Abstract
Sex hormones and white (and grey) matter in the limbic system, cortex and other brain regions undergo changes during adolescence. Some of these changes include ongoing white matter myelination and sexually dimorphic features in grey and white matter. Adolescence is also a period of vulnerability when many are first exposed to alcohol and cannabis, which appear to influence the developing brain. Neuropsychological studies have provided considerable understanding of the effects of alcohol and cannabis on the brain. Advances in neuroimaging have allowed examination of neuroanatomic changes, metabolic and neurotransmitter activity, and neuronal activation during adolescent brain development and substance use. In this review, we examine major differences in brain development between users and non-users, and recent findings on the influence of cannabis and alcohol on the adolescent brain. We also discuss associations that appear to resolve following short-term abstinence, and attentional deficits that appear to persist. These findings can be useful in guiding earlier educational interventions for adolescents, and clarifying the neural sequelae of early alcohol and cannabis use to the general public.
Keywords: cannabis, alcohol, adolescence, developing brain, positron emission tomography (PET), blood oxygen dependent level (BOLD), neuroimaging, neuropsychological studies
1. Introduction
Sex hormones and white (and grey) matter in the limbic system, cortex and other brain regions undergo changes during adolescence [1]. Increasingly high resolution and rapid acquisition neuroimaging techniques have allowed us to examine neuromaturation more carefully than in the past, permitting the identification of sex similarities and differences in brain development.
2. White matter development in adolescence
White matter development and pubertal staging are highly correlated [2]. White matter, consisting of myelin-coated axons, is primarily concentrated in the inner brain and facilitates communication between regions to create neural networks such as the cortico-cortical and cortico-subcortical connections [2]. These cortico-cortical and cortico-subcortical connections are critical in serving cognitive and mood functions [2].
Whole brain analyses have demonstrated age-related increases in white matter volume and fractional anisotropy (FA) bilaterally in a number of fiber tracts including the arcuate fasciculus and corticospinal tract [3]. FA changes are thought to be a consequence of increases in the diameter of axons forming fiber tracts resulting in increases in parallel diffusivity [3]. These observations have occurred in areas supporting speech and motor function (i.e., arcuate fasciculus and corticospinal tract) and are thought to enhance long distance connectivities in white matter tracts [3].
3. Grey matter development in adolescence
Grey matter, consisting predominantly of neuronal cell bodies, dendrites, synapses, and unmyelinated axons, serves to process information by means of directing sensory stimuli to nerve cells [4]. Grey matter also contains glial cells that function to transport nutrients and energy to neurons [4].
Grey matter and intelligence have demonstrated some very interesting correlations. Intelligence quotient (IQ) is positively correlated with grey matter cortical volume in the prefrontal cortex in adolescents [5–7]. Remodeling and ongoing maturation of the central nervous system in adolescence is thought to be mediated in part by age-related grey matter volume changes [3,8]. Overall reductions in cortical grey matter volume have been observed during adolescence [3]. This process has been described as grey matter undergoing synaptic pruning [3]. Synaptic pruning may result in more specialized functional networks and more efficient processing of information as some have suggested [9]. However, others have suggested that cortical volume changes of the scale detected by magnetic resonance imaging (MRI) are unlikely due primarily to synaptic pruning and instead may be the result of increased myelination of intra-cortical axons [10]. For girls, subcortical grey matter forebrain structures have been found to be at adult volumes. For boys, volumes of the same subcortical gray matter structures have been found to be larger than for adult males and are thought to undergo regression into adulthood [11,12]. Girls have significantly smaller volumes of cortical grey matter, but greater extraventricular cerebrospinal fluid (CSF) than boys [8].
Refined analytic approaches show that most brain regions, including mean cortical thickness, appear to demonstrate a linear, monotonic decrease in cortical thickness after 5 years of age and through adolescence [13]. A minority of areas, the bilateral temporo-parietal regions and the right prefrontal cortex, demonstrated cubic trajectories with peaks in cortical thickness at or before age 8 [13]. Males demonstrated faster occipital thinning than females [13].
The limbic system is well known to play an important role in emotion, behavior, motivation, long-term memory, and olfaction [14]. During adolescence, amygdala and hippocampal volume increase for both males and females [15]. However, amygdala volume increases significantly more in males than females while hippocampal volume increases more in females than males [15].
