Abstract
Exposure of rodents to the Sertoli cell (SC) toxicant mono-(2-ethylhexyl) phthalate (MEHP) has been reported to trigger an infiltration of macrophages into the testis in an age- and species-dependent manner. Here we challenge the hypothesis that the peripubertal rat-specific infiltration of macrophages after MEHP exposure is due, in part, to an increase in SC-specific inflammatory cytokine expression. To rule out that GC apoptosis itself is responsible for macrophage recruitment, rats were exposed to a direct germ cell toxicant, methoxy acetic acid (MAA), but no infiltration of macrophages was observed. Next, mRNA levels of inflammatory cytokines were evaluated after MEHP exposure. IL-1α, IL-6, and MCP-1 expression was increased in vivo and correlated with macrophage infiltration in a species-specific manner. Additionally, IL-6 and MCP-1 expression was increased in SC-GC co-cultures and ASC-17D SCs. These results indicate that MEHP-injury in pubertal rats specifically stimulates secretion of pro-inflammatory cytokines and alters the immune microenvironment.
Keywords: Mono-(2-ethylhexyl) phthalate, Sertoli cells, cytokines, testicular macrophages
1. INTRODUCTION1
The testicular immune environment and the process of spermatogenesis itself are facilitated by both pro- and anti-inflammatory cytokines. Under normal physiological conditions cytokines are produced by testicular macrophages (TMs) in the interstitial space, and within the testicular seminiferous epithelium, the Sertoli cells (SCs), and the germ cells (GCs) [1]. As these immune cell signaling molecules are necessary to maintain proper testicular function, the immune microenvironment must remain carefully modulated. When cytokines are dysregulated in the testis, as common in conditions of inflammation, spermatogenesis can become disrupted. Epidemiological studies have demonstrated that cohorts of infertile men suffer from acute or chronic inflammation [2] indicating that disruption of the physiologic levels of cytokines within the testis may contribute to idiopathic infertility.
SCs produce both cytokines (e.g., IL-1α, IL-6) that support GC survival and development [1] and immunosuppressive factors (Activin A [3], Jagged1 [4] Serpin G1/E1 [5]) that promote immune tolerance. SCs exhibit the innate ability to promote immune tolerance, even when transplanted to remote tissues [6]. Resident TMs (CD68-, CD163+) play a key supportive role in maintaining the immune tolerant environment within the testis due to their high basal expression of the potent immunosuppressive cytokine IL-10 and a blunted response to inflammatory stimuli [7]. GCs secrete TNFα, which influence SCs through a feedback mechanism during GC development [8], causing reorganization of the SC cytoskeleton, tight junctions between SCs, and the ectoplasmic specialization junctions between SCs and GCs [8]. Together, these cells coordinate a complex signaling network to maintain the testicular immune tolerant environment.
We recently reported that peripubertal male rats exposed to an acute dose of the testicular toxicant mono (2-ethylhexyl) phthalate (MEHP), the primary active metabolite of di (2-ethylhexyl) phthalate (DEHP), displays testicular inflammation characterized by macrophage infiltration into the testis in an age- and species-specific manner [9]. Specifically, young peripubertal aged male Fisher rats (PND 21, 28) show high levels of GC apoptosis accompanied by macrophage infiltration as compared to adult (PND 56) rats or peripubertal mice, both of which show low increases in GC apoptosis and neither have accompanying macrophage infiltration [9]. The peak of macrophage infiltration in peripubertal rats occurred at time points concurrent with a robust increase in monocyte chemoattractant protein-1 (MCP-1) in peritubular myoid cells (PTMCs). The expression of MCP-1 by PTMCs [9] suggests active recruitment of macrophages after injury in the sensitive age and species.
Phthalates continue to be some of the most studied endocrine disruptors and reproductive toxicants, yet, the exact mechanism of action remains unknown [10]. The extensive literature on phthalate effects on the testis is generally divided into anti-androgen effects on the Leydig cells, and seminiferous tubule effects resulting in germ cell apoptosis and/or multinucleated germ cells; where these effects are regarded as independent of one another [11, 12]. Peripubertal rat exposure to high acute levels of MEHP has been shown to result in seminiferous tubule effects resulting in Sertoli cell alterations and germ cell apoptosis [13, 14]. Peripubertal rats have the highest amounts of germ cell apoptosis after exposure in this paradigm, suggesting they are more sensitive to the seminiferous tubule effects than adults [15].
The unique infiltration of macrophages displayed by rats around the pubertal period [9] suggest that sensitivity of peripubertal rats may be due to differences in the testicular immune environment during this developmental period. The peripubertal phase is appreciated to be a crucial time for the immunoregulatory functions of the testis to be established. As the blood testis barrier (BTB) becomes fully established and activated [16], the SCs begin to produce cytokines [17], and the residential population of TMs is established [18]. Although both the establishment of immunoregulatory functions within the testis and initiation of spermatogenesis have been well described, the interaction of immune and germ cells during this crucial developmental period remains unknown.
Here we challenge the hypothesis that the rat-specific infiltration of macrophages after MEHP exposure around puberty is due, in part, to an increase in SC-specific cytokine expression. These cytokines could underlie the mechanism to trigger the release of the chemokine MCP-1 from the adjacent PTMCs and account for the subsequent infiltration of pro-inflammatory macrophages into the testis. To test the alternate hypothesis that the process of GC apoptosis itself is responsible for macrophage recruitment, the infiltration of macrophages was assessed after exposure to a toxicant, methoxy acetic acid (MAA), that induces GC apoptosis through a different mechanism [19, 20]. Then, a MEHP-specific mechanism involving inflammatory cytokines IL-1α and IL-6, that may trigger MCP-1 [21] was investigated by measuring mRNA both in vivo and in vitro after MEHP treatment. The results herein suggest that the production of IL-1α and IL-6 are increased by MEHP exposure as a possible mechanism of immune infiltration in a species-specific way during the peripubertal period. Furthermore, the MEHP exposure model can be utilized to understand how disruption of testicular immune regulatory mechanisms contribute to GC apoptosis, and infertility.
2. MATERIALS AND METHODS
2.1. Cell Line
The adult rat (Sprague-Dawley) derived SC line, ASC-17D (a gift from Ann Clark, Serono Research Institute, Rockland, MA; originally created by the laboratory of Dr. Ken Roberts; [22]) was cultivated at 33°C in cell culture medium consisting of equal volumes of Dulbecco’s modified Eagle’s media and Ham’s F-12 supplemented with 4% fetal bovine serum and 1% antibiotic penicillin-streptomycin (Thermo Scientific, Waltham, MA). ASC-17D cells were created with a temperature-sensitive mutant of the SV40 virus that allows for the propagation of these cells at a permissive temperature (33°C) and differentiation at an elevated temperature (40°C). Cells were seeded and allowed to adhere to the dish at 33 °C for 24 h and then transferred to 40°C for 48 h prior to experimental initiation.
