for within and between individual variability in cortisol measures avoiding the use of aggregate means that are less informative. The timing of cortisol samples and daily diary reports were verified electronically thereby increasing our confidence in the validity and reliability of the data.
There are also several limitations to note. Foremost, there was no healthy control group for comparison, making it difficult to know the extent to which these results differ from the norm, nonetheless, the significant difference between groups using a WOMAC pain subscale cut score of 9 is informative. However, it should be noted that this was the median value from this small sample as there is no known cut point for WOMAC pain severity applicable to a general OA population and hence it may not be generalizable. Previous studies have shown women with OA to have similar pain ratings and cortisol levels to women with rheumatoid arthritis,7 however the literature in people with RA indicates that cortisol levels are variable in response to psychophysical stressors43. The cortisol awakening response was not assessed to limit participant burden and study expenses. It has been suggested that samples taken upon waking are a preferred estimate to anchor the diurnal pattern44. There were design issues regarding the measurement of pain that we were unable to address. The timing of the WOMAC pain measurement was taken within an average of 7 days after daily diary and cortisol measurements were completed and may be an issue. However, the disease process was clearly pre-existing and it is known that symptoms of OA are slow to progress, remaining fairly consistent for years45. Further, we accounted for the daily pain experience and the absence of mediation by daily pain indicates that it was not significantly associated with our outcome. While the sample size of cortisol measures was sufficient (570), it did utilize 31 sets of mean scores. Finally, there is evidence that variation in cortisol responses may exist in different ethnic groups. Although the larger study enrolled people of many ethnicities, our subsample was comprised of only Caucasians and this may affect the generalizability of our results.
In summary, we have provided preliminary results exploring the possible association of OA pain in women with cortisol levels and the diurnal pattern. A modest significant association of cortisol found in women with greater pain provides grounds for future exploration. Replication of these findings and comparison with healthy controls and between the sexes in a larger study with closer concordance between measurements is suggested. The absence of association of daily experience variables in the pathway between pain and cortisol warrants the exploration of other factors, including the potential roles of pro-inflammatory cytokines and glucose levels as they are both impacted by cortisol levels and related to the disease process in OA.
Acknowledgments
Role of the funding source: LC is supported by a Fellowship award from the Canadian Institutes of Health Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
- the conception and design of the study, or acquisition of data, or analysis and interpretation of data
- drafting the article or revising it critically for important intellectual content
- final approval of the version to be submitted
Conflict of interest: None declared.
Contributor Information
Lisa C. Carlesso, Assistant Professor, School of Rehabilitation, Faculty of Medicine, Université de Montréal, Maisonneuve-Rosemont Hospital Research Centre, Montréal, Quebec, Canada
John A. Sturgeon, Stanford University School of Medicine, Department of Anesthesiology, Perioperative, and Pain Medicine, Stanford Systems Neuroscience and Pain Laboratory, Palo Alto, CA, USA
Alex J. Zautra, Professor, Department of Psychology, Arizona State University, Tempe, Arizona
References
- 1.Tennant F. The physiologic effects of pain on the endocrine system. Pain Ther. 2013;2:75–86. doi: 10.1007/s40122-013-0015-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nicolson NA. Measurement of cortisol. In: Luecken LJ, Gallo LC, editors. Handbook of physiological research methods in health psychology. Thousand Oaks, CA, US: Sage Publications, Inc; 2008. pp. 37–74. [Google Scholar]
- 3.Colloca L, Benedetti F. Nocebo hyperalgesia: how anxiety is turned into pain. Curr Opin Anaesthesiol. 2007;20:435–9. doi: 10.1097/ACO.0b013e3282b972fb. [DOI] [PubMed] [Google Scholar]
