Abstract
Allergens are foreign proteins or glycoproteins that are the target of IgE antibody responses in humans. The relationship between subsequent exposure and the allergic symptoms is often or usually obvious; however, there is increasing evidence that in asthma, atopic dermatitis and some forms of food allergy the induction of symptoms is delayed or chronic. The primary exposure to inhaled allergens is to the particles, which are capable of carrying allergens in the air. Thus, the response reflects not only the properties of the proteins, but also the biological properties of the other constituents of the particle. This is best understood in relation to the mite fecal particles in which the contents include many different immunologically active substances. Allergic disease first became a major problem over 100 years ago, and for many years sensitization to pollens was the dominant form of these diseases. The rise in pediatric asthma correlates best with the move of children indoors, which started in 1960 and was primarily driven by indoor entertainment for children. While the causes of the increase are not simple they include both a major increase in sensitization to indoor allergens and the complex consequences of inactivity. Most recently, there has also been an increase in food allergy. Understanding this has required a reappraisal of the importance of the skin as a route for sensitization. Overall, understanding allergic diseases requires knowing about the sources, the particles and the routes of exposure as well as the properties of the individual allergens.
Keywords: Allergen immunochemistry, Hay fever, Hygiene, IgE antibody titer, Pediatric asthma
Introduction
In public usage an allergy is a reaction that follows exposure to a foreign agent with a predictable time relationship. In many cases, this implies a rapid or immediate relationship but patients can be “allergic” to poison ivy, which takes 6–24 h, or to red meat in the alpha-gal syndrome which takes 3–6 h.1,2 Allergic diseases can be characterized by the nature of the immune response that gives rise to the unwanted symptoms or by the form of the clinical presentation (Table 1). Many of these diseases have rapid onset after exposure, which greatly simplifies identifying the cause. Thus, exposure to a cat can cause sneezing, eye itching and wheezing within a few minutes; similarly the sting of a wasp or yellow jacket can cause generalized hives within 10–15 min, and eating peanuts can cause full-blown anaphylaxis within 20 min. On the other hand, there are many allergic diseases for which the symptoms take longer to develop, and the symptoms may be much less obviously related to the relevant exposure. In addition, there are now several situations in which the route of exposure that gives rise to sensitization is not the same as the route that precipitates symptoms (Table 2)
Table 1.
Allergic diseases.
| Source | Mechanism/Exposure | |
|---|---|---|
| Immediate reactions: | ||
| Venom anaphylaxis | Bee, wasp or fire ant stings | IgE + injected |
| Penicillin anaphylaxis | Penicillins (oral or injected) | IgE + injected or swallowed |
| Peanut anaphylaxis (Other foods) | Peanut products (oral or skin) | IgE + swallowed |
| Immediate + Delayed or chronic reactions: | ||
| Seasonal allergic rhinitis | Pollen grains | IgE + inhaled |
| Allergic asthma | Dust mite, cockroach, cat, dog, Alternaria | IgE + inhaled |
| Non-immediate reactions: | ||
| Atopic dermatitis | Many allergens (both food and inhalant) + Infection of the skin | IgE and T cells + multiple routes of exposure |
| Poison ivy | Chemicals in the plant | T cells + contact |
| Delayed anaphylaxis to red meat | Tick bites give sensitization to alpha-gal | IgE + oral mammalian meat |
Table 2.
Alternate routes of exposure.
| Source | Route | IgE response | Syndrome |
|---|---|---|---|
| Tick bites | Skin | IgE to alpha-gal | Delayed anaphylaxis to red meat |
| Peanuts (e.g., peanut butter) | Skin | IgE to Ara h 1 and Ara h 2 | Immediate reactions to oral peanut |
| Wheat in soap | Skin of face | IgE to wheat | Wheat dependent exercise induced anaphylaxis |
The three diseases included in Table 2 illustrate the fact that the skin is an excellent route for inducing IgE antibodies. The first example was actually schistosomiasis. In this case, Taliafero and Taliafero demonstrated in 1931 that the serum of patients with the disease could transfer skin sensitivity into the skin of non-infected individuals.3 The importance of the skin as a route of sensitization has now been established for three diseases: i) peanut allergy4; ii) delayed anaphylaxis to red meat in patients with IgE to galactose alpha-1, 3-galactose5; and iii) wheat sensitization related to wheat dependent exercise induced anaphylaxis6,7 (Table 2). It should also be noted that many of the patients with diseases shown in Table 1 do not describe an immediate response to exposure. In some cases, this may be because the inflammation in the tissues is induced by a T cell mechanism, but the apparent delay may also represent either a delayed inflammatory response following mast cell mediator release or the effects of chronic low dose exposure. There is also another allergic disease characterized by IgE to milk for which a diet avoiding cow’s milk can be an effective treatment; however, the relevance of the IgE antibodies to the disease is not clear.8–10 This disease is eosinophilic esophagitis, which presents a special challenge because the disease includes a progressive eosinophil-rich inflammation without any obvious immediate phase.
Size of particles and proteins: relevance to sensitization
Particles and the delivery of inhalant allergens
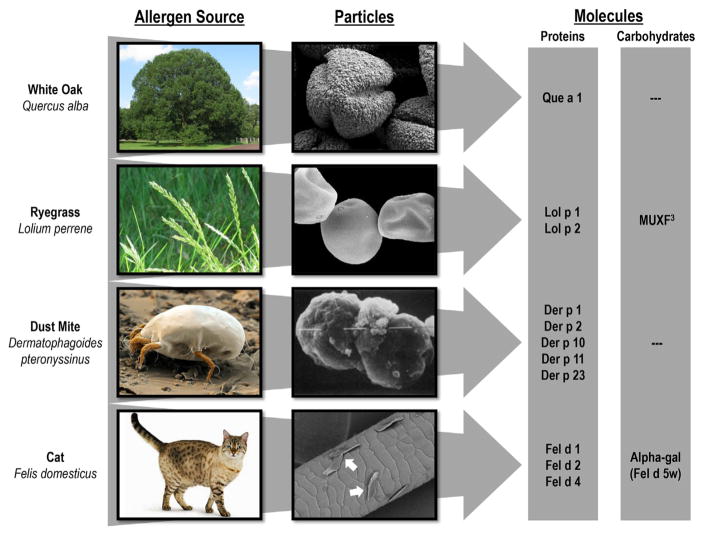
The purification of allergens started in the 1960’s with ragweed and grass pollen, which were the major causes of hay fever in the USA and UK respectively.11–13 At that time it was already recognized that exposure depended on both the nature of the proteins and the properties of the particles carrying them. It cannot be stressed too strongly that there is no such thing as airborne protein molecules—the saturated vapor pressure of molecules that are ≥5 kDa is close to zero. Thus the only relevant source of airborne allergens is on particles that are capable of becoming airborne (Fig. 1). For the outdoor exposures, it is possible both to count and identify pollen grains or fungal spores. However, this counting depends on the fact that these particles stay airborne. By contrast, the indoor environment is characterized by very low rates of air movement and particles as big as mite fecal particles or pollen grains fall rapidly. There are major differences between the particles on which dust mite and cat allergens become airborne.14–16 The way in which small particles or flakes of dander remain airborne leads to very different calculations about the quantity of allergen that can be inhaled. However, it is worth remembering that a large proportion of the particles inhaled through the mouth will impact on the back of the throat, and are subsequently swallowed.
Fig. 1.
White oak, ryegrass, dust mite, and cat allergens including the relevant particles and molecules.