4. Sex differences in adolescent brain development
A number of sex differences in the developing brain have been found. Males have demonstrated volumetric increases in white matter compared to females [8]. However, males have shown faster occipital thinning than females. Differences in total cerebral volume between boys and girls have been noted with 10% larger volume in boys and primarily in the form of increased cortical gray matter [8]. However, after 5 years of age, neither boys nor girls show any major change in total cerebral volume [8]. These findings support the hypothesis that sex differences in brain size are related to cortical neuronal differences. Subcortical nuclei size was found to be the same between boys and girls. However, girls have proportionally greater extraventricular CSF but not ventricular CSF [8,15] compared to boys. Cerebral volumes adjusted for intracranial vault size have been found to differ in the mean volume of the caudate and globus pallidus between males and females; the volume of the caudate has been found to be larger in females whereas the volume of the globus pallidus has been found to be larger in males [15]. Lateral ventricles demonstrated increases in size in males but not in females during development after age 11 [15]. Boys have demonstrated faster increases in white matter volume than girls during adolescence [16]. Using diffusion tensor imaging, greater FA has been noted in white matter regions (including frontal lobe) in boys whereas greater FA has been noted in the splenium of the corpus callosum for girls [17].
In addition, greater mean diffusivity (MD) in boys has been seen in the corticospinal tract and frontal white matter in the right hemisphere [17]. In contrast, girls have shown greater MD in the occipito-parietal lobes and superior aspect of the corticospinal tract of the right hemisphere [17]. Mean diffusivity reflects the rate of water diffusion, independent of directionality and is an inverse measure of membrane density. Mean diffusivity of white matter has been found to correlate with cognitive performance (IQ, executive function) [17]. Mean diffusivity of the corticospinal tract was found to correlate with clinical scores related to motor function [17]. Finally, girls demonstrated greater fiber density increases with age than boys in associative regions based on MD values [11,12,16]. These sex differences may be responsible in part for many of the differences observed in prevalence, onset, and symptoms of psychiatric disorders of adolescence.
5. Effects of cannabis on adolescent brain development
Cannabis has been most well studied in heavy users rather than those who use sporadically where the effect of use is unclear. The most common form of cannabis, smoked, has seen an evolution in its composition over the past few decades. There has been an increase in the psychoactive component (delta-9-tetrahydrocannabinol or THC) and a decrease in the therapeutic component (cannabidiol). Some experts have suggested this has led to an increase in the persistence of neuroanatomic changes in the brain during adolescence [18,19]. We discuss some of these changes here, in addition to alterations in volumetric, grey matter density, and positron emission tomography activity recently observed in the literature.
5.1. Neuropsychological changes: Attention, memory, processing speed, and visuospatial functioning
Jacobus and colleagues [45] have found that heavy cannabis use in adolescence was linked to a number of neurocognitive deficits when studied in cannabis users whom also had co-occurring alcohol use. Extent of usage was quantified using the Customary Drinking and Drug Use Record, which allows assessment of lifetime alcohol, marijuana, cigarette, and other drug use [20]. Specifically, deficits were found in attention, memory, processing speed, and visuospatial functioning [21]. Medina and colleagues [22] also found that cannabis use was associated with slower psychomotor processing, poorer attention, story memory, and planning and sequencing ability. Cousijn et al. [21] found that working memory network functionality was not associated with moderate to heavy levels of cannabis use over a 3 year period. Number of lifetime cannabis use episodes was also associated with poorer cognitive function in spite of controlling for lifetime alcohol use. After one month of abstinence, adolescent cannabis users still showed subtle neuropsychological deficits compared to nonusers [22]. Hanson and colleagues [23] found that poorer verbal learning and working memory improved following 3 weeks of abstinence whereas attention deficits remained. They suggested that changes in the hippocampus, subcortical, and prefrontal cortex might be responsible for these findings [23]. Earlier onset of cannabis use was also associated with decreased processing speed and executive function 3 years later [21].
5.2. Neuroanatomic changes: Cortical thickness, volume, and white and grey matter changes
A number of neuroanatomic changes have been observed in heavy, long-term cannabis users. Cannabis users have been found to have thicker cortices in the left entorhinal cortex, even after 28 days of abstinence (see Figure 1). In addition, more lifetime cannabis use was found to be associated with thinner temporal and frontal cortices [24]. Regular cannabis users compared to controls were also found to have reduced hippocampal, prefrontal cortex, and amygdala volumes, in addition to larger cerebellum and striatum volumes [25]. These regions are thought to be high in cannabinoid type 1 (CB1) receptors where THC binds; the hippocampus has one of the highest densities of cannabinoid receptors [24]. THC is thought to accumulate in neurons and to become neurotoxic with chronic use [24]. There is also some evidence that changes in the hippocampus, cerebellum, prefrontal and lingual regions were associated with THC and cannabidiol levels, suggesting toxic and protective effects respectively [24]. Some studies have demonstrated that THC induces apoptosis in cultured cortical neurons [26]. Chronic THC use in animals has shown widespread CB1 receptor down-regulation and desensitization [27]. Greater dose and earlier starting age of use were also found to be associated with these changes [18]. In small preliminary studies, adolescents with heavy cannabis use have been found to have reduced fractional anisotropy, increased radial diffusivity, and increased trace in the frontotemporal connection via the arcuate fasciculus [28]. Lastly, higher gray matter density or a reduction in grey matter volume has been found in the amygdala, prefrontal cortex, parietal cortex, and striatum with THC use compared to controls [18,29].