- 4.Benedetti F, Amanzio M, Vighetti S, Asteggiano G. The biochemical and neuroendocrine bases of the hyperalgesic nocebo effect. J Neurosci. 2006;26:12014–22. doi: 10.1523/JNEUROSCI.2947-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaab J, Baumann S, Budnoik A, Gmunder H, Hottinger N, Ehlert U. Reduced reactivity and enhanced negative feedback sensitivity of the hypothalamus-pituitary-adrenal axis in chronic whiplash-associated disorder. Pain. 2005;119:219–24. doi: 10.1016/j.pain.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Muhtz C, Rodriguez-Raecke R, Hinkelmann K, Moeller-Bertram T, Kiefer F, Wiedemann K, et al. Cortisol response to experimental pain in patients with chronic low back pain and patients with major depression. Pain Med. 2013;14:498–503. doi: 10.1111/j.1526-4637.2012.01514.x. [DOI] [PubMed] [Google Scholar]
- 7.Zautra AJ, Burleson MH, Matt KS, Roth S, Burrows L. Interpersonal stress, depression, and disease activity in rheumatoid arthritis and osteoarthritis patients. Health Psychol. 1994;13:139–48. doi: 10.1037//0278-6133.13.2.139. [DOI] [PubMed] [Google Scholar]
- 8.Martocchia A, Stefanelli M, Falaschi GM, Toussan L, Ferri C, Falaschi P. Recent advances in the role of cortisol and metabolic syndrome in age-related degenerative diseases. Aging Clin Exp Res. 2015 doi: 10.1007/s40520-015-0353-0. [DOI] [PubMed] [Google Scholar]
- 9.Wang X, Hunter D, Xu J, Ding C. Metabolic triggered inflammation in osteoarthritis. Osteoarthritis Cartilage. 2015;23:22–30. doi: 10.1016/j.joca.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 10.Hawker G, Davis A, French M, Cibere J, Jordan J, March L, et al. Development and preliminary psychometric testing of a new OA pain measure - an OARSI/OMERACT initiative. Osteoarthritis Cartilage. 2008;16:409–14. doi: 10.1016/j.joca.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iannone F, Lapadula G. Obesity and inflammation–targets for OA therapy. Curr Drug Targets. 2010;11:586–98. doi: 10.2174/138945010791011857. [DOI] [PubMed] [Google Scholar]
- 12.Strittmatter M, Bianchi O, Ostertag D, Grauer M, Paulus C, Fischer C, et al. Altered function of the hypothalamic-pituitary-adrenal axis in patients with acute, chronic and episodic pain. Schmerz. 2005;19:109–16. doi: 10.1007/s00482-004-0330-6. [DOI] [PubMed] [Google Scholar]
- 13.Khoromi S, Muniyappa R, Nackers L, Gray N, Baldwin H, Wong KA, et al. Effects of chronic osteoarthritis pain on neuroendocrine function in men. J Clin Endocrinol Metab. 2006;91:4313–8. doi: 10.1210/jc.2006-1122. [DOI] [PubMed] [Google Scholar]
- 14.Kudielka BM, Kirschbaum C. Sex differences in HPA axis responses to stress: a review. Biol Psychol. 2005;69:113–32. doi: 10.1016/j.biopsycho.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 15.Goel N, Workman JL, Lee TT, Innala L, Viau V. Sex differences in the HPA axis. Comprehensive Physiology. 2014;4:1121–55. doi: 10.1002/cphy.c130054. [DOI] [PubMed] [Google Scholar]
- 16.Bartley EJ, Fillingim RB. Sex differences in pain: a brief review of clinical and experimental findings. Br J Anaesth. 2013;111:52–8. doi: 10.1093/bja/aet127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Juster RP, Raymond C, Desrochers AB, Bourdon O, Durand N, Wan N, et al. Sex hormones adjust “sex-specific” reactive and diurnal cortisol profiles. Psychoneuroendocrinology. 2016;63:282–90. doi: 10.1016/j.psyneuen.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 18.Hunter DJ, Guermazi A, Roemer F, Zhang Y, Neogi T. Structural correlates of pain in joints with osteoarthritis. Osteoarthritis Cartilage. 2013;21:1170–8. doi: 10.1016/j.joca.2013.05.017. [DOI] [PubMed] [Google Scholar]
- 19.Srikanth VK, Fryer JL, Zhai G, Winzenberg TM, Hosmer D, Jones G. A meta-analysis of sex differences prevalence, incidence and severity of osteoarthritis. Osteoarthritis Cartilage. 2005;13:769–81. doi: 10.1016/j.joca.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 20.Sulon J, Demey-Ponsart L, Beauduin P, Sodoyez JC. Radioimmunoassay of corticosterone, cortisol and cortisone: their application to human cord and maternal plasma. J Steroid Biochem. 1978;9:671–6. doi: 10.1016/0022-4731(78)90180-2. [DOI] [PubMed] [Google Scholar]
- 21.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15:1833–40. [PubMed] [Google Scholar]
- 22.Keefe FJ, Brown GK, Wallston KA, Caldwell DS. Coping with rheumatoid arthritis pain: catastrophizing as a maladaptive strategy. Pain. 1989;37:51–6. doi: 10.1016/0304-3959(89)90152-8. [DOI] [PubMed] [Google Scholar]
- 23.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54:1063–70. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 24.Raudenbush S, Bryk A. Hierarchical LinearModels: Applications and Data Analysis Methods. 2. Thousand Oaks, CA: Sage; 2002. [Google Scholar]
- 25.MacKinnon DP, Fritz MS, Williams J, CM L. Distribution of the product confidence limits for the indirect effect: Program PRODCLIN. Behav Res Methods. 2007:384–9. doi: 10.3758/bf03193007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kenny DA, Korchmaros JD, B N. Lower level mediation in multilevel models. Psychol Methods. 2003:115–28. doi: 10.1037/1082-989x.8.2.115. [DOI] [PubMed] [Google Scholar]