The best studied of the allergen particles are the fecal particles of dust mites. After the purification of the group I and group II proteins it rapidly became clear that the major form in which they become airborne was on these particles.17,18 In addition, it was already suggested that Der p 1 was a digestive enzyme. With the cloning of Der p 1, it became clear that it had major homology with cysteine proteases.1,19 A whole series of studies were carried out on the relevance of the enzymatic activity, and this was followed by the increasing realization that the fecal particles were a veritable treasure trove of toll-like receptor ligands, as well as foreign proteins (Table 3). The message is clearly that these particles have great potential to induce an IgE response, and also that it is very unlikely that the response can be attributed to one of these constituents alone.
Table 3.
Immunostimulating effects of mite fecal particles: allergens and pathogen-associated molecular patterns [PAMPS].
| I | The dust mite allergen Der p 1 is a cysteine protease:
|
| II | Der p 2 is a homolog of the adapter protein MD-2 and can facilitate LPS-mediated signaling through TLR4 |
| III | Other PAMPs and their targets:
|
Cat dander particles appear to be “sticky” and can be found on walls and clothing, as well as distributed widely in buildings which do not have an animal present. Again, this has nothing to do with the properties of Fel d 1. However, it is clear that many children, who have never lived in a house with a cat, become sensitized to Fel d 1. This raises the question about how much community exposure is influenced by the prevalence of cat ownership in the community.20,21 Clearly, there must be a difference between a community such as the low income area of Atlanta where less than 5% of homes have a cat and New Zealand where over 50% of homes have a cat. There is good evidence that children who live in a house with a cat are less likely to become sensitized. Indeed, a major birth cohort in Detroit reported that children who had a cat at home during the first year of life were still less likely to be sensitized to cat allergens at the age of 18 years.22 In the OLIN cohort in Norbotten, where ~25% of the homes have a cat, it appears that 80% of the cat sensitized children at age 12 did not currently live in a house with a cat.23 At age 19, 77% of the subjects with high titer IgE to cat did not live in a house with a cat. With this model, it is not surprising that cat sensitization is actually less common in New Zealand where 50% of the homes have cats.24,25
Proteins and glycoproteins
Purified allergens were initially defined by their molecular weight and by specific antibodies. This was followed by the production of monoclonal antibodies, sequencing and x-ray crystallography. An important development came with the definition of the nomenclature and the establishment of an official IUIS subcommittee (see www.allergen.org).26 Today, we know the full structure of many hundreds of allergens, and it is increasingly possible to assay IgE antibodies to specific proteins rather than to the extract.27 For some allergen sources there is enough evidence to recommend assay of IgE to specific proteins in order to define the relevance of the IgE response. This is best recognized for peanut allergy, for cat, and for the new syndromes related to meat allergy.28,29 In addition, there are important cross-reactivities such as those between the tropomyosins, the lipocalins, and the albumins that can only be correctly understood using component assays (Table 4A–C). It is important to remember that identifying component specific sensitivity is only practical using in vitro IgE assays.
Table 4A.
Component analysis of IgE response to peanut and birch.
| Allergen | Peanut anaphylaxis | Oral allergy syndrome |
|---|---|---|
| Peanut | 139 | 16.7 |
| Ara h 1 | 20.5 | <0.35 |
| Ara h 2 | 90.3 | <0.35 |
| Ara h 3 | 16 | <0.35 |
| Ara h 8 | 0.6 | 44.6 |
| Birch pollen | 0.8 | 62 |
| Bet v 1 | 1.0 | 85 |
The bold numbers are the positive values.
Table 4C.
Comparison of total IgE and specific IgE titers in three syndromes.
| Allergen | Eosinophilic esophagitis† | Eosinophilic esophagitis† | Eosinophilic esophagitis | Peanut anaphylaxis† | Alpha-gal |
|---|---|---|---|---|---|
| Wheat | 0.85 | 0.41 | 1.18 | 2.04 | <0.35 |
| Milk | 1.68 | 2.32 | <0.35 | 3.62 | <0.35 |
| Egg | 0.68 | <0.35 | <0.35 | 3.49 | <0.35 |
| Soybean | <0.35 | 0.39 | <0.35 | 16.8 | <0.35 |
| Peanut | <0.35 | <0.35 | <0.35 | 473 | <0.35 |
| Alpha-gal | <0.35 | <0.35 | <0.35 | <0.35 | 32.3 |
| Total IgE | 63.7 | 141 | 19.2 | 1284 | 180 |
Each column represents an individual case.
The bold numbers are the positive values.
Denotes pediatric case.
Most allergens identified to date are water-soluble proteins with a molecular weight between 5 kDa and 50 kDa. While many of these proteins are glycosylated, in general, this glycosylation does not play a significant role in their allergenicity. It was traditionally considered that the basement membrane of the nose was only permeable to molecules <60 kDa. However, it is now clear that dendritic cells can sample proteins by extending their dendrites across the membrane, and thus, may be able to collect larger molecules. On the other hand, a recent study found that sensitization to Der p 11, which has a molecular weight of ~100 kDa, but forms aggregates of even higher molecular weight, was strongly associated with atopic dermatitis.29 This suggests that molecules of this size are more likely to give rise to sensitization than smaller molecules, if they can penetrate the skin.
The use of component assays
Peanut sensitization (Table 4A)
Peanut allergy has dramatically increased in prevalence and the severity of the clinical reactions correlates broadly with high titer IgE to Ara h 1 and Ara h 2 28. In some climatic areas there is an important cross-reactivity that can confuse diagnosis. Specifically, the peanut protein Ara h 8, cross-reacts extensively with the birch pollen allergen Bet v 1, and readily gives rise to moderate levels of IgE antibody to peanut.30 However, this IgE is generally associated with oral allergy syndrome and not anaphylaxis (See examples in Table 4A). Because Ara h 8 is only a minor component of the peanut extract this may give rise to a confusing situation where the level of IgE to Ara h 8 is higher than the level of IgE to peanut extract.
Red meat cross-reactivity (Table 4B)
Table 4B.
Four syndromes in which sensitivity to mammalian allergens may require component analysis.
| Allergen | Cat-allergic asthma | Pork cat | Alpha-gal | Meat anaphylaxis* |
|---|---|---|---|---|
| Cat extract | 85 | 4.3 | 33 | <0.35 |
| Fel d 1 | 90 | <0.35 | <0.35 | <0.35 |
| Cat albumin | <0.35 | 6.2 | <0.35 | <0.35 |
| Cat IgA | ND | <0.35 | 6.8 | <0.35 |
| Alpha gal | <0.35 | <0.35 | 23 | <0.35 |
| Milk protein | <0.35 | <0.35 | 14 | 16.3 |
| Bos d 5 | <0.35 | <0.35 | <0.35 | 8.4 |
| Pork | <0.35 | 3.6 | 6.2 | 8.7 |
| Beef | <0.35 | 0.8 | 7.3 | 6.2 |
| Asthma | Yes | No | No | No |
| Allergic reactions: | ||||
| To pork | No | Yes | Yes | ± |
| To beef | No | No | Yes | Yes |
The asterisk indicates that the patient is pediatric.
The bold numbers are the positive values.
The earliest descriptions of cross-reactivity leading to allergic reactions to meat relate to cow’s milk. Those children who present with allergic reactions to beef are in general already known to be allergic to cow’s milk.31 More recently, another syndrome was recognized in which patients who had positive skin tests and/or serum IgE assays for cat extract, presented with severe allergic reactions to pork. In these cases, the cross-reactivity relates to cat albumin and pork serum albumin.32,33 Interestingly, this sensitivity appears to relate primarily to exposure to a cat at home, but is not generally associated with asthma. The next syndrome also includes sensitivity to cat proteins, but in this case the relevant protein is cat IgA and the epitope is the oligosaccharide galactose alpha-1, 3-galactose (or alpha-gal). This oligosaccharide is present on many different mammalian proteins. Sensitization to alpha-gal is associated with two forms of anaphylaxis: i) immediate and often severe reactions to the monoclonal antibody cetuximab34 and ii) urticarial or anaphylactic reactions to red meat that start 3–6 h after eating.35 The nature of the response and the reasons for this IgE response to alpha-gal will be discussed later. Finally in Table 4B, we include a case of allergic asthma related to IgE to cat. In this case, the IgE is primarily directed at Fel d 1, which does not cross-react with any meat proteins.