Figure 1.
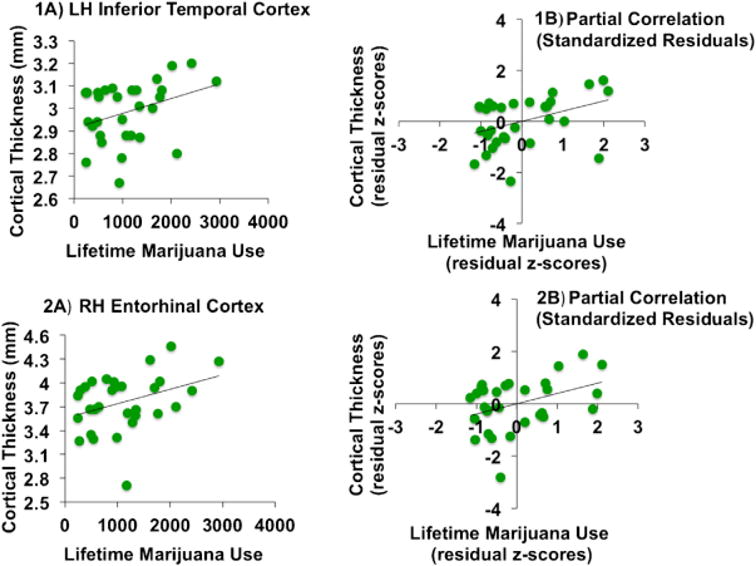
Bivariate relationships (A) and corresponding partial correlations (B) with lifetime marijuana use and cortical thickness, measured in millimeters (mm) (left (LH) and right (RH) hemisphere), controlling for lifetime alcohol use (n = 30) [21].*
*reprinted with permission of author, Joanna Jacobus, from her original paper [44]
5.3. Neurotransmitter functioning
Many studies using positron emission tomography (PET) have found that the administration of THC led to increased activity of the frontal and paralimbic regions, in addition to the cerebellum. Some authors have proposed that the pleasurable effects of THC are mediated by increases in activation of the paralimbic brain regions whereas distortions of time perception are mediated by activation of the cerebellum [30]. In chronic cannabis users, decreased resting perfusion of the cerebellum and frontal regions has been observed [30]. Iverson [31] explains this decrease in activity as a down-regulation of CB1 receptors and consequential inhibition of neurotransmitters such as gamma-aminobutyric acid (GABA), glutamate, dopamine, serotonin, and acetylcholine.
5.4 Summary of cannabis-related findings
The effects of cannabis on adolescent brain development span numerous domains. Neuropsychological deficits have been noted in attention, memory, processing speed, and visuospatial functioning [21]. Even after three weeks to one month of abstinence, cannabis users still showed poorer verbal learning and working memory than nonusers [22,23]. Neuroanatomic differences in cannabis users compared to non-users include thicker left entorhinal cortices and longer lifetime cannabis use was associated with thinner temporal and frontal cortices [24]. Reduced hippocampal, prefrontal cortex, and amygdala volumes and larger cerebellum and striatum volumes were also noted in regular cannabis users. Finally, a reduction in grey matter volume has been found in the amygdala, prefrontal cortex, parietal cortex, and striatum of cannabis users [18,29]. PET studies of neurotransmitter functioning have revealed increased activity of the frontal and paralimbic regions, in addition to the cerebellum following THC administration [30]. These findings have been summarized in Table 1 and Figure 1.
Table 1.