2.2. Preparation of Primary SC-GC Co-cultures
Primary SC-GC co-cultures were prepared from PND 21–28 day old Fisher F344 rat testis (pooled at least 3 rats per collection, 3 separate collections) as previously described [23]. Briefly, testes were detunicated, seminiferous tubules were gently teased apart using forceps, and then the tubules were subjected to a serial process of enzymatic digestions. Cells (1 × 106) were seeded onto laminin (1.5ng) coated 6-well plates containing Dulbecco’s modified Eagle’s/Ham’s F-12 media supplemented with 1 ng/ml of epidermal growth factor (Sigma), 10 μg/ml of ITS Plus Premix (insulin, transferrin, selenious acid, bovine serum albumin, and linoleic acid; BD Biosciences), and 50 μg/ml of gentamicin (Thermo Scientific, Waltham, MA). Primary co-cultures were maintained at 37°C for 72 h prior to treatment.
2.3. Animals
Male Fischer rats were purchased from Harlan Laboratories, Inc. (Houston, TX). A/J mice and breeding pairs of C57 mice were purchased from Jackson labs (Bar Harbor, ME). Animals were maintained in a controlled temperature (22°C ± 0.5°C) and light (12 L:12 D) environment and allowed to acclimate for 1 week prior to experimental challenge. Standard lab chow (Purina Mills LabDiet #5LL2, St. Louis, MO) and tap water were supplied ad libitum. All animal procedures were performed in accordance with the guidelines and approval of The University of Texas at Austin’s Institutional Animal Care and Use Committee, which abides by NIH Publications No. 8023, revised 1978.
2.4. In Vitro MEHP Treatment
Primary co-cultures or ASC-17D cells were treated with 200 μM MEHP (97.3% purity; Wako Chemicals, Richmond, VA) [23, 24] diluted in dimethyl sulfoxide (DMSO, final concentration: 0.04%) or equivalent volume of DMSO only, for various time periods (3, 6, 12, 24 h) that precede peak CD11b+ infiltration (24h) [9]. Determination of the dose was based on previous experiments that utilized 200 μM MEHP in vitro to replicate the high exposure of MEHP in vivo [23–26]. This dose was found to not inhibit survival in either primary cell cultures or ASC-17D cell cultures via the trypan blue exclusion method [27]. At each time point cells were washed with phosphate buffered saline (PBS), then lysed and immediately processed for RNA as described below.
2.5. In Vivo MAA or MEHP Treatment
Based on previous studies, male Fischer F344 rats of exact PND 28 were pretreated with a single oral dose of MAA (n=3 per treatment) (650 mg/kg dissolved in 0.9% NaCl), or equivalent volume of vehicle (5.5 ml/kg NaCl) [27], MEHP (667 mg/kg in corn oil p.o.) or equivalent volume of vehicle (n=6 per treatment per time point) (corn oil, 2 ml/kg p.o.) [9]. As previously described, male C57BL/6J and A/J mice PND 28 were treated with MEHP or corn oil vehicle (800 mg/kg p.o., 4 ml/kg p.o.) for 12 h [9]. Twenty-four hours after the MAA treatment [28], or 3, 6, 12, and 24 hours after MEHP treatment, animals were anesthetized using a ketamine (100 mg/ml) and xylazine (20 mg/ml) cocktail (0.125 mg/ g body weight i.p.; Animal Health International, Westlake, TX) and perfused with phosphate buffered saline (PBS) and heparin (10 U/ml) until complete exsanguination. The testes of rats were removed and weighed, then one testis was snap frozen in liquid nitrogen and stored at −80°C (immunostaining and RNA isolation) while the other testis was placed in PBS (3 ml) on ice until interstitial cell and seminiferous tubule collection.
2.6. Interstitial Cell Collection, Flow Cytometry, and Seminiferous Tubule isolation
Single-cell suspensions from the interstitial space of rat testis were obtained by decapsulating one testis per rat in 3 ml of ice-cold PBS. After removal of the tunica albuginea, 1 ml of DMEM + 1% collagenase was added and seminiferous tubules were gently teased apart using fine forceps. Seminiferous tubules were then rinsed with 1 ml of PBS, snap frozen and stored at −80 °C until use. Interstitial cells were centrifuged (5 min, 1200 rpm, 4°C Beckman table top), filtered through a 70 μm cell strainer, resuspended in 1 ml of PBS, and adjusted to approximately 1 × 106 cells/ml. Cells were centrifuged (1200 rpm at 4°C) for 5 min, resuspended in 200 μl of FACS buffer (PBS + 2% inactivated bovine serum) with anti-CD11b-APC (1:200; eBiosinces, San Diego, CA). Cells were incubated on ice in the dark for 30 min, then washed with 3 ml of FACS buffer, centrifuged at 1200 rpm for 5 min at 4°C and resuspended in 0.5 ml FACS buffer containing propidium iodide (2 μg/ml). For each sample, 50,000 cells were analyzed on a BD LRSFortessa flow cytometer with FACSDiva software and interpreted with FlowJo software. Additionally, single-cell suspensions from lymph nodes were reacted with or without antibodies to set gates, compensation, and autofluorescence. Only live cells were used for analysis. The total numbers of CD11b+ cells (leukocytes) were determined for each rodent based on cell counts.
2.7. Terminal Deoxynucleotidyl Transferase-Mediated Digoxigenin-dUTP Nick End Labeling (TUNEL) Assay
The presence of apoptotic fragmentation of DNA in frozen testis cross sections was determined by TUNEL analysis using the ApopTag kit (EMD Millipore, Billerica, MA). According to previous studies [23, 25, 26] the apoptotic index (AI) was calculated as the percentage of essentially round seminiferous tubules containing more than three TUNEL-positive GCs in each cross section. For each rat, at least 2 cross sections and at least 100 seminiferous tubules were analyzed.
2.8. Quantitative PCR
To measure the level of mRNA expression in co-cultures, ASC-17D cells, and seminiferous tubule tissue, quantitative -PCR (qPCR) was performed. Tissue RNA was isolated using TRIzol reagent (Thermo Scientific, Waltham, MA) and cell culture total RNA was isolated using lysis buffer from PureLink RNA mini kit (Thermo Scientific, Waltham, MA) per manufacturers protocol, then stored at −80°C. RNA quantity and quality were determined using a ThermoScientific Nanodrop 1000. First strand cDNA was prepared using 2.5 or 1 μg (tissue or cell culture, respectively) of total RNA with Multiscribe reverse transcriptase and random hexamers (Thermo Scientific, Waltham, MA) into a 25 μl reaction volume (100ng/μl tissue, 40ng/μl cells). The resulting cDNA was analyzed in 10 μl volumes containing 1 μl cDNA, 5 μl of iTaq Universal SYBR Green Supermix (BioRad, Hercules, CA), and 250 μM (each) forward and reverse primers. Melt curves and Ct values were obtained using the Bio-Rad CFX96 qPCR detection system. Forward and reverse primers used to detect expression levels of mRNA are in Table 1. Each target gene was normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) levels and performed in duplicate. Ct values were analyzed using the “Delta Delta Ct method”. Because treated and control animals treated in vivo were different individuals, in vivo treatments at each time point were compared to the average control treated animals to find fold change in mRNA expression. In vitro, each of the isolations of primary cells (or ASC-17D passage) had control and treated groups on cells from the same individual animal (or pool of animal tissue), therefore each treatment was compared to its own respective DMSO control (DMSO equals 1 in each experiment, thus no SEM) to find fold change in mRNA expression.