Oligosaccharide IgE responses and their relevance to allergic symptoms
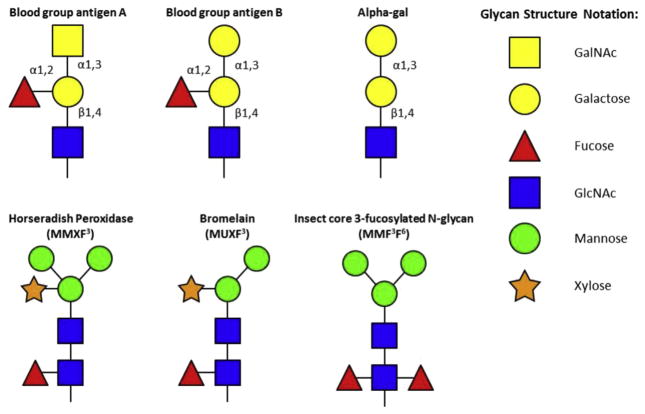
There have been occasional reports of IgE responses to oligosaccharides such as dextran, but the cases were not common enough for an analysis of reasons why subjects became sensitized. Around 1990, it became clear that many patients who were allergic to pollen had a proportion of their IgE directed against glycosylation motifs on the pollen proteins (Fig. 2).36,37 These IgE antibodies bind to many different proteins and can cause confusion in diagnosis. Furthermore, despite extensive investigation, no evidence could be found that IgE antibodies to these cross-reactive carbohydrate determinants (CCD’s), contributed to symptoms.38
Fig. 2.
Glycosylation motifs of mammalian blood group antigens as well as common insect- and plant-related CCDs. In contrast to insect- and plant-related CCDs, mammalian glycoproteins do not contain alpha-1,3-fucose or xylose moieties.
As we have already mentioned, there are also IgE antibodies to the oligosaccharide alpha-gal. These antibodies are present in individuals living in coastal New South Wales, Germany, Japan, France and Sweden, as well as a large area of the United States.39–43 In America these IgE antibodies were known to be common in NC, TN, AK, MI, and VA but were also present in surrounding states.5 This geographic localization initially led us investigate the possible role of ticks, and to follow the response to tick bites. In the USA, the tick involved is Amblyomma americanum (commonly known as the Lone Star Tick). In Australia, Ixodes holocyclus is responsible for these responses, and in Europe the relevant tick is Ixodes ricinus.39,42 In some cases, the response to these bites occurs within weeks and can dramatically increase the total IgE. In keeping with this, IgE antibodies to alpha-gal often make up a large proportion of the total IgE.5
In Japan and Singapore, another oligosaccharide-specific IgE response has been identified which may also be induced by a primary exposure through the skin. In Japan, subjects were identified because they had severe reactions to a soft drink that had been fortified with the prebiotic galacto-oligosaccharide (GOS).43 However, the subjects all worked as oyster shuckers and had been exposed to a specific sea squirt.43 Thus, in keeping with the role of tick bites, it is likely that sensitization in this case went through the skin. Sensitization to GOS has also been recognized in Singapore. In this case, it was baby food fortified with GOS that identified the cases, but the cause of the sensitization in that area is not clear.44
The sequence of allergic diseases in “post-hygiene” countries (Table 5)
Table 5.
Allergy epidemics of the last 100 years.
| Date | Relevant changes | Epidemic |
|---|---|---|
| 1870–1950 | Clean water and helminth eradication Increase in dairy herds and in the pollen of grass and ragweed |
Seasonal allergy |
| 1955–2000 | Onset of indoor lifestyle:
|
Pediatric asthma |
| ~1955 | Changes in peanut products:
|
Peanut allergy |
| ~2005 | Increase in tick bites secondary to the rising population of deer Free access of deer to suburban areas because of leash laws for dogs |
Delayed anaphylaxis to red meat (Alpha-gal) |
The primary measures of hygiene are clean water, control of helminth infections, shoes and probably some limited separation from farm animals.11 The major elements of these changes were achieved in London, Berlin, Munich, New York and Chicago by 1920 at the latest.45 In keeping with that, there were dramatic declines in infection disease mortality, particularly for cholera and typhoid.46 In the UK, Northern Germany, and New England there were also progressive rises in hay-fever related primarily to grass pollen in Europe and ragweed in the USA.11 The important thing here is that there was only modest awareness of the importance of the role of house dust before the 1960’s. Furthermore, the increase in pediatric asthma did not start until ~1960. Thus the second “epidemic” of allergic disease started long after allergic rhinitis was fully established.47 The rise in asthma was observed primarily in children and this disease had been uncommon among children before that time. Pediatric asthma has now been recognized as a problem in many different countries but this is only in countries, which are “post-hygiene”. Thus in the villages of Kenya, Papua New Guinea or Ecuador and the poor areas of the city of Kumasi in Uganda, allergic asthma in children is not yet a problem.48–50 Initially, the increase in asthma had been observed in countries where dust mites were being recognized as the primary source of perennial/indoor allergens. Thus, the increase was seen in the UK, Australia, New Zealand and Japan, long before it became clear that the same increase but on a smaller scale had been occurring in countries such as Finland and Sweden where the primary allergens related to asthma were those from animal dander.11,51,52 Equally, it was not until 1990 that it became clear that cockroach allergens were an important cause of sensitization among patients with asthma living in poverty in the USA.53–55
The common feature of pediatric asthma in different communities is that sensitization is primarily to indoor allergens. This can be seen very clearly in countries which have become westernized relatively recently such as Singapore and Costa Rica.48,56 The obvious reasons would be changes in housing conditions, more time spent indoors and dramatically decreased time spent outdoors. The move indoors was induced or made possible by the rise in indoor entertainment. This move has had multiple health consequences most of which have been harmful, with time spent in front of a screen being a major feature. So the primary contributors to asthma are a dramatic increase in the sensitization to indoor allergens and prolonged periods of time sitting still with inadequate expansion of the lungs11,57–59 (Table 5).
The most recent “epidemic” is the rise in food allergy, most notably to peanuts. Here the important observations are now clear: first, that sensitization to peanuts can occur through the skin; and second, that avoidance of peanut exposure in early childhood is likely to make the situation worse.4,60,61 However, the more relevant question here is whether regular washing of the skin, especially with detergents, has interfered with some element of the skin barrier. At present, there is good evidence that defects in the structural protein, filaggrin, can enhance sensitization through the skin, but this is most closely linked to eczema.62 Further work is warranted to test whether repeated washing compromises skin barrier integrity in a similar fashion.
Why do we make IgE?