Summary of major findings of effects of cannabis and alcohol on developing, adolescent brain
| Neuropsychological | Neuroanatomical | Neurotransmitter | Brain response | |
|---|---|---|---|---|
| Cannabis | - ⬇ performance in attention, memory, processing speed, and visuospatial functioning; decreased processing speed and executive function (Jacobus et al. 2015) - ⬇ psychomotor processing, attention, story memory, and planning and sequencing ability; with abstinence, subtle neuropsychological deficits remained [22] - ⬇verbal learning and working memory improved following 3 weeks of abstinence whereas attention deficits remained [23] |
- ⬆ thickness of cortices in left entorhinal cortex; thinner temporal and frontal cortices (Jacobus et al. 2014) - ⬇hippocampal, prefrontal cortex, and amygdala volumes and larger cerebellum and striatum volumes (Lorenzetti, Solowij, and Yücel 2016) - ⬇ grey matter volume in amygdala, prefrontal cortex, parietal cortex, and striatum (Battistella et al. 2014) |
- ⬆activity of frontal and paralimbic regions, in addition to cerebellum (Chang and Chronicle 2007) - ⬇resting function of cerebellum and frontal regions (Iversen 2003) |
|
| Alcohol | ⬇ attention and executive function (Thoma et al. 2011) ⬆emotional reactivity, distress tolerance recovered with abstinence (Winward et al. 2014) -⬇ verbal memory and visuospatial ability, and more post-drinking effects and drug use were associated with slower psychomotor speed [42] - ⬇ visuospatial performance for females with more past year drinking [43] - ⬇ sustained attention for males with more past year hangover symptoms [43] |
-⬇left cingulate decreased cerebellar volumes and cerebellar activity during reward processing (Lisdahl et al. 2013; Cservenka Jones, and Nage 2015) -⬆ thickness of cortices in all four lobes (Jacobus et al. 2014) -⬇volume of left ventra diencephalon, left inferior and middle temporal gyrus, and left caudate and brain stem (Squeglia et al 2014) - ⬇ grey matter volume in lateral frontal and temporal regions ⬇ rates of white matter growth in corpus callosum and pons [40] |
- differences in response in bilateral caudate, orbitofrontal cortex, medial frontal cortex/anterior cingulate and left insula (Dager et al. 2014) - changes in response for spatial working memory tasks despite adequate performance (Tapert et al. 2004) - ⬆response in bilateral parietal cortices and diminished response in other regions (left precentral gyrus, bilateral cerebellum) (Tapert et al. 2004) - ⬆BOLD activation in the bilateral striatum/globus pallidus, left anterior cingulate, bilateral cerebellum, and parahippocampal gyrus extending to the thalamus/substantia nigra; increased BOLD activation in response to alcohol cues in left anterior cingulate cortex and in right cerebellar region, though activation reduced to non-significance following abstinence [41] |
6. Effects of alcohol on adolescent brain development
The effects of alcohol observed depend on the severity of drinking during adolescence. In these studies, binge drinking was defined as ≥4 drinks/occasion for females, ≥5 drinks/occasion for males, moderate drinking encompassed binge drinking <13 of the previous 26 weeks, averaging ≤30 drinks/month, and heavy drinking included ≥30 drinks and ≥4 binges in a month [31]. Our discussion will focus on heavy drinkers as these compose most adolescent problematic drinkers.
6.1. Neuropsychological changes: Attention, executive function, emotional reactivity, distress tolerance
Heavy adolescent drinkers in general have been found to have overall poorer attention and executive function [32]. Heavy adolescent drinkers have also demonstrated heightened emotional reactivity and poorer distress tolerance than nondrinkers; however, these were recovered with abstinence [33]. Nguyen-Louie [35] and colleagues found that a greater number of alcohol use days was associated with worse verbal memory and visuospatial ability, and more post-drinking effects and drug use were associated with slower psychomotor speed compared to controls. Squeglia [36] and colleagues found that more drinking days in the past year in females was associated with worsened visuospatial performance whereas for boys, more past year hangover symptoms predicted worse sustained attention.
6.2. Neuroanatomic changes: Cerebellum, cortical thickness, and other regions
Outcome drinking classification was based on Cahalan et al. [34] and modified based on the distribution of drinking characteristics of adolescent males and females observed in the first two years of this project [35,36]. Heavy episodic drinking in adolescents has been associated with smaller cerebellar volumes and reduced cerebellar activity during reward processing [37,38] compared to controls. Heavy episodic drinking and lifetime alcohol use has been linked to thicker cortices in all four lobes [24]. Those subjects that transitioned into heavy drinking demonstrated smaller left cingulate, pars triangularis, and rostral anterior cingulate volume, and less right cerebellar white matter volumes compared to nondrinkers [39]. Those who drank heavily over the long term demonstrated greater reduction in volume of the left ventral diencephalon, left inferior and middle temporal gyrus, and left caudate and brain stem compared to nondrinkers [39]. Squeglia and colleagues also found that heavy drinking adolescents had increased rates of grey matter volume loss in the cortico-lateral frontal and temporal regions, in addition to decreased rates of white matter growth in the corpus callosum and pons compared to non-drinkers [40].