TABLE 1.
Primers used for qPCR.
| TARGET | PRIMER SEQUENCE (5′–>3) | LENGTH (BP) |
PRODUCT LENGTH |
|---|---|---|---|
| IL-1α (RAT) | CCTCGTCCTAAGTCACTCGC GGCTGGTTCCACTAGGCTTT |
20 20 |
102 |
| IL-6 (RAT) | TTCTCTCCGCAAGAGACTTCC TCTCCTCTCCGGACTTGTGAA |
21 21 |
94 |
| IL-6 (MOUSE) | TCTCTGCAAGAGACTTCCATCC AGTCTCCTCTCCGGACTTGT |
22 20 |
93 |
| MCP-1 (RAT) | TAGCATCCACGTGCTGTCTC CAGCCGACTCATTGGGATCA |
20 20 |
94 |
| MCP-1 (MOUSE) | TGACCCCAAGAAGGAATGGG ACCTTAGGGCAGATGCAGTT |
20 20 |
104 |
| GAPDH (RAT) | ATGATTCTACCCACGGCAAG CTGGAAGATGGTGATGGGTT |
20 20 |
89 |
| GAPDH (MOUSE) | TGTAGTGGCAAAGTGGAGATT GTGGTGCAGGATGCATTGCT |
21 19 |
392 |
2.9. Immunofluorescence of ASC-17D
ASC-17D (0.25 × 105) cells were seeded in 4-well chamber slides. Cells were treated with DMSO or MEHP (200 μM) for 24 h, then washed with PBS and fixed with 3.7% paraformaldehyde for 10 minutes. Cells were washed in PBS then incubated in blocking buffer (3% Bovine Serum Albumin; Sigma). Cells were incubated with rabbit anti-IL-6 (1:500; Abcam, Cambridge, MA) for 1 h at room temperature. Then incubated in Alexa Fluor 488 conjugated anti-rabbit antibody (1:500; Thermo Scientific, Waltham, MA) for 1 h and mounted with Vectashield Mounting Medium containing propidium iodine (Vector Labs, Burlingame, CA). Cells were seeded and treated 3 times/treatment and fluorescent signals were detected using excitation/emission wavelengths of 495 nm/519 nm, respectively. All sections were imaged using Nikon Eclipse microscope and captured with Nikon Cool-SNAP digital camera. Images were processed and analyzed using NIS Elements software.
2.10. Statistical Analysis
The data are reported as mean ± SEM. Data were subjected to Student’s t-test, when applicable, or Two-way ANOVA followed by Sidak’s multiple comparison test was performed using GraphPad Prism version 7 (GraphPad, La Jolla, USA). The in vitro experiments were analyzed according to the matched pair design, whereas the in vivo experiments were analyzed as unmatched data. P values of 0.05 or less were considered statistically significant.
3. RESULTS
3.1. GC apoptosis does not directly induce macrophage infiltration
3.1.1. MAA exposure for 24 hours increases GC apoptosis
Macrophages infiltrate into the testis after MEHP exposure specifically in peripubertal rats [9]. It remains unknown whether the macrophages are actively recruited to the testis through a MEHP-specific SC mechanism, or simply in response to the process of GCs undergoing apoptosis in the testis. Therefore, MAA, a toxicant that induces GC apoptosis through a different mechanism [19, 20], was given to rats (PND 28) at a dose (650 mg/kg BW) shown previously to induce similar levels of apoptosis at a similar time frame as that seen after MEHP exposure [28]. GC apoptosis (MAA- 53.3% ± 6.8%; control- 4.6% ± 0.5%) after 24 h was significantly increased over controls (Figure 1A).
FIGURE 1. MAA causes extensive germ cell apoptosis but does not result in macrophage infiltration.
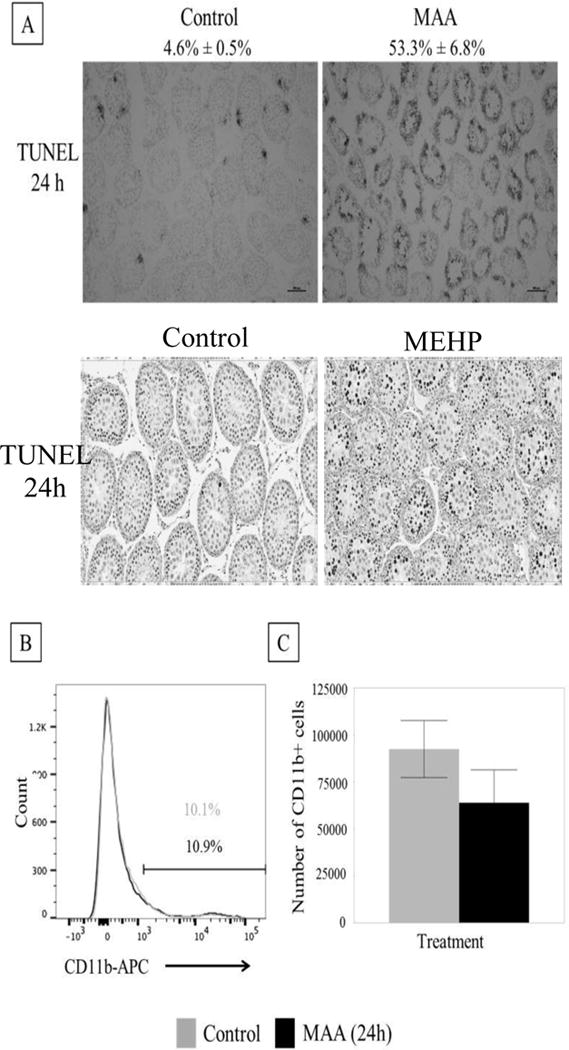
(A) Top: Representative photomicrographs of TUNEL staining on frozen MAA (650 mg/kg, p.o.) or equivalent volume control (5.5 ml/kg, NaCl) treated PND 28 rat testis after 24h exposure (n=3 per treatment). MAA induced a significant increase in apoptotic index (% seminiferous tubules with >3 TUNEL positive germ cells/total number of seminiferous tubules) from 4.6% ± 0.5% to 53.3% ± 6.8%. Bottom: Representative photomicrographs of TUNEL staining on paraffin embedded MEHP (667 mg/kg, p.o.) or equivalent control (2ml/kg corn oil) treated PND 28 rat testis after 24 h exposure. MEHP induced a significant increase in apoptotic index nearly 100 fold [9]. (B) Histogram of MAA or control treated rat interstitial cells (after 24h exposure). The CD11b+ population has high intensity fluorescence and is on the right of the graph. This population does not change between treatment (black bar) and control (grey bar). The population on the left is the CD11b- population. (C) When the percentage CD11b+ found from flow were applied to the total number of cells collected from the interstitium, there were no differences in the total number of CD11b+ cells. Statistical analysis was performed using Student’s t-tests.