- 27.Turner-Cobb JM, Osborn M, da Silva L, Keogh E, Jessop DS. Sex differences in hypothalamic-pituitary-adrenal axis function in patients with chronic pain syndrome. Stress. 2010;13:292–300. doi: 10.3109/10253890903524785. [DOI] [PubMed] [Google Scholar]
- 28.Somers TJ, Keefe FJ, Godiwala N, Hoyler GH. Psychosocial factors and the pain experience of osteoarthritis patients: new findings and new directions. Curr Opin Rheumatol. 2009;21:501–6. doi: 10.1097/BOR.0b013e32832ed704. [DOI] [PubMed] [Google Scholar]
- 29.Finan P, Quartana P, Smith M. Positive and negative affect dimensions in chronic knee osteoarthritis: effects on clinical and laboratory pain. Psychosom Med. 2013 Jun;75:463–70. doi: 10.1097/PSY.0b013e31828ef1d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clark LA, Watson D, Mineka S. Temperament, personality, and the mood and anxiety disorders. J Abnorm Psychol. 1994;103:103–16. [PubMed] [Google Scholar]
- 31.Hawker GA, Gignac MA, Badley E, Davis AM, French MR, Li Y, et al. A longitudinal study to explain the pain-depression link in older adults with osteoarthritis. Arthritis Care Res (Hoboken) 2011;63:1382–90. doi: 10.1002/acr.20298. [DOI] [PubMed] [Google Scholar]
- 32.Somers TJ, Keefe FJ, Carson JW, Pells JJ, Lacaille L. Pain catastrophizing in borderline morbidly obese and morbidly obese individuals with osteoarthritic knee pain. Pain Res Manag. 2008;13:401–6. doi: 10.1155/2008/652453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cowen P. Cortisol, serotonin and depression: all stressed out? Br J Psychiatry. 2002:99–100. doi: 10.1192/bjp.180.2.99. [DOI] [PubMed] [Google Scholar]
- 34.Evans KD, Douglas B, Bruce N, Drummond PD. An exploratory study of changes in salivary cortisol, depression, and pain intensity after treatment for chronic pain. Pain Med. 2008;9:752–8. [Google Scholar]
- 35.Quartana PJ, Buenaver LF, Edwards RR, Klick B, Haythornthwaite JA, Smith MT. Pain catastrophizing and salivary cortisol responses to laboratory pain testing in temporomandibular disorder and healthy participants. J Pain. 2010;11:186–94. doi: 10.1016/j.jpain.2009.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vachon-Presseau E, Roy M, Martel MO, Caron E, Marin MF, Chen J, et al. The stress model of chronic pain: evidence from basal cortisol and hippocampal structure and function in humans. Brain. 2013;136:815–27. doi: 10.1093/brain/aws371. [DOI] [PubMed] [Google Scholar]
- 37.Allen KD, Coffman CJ, Golightly YM, Stechuchak KM, Voils CI, Keefe FJ. Comparison of pain measures among patients with osteoarthritis. J Pain. 2010;11:522–7. doi: 10.1016/j.jpain.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 38.Cimmino MA, Scarpa R, Caporali R, Parazzini F, Zaninelli A, Sarzi-Puttini P. Body mass and osteoarthritic pain: results from a study in general practice. Clin Exp Rheumatol. 2013;31:843–9. [PubMed] [Google Scholar]
- 39.Courties A, Gualillo O, Berenbaum F, Sellam J. Metabolic stress-induced joint inflammation and osteoarthritis. Osteoarthritis Cartilage. 2015 doi: 10.1016/j.joca.2015.05.016. [DOI] [PubMed] [Google Scholar]
- 40.Schaible H. Nociceptive neurons detect cytokines in arthritis. Arthritis Res Ther. 2014;16:470. doi: 10.1186/s13075-014-0470-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Edwards RR, Kronfli T, Haythornthwaite JA, Smith MT, McGuire L, Page GG. Association of catastrophizing with interleukin-6 responses to acute pain. Pain. 2008;140:135–44. doi: 10.1016/j.pain.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sturgeon JA, Darnall BD, Zwickey HL, Wood LJ, Hanes DA, Zava DT, et al. Proinflammatory cytokines and DHEA-S in women with fibromyalgia: impact of psychological distress and menopausal status. J Pain Res. 2014;7:707–16. doi: 10.2147/JPR.S71344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.de Brouwer SJ, Kraaimaat FW, Sweep FC, Creemers MC, Radstake TR, van Laarhoven AI, et al. Experimental stress in inflammatory rheumatic diseases: a review of psychophysiological stress responses. Arthritis Res Ther. 2010;12:R89. doi: 10.1186/ar3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kraemer HC, Giese-Davis J, Yutsis M, O’Hara R, Neri E, Gallagher-Thompson D, et al. Design decisions to optimize reliability of daytime cortisol slopes in an older population. Am J Geriatr Psychiatry. 2006;14:325–33. doi: 10.1097/01.JGP.0000201816.26786.5b. [DOI] [PubMed] [Google Scholar]
- 45.Collins JE, Katz JN, Dervan EE, Losina E. Trajectories and risk profiles of pain in persons with radiographic, symptomatic knee osteoarthritis: data from the osteoarthritis initiative. Osteoarthritis Cartilage. 2014;22:622–30. doi: 10.1016/j.joca.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]