6.3. Brain activity
Blood oxygen dependent level (BOLD) activity has been found to predict escalating drinking and alcohol-related problems [31]. Those that transitioned into heavy drinking compared to moderate and heavy drinkers demonstrated group differences in BOLD response in the bilateral caudate, orbitofrontal cortex, medial frontal cortex/anterior cingulate and left insula [31]. BOLD response predicted best over family history of alcoholism or impulsivity [31]. Brumback and colleagues found that heavy drinkers also exhibited greater BOLD activation in the bilateral striatum/globus pallidus, left anterior cingulate, bilateral cerebellum, and parahippocampal gyrus extending to the thalamus/substantia nigra compared to nondrinkers over the course of one month. Increased BOLD activation was found in response to alcohol cues by heavy drinkers versus non-drinkers in the left anterior cingulate cortex and in the right cerebellar region, though this activation was reduced to non-significance following one month of abstinence [41].
Those with alcohol use disorders have also been found to have changes in BOLD response to spatial working memory tasks despite adequate performance compared to controls [36]. Those with alcohol use disorder demonstrated increased brain response during the task in the bilateral parietal cortices and diminished response in other regions (left precentral gyrus, bilateral cerebellum) compared to controls [36]. Some have suggested this is related to neuronal re-organization that occurs with early drinking [36].
6.4 Summary of alcohol-related findings
The observed associations of alcohol on the developing adolescent are many. Heavy drinking has been found to be associated with neuropsychological changes including poorer attention and executive function [32], heightened emotional reactivity [33], and poorer distress tolerance [33]. Neuroanatomic changes such as smaller cerebellar volumes and reduced cerebellar activity during reward processing have also been found in heavy drinkers [37,38]; thicker cortices in all four lobes in heavy drinkers have also been found [24]. Increased rates of grey matter volume loss in the cortical lateral frontal and temporal regions, in addition to decreased rates of white matter growth in the corpus callosum and pons have also been found in heavy drinkers compared to nondrinkers [40]. Finally, BOLD activity has been found to predict escalating drinking and alcohol-related problems. BOLD response predicted best over family history of alcoholism or impulsivity [31]. Heavy drinkers have also been found to have changes in BOLD response for spatial working memory tasks despite adequate performance [36]. These findings have been summarized in Table 1.
6.5. Caveats
A major caveat in interpreting these neural findings is that it is difficult to tease apart whether some of the changes observed are related to the development of alcohol use disorder versus a consequence of heavy alcohol use during critical periods of brain development; there is also some overlap in findings between these two. Squeglia and colleagues [40] have suggested that use of animal models may clarify these relationships. One animal model cited has been an example of rodents provided with high, intermittent dosing of alcohol that were found to exhibit possible neuroadaptation in response [40]. These rodents were found to have increased neuroimmune receptor expression for advanced glycation end products in the prefrontal cortex following extended periods in the absence of alcohol, but not in the interim period [40]. Similar caveats apply to cannabis use disorders. Brumback and colleagues [41] have also argued that the anterior cingulate cortex and cerebellar regions are associated with cue reactivity in those with alcohol and other substance use disorders. Whether these regions are also implicated as consequences of use during adolescent brain development is not clear but highlights the importance of considering overlap in regions identified [41].
7. Conclusions
Brain development in the growing adolescent is a very active time with major changes observed in the limbic system, cerebral white matter, grey matter, and a number of sex based differences. The influence of cannabis and alcohol on brain development during adolescence is significant across a number of areas that have been studied.
Adolescent cannabis users tend to perform more poorly than non-users on tasks requiring attention, memory, processing speed, visuospatial functioning, and executive functioning, while young alcohol users show poorer performance than non-drinkers on tasks of attention and executive functioning. Cannabis users show thicker cortices in the left entorhinal cortex, and thinner temporal and frontal cortices; in contrast, alcohol users demonstrate thicker cortices in all four lobes. Both cannabis and alcohol users show less brain volumes in many areas than non-users. In some studies, cannabis users have shown lower hippocampal, prefrontal cortex, and amygdala volumes than non-users, and less grey matter volume in the amygdala, prefrontal cortex, parietal cortex, and striatum than non-users. Alcohol users show smaller volumes of the left ventral diencephalon, left inferior and middle temporal gyrus, and left caudate and brain stem volumes than non-drinkers. Lastly, cannabis users show more PET activity of frontal and paralimbic regions and the cerebellum than non-users, while chronic cannabis users show lower resting function of the cerebellum and frontal regions than non-users. Heavy drinkers show differences in BOLD response in the bilateral caudate, orbitofrontal cortex, and medial frontal cortex/anterior cingulate and left insula, as compared to non-users.
A critical issue is if the differences between groups are provoked by exposure of the developing brain to alcohol and/or cannabis, or if these abnormalities were present prior to the onset of substance use. Only carefully designed longitudinal studies that initially characterized youth prior to the onset of any substance use, and with high follow-up rates and large enough samples sizes from which to observe transitions into various types and levels of substance use, can help evaluate the relationships between substance use and brain development.