3.1.2. MAA exposure for 24 hours does not increase CD11b+ cells
Flow cytometric analysis of interstitial cells of vehicle treated (NaCl) and MAA (650 mg/kg) treated PND 28 rats showed no difference in the percentage of CD11b+ cells in the interstitium (MAA- 10.1%; control- 10.9%) (Figure 1B) or total number of CD11b+ cells in the interstitium (Figure 1C). These results suggest that germ cell apoptosis in itself does not result in macrophage infiltration into the testis.
3.2. MEHP-induced cytokine changes
3.2.1. In vivo seminiferous tubule expression of IL-1α and IL-6 mRNA increase after MEHP treatment
Within the rat testis, IL-1α and IL-6 are the key cytokines produced by SCs, where IL-1α has been shown to increase MCP-1 expression in PTMCs [21]. To ascertain a MEHP-specific mechanism that may be responsible for the species specific infiltration of macrophages, analysis of IL-1α and IL-6 mRNA, in isolated preparations of seminiferous tubules of PND 28 rats treated with MEHP (667 mg/kg) or control was performed. There was a significant increase in IL-1α mRNA expression at 3 h (Figure 2A). IL-6 mRNA expression increased with MEHP treatment at 6 h (Figure 2B), which was also seen in vitro in Figure 2B. MCP-1 mRNA expression increased at 12 h (Figure 2C) which aligns with previous ELISA protein analysis, also showing an increase in MCP1 at 12 h [9].
FIGURE 2. Cytokine RNA expression changes in in vivo seminiferous tubule preparations from PND 28 rat treated in vivo with MEHP.
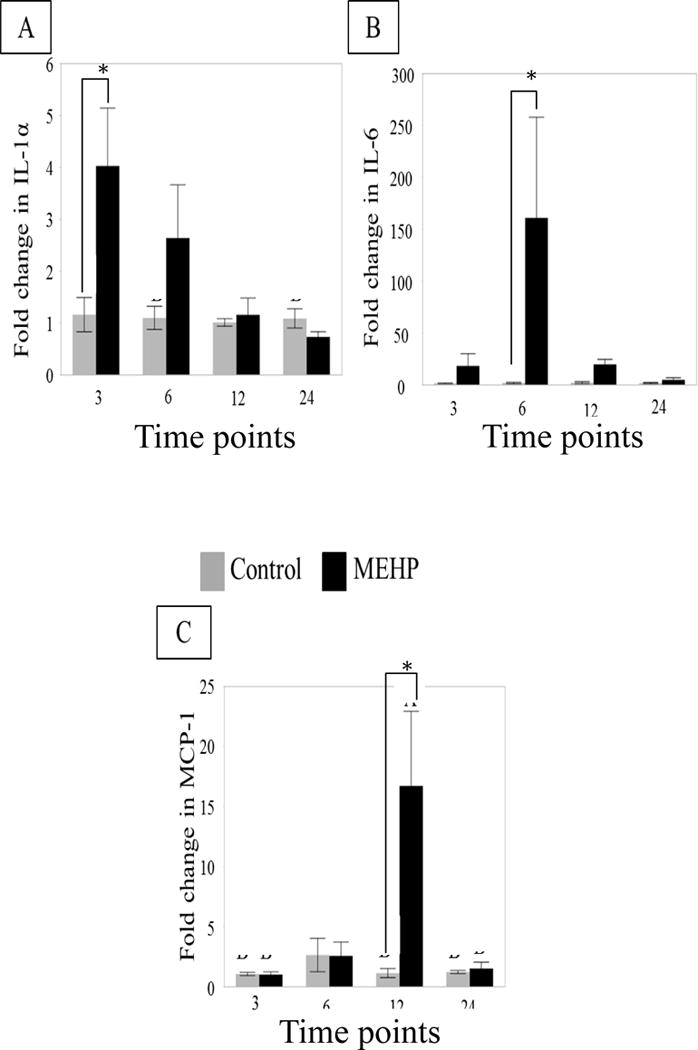
Changes in mRNA expression of (A) IL-1α and (B) IL-6 (C) MCP-1 in MEHP (667 mg/kg p.o, black bars) or control (2 ml/kg corn oil, grey bars) treated rat seminiferous tubules at 3, 6, 12, and 24 h (n=6 per treatment per time point). IL-1α expression was significantly increased at 3 h. IL-6 expression was found to be significantly increased at 6 h. MCP- mRNA expression was significantly increased at 12 h. Statistical analysis was performed using two-way ANOVA for unmatched data with Sidak’s multiple comparison test and each MEHP-treated sample was compared to its time point control.
3.2.2. MEHP-induced changes in primary rat co-culture mRNA expression
To investigate whether the observed changes in the IL-1α/IL-6 signaling pathways also occur in vitro, cytokine transcription in rat SC-GC co-culture was analyzed. IL-1α (Figure 3A) mRNA was not significantly changed due to MEHP (200μM) exposure, but IL-6 (Figure 3B) mRNA was increased significantly at 6 h.
FIGURE 3. Cytokine changes in primary rat co-cultures after in vitro exposure to MEHP.
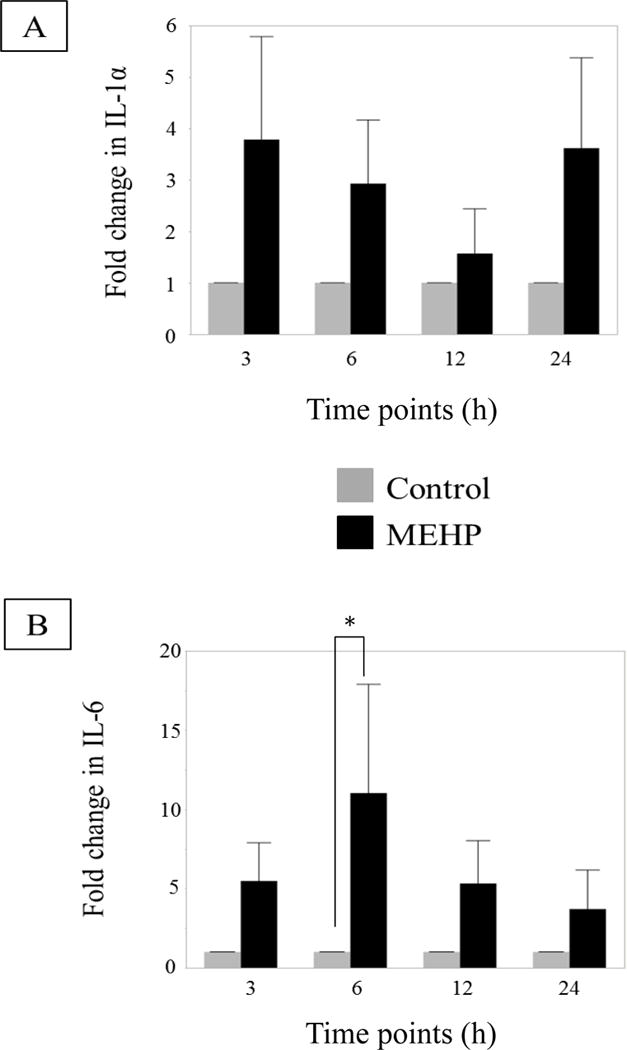
(A) Changes in IL-1α (B) and IL-6 mRNA levels primary SC-GC co-cultures after exposure to control (0.04% DMSO, grey bar), MEHP (200 μM, black bar), at selected time points (n=3 per treatment per time point, each n is a separate collection and pooling of at least 3 animals) (h). MEHP induced a significant increase of IL-6 mRNA levels at 6 h. Statistical analysis was performed using two-way ANOVA for matched pairs with Sidak’s multiple comparison test and each MEHP-treated sample was compared to its time point control.