In studies on children in tropical pre-hygiene villages or towns, the total serum IgE values are routinely over 400 IU/ml with mean values closer to 1000 IU/ml.48,49 In addition, it is well recognized that helminth infections such as ascariasis, schistosomiasis, anisakiasis and echinococcosis give rise to IgE responses. Following the establishment of clean water and helminth eradication, the major documented IgE responses have been to inhalant allergens such as pollen, dust mite and cat.1,11,25 Because of this, much of the analysis of IgE responses, including the genetics, has related to proteins derived from these sources. The primary conclusion is that proteins on particles inhaled through the nose or mouth can induce IgE responses in genetically predisposed individuals. For dust mite and the pollens, high titer IgE responses are common in areas where these allergens are common. Interestingly, high titer IgE responses to cat allergens are less common and this is particularly true among children raised in a house with a cat. Indeed, in communities where 20–30% of the homes have a cat, three quarters of the children with high titer IgE to Fel d 1 do not live in a house with a cat.23
As discussed earlier, high titer IgE responses can be initiated through the skin. In our practice in Virginia, high titer IgE responses are common in relation to peanut and also to alpha-gal, both of which are considered to go through the skin. In keeping with this, IgE antibodies are considered to have a direct mechanistic role in the diseases. In complete contrast, children and adults with eosinophilic esophagitis have low or very low titers of IgE to cow’s milk and wheat proteins. In this case, although cow’s milk and wheat appear to be important causes of the disease, there is very little evidence that IgE antibodies are mechanistically involved in the disease (Table 4C).
The ability to make IgE responses depends on initial recognition of allergen in the context of its associated molecules and particles, leading ultimately to activation of dendritic cells (DCs) or other antigen presenting cells (APC). In turn, this leads to priming of T cells that are capable of providing help to B cells for IgE production. This latter event involves the switch of B cells from IgM to IgE, which is mediated by IL-4 produced by Th2 cells. This event can occur within an organized germinal center (GC), or else by direct switch outside a GC.
Initial recognition of allergens and the activation or priming of dendritic cells and T cells
As has already been emphasized, we are not exposed to allergens as individual molecules. Thus the correct question is whether the overall content of a particle, or source, includes molecules that could have biological effect that would be expected to influence the IgE response. Several allergens have been shown to exert their effects via binding to pattern recognition receptors capable of triggering Th2 responses, and we list three examples:
Ara h 1 in its native form can induce Th2 skewing in naïve T cells, and this is dependent on glycosylation of the molecule, which facilitates binding to the C-type lectin receptor, DC-SIGN, on DCs.63
Der p 2 functions as an analog of MD2, acting to bind LPS to the toll-like receptor-4 molecule.64
Bermuda grass pollen binds DC-SIGN to initiate activation of DC’s, resulting in the production of TNF-alpha.65
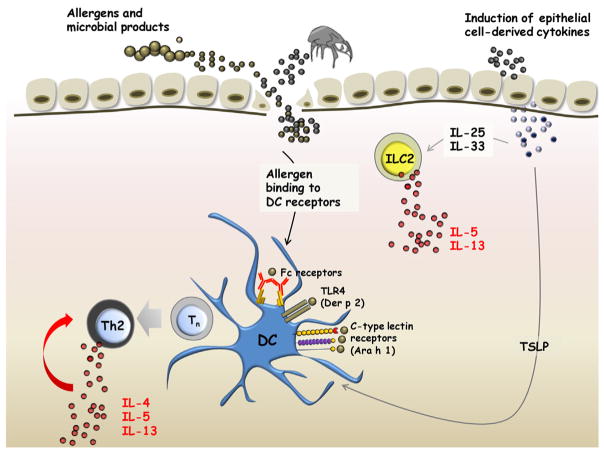
The evidence that different allergens can have significant pro-Th2 effects continues to accumulate. However, it is equally clear that an extremely diverse range of molecules are involved. Nonetheless, the dust mite remains one of the most impressive triggers of high titer IgE antibody responses. There are many studies where IgE to dust mites, including titers over 50 IU/ml (Class 5), are very strongly associated with asthma. This includes places as diverse as Singapore, Costa Rica, Taiwan, New Zealand and the UK, as well as Virginia in the USA.56,57,66,67 The range of biological effects that can occur in response to the contents of mite feces is so diverse that attributing the overall phenomenon to one constituent seems foolish (See Table 3, Fig. 3).
Fig. 3.
Role of allergens and epithelial cell-derived cytokines in induction of type 2 cytokines. Allergens trigger the release of TSLP, IL-25 and IL-33 from epithelial cells by binding to pattern recognition receptors. Allergens can also penetrate the subepithelial space through defects in the epithelial barrier. These defects may be structural (e.g., filaggrin deficiency) or else allergen-induced (eg. disrupted tight junctions arising from cleavage of occludin by Der p 1). Epithelial cell-derived cytokines induce IL-5 and IL-13 secretion by innate lymphoid cells, and, in conjunction with allergen, promote Th2 differentiation via dendritic cell-mediated pathways.
The role of epithelial cell-derived cytokines in Th2 responses
There is now extensive evidence that a broad range of allergens can act directly on epithelial cells to induce the production of a variety of Th2-promoting cytokines including TSLP, IL-25 and IL-3368(Fig. 3). Both in humans and in mice these molecules play a significant role in stimulating the activation of DCs and the development of Th2 cells from naïve T cells. More recently, these molecules have been implicated in the activation of innate lymphoid cells at epithelial surfaces that also serve to promote Th2 responses via production of the type 2 cytokines, IL-5 and IL-13.69 In relation to TSLP, experiments in mice engineered to overexpress TSLP or else knockout its receptor, strongly support its role in allergic inflammation.70,71 In humans, the evidence comes from the ability for TSLP-primed DCs to induce Th2 differentiation,72 and increased levels of TSLP in the bronchial mucosa of asthmatics and in skin lesions of patients with atopic dermatitis.73,74 Furthermore, there is in vitro evidence to indicate that optimal priming of DCs for Th2 responses occurs when TSLP and foreign antigen are combined, through a mechanism that involves antigen-mediated upregulation of the TSLP receptor.73,75
The possible mechanisms influencing the response to exposure via the skin, gastrointestinal tract or nose
Priming of DCs in the skin is highly relevant for antigens such as bee stings, tick bites and schistosomes. The skin may also be relevant for peanut, dust mite and cat allergens particularly in patients with eczema and/or a defect in the barrier function of the skin. The evidence about tick bites inducing IgE to alpha-gal, and peanut allergy in children avoiding oral exposure, is in keeping with the skin being an excellent site for priming of DCs and induction of Th2 cells. However, there is also extensive evidence that the skin is rich in Th1 and Th17 cells as well as Th2 cells.76,77 The obvious implication is that exposure through the skin is not inevitably a source of IgE rich responses. Indeed poison ivy, which is a very effective method of inducing a T cell response capable of recruiting eosinophils to the skin, does not include an IgE response. Similarly, the live viral vaccines applied to the skin, most obviously vaccinia itself, are remarkably effective at inducing long term immunity, but do not induce immediate hypersensitivity. Again, these aspects highlight the important contribution of the antigen itself and its associated molecules and particles.
The recent results on the “protective” effects of early oral exposure to peanut allergens confirmed that oral exposure can be, or normally is, a very effective method of inducing tolerance.61 Oral tolerance was well established using dinitrochlorobenzene (DNCB), which can dramatically inhibit sensitization through the skin in mice.78 Anecdotally the same thing is true for poison ivy in humans. The question then is, what is the mechanism for oral tolerance and is it similar for all food allergens? Sites have been identified in the oral cavity that have increased populations of T cells that preferentially express TGF-beta, IL-10, IFN-gamma and IL-17. The same sites have DCs expressing TLR-2 and TLR-4.79 The implication is that sublingual immunotherapy can specifically induce T-cell-mediated tolerance. Consistent with this notion, sublingual grass pollen (Phl p 5) has been shown to induce “tolerogenic” properties in Langerhans cells of the oral mucosa.80 Similarly, sublingual mite has been reported to increase circulating regulatory T cells.81 At present the functional and phenotypic differences between DCs in the oral cavity or gut compared to those in the skin, are not well established.