The findings reviewed here suggest that developing brain in adolescence is sensitive to the use of cannabis and alcohol. Some effects appear to resolve following abstinence, whereas others appear to persist. For example, cannabis users have been found to have thicker cortices in the left entorhinal cortex even after 28 days of abstinence. However, lifetime cannabis use has been associated with thinner temporal and frontal cortices. While heavy drinkers have been found to have poorer attention and executive function long term, though emotional reactivity and distress tolerance are recovered with abstinence. These findings can help guide clinicians, parents, and adolescents about the consequences of alcohol and cannabis use.
Acknowledgments
This work was made possible in part by a training grant NIMH R25 MH101072 (PI: Swerdlow) that provided salary support for research track Resident Alejandro Meruelo, as well as a NIDA-AACAP Resident Training Award in Substance Abuse and Addiction (PI: Meruelo) that provided salary support for research assistant Norma Castro. Data collection was funded by grant 2R01 AA013419 (PI: Tapert).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: The authors above have no financial conflicts of interest to declare.
References
- 1.Arain M, Haque M, Johal L, Mathur P, Nel W, Rais A, Sandhu R, Sharma S. Maturation of the adolescent brain. Neuropsychiatr Dis Treat. 2013;9:449–461. doi: 10.2147/NDT.S39776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ladouceur CD, Peper JS, Crone EA, Dahl RE. White matter development in adolescence: The influence of puberty and implications for affective disorders. Dev Cogn Neurosci. 2012;2:36–54. doi: 10.1016/j.dcn.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giorgio A, Watkins KE, Chadwick M, James S, Winmill L, Douaud G, De Stefano N, Matthews PM, Smith SM, Johansen-Berg H, James AC. Longitudinal changes in grey and white matter during adolescence. NeuroImage. 2010;49:94–103. doi: 10.1016/j.neuroimage.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 4.Purves D, George A, David F, William H, LaMantia AS, McNamara J, White L. Neuroscience. 4th. Sinauer Associates; 2008. [Google Scholar]
- 5.Menary K, Collins PF, Porter JN, Muetzel R, Olson EA, Kumar V, Steinbach M, Lim KO, Luciana M. Associations between cortical thickness and general intelligence in children, adolescents and young adults. Intelligence. 2013;41:597–606. doi: 10.1016/j.intell.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frangou S, Chitins X, Williams SCR. Mapping IQ and gray matter density in healthy young people. NeuroImage. 2004;23:800–805. doi: 10.1016/j.neuroimage.2004.05.027. [DOI] [PubMed] [Google Scholar]
- 7.Wilke M, Kraegeloh-Mann I, Holland SK. Global and local development of gray and white matter volume in normal children and adolescents. Exp Brain Res. 2007;178:296–307. doi: 10.1007/s00221-006-0732-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reiss AL, Abrams MT, Singer HS, Ross JL, Denckla MB. Brain development, gender and IQ in children. A volumetric imaging study. Brain J Neurol. 1996;119(Pt 5):1763–1774. doi: 10.1093/brain/119.5.1763. [DOI] [PubMed] [Google Scholar]
- 9.Blakemore SJ. The social brain in adolescence. Nat Rev Neurosci. 2008;9:267–277. doi: 10.1038/nrn2353. [DOI] [PubMed] [Google Scholar]
- 10.Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci. 2008;9:947–957. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caviness VS, Kennedy DN, Richelme C, Rademacher J, Filipek PA. The Human Brain Age 7–11 Years: A Volumetric Analysis Based on Magnetic Resonance Images. Cereb Cortex. 1996;6:726–736. doi: 10.1093/cercor/6.5.726. [DOI] [PubMed] [Google Scholar]
- 12.De Bellis MD, Keshavan MS, Beers SR, Hall J, Frustaci K, Masalehdan A, Noll J, Boring AM. Sex differences in brain maturation during childhood and adolescence. Cereb Cortex N Y N 1991. 2001;11:552–557. doi: 10.1093/cercor/11.6.552. [DOI] [PubMed] [Google Scholar]