3.3. ASC-17D cells respond differently than other model systems after MEHP exposure
3.3.1. MEHP-increased IL6 mRNA in the rat Sertoli cell line
To further investigate the induction of the cytokines by MEHP in a SC-specific mechanism, the rat SC line (ASC-17D) was utilized to remove the potential alternative cell (PTMC, GC) contribution in the co-culture system. MEHP (200μM) induced a significant increase of IL-6 mRNA levels compared to controls at 12 h (Figure 4A). ASC-17D cells also had a relative increase in IL-6 immunofluorescence after MEHP exposure (Figure 4B) suggesting that IL-6 is increased in SC after MEHP exposure.
FIGURE 4. IL-6 increases in Sertoli cell rat line ASC-17D cells after in vitro exposure to MEHP.
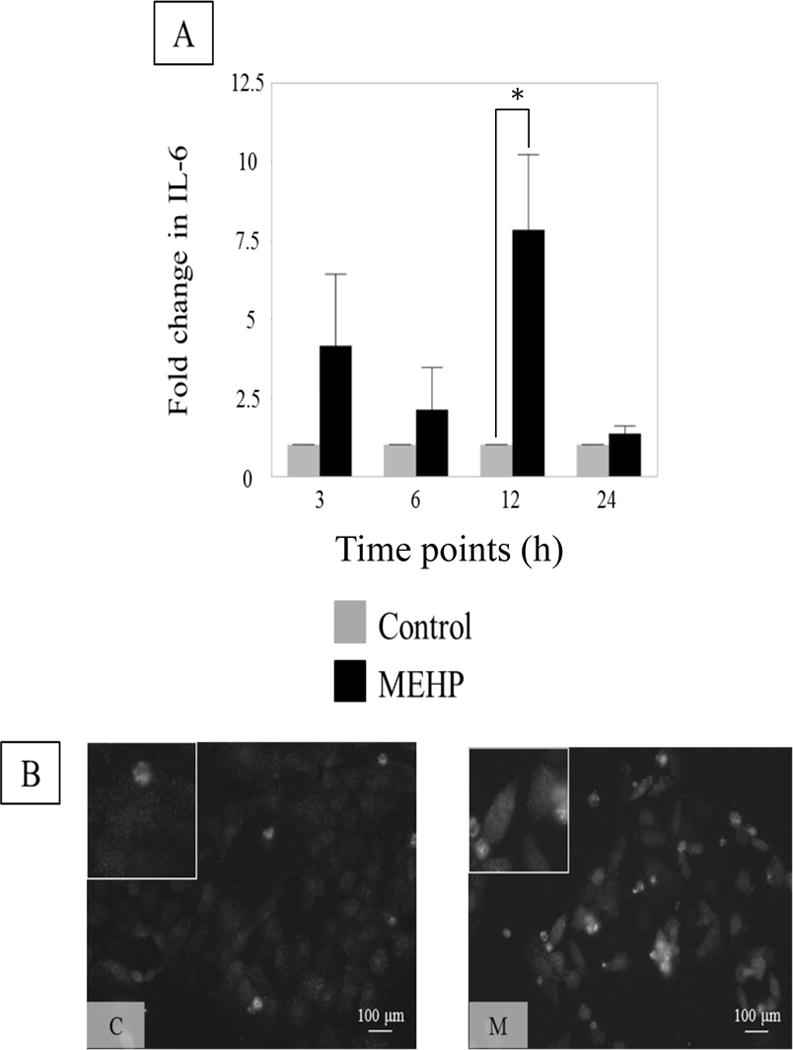
(A) IL-6 expression in control (0.04% DMSO, control, grey) or MEHP (200 μM, black) treated ASC-17D cells at 3, 6, 12 and 24 h. MEHP induced a significant increase of IL-6 mRNA levels at 12 h. Statistical analysis was performed using two-way ANOVA for matched pairs with Sidak’s multiple comparison test and each MEHP-treated sample was compared to its time-point control. Representative microphotographs (B) of ASC-17D SC line immunostained with IL-6 (green, AlexaFlour488) after exposure to C (DMSO, control) or M (MEHP) for 24 h.
3.3.2. MEHP exposure does not increase IL-1α, but does increase MCP-1 mRNA
IL-1α was not significantly changed in ASC-17D cells treated with MEHP (200 μM) for 3, 6, 12, and 24 hours (Figure 5A). Interestingly, MCP-1 mRNA, which is normally expressed by PTMCs in peripubertal rats [9, 21], was increased in the ASC-17D cells (Figure 5B).
FIGURE 5. No change in IL-1α, but MCP-1 does increase in rat Sertoli cell line ASC-17D.
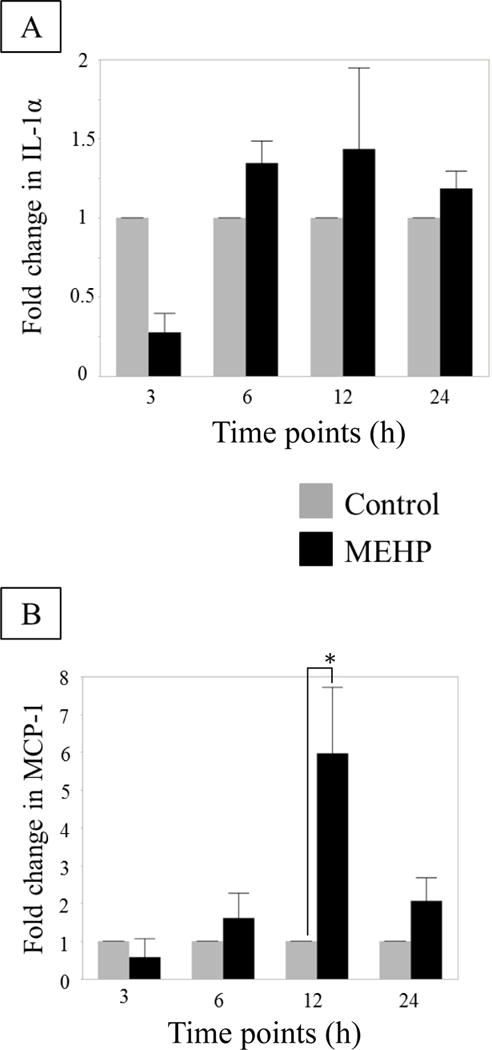
(A) IL-1α and (B) MCP-1 mRNA expression by ASC-17D SC line at 3, 6, 12 and 24 h after MEHP (200 μM, black) or control (DMSO, grey) exposure (n=3 per treatment per time point). No statistical differences were found in IL-1α mRNA expression. MEHP induced a significant increase in MCP-1 mRNA compared to control at 12 h. Statistical analysis was performed using two-way ANOVA for matched pairs with Sidak’s multiple comparison test and each MEHP-treated sample was compared to its time-point control.