There are two questions related to oral tolerance that may be highly relevant to the control of allergic disease.
Firstly, is the tolerance to cat allergens that is common among children raised in a house with a cat, induced by exposure to cat allergen in the mouth or by swallowing?.23,82 Interestingly, students spending one or two years in a low allergen university setting, who had come from a house with a cat, had a highly significant fall in IgG antibodies to Fel d 1 with little change in their IgE antibodies to cat allergen.83 Thus, progressive decline in IgG antibodies may be a marker of loss of tolerance during periods of decreased exposure.
Secondly, it is well established that both allergen specific immunotherapy and seasonal increases in bee stings can induce enhanced production of IL-10 by circulating T cells. This IL-10 production is thought to be an effective enhancer of IgG4 antibodies while suppressing the switch to IgE.84 Whether oral exposure can achieve an equivalent degree of IL-10 production or tolerance it still not clear.
Summary and conclusions
We now have a truly remarkable amount of information about the allergens, which are the target for IgE antibodies. Protein or glycoprotein allergens from hundreds of sources have been purified, cloned, sequenced and crystallized (www.allergen.org). In addition, the biological properties of these allergens have been studied extensively. There are several properties of these allergens that stand out:
First, they are all foreign, for the pollens, fungi, insects and mites, we have been evolutionarily separate for 300 million years or more. Thus of all the sources only the mammals could be considered “close relatives”.
Second, although almost all the allergens are water-soluble proteins of small to moderate size, the inhalant allergens are always inhaled on particles. Thus it is the physical properties of these particles that allow them to become and remain airborne, but it is the cumulative biological and immunological effects of the contents of the particles that make the IgE response possible.
Third, for the main inhalant allergens the exposure that induces the immune response is similar to the exposure that causes symptoms. In the case of asthma, there is the added element that although exposure of the nose can induce an IgE ab response, the particles need to enter the lungs in order to cause the inflammation that underlies allergic asthma.
Fourth, in recent years, it has become clear that there are many other routes for exposure, and the skin in particular may be an excellent route for inducing IgE responses. This is not only true for insects, helminths, ticks and scabies mites that penetrate the skin, but also for allergens from peanut, wheat, cat and mite that penetrate the skin if the barrier function is disturbed. Same defects in barrier function are genetic, e.g. filaggrin, but in addition barriers can be disrupted by eczema or by excessive washing.
The increase in allergic disease over the last 100 years has not been unimodal. Initially, hay fever dominated our specialty and was epidemic by 1935. This increase related to the onset of hygiene, i.e., pathogen free water and helminth eradication, but also involved an increase in seasonal pollen. The rise in pediatric asthma started around 1960 and is best explained by the multiple effects of the change to an indoor life style. This included both increased exposure to and increased sensitization to the major perennial indoor allergens but also the disastrous effects of inactivity. Most recently many western countries have experienced a dramatic rise in food allergy, most obviously peanut. The primary causes of this are not yet clear. However, it is clear that avoidance of oral peanut exposure is not a method of preventing sensitization. What is increasingly clear is that all these epidemics relate to changes in human lifestyle.11 It is also probable that we will continue to experience changes in allergic disease and equally inevitable that we will not successfully predict the consequences of future changes in human behavior.
Acknowledgments
The research reported here was funded by NIH/NIAID, RO1-AI20565.
Abbreviations
- USA
United States of America
- UK
United Kingdom
- kDa
kilodalton
- OLIN
Obstructive Lung Disease in Northern Sweden Studies
- NC
North Carolina
- TN
Tennessee
- AK
Arkansas
- MI
Missouri
- VA
Virginia
- GOS
galacto-oligosaccharide
- IU/mL
international units per milliliter
- APC
antigen presenting cell
- DCs
dendritic cells
- IL
interleukin
- GC
germinal center
- DC-SIGN
dendritic cell-specific intercellular adhesion molecule-3 grabbing non-integrin
- MD2
lymphocyte antigen 96
- Th2
Type 2 (helper T cells)
- TSLP
thymic stromal lymphopoietin
- DNCB
dinitrochlorobenzene
- TGF
transforming growth factor
- TLR
Toll-like receptors
- IFN
interferon
- CCD
carbohydrate cross-reactive determinants
- MMXF3
horseradish peroxidase
- MUXF3
Bromelain
- MMF3F6
Insect core 3-fucosylated N-glycan
- GalNAc
N-Acetylgalactosamine
- GlcNAc
N-Acetylglucosamine
Footnotes
Peer review under responsibility of Japanese Society of Allergology.
Conflict of interest
The authors have no conflict of interest to declare.
References
- 1.Platts-Mills TA, Woodfolk JA. Allergens and their role in the allergic immune response. Immunol Rev. 2011;242:51–68. doi: 10.1111/j.1600-065X.2011.01021.x. [DOI] [PubMed] [Google Scholar]
- 2.Platts-Mills TA, Commins SP. Emerging antigens involved in allergic responses. Curr Opin Immunol. 2013;25:769–74. doi: 10.1016/j.coi.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taliafero WH, Taliafero LG. Skin reaction in persons infected with Schistosoma Mansoni. P R J Publ Health Trop Med. 1931;7:23. [Google Scholar]
- 4.Lack G, Fox D, Northstone K, Golding J. Factors associated with the development of peanut allergy in childhood. N Engl J Med. 2003;348:977–85. doi: 10.1056/NEJMoa013536. [DOI] [PubMed] [Google Scholar]
- 5.Commins S, James H, Kelly E, Pochan S, Workman L, Perzanowski M, et al. The relevance of tick bites to the production of IgE antibodies to the mammalian oligosaccharide galactose-α-1,3-galactose. J Allergy Clin Immunol. 2011;127:1286–93. doi: 10.1016/j.jaci.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yokooji T, Kurihara S, Murakami T, Chinuki Y, Takahashi H, Morita E. Characterization of causative allergens for wheat-dependent exercise-induced anaphylaxis sensitized with hydrolyzed wheat proteins in facial soap. Allergol Int. 2013;64:435–45. doi: 10.2332/allergolint.13-OA-0561. [DOI] [PubMed] [Google Scholar]
- 7.Tripathi A, Commins SP, Heymann PW, Platts-Mills TA. Diagnostic and experimental food challenges in patients with nonimmediate reactions to food. J Allergy Clin Immunol. 2015;135:985–7. doi: 10.1016/j.jaci.2014.11.032. [DOI] [PubMed] [Google Scholar]