- 13.Ducharme S, Albaugh MD, Nguyen T-V, Hudziak JJ, Mateos-Pérez JM, Labbe A, Evans AC, Karama S. Brain Development Cooperative Group, Trajectories of cortical thickness maturation in normal brain development–The importance of quality control procedures. NeuroImage. 2016;125:267–279. doi: 10.1016/j.neuroimage.2015.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morgane PJ, Galler JR, Mokler DJ. A review of systems and networks of the limbic forebrain/limbic midbrain. Prog Neurobiol. 2005;75:143–160. doi: 10.1016/j.pneurobio.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 15.Giedd JN, Castellanos FX, Rajapakse JC, Vaituzis AC, Rapoport JL. Sexual dimorphism of the developing human brain. Prog Neuropsychopharmacol Biol Psychiatry. 1997;21:1185–1201. doi: 10.1016/s0278-5846(97)00158-9. [DOI] [PubMed] [Google Scholar]
- 16.Schmithorst VJ, Holland SK, Dardzinski BJ. Developmental differences in white matter architecture between boys and girls. Hum Brain Mapp. 2008;29:696–710. doi: 10.1002/hbm.20431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alexander AL, Lee JE, Lazar M, Field AS. Diffusion Tensor Imaging of the Brain. Neurother J Am Soc Exp Neurother. 2007;4:316–329. doi: 10.1016/j.nurt.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lorenzetti V, Solowij N, Yücel M. The Role of Cannabinoids in Neuroanatomic Alterations in Cannabis Users. Biol Psychiatry. 2016;79:e17–e31. doi: 10.1016/j.biopsych.2015.11.013. [DOI] [PubMed] [Google Scholar]
- 19.Lorenzetti V, Solowij N, Fornito A, Lubman DI, Yucel M. The association between regular cannabis exposure and alterations of human brain morphology: an updated review of the literature. Curr Pharm Des. 2014;20:2138–2167. doi: 10.2174/13816128113199990435. [DOI] [PubMed] [Google Scholar]
- 20.Brown SA, Myers MG, Lippke L, Tapert SF, Stewart DG, Vik PW. Psychometric evaluation of the Customary Drinking and Drug Use Record (CDDR): a measure of adolescent alcohol and drug involvement. J Stud Alcohol. 1998;59:427–438. doi: 10.15288/jsa.1998.59.427. [DOI] [PubMed] [Google Scholar]
- 21.Jacobus J, Squeglia LM, Infante MA, Castro N, Brumback T, Meruelo AD, Tapert SF. Neuropsychological Performance in Adolescent Marijuana Users with Co-Occurring Alcohol Use: A Three-Year Longitudinal Study. Neuropsychology. 2015;29:829–843. doi: 10.1037/neu0000203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Medina KL, Hanson KL, Schweinsburg AD, Cohen-Zion M, Nagel BJ, Tapert SF. Neuropsychological functioning in adolescent marijuana users: subtle deficits detectable after a month of abstinence. J Int Neuropsychol Soc JINS. 2007;13:807–820. doi: 10.1017/S1355617707071032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanson KL, Winward JL, Schweinsburg AD, Medina KL, Brown SA, Tapert SF. Longitudinal study of cognition among adolescent marijuana users over three weeks of abstinence. Addict Behav. 2010;35:970–976. doi: 10.1016/j.addbeh.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jacobus J, Squeglia LM, Sorg SF, Nguyen-Louie TT, Tapert SF. Cortical Thickness and Neurocognition in Adolescent Marijuana and Alcohol Users Following 28 Days of Monitored Abstinence. J Stud Alcohol Drugs. 2014;75:729–743. doi: 10.15288/jsad.2014.75.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cousijn J, Wiers RW, Ridderinkhof KR, van den Brink W, Veltman DJ, Goudriaan AE. Grey matter alterations associated with cannabis use: results of a VBM study in heavy cannabis users and healthy controls. NeuroImage. 2012;59:3845–3851. doi: 10.1016/j.neuroimage.2011.09.046. [DOI] [PubMed] [Google Scholar]
- 26.Downer EJ, Fogarty MP, Campbell VA. Tetrahydrocannabinol-induced neurotoxicity depends on CB1 receptor-mediated c-Jun N-terminal kinase activation in cultured cortical neurons. Br J Pharmacol. 2003;140:547–557. doi: 10.1038/sj.bjp.0705464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sim-Selley LJ. Regulation of cannabinoid CB1 receptors in the central nervous system by chronic cannabinoids. Crit Rev Neurobiol. 2003;15:91–119. doi: 10.1615/critrevneurobiol.v15.i2.10. [DOI] [PubMed] [Google Scholar]
- 28.Ashtari M, Cervellione KL, Hasan KM, Wu J, McIlree C, Kester H, Ardekani BA, Roofeh D, Szeszko PR, Kumra S. White matter development during late adolescence in healthy males: a cross-sectional diffusion tensor imaging study. NeuroImage. 2007;35:501–510. doi: 10.1016/j.neuroimage.2006.10.047. [DOI] [PubMed] [Google Scholar]