3.4. MEHP-induced cytokine changes are rat-specific
3.4.1. IL-6 and MCP-1 are increased in rat compared with controls, but not mouse seminiferous tubules after MEHP exposure
The timing of cytokine mRNA occurs in a time- dependent manner that aligns with a possible instigating mechanism of macrophage infiltration. To see whether the increase in cytokines aligns with the species-dependent infiltration in macrophages, we compared rats, which have macrophage infiltration after MEHP exposure, to mice, which do not have macrophage infiltration after exposure [9]. Two strains of mice were analyzed: C57BL6/J mice are more resistant to autoimmune orchitis, and A/J that are more susceptible to autoimmune orchitis [29]. MCP-1 mRNA levels increased 12 h after exposure in rat seminiferous tubules (MEHP 667 mg/kg), but not in either C57BL/6J or A/J mice (MEHP 800mg/kg) (Figure 6A), corresponding to their lack of macrophage infiltration shown previously [9]. Additionally, neither C57BL/6J nor A/J mice showed increases in IL-6 mRNA (Figure 6B).
FIGURE 6. Differential expression of cytokines after in vivo exposure of Fisher rats, C57Bl/6J and A/J mice.
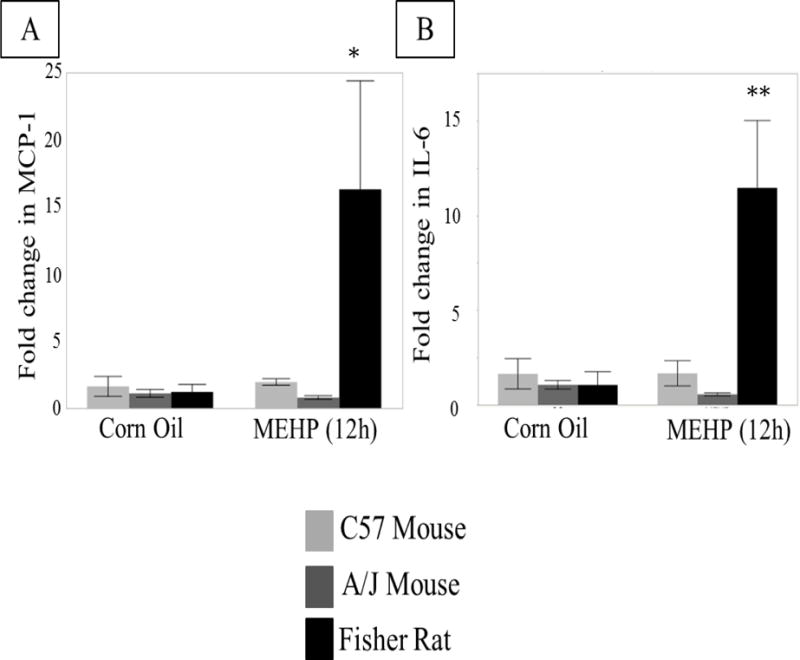
(A) MCP-1 and (B) IL-6 mRNA expression in 12 h MEHP or control treated Fisher Rats (667 mg/kg p.o., light grey), C57BL/6J (800 mg/kg p.o., black) and A/J mice (800 mg/kg p.o., dark grey) (n=3 per treatment per species/strain). Only Fisher rats showed increased cytokine mRNA expression as a result of MEHP treatment. Statistical analysis was performed using two-way ANOVA for unmatched data with Sidak’s multiple comparison test and significance is indicated for each MEHP-treated animal compared to its control. * p < 0.05, ** p < 0.01.
4. DISCUSSION
The results from this study support our hypothesis that MEHP exposure alters inflammatory cytokine expression and correlates with the infiltration of macrophages into the testis. Changes in IL-1α cytokine gene expression was observed only in vivo (Figure 3), whereas IL-6 increases both in vivo and in vitro (Figure 3, 4). MCP-1 increases in vivo, and the timing suggests that the signaling cascade aligns with previously established mechanisms in which IL-1α is upstream of IL-6 [30, 31], and both are increased before MCP-1 increases. The increase in these cytokines is species dependent (Figure 6), further supporting our hypothesis that SC- specific inflammatory cytokine signaling in response to MEHP causes macrophage infiltration, and underscores a unique testicular immune environment in the rat that results in the sensitivity to MEHP.
Acute high dose exposures to MEHP in the peripubertal age range show seminiferous tubule effects [23–26], although repeated dosing around puberty suggests Leydig cell function can also be hindered [32, 33]. The MEHP-induced testicular toxicity within the seminiferous tubule in the dosing paradigm used in this study primarily result from changes in the SCs [13, 14, 34]. SCs function in a complex interaction with the other cells in the seminiferous tubules, and may require the presence of these other cell types to function properly in response to toxicants. This may explain why our in vitro cultures did not completely copy the results obtained in vivo. The primary co-culture system was obtained from seminiferous tubules homogenates, and includes SCs and GCs, and may also contain PTMCs, whereas the ASC-17D cells, a Sertoli cell line, was used with the purpose of specifically dissecting SC contribution to cytokine expression in the seminiferous tubules of peripubertal rats in response to MEHP. Neither of the in vitro systems repeated the early increase in IL-1α gene expression measured in vivo in response to MEHP, suggesting that IL-1α is not increased by SCs directly in response to MEHP, but may require the presence of other cells to induce an IL-1α response. Alternatively, other cells, e.g. PTMCs, might be responsible for the increase in IL-1α gene expression.
Interestingly, MEHP treatment of ASC-17D cells also increased mRNA expression of MCP-1, which is contradictory to our previous findings that MCP-1 protein is primarily up-regulated by PTMCs in the acute pubertal MEHP exposure paradigm [9]. It is possible that both SCs and PTMCs upregulate MCP-1 as a result of MEHP exposure, but that the contribution of PTMCs is the largest. On the other hand, the ASC-17D cells may also act differently to MEHP exposure than the other model systems because they were harvested from an adult rat, and then immortalized using a viral large T antigen to drive proliferation [22]. In pubertal rats MCP-1 is primarily localized to PTMC, but in adult rats, there is a more diffuse localization with many types of cells in the testis expressing MCP-1, including the SC [9]. Given the finding here that ASC-17D cells increase MCP-1 gene expression after MEHP exposure, this may suggest that these cells still retain a more adult phenotype. Taking these differences between the ASC-17D cells and the in vivo results into consideration, the ASC-17D SC line does not seem to accurately replicate a pubertal response to MEHP and therefore may not act as a good system to investigate the mechanism that underlies the phenotypes illustrated in this type of exposure.
Despite the observed increases in IL-1α and IL-6 after MEHP exposure, it remains unclear how these cytokines may participate in the mechanism leading to macrophage infiltration into the testis. Increased expression of IL-1α and IL-6 within co-cultures and the in vivo seminiferous tubule preparations correlate tightly with MCP-1 expression. MEHP treated rats showed a concurrent increase in expression of MCP-1 and number of macrophages in the interstitium [9]. This correlation is strengthened by the findings that C57BL/6J and A/J mice, previously shown not to display macrophage infiltration in the acute pubertal MEHP exposure paradigm [9], did not present with increases in any of these cytokines or chemokines (Figure 6) after acute pubertal MEHP exposure.