- 8.Spergel JM, Brown-Whitehorn TF, Cianferoni A, Shuker M, Wang ML, Verma R, et al. Identification of causative foods in children with eosinophilic esophagitis treated with an elimination diet. J Allergy Clin Immunol. 2012;130:461–7. e5. doi: 10.1016/j.jaci.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 9.Erwin EA, James HR, Gutekunst HM, Russo JM, Kelleher KJ, Platts-Mills TA. Serum IgE measurement and detection of food allergy in pediatric patients with eosinophilic esophagitis. Ann Allergy Asthma Immunol. 2010;104:496–502. doi: 10.1016/j.anai.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kruszewski PG, Russo JM, Franciosi JP, Vrni JW, Platts-Mills TA, Erwin EA. Prospective, comparative effectiveness trial of cow’s milk elimination and swallowed fluticasone for pediatric eosinophilic esophagitis. Dis Esophagus. 2015 doi: 10.1111/dote.12339. http://dx.doi.org/10.1111/dote.12339. [DOI] [PubMed]
- 11.Platts-Mills T. The allergy epidemics: 1870–2010. J Allergy Clin Immunol. 2015;136:3–13. doi: 10.1016/j.jaci.2015.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.King T, Norman P. Isolation studies of allergens from ragweed pollen. Biochemistry. 1962;1:709. doi: 10.1021/bi00910a027. [DOI] [PubMed] [Google Scholar]
- 13.Johnson P, Marsh DG. ‘Isoallergens’ from rye grass pollen. Nature. 1965;206:935–7. doi: 10.1038/206935b0. [DOI] [PubMed] [Google Scholar]
- 14.Tovey ER, Chapman MD, Wells CW, Platts-Mills TA. The distribution of dust mite allergen in the houses of patients with asthma. Am Rev Respir Dis. 1981;124:630–5. doi: 10.1164/arrd.1981.124.5.630. [DOI] [PubMed] [Google Scholar]
- 15.Luczynska CM, Li Y, Chapman MD, Platts-Mills TA. Airborne concentrations and particle size distribution of allergen derived from domestic cats (Felis domesticus). Measurements using cascade impactor, liquid impinger, and a two-site monoclonal antibody assay for Fel d I. Am Rev Respir Dis. 1990;141:361–7. doi: 10.1164/ajrccm/141.2.361. [DOI] [PubMed] [Google Scholar]
- 16.de Blay F, Sanchez J, Hedelin G, Perez-Infante A, Verot A, Chapman MD, et al. Dust and airborne exposure to allergens derived from cockroah (Blattella germanica) in low-cost public housing in Strasbourg (France) J Allergy Clin Immunol. 1997;99:107–12. doi: 10.1016/s0091-6749(97)70307-5. [DOI] [PubMed] [Google Scholar]
- 17.Tovey ER, Chapman MD, Platts-Mills TA. Mite faeces are a major source of house dust allergens. Nature. 1981;289:592–3. doi: 10.1038/289592a0. [DOI] [PubMed] [Google Scholar]
- 18.de Blay F, Heymann PW, Chapman MD, Platts-Mills TA. Airborne dust mite allergens: comparison of group II allergens with group I mite allergen and cat-allergen Fel d I. J Allergy Clin Immunol. 1991;88:919–26. doi: 10.1016/0091-6749(91)90249-n. [DOI] [PubMed] [Google Scholar]
- 19.Chua KY, Stewart GA, Thomas WR, Simpson RJ, Dilworth RJ, Plozza TM, et al. Sequence analysis of cDNA coding for a major house dust mite allergen, Der p 1. Homology with cysteine proteases. J Exp Med. 1988;167:175–82. doi: 10.1084/jem.167.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Konradsen JR, Fujisawa T, van Hage M, Hedlin G, Hilger C, Kleine-Tebbe J, et al. Allergy to furry animals: new insights, diagnostic approaches, and challenges. J Allergy Clin Immunol. 2015;135:616–25. doi: 10.1016/j.jaci.2014.08.026. [DOI] [PubMed] [Google Scholar]
- 21.Svanes C, Heinrich J, Jarvis D, Chinn S, Omenaas E, Gulsvik A, et al. Pet-keeping in childhood and adult asthma and hay fever: European community respiratory health survey. J Allergy Clin Immunol. 2003;112:289–300. doi: 10.1067/mai.2003.1596. [DOI] [PubMed] [Google Scholar]
- 22.Wegienka G, Johnson CC, Havstad S, Ownby DR, Nicholas C, Zoratti EM. Lifetime dog and cat exposure and dog- and cat-specific sensitization at age 18 years. Clin Exp Allergy. 2011;41:979–86. doi: 10.1111/j.1365-2222.2011.03747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perzanowski MS, Ronmark E, Platts-Mills TA, Lundback B. Effect of cat and dog ownership on sensitization and development of asthma among preteenage children. Am J Respir Crit Care Med. 2002;166:696–702. doi: 10.1164/rccm.2201035. [DOI] [PubMed] [Google Scholar]
- 24.Erwin EA, Ronmark E, Wickens K, Perzanowski MS, Barry D, Lundback B. Contribution of dust mite and cat specific IgE to total IgE: relevance to asthma prevalence. J Allergy Clin Immunol. 2007;119:359–65. doi: 10.1016/j.jaci.2006.12.648. [DOI] [PubMed] [Google Scholar]
- 25.Sears MR, Herbison GP, Holdaway MD, Hewitt CJ, Flannery EM, Silva PA. The relative risks of sensitivity to grass pollen, house dust mite and cat dander in the development of childhood asthma. Clin Exp Allergy. 1989;19:419–24. doi: 10.1111/j.1365-2222.1989.tb02408.x. [DOI] [PubMed] [Google Scholar]
- 26.King TP, Hoffman D, Lowenstein H, Marsh DG, Platts-Mills TA, Thomas W. Allergen nomenclature. WHO/IUIS allergen nomenclature subcommittee. Int Arch Allergy Immunol. 1994;105:224–33. doi: 10.1159/000236761. [DOI] [PubMed] [Google Scholar]
- 27.Lidholm J, Ballmer-Weber BK, Mari A, Vieths S. Component-resolved diagnostics in food allergy. Curr Opin Allergy Clin Immunol. 2006;6:234–40. doi: 10.1097/01.all.0000225166.90768.d6. [DOI] [PubMed] [Google Scholar]
- 28.Santos A, James L, Bahnson H, Shamji M, Couto-Francisco N, Islam S, et al. IgG4 inhibits peanut-induced basophil and mast cell activation in peanut-tolerant children sensitized to peanut major allergens. J Allergy Clin Immunol. 2015;135:1249–56. doi: 10.1016/j.jaci.2015.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Banerjee S, Resch Y, Chen K, Swoboda I, Focke-Tejkl M, Batt K, et al. Der p 11 is a major allergen for house dust mite-allergic patients suffering from atopic dermatitis. J Invest Dermatol. 2015;135:102–9. doi: 10.1038/jid.2014.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hurlburt B, Cheng H, Offermann L, Chruszcz M, Santos A, Lack G, et al. Peanut panallergen Ara h 8 IgE and IgG4 epitopes. Clin Transl Allergy. 2014;4(Suppl 2):30. [Google Scholar]
- 31.Martelli A, De Chiara A, Corvo M, Restani P, Fiocchi A. Beef allergy in children with cow’s milk allergy; cow’s milk allergy in children with beef allergy. Ann Allergy Asthma Immunol. 2002;89:38–43. doi: 10.1016/s1081-1206(10)62121-7. [DOI] [PubMed] [Google Scholar]
- 32.Hilger C, Kohnen M, Grigioni F, Lehners C, Hentges F. Allergic cross-reactions between cat and pig serum albumin. Study at the protein and DNA levels. Allergy. 1997;52:179–87. doi: 10.1111/j.1398-9995.1997.tb00972.x. [DOI] [PubMed] [Google Scholar]