- 29.Battistella G, Fornari E, Annoni JM, Chtioui H, Dao K, Fabritius M, Favrat B, Mall JF, Maeder P, Giroud C. Long-Term Effects of Cannabis on Brain Structure. Neuropsychopharmacology. 2014;39:2041–2048. doi: 10.1038/npp.2014.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang L, Chronicle EP. Functional imaging studies in cannabis users. Neurosci Rev J Bringing Neurobiol Neurol Psychiatry. 2007;13:422–432. doi: 10.1177/1073858406296601. [DOI] [PubMed] [Google Scholar]
- 31.Dager AD, Anderson BM, Rosen R, Khadka S, Sawyer B, Jiantonio-Kelly RE, Austad CS, Raskin SA, Tennen H, Wood RM, Fallahi CR, Pearlson GD. fMRI Response to Alcohol Pictures Predicts Subsequent Transition to Heavy Drinking in College Students. Addict Abingdon Engl. 2014;109:585–595. doi: 10.1111/add.12437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thoma RJ, Monnig MA, Lysne PA, Ruhl DA, Pommy JA, Bogenschutz M, Tonigan JS, Yeo RA. Adolescent Substance Abuse: The Effects of Alcohol and Marijuana on Neuropsychological Performance. Alcohol Clin Exp Res. 2011;35:39–46. doi: 10.1111/j.1530-0277.2010.01320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Winward JL, Bekman NM, Hanson KL, Lejuez CW, Brown SA. Changes in emotional reactivity and distress tolerance among heavy drinking adolescents during sustained abstinence. Alcohol Clin Exp Res. 2014;38:1761–1769. doi: 10.1111/acer.12415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cahalan D, Cisin IH, Crossley HM. American drinking practices: a national study of drinking behavior and attitudes, Publications Division, Rutgers Center of Alcohol Studies; distributed by College & University Press, New Haven, Conn. 1969 https://books.google.co.in/books?id=vd5rAAAAIAAJ.
- 35.Schweinsburg AD, Schweinsburg BC, Cheung EH, Brown GG, Brown SA, Tapert SF. fMRI response to spatial working memory in adolescents with comorbid marijuana and alcohol use disorders. Drug Alcohol Depend. 2005;79:201–210. doi: 10.1016/j.drugalcdep.2005.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tapert SF, Schweinsburg AD, Barlett VC, Brown SA, Frank LR, Brown GG, Meloy MJ. Blood oxygen level dependent response and spatial working memory in adolescents with alcohol use disorders. Alcohol Clin Exp Res. 2004;28:1577–1586. doi: 10.1097/01.alc.0000141812.81234.a6. [DOI] [PubMed] [Google Scholar]
- 37.Lisdahl KM, Thayer R, Squeglia LM, McQueeny TM, Tapert SF. Recent binge drinking predicts smaller cerebellar volumes in adolescents. Psychiatry Res. 2013;211:17–23. doi: 10.1016/j.pscychresns.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cservenka A, Jones SA, Nagel BJ. Reduced cerebellar brain activity during reward processing in adolescent binge drinkers. Dev Cogn Neurosci. 2015;16:110–120. doi: 10.1016/j.dcn.2015.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Squeglia LM, Rinker DA, Bartsch H, Castro N, Chung Y, Dale AM, Jernigan TL, Tapert SF. Brain volume reductions in adolescent heavy drinkers. Dev Cogn Neurosci. 2014;9:117–125. doi: 10.1016/j.dcn.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Squeglia LM, Tapert SF, Sullivan EV, Jacobus J, Meloy MJ, Rohlfing T, Pfefferbaum A. Brain Development in Heavy Drinking Adolescents. Am J Psychiatry. 2015;172:531–542. doi: 10.1176/appi.ajp.2015.14101249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brumback T, Squeglia LM, Jacobus J, Pulido C, Tapert SF, Brown SA. Adolescent heavy drinkers’ amplified brain responses to alcohol cues decrease over one month of abstinence. Addict Behav. 2015;46:45–52. doi: 10.1016/j.addbeh.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nguyen-Louie TT, Castro N, Matt GE, Squeglia LM, Brumback T, Tapert SF. Effects of Emerging Alcohol and Marijuana Use Behaviors on Adolescents’ Neuropsychological Functioning Over Four Years. J Stud Alcohol Drugs. 2015;76:738–748. doi: 10.15288/jsad.2015.76.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Squeglia LM, Spadoni AD, Infante MA, Myers MG, Tapert SF. Initiating moderate to heavy alcohol use predicts changes in neuropsychological functioning for adolescent girls and boys. Psychol Addict Behav J Soc Psychol Addict Behav. 2009;23:715–722. doi: 10.1037/a0016516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jacobus J, Squeglia LM, Meruelo AD, Castro N, Brumback T, Giedd JN, Tapert SF. Cortical thickness in adolescent marijuana and alcohol users: A three-year prospective study from adolescence to young adulthood. Dev Cogn Neurosci. 2015;16:101–109. doi: 10.1016/j.dcn.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]