In addition to their sensitivity to macrophage infiltration, peripubertal rats are also more sensitive to MEHP-induced GC apoptosis as a result of an acute high dose exposure to MEHP than mice. Previous studies in mice have found that the primary cellular target of MEHP is thought to be the SC and the mechanism of injury is multifaceted including disruption of BTB [35], increase in the expression of the pro-apoptotic protein FasL [13], and disruption of vimentin filaments [14]. Thus, the previously established mechanism of injury to the SC does not seem to be specific to the rat [13, 14, 23, 25, 26] and a new species specific mechanism of injury that results in immune disruption must be identified.
The results from this study indicated that MEHP-injury in pubertal rats specifically stimulates secretion of pro-inflammatory cytokines and alters the immune microenvironment. MEHP exposure in vivo and in vitro increases IL-1α and IL-6 mRNA levels. Although these cytokines are used by SCs in many homeostatic functions, the exact cell source and role of these cytokines in macrophage infiltration remains unclear. Future investigations will focus on inhibiting the expression of these cytokines in specific testicular cell types in order to test their functional significance in the induction of germ cell apoptosis and/or macrophage infiltration after toxicant-induced SC injury. Furthermore, the present findings indicate that the MEHP exposure model is useful for understanding the unique biology of immune development in the testis during the peripubertal period and for deciphering how immune balance in the testis is regulated in order to maintain functional spermatogenesis.
Highlights.
Germ cell apoptosis itself does not instigate macrophage infiltration into the testis.
MCP-1, IL-1α and IL-6 mRNA expression is increased in the seminiferous tubule after MEHP exposure.
MEHP-induced IL-6 and MCP-1 mRNA expression is species-dependent.
MEHP-induced cytokines correlate with macrophage infiltration.
Acknowledgments
Funding:
This work was funded, in part, by a grant from the National Institute of Environmental Health Sciences (NIEHS/NIH; ES016591) and the Center for Molecular Carcinogenesis and Toxicology. These funding sources did not influence the experimental design, collection or analysis of the data in this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
SC: Sertoli cell, GC: germ cell, TM: testicular macrophage, LC: Leydig cell, PTMC: peritubular myoid cell, MEHP: mono-(2-ethylhexyl) phthalate, DEHP: di-(2-ethylhexyl) phthalate, MAA: methoxy acetic acid, BTB: blood testis barrier, PND: post-natal day, FasL: Fas ligand
References
- 1.O’Bryan MK, Hedger MP. Inflammatory networks in the control of spermatogenesis : chronic inflammation in an immunologically privileged tissue? Adv Exp Med Biol. 2008;636:92–114. doi: 10.1007/978-0-387-09597-4_6. [DOI] [PubMed] [Google Scholar]
- 2.Hedger MP. Immunophysiology and pathology of inflammation in the testis and epididymis. J Androl. 2011 Nov-Dec;32(6):625–40. doi: 10.2164/jandrol.111.012989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hedger MP, Winnall WR. Regulation of activin and inhibin in the adult testis and the evidence for functional roles in spermatogenesis and immunoregulation. Mol Cell Endocrinol. 2012 Aug 15;359(1–2):30–42. doi: 10.1016/j.mce.2011.09.031. [DOI] [PubMed] [Google Scholar]
- 4.Campese AF, Grazioli P, de Cesaris P, Riccioli A, Bellavia D, Pelullo M, et al. Mouse Sertoli cells sustain de novo generation of regulatory T cells by triggering the notch pathway through soluble JAGGED1. Biol Reprod. 2014 Mar;90(3):53. doi: 10.1095/biolreprod.113.113803. [DOI] [PubMed] [Google Scholar]
- 5.Doyle TJ, Kaur G, Putrevu SM, Dyson EL, Dyson M, McCunniff WT, et al. Immunoprotective properties of primary Sertoli cells in mice: potential functional pathways that confer immune privilege. Biol Reprod. 2012 Jan;86(1):1–14. doi: 10.1095/biolreprod.110.089425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dufour JM, Rajotte RV, Kin T, Korbutt GS. Immunoprotection of rat islet xenografts by cotransplantation with sertoli cells and a single injection of antilymphocyte serum. Transplantation. 2003 May 15;75(9):1594–6. doi: 10.1097/01.TP.0000058748.00707.88. [DOI] [PubMed] [Google Scholar]
- 7.Winnall WR, Muir JA, Hedger MP. Rat resident testicular macrophages have an alternatively activated phenotype and constitutively produce interleukin-10 in vitro. Journal of leukocyte biology. 2011 Jul;90(1):133–43. doi: 10.1189/jlb.1010557. [DOI] [PubMed] [Google Scholar]
- 8.Li MW, Mruk DD, Lee WM, Cheng CY. Cytokines and junction restructuring events during spermatogenesis in the testis: an emerging concept of regulation. Cytokine & growth factor reviews. 2009 Aug;20(4):329–38. doi: 10.1016/j.cytogfr.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murphy CJ, Stermer AR, Richburg JH. Age- and species-dependent infiltration of macrophages into the testis of rats and mice exposed to mono-(2-Ethylhexyl) phthalate (MEHP) Biology of reproduction. 2014;91(1):18. doi: 10.1095/biolreprod.113.115527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hannas BR, Lambright CS, Furr J, Evans N, Foster PM, Gray EL, Wilson VS. Genomic biomarkers of phthalate-induced male reproductive developmental toxicity: a targeted RT-PCR array approach for defining relative potency. Toxicol Sci. 2012 Feb;125(2):544–57. doi: 10.1093/toxsci/kfr315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaido KW, Hensley JB, Liu D, Wallace DG, Borghoff S, Johnson KJ, et al. Fetal mouse phthalate exposure shows that Gonocyte multinucleation is not associated with decreased testicular testosterone. Toxicol Sci. 2007;97(2):491–503. doi: 10.1093/toxsci/kfm049. [DOI] [PubMed] [Google Scholar]
- 12.Scott HM, Hutchison GR, Mahood IK, Hallmark N, Welsh M, De Gendt K, et al. Role of androgens in fetal testis development and dysgenesis. Endocrinology. 2007;148(5):2027–36. doi: 10.1210/en.2006-1622. [DOI] [PubMed] [Google Scholar]
- 13.Lee J, Richburg JH, Younkin SC, Boekelheide K. The Fas system is a key regulator of germ cell apoptosis in the testis. Endocrinology. 1997;138(5):2081–8. doi: 10.1210/endo.138.5.5110. [DOI] [PubMed] [Google Scholar]