- 33.Posthumus J, James HR, Lane CJ, Matos LA, Platts-Mills TA, Commins SP. Initial description of pork-cat syndrome in the United States. J Allergy Clin Immunol. 2013;131:923–5. doi: 10.1016/j.jaci.2012.12.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chung CH, Mirakhur B, Chan E, Le QT, Berlin J, Morse M, et al. Cetuximab-induced anaphylaxis and IgE specific for galactose-alpha-1,3-galactose. N Engl J Med. 2008;358:1109–17. doi: 10.1056/NEJMoa074943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Commins SP, Satinover SM, Hosen J, Mozena J, Borish L, Lewis BD, et al. Delayed anaphylaxis, angioedema, or urticaria after consumption of red meat in patients with IgE antibodies specific for galactose-alpha-1,3-galactose. J Allergy Clin Immunol. 2009;123:426–33. doi: 10.1016/j.jaci.2008.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aalberse RC, van Ree R. Cross-reactive carbohydrate determinants. Monogr Allergy. 1996;32:78–83. [PubMed] [Google Scholar]
- 37.Mittermann I, Zidarn M, Silar M, Markovic-Housley Z, Aberer W, Korosec P. Recombinant allergen-based IgE testing to distinguish been and wasp allergy. J Allergy Clin Immunol. 2010;125:1300–7. doi: 10.1016/j.jaci.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 38.Mari A. IgE to cross-reactive carbohydrate determinants: analysis of the distribution and appraisal of the in vivo and in vitro reactivity. Int Arch Allergy Immunol. 2002;129:286–95. doi: 10.1159/000067591. [DOI] [PubMed] [Google Scholar]
- 39.Van Nunen SA, O’Connor KS, Clarke LR, Boyle RX, Fernando SL. An association between tick bite reactions and red meat allergy in humans. Med J Aust. 2009;190:510–1. doi: 10.5694/j.1326-5377.2009.tb02533.x. [DOI] [PubMed] [Google Scholar]
- 40.Morisset M, Richard C, Astier C, Jacquenet S, Croizier A, Beaudouin E, et al. Anaphylaxis to pork kidney is related to IgE antibodies specific for galactose-alpha-1,3-galactose. Allergy. 2012;67:699–704. doi: 10.1111/j.1398-9995.2012.02799.x. [DOI] [PubMed] [Google Scholar]
- 41.Fischer J, Hebsaker J, Caponetto P, Platts-Mills TA, Biedermann T. Galactose-alpha-1,3-galactose sensitization is a prerequisite for pork-kidney allergy and cofactor-related mammalian meat anaphylaxis. J Allergy Clin Immunol. 2014;133:755–9. e1. doi: 10.1016/j.jaci.2014.05.051. [DOI] [PubMed] [Google Scholar]
- 42.Hamsten C, Tran T, Starkhammar M, Brauner A, Commins SP, Platts-Mills TA, et al. Red meat allergy in Sweden: association with tick sensitization and B-negative blood groups. J Allergy Clin Immunol. 2013;132:1431–4. doi: 10.1016/j.jaci.2013.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jyo T, Kuwabara M, Kodommari Y, Tanemori N, Asaoku Y, Katsutani T, et al. Cases of immediate-type allergy in oyster shuckers due to galacto-oligosac-charide. J Hirosmima Med Ass. 1993;25:19–26. (in Japanese) [Google Scholar]
- 44.Chiang WC, Huang CH, Llanora GV, Gerez I, Goh SH, Shek LP, et al. Anaphylaxis to cow’s milk formula containing short-chain galacto-oligosaccharide. J Allergy Clin Immunol. 2012;130:1361–7. doi: 10.1016/j.jaci.2012.08.048. [DOI] [PubMed] [Google Scholar]
- 45.Reynolds A, Hazen A. The Water-Supply of Chicago: Its Souorce and Sanitary aspects. Public Health Pap. 1893;19:146–51. [PMC free article] [PubMed] [Google Scholar]
- 46.Armstrong GL, Conn LA, Pinner RW. Trends in infectious disease mortality in the United States during the 20th century. JAMA. 1999;281:61–6. doi: 10.1001/jama.281.1.61. [DOI] [PubMed] [Google Scholar]
- 47.Smith JM, Disney ME, Williams JD, Goels ZA. Clinical significance of skin reactions to mite extracts in children with asthma. Br Med J. 1969;2:723–6. doi: 10.1136/bmj.2.5659.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Perzanowski MS, Ng’ang’a LW, Carter MC, Odhiambo J, Ngari P, Vaughan JW, et al. Atopy, asthma, and antibodies to Ascaris among rural and urban children in Kenya. J Pediatr. 2002;140:582–8. doi: 10.1067/mpd.2002.122937. [DOI] [PubMed] [Google Scholar]
- 49.Endara P, Vaca M, Platts-Mills TA, Workman L, Chico ME, Barreto ML, et al. Effect of urban versus rural residence on the association between atopy and wheeze in Latin America: findings from a case-control analysis. Clin Exp Allergy. 2015;45:438–47. doi: 10.1111/cea.12399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stevens W, Addo-Yobo E, Roper J, Woodcock A, James H, Platts-Mills T, et al. Differences in both prevalence and titre of specific immunoglobulin E among children with asthma in affluent and poor communities within a large town in Ghana. Clin Exp Allergy. 2011;41:1587–94. doi: 10.1111/j.1365-2222.2011.03832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Haahtela T, Lindholm H, Bjorksten F, Koskenvuo K, Laitinen LA. Prevalence of asthma in Finnish young men. BMJ. 1990;301:266–8. doi: 10.1136/bmj.301.6746.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Braback L, Hjern A, Rasmussen F. Trends in asthma, allergic rhinitis and eczema among Swedish conscripts from farming and non-farming environments. A nationwide study over three decades. Clin Exp Allergy. 2004;34:38–43. doi: 10.1111/j.1365-2222.2004.01841.x. [DOI] [PubMed] [Google Scholar]
- 53.Gelber L, Seltzer L, Bouzoukis J, Pollart S, Chapman M, Platts-Mills T. Sensitization and exposure to indoor allergens as risk factors for asthma among patients presenting to hospital. Am Rev Respir Dis. 1993;147:573–8. doi: 10.1164/ajrccm/147.3.573. [DOI] [PubMed] [Google Scholar]
- 54.Hulett A, Dockhorn R. House dust, mite (D. farinae) and cockroach allergy in a midwestern population. Ann Allergy Asthma Immunol. 1979;43:160–5. [PubMed] [Google Scholar]
- 55.Rosenstreich DL, Eggleston P, Kattan M, Baker D, Slavin RG, Gergen P, et al. The role of cockroach allergy and exposure to cockroach allergen in causing morbidity among inner-city children with asthma. N Engl J Med. 1997;336:1356–63. doi: 10.1056/NEJM199705083361904. [DOI] [PubMed] [Google Scholar]
- 56.Andiappan AK, Puan KJ, Lee B, Nardin A, Poidinger M, Connollhy J, et al. Allergic airway diseases in a tropical urban environment are driven by dominant mono-specific sensitization against house dust mites. Allergy. 2014;69:501–9. doi: 10.1111/all.12364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Soto-Quiros M, Avila L, Platts-Mills T, Hunt J, Erdman D, Carper H, et al. High titers of IgE antibody to dust mite allergen and risk for wheezing among asthmatic children infected with rhinovirus. J Allergy Clin Immunol. 2012;129:1499–505. doi: 10.1016/j.jaci.2012.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Skloot G, Permutt S, Togias A. Airway hyperresponsiveness in asthma: a problem of limited smooth muscle relaxation with inspiration. J Clin Invest. 1995;96:2393–403. doi: 10.1172/JCI118296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fredberg J. Airway smooth muscle in asthma: flirting with disaster. Eur Respir J. 1998;12:1252–6. doi: 10.1183/09031936.98.12061252. [DOI] [PubMed] [Google Scholar]
- 60.Sicherer S, Munoz-Furlong A, Godbold J, Sampson H. US prevalence of self-reported peanut, tree nut, and sesame allergy: 11-year follow-up. J Allergy Clin Immunol Pract. 2010;125:1322–6. doi: 10.1016/j.jaci.2010.03.029. [DOI] [PubMed] [Google Scholar]