- 14.Richburg JH, Boekelheide K. Mono (2-ethylhexyl) phthalate rapidly alters both Sertoli cell vimentin filaments and germ cell apoptosis in young rat testes. Toxicol Appl Pharmacol. 1996 Mar;137(1):42–50. doi: 10.1006/taap.1996.0055. 1996. [DOI] [PubMed] [Google Scholar]
- 15.Sjoberg P, Lindqvist NG, Ploen L. Age-dependent response of the rat testes to di(2-ethylhexyl) phthalate. Environ Health Perspect. 1986 Mar;65:237–42. doi: 10.1289/ehp.65-1474698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Franca LR, Auharek SA, Hess RA, Dufour JM, Hinton BT. Blood-tissue barriers: morphofunctional and immunological aspects of the blood-testis and blood-epididymal barriers. Adv Exp Med Biol. 2012;763:237–59. [PubMed] [Google Scholar]
- 17.Petersen C, Soder O. The Sertoli cell− a hormonal target and ‘super’ nurse for germ cells that determines testicular size. Horm Res. 2006;66(4):153–61. doi: 10.1159/000094142. [DOI] [PubMed] [Google Scholar]
- 18.Hutson JC. Changes in the concentration and size of testicular macrophages during development. Biol Reprod. 1990 Nov;43(5):885–90. doi: 10.1095/biolreprod43.5.885. [DOI] [PubMed] [Google Scholar]
- 19.Li LH, Wine RN, Miller DS, Reece JM, Smith M, Chapin RE. Protection against Methoxyacetic-Acid-Induced Spermatocyte Apoptosis with Calcium Channel Blockers in Cultured Rat Seminiferous Tubules: Possible Mechanisms. Toxicology and Applied Pharmacology. 1997;144:105–19. doi: 10.1006/taap.1997.8129. [DOI] [PubMed] [Google Scholar]
- 20.Wang W, Chapin RE. Differential Gene Expression Detected by Suppression Subtractive Hybridization in the Ethylene Glycol Monomethyl Ether-Induced Testicular Lesion. Toxicological Sciences. 2000;56:165–74. doi: 10.1093/toxsci/56.1.165. [DOI] [PubMed] [Google Scholar]
- 21.Aubry F, Habasque C, Satie AP, Jegrou B, Samson M. Expression and regulation of the cc-chemokine monocyte chemoattractant protein-1 in rat testicular cells in primary culture. Biol Reprod. 2000 May;62(5):1427–35. doi: 10.1095/biolreprod62.5.1427. [DOI] [PubMed] [Google Scholar]
- 22.Roberts KP, Banerjee PP, Tindall JW, Zirkin BR. Immortalization and characterization of a Sertoli cell line from the adult rat. Biol Reprod. 1995 Dec;53(6):1446–53. doi: 10.1095/biolreprod53.6.1446. [DOI] [PubMed] [Google Scholar]
- 23.Yao PL, Lin YC, Richburg JH. TNF alpha-mediated disruption of spermatogenesis in response to Sertoli cell injury in rodents is partially regulated by MMP2. Biol Reprod. 2009 Mar;80(3):581–9. doi: 10.1095/biolreprod.108.073122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moss EJ, Cook MW, Thomas LV, Gray TJ. The effect of mono-(2-ethylhexyl) phthalate and other phthalate esters on lactate production by Sertoli cells in vitro. Toxicology letters. 1988;40(1):77–84. doi: 10.1016/0378-4274(88)90185-3. [DOI] [PubMed] [Google Scholar]
- 25.Yao PL, Lin YC, Sawhney P, Richburg JH. Transcriptional regulation of FasL expression and participation of sTNF-alpha in response to sertoli cell injury. J Biol Chem. 2007 Feb;282(8):23. 5420–31. doi: 10.1074/jbc.M609068200. [DOI] [PubMed] [Google Scholar]
- 26.Lin YC, Yao PL, Richburg JH. FasL gene-deficient mice display a limited disruption in spermatogenesis and inhibition of mono-(2-ethylhexyl) phthalate-induced germ cell apoptosis. Toxicol Sci. 2010 Apr;114(2):335–45. doi: 10.1093/toxsci/kfq015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karzai AW, Wright WW. Regulation of the synthesis and secretion of transferrin and cyclic protein-2/cathepsin L by mature rat Sertoli cells in culture. Biol Reprod. 1992 Nov;47(5):823–31. doi: 10.1095/biolreprod47.5.823. [DOI] [PubMed] [Google Scholar]
- 28.Tirado OM, Selva DM, Toràn N, Suárez-Quian CA, Jansen M, McDonnell DP, Reventós J, Munell F. Increased expression of estrogen receptor beta in pachytene spermatocytes after short-term methoxyacetic acid administration. J Androl. 2004 Jan-Feb;25(1):84–94. doi: 10.1002/j.1939-4640.2004.tb02762.x. [DOI] [PubMed] [Google Scholar]
- 29.Wheeler K, Tardif S, Rival C, Luu B, Bui E, Del Rio R, et al. Regulatory T cells control tolerogenic versus autoimmune response to sperm in vasectomy. Proceedings of the National Academy of Sciences of the United States of America. 2011 May;108(18):3. 7511–6. doi: 10.1073/pnas.1017615108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Syed V, Stephan JP, Gerard N, Legrand A, Parvinen M, Bardin CW, Jegou B. Residual bodies activate Sertoli cell interleukin-1 alpha (IL-1 alpha) release, which triggers IL-6 production by an autocrine mechanism, through the lipoxygenase pathway. Endocrinology. 1995 Jul;136(7):3070–8. doi: 10.1210/endo.136.7.7789334. [DOI] [PubMed] [Google Scholar]
- 31.Stephan JP, Syed V, Jegou B. Regulation of Sertoli cell IL-1 and IL-6 production in vitro. Mol Cell Endocrinol. 1997 Nov;134(2):15. 109–18. doi: 10.1016/s0303-7207(97)00172-x. [DOI] [PubMed] [Google Scholar]
- 32.Ge RS, Chen GR, Dong Q, Akingbemi B, Sottas CM, Santos M, et al. Biphasic effects of postnatal exposure to diethylhexylphthalate on the timing of puberty in male rats. J Androl. 2007;28(4):513–20. doi: 10.2164/jandrol.106.001909. [DOI] [PubMed] [Google Scholar]
- 33.Noriega NC, Howdeshell KL, Furr J, Lambright CR, Wilson VS, Gray LE., Jr Pubertal administration of DEHP delays puberty, suppresses testosterone production, and inhibits reproductive tract development in male Sprague-Dawley and Long-Evans rats. Toxicological sciences : an official journal of the Society of Toxicology. 2009;111(1):163–78. doi: 10.1093/toxsci/kfp129. [DOI] [PubMed] [Google Scholar]
- 34.Heindel JJ, Chapin RE. Inhibition of FSH-stimulated cAMP accumulation by mono(2-ethylhexyl) phthalate in primary rat Sertoli cell cultures. Toxicol Appl Pharmacol. 1989 Feb;97(2):377–85. doi: 10.1016/0041-008x(89)90342-6. [DOI] [PubMed] [Google Scholar]
- 35.Yao PL, Lin YC, Richburg JH. Mono-(2-ethylhexyl) phthalate-induced disruption of junctional complexes in the seminiferous epithelium of the rodent testis is mediated by MMP2. Biol Reprod. 2010 Mar;82(3):516–27. doi: 10.1095/biolreprod.109.080374. [DOI] [PMC free article] [PubMed] [Google Scholar]