- 61.Du Toit G, Roberts G, Sayre P, Bahnson H, Radulovic S, Santos A, et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. 2015;372:803–13. doi: 10.1056/NEJMoa1414850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brough H, Simpson A, Makinson K, Hankinson J, Brown S, Douiri A, et al. Peanut allergy: effect of environmental peanut exposure in children with filaggrin loss-of-function mutations. J Allergy Clin Immunol Pract. 2014;134:867–75. doi: 10.1016/j.jaci.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shreffler WG, Castro RR, Kucuk ZY, Charlop-Powers Z, Grishina G, Yoo S, et al. The major glycoprotein allergen from Arachis hypogaea, Ara h 1, is a ligand of dendritic cell-specific ICAM-grabbing nonintegrin and acts as a Th2 adjuvant in vitro. J Immunol. 2006;177:3677–85. doi: 10.4049/jimmunol.177.6.3677. [DOI] [PubMed] [Google Scholar]
- 64.Trompette A, Divanovic S, Visintin A, Blanchard C, Hegde RS, Madan R, et al. Allergenicity resulting from functional mimicry of a Toll-like receptor complex protein. Nature. 2009;457:585–8. doi: 10.1038/nature07548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hsu SC, Chen CH, Tsai SH, Kawasaki H, Hung CH, Chu YT, et al. Functional interaction of common allergens and a C-type lectin receptor, dendritic cell-specific ICAM3-grabbing non-integrin (DC-SIGN), on human dendritic cells. J Biol Chem. 2010;285:7903–10. doi: 10.1074/jbc.M109.058370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Squillace SP, Sporik RB, Rakes G, Couture N, Lawrence A, Merriam S, et al. Sensitization to dust mites as a dominant risk factor for asthma among adolescents living in central Virginia. Multiple regression analysis of a population-based study. Am J Respir Crit Care Med. 1997;156:1760–4. doi: 10.1164/ajrccm.156.6.9704026. [DOI] [PubMed] [Google Scholar]
- 67.Erwin EA, Wickens K, Custis NJ, Siebers R, Woodfolk J, Barry D, et al. Cat and dust mite sensitivity and tolerance in relation to wheezing among children raised with high exposure to both allergens. J Allergy Clin Immunol. 2005;115:74–9. doi: 10.1016/j.jaci.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 68.Papazian D, Hansen S, Wurtzen P. Airway responses towards allergens - from the airway epithelium to T cells. Clin Exp Allergy. 2014;14:12451. doi: 10.1111/cea.12451. [DOI] [PubMed] [Google Scholar]
- 69.Licona-Limon P, Kim L, Palm N, Flavell R. TH2, allergy and group 2 innate lymphoid cells. Nat Immunol. 2013;14:536–42. doi: 10.1038/ni.2617. [DOI] [PubMed] [Google Scholar]
- 70.Al-Shami A, Spolski R, Kelly J, Keane-Myers A, Leonard WJ. A role for TSLP in the development of inflammation in an asthma model. J Exp Med. 2005;202:829–39. doi: 10.1084/jem.20050199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhou B, Comeau MR, De Smedt T, Liggitt HD, Dahl ME, Lewis DB, et al. Thymic stromal lymphopoietin as a key initiator of allergic airway inflammation in mice. Nat Immunol. 2005;6:1047–53. doi: 10.1038/ni1247. [DOI] [PubMed] [Google Scholar]
- 72.Ito T, Wang YH, Duramad O, Hori T, Delespesse GJ, Watanabe N, et al. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J Exp Med. 2005;202:1213–23. doi: 10.1084/jem.20051135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Agrawal R, Wisniewski J, Yu M, Kennedy J, Platts-Mills T, Heymann P, et al. Infection with human rhinovirus 16 promotes enhanced IgE responsiveness in basophils of atopic athmatics. Clin Exp Allergy. 2014;44:1266–73. doi: 10.1111/cea.12390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Soumelis V, Reche PA, Kanzler H, Yuan W, Edward G, Homey B, et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol. 2002;3:673–80. doi: 10.1038/ni805. [DOI] [PubMed] [Google Scholar]
- 75.Hulse KE, Reefer AJ, Engelhard VH, Patrie JT, Ziegler SF, Chapman MD, et al. Targeting allergen to FcgammaRI reveals a novel T(H)2 regulatory pathway linked to thymic stromal lymphopoietin receptor. J Allergy Clin Immunol. 2010;125:247–56. e1–8. doi: 10.1016/j.jaci.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Thepen T, Langeveld-Wildschut EG, Bihari IC, van Wichen DF, van Reijsen FC, Mudde GC, et al. Biphasic response against aeroallergen in atopic dermatitis showing a switch from an initial TH2 response to a TH1 response in situ: an immunocytochemical study. J Allergy Clin Immunol. 1996;97:828–37. doi: 10.1016/s0091-6749(96)80161-8. [DOI] [PubMed] [Google Scholar]
- 77.Grewe M, Bruijnzeel-Koomen CA, Schopf E, Thepen T, Langeveld-Wildschut AG, Ruzicka T, et al. A role for Th1 and Th2 cells in the immunopathogenesis of atopic dermatitis. Immunol Today. 1998;19:359–61. doi: 10.1016/s0167-5699(98)01285-7. [DOI] [PubMed] [Google Scholar]
- 78.Asherson G, Zembala M, Perera M, Mayhew B, Thomas W. Production of immunity and unresponsiveness in the mouse by feeding contact sensitizing agents and the role of suppressor cells in the peyer’s patches, mesenteric lymph nodes and other lymphoid tissues. Cell Immunol. 1977;33:134–55. doi: 10.1016/0008-8749(77)90142-3. [DOI] [PubMed] [Google Scholar]
- 79.Allam JP, Duan Y, Winter J, Stojanovski G, Fronhoffs F, Wenghoefer M, et al. Tolerogenic T cells, Th1/Th17 cytokines and TLR2/TLR4 expressing dendritic cells predominate the microenvironment within distinct oral mucosal sites. Allergy. 2011;66:532–9. doi: 10.1111/j.1398-9995.2010.02510.x. [DOI] [PubMed] [Google Scholar]
- 80.Allam JP, Wurtzen PA, Reinartz M, Winter J, Vrtala S, Chen KW, et al. Phl p 5 resorption in human oral mucosa leads to dose-dependent and time-dependent allergen binding by oral mucosal Langerhans cells, attenuates their maturation, and enhances their migratory and TGF-beta1 and IL-10-producing properties. J Allergy Clin Immunol. 2010;126:638–45. e1. doi: 10.1016/j.jaci.2010.04.039. [DOI] [PubMed] [Google Scholar]
- 81.O’Hehir RE, Gardner LM, de Leon MP, Hales BJ, Biondo M, Douglass JA, et al. House dust mite sublingual immunotherapy: the role for transforming growth factor-beta and functional regulatory T cells. Am J Respir Crit Care Med. 2009;180:936–47. doi: 10.1164/rccm.200905-0686OC. [DOI] [PubMed] [Google Scholar]
- 82.Platts-Mills T, Vaughan J, Squillace S, Woodfolk J, Sporik R. Sensitisation, asthma, and a modified Th2 response in children exposed to cat allergen: a population-based cross-sectional study. Lancet. 2001;357:752–6. doi: 10.1016/S0140-6736(00)04168-4. [DOI] [PubMed] [Google Scholar]
- 83.Erwin E, Woodfolk J, James H, Satinover S, Platts-Mills TA. Changes in cat specific IgE and IgG antibodies with decreased cat exposure. Ann Allergy Asthma Immunol. 2014;112:545–50. doi: 10.1016/j.anai.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Akdis CA, Blesken T, Akdis M, Wuthrich B, Blaser K. Role of interleukin 10 in specific immunotherapy. J Clin Invest. 1998;102:98–106. doi: 10.1172/JCI2250. [DOI] [PMC free article] [PubMed] [Google Scholar]