Abstract
Human cannabinoid receptor CB2 belongs to the class A of G protein-coupled receptor (GPCR). High resolution structural studies of CB2 require milligram quantities of purified, structurally intact protein. Here we describe an efficient protocol for purification of this protein using the Twin-Strep-tag/Strep-Tactin XT system.
To improve the affinity of interaction of the recombinant CB2 with the resin, the double repeat of the Strep-tag was attached either to the N- or C-terminus of CB2 via a short linker. The CB2 was isolated at high purity from dilute solutions containing high concentrations of detergents, glycerol and salts, by capturing onto the Strep-Tactin XT resin, and was eluted from the resin under mild conditions upon addition of biotin.
Surface plasmon resonance studies performed demonstrate the high affinity of interaction between the Twin-Strep-tag fused to the CB2 and Strep-Tactin XT with an estimated Kd in the low nanomolar range. The affinity of binding did not vary significantly in response to the position of the tag at either N- or C-termini of the fusion. The variation in the length of the linker between the double repeats of the Strep-tag from 6 to 12 amino acid residues did not significantly affect the binding.
The novel purification protocol reported here enables efficient isolation of a recombinant GPCR expressed at low titers in host cells. This procedure is suitable for preparation of milligram quantities of stable isotope-labelled receptor for high-resolution NMR studies.
Introduction
Human cannabinoid receptor CB2 is an integral membrane G protein-coupled receptor (GPCR) predominantly expressed in cells of immune origin. It is implicated in regulation of metabolic pathways of inflammation, neurodegenerative disorders, obesity and pain sensing and thus is an important target for pharmaceutical drug development.(1, 2) Rational design of novel drug candidates specifically targeting this receptor relies on studies of its structure, binding with specific ligands, and activation of the interacting partners (G protein, β-arrestin) etc. The biophysical methods that yield high structural resolution require purified, structurally intact receptor. We previously reported on the methodology for expression of CB2 and its purification and stabilization in a functional state. (3-7)
With the notable exception of rhodopsin, GPCR are present at low levels in native tissues and recombinant expression is required to generate milligrams quantities of protein sample for structural characterization. For studies by NMR the target protein must be labelled by stable isotopes either uniformly or at selected positions (6). This latter condition can be met by expressing the protein in a medium of a defined composition supplemented with labelled amino acid(s). However, cultivation in minimal medium typically yields lower titers of the target protein which, in the case of CB2 does not exceed 0.1% of the total protein in the cell. Thus, a very efficient method of purification is required to recover the recombinant receptor from dilute extracts.
High concentrations of detergents required to solubilize the GPCR from cell membranes impose further restrictions on downstream purification methods. Conventional chromatographic methods such as ion exchange or hydrophobic chromatography are not effective for high-level purification of large quantities of proteins from dilute, detergent-containing solutions.(4, 8) A more practical alternative approach is the use of small affinity tag(s), provided that they do not alter the structural stability and function of the target protein.(9, 10)A large number of fusion protein tags have been developed in the past two decades to enhance expression(11, 12), facilitate proper folding and solubility of the target protein(11) as well as enable detection, surface capture, purification and studies of protein-protein interaction, and these studies were thoroughly analysed in several excellent reviews.(10, 12-17) Some of the tags can serve several purposes, such as detection and purification of the target protein. These include c-myc, HA, FLAG, 1D4, polyArg and polyHis, Strep-tag, HaloTag, S-tag, to name a few.(12, 13, 17, 18) While the use of affinity tags for protein purification is currently widespread, the application of this technology to preparation of membrane proteins for structural studies has to satisfy a number of stringent requirements:
(i) The affinity resin should be relatively inexpensive, to allow purification of milligram quantities of the target protein required for structural studies.
(ii) Resin matrices should be sufficiently robust to withstand multiple cycles of regeneration
(iii) It is often advantageous not to remove the affinity tag from the target protein after purification, since the tag can be subsequently used for immobilization on a solid support for detergent/ ligand exchange and biophysical studies. Thus, the tag should be relatively small and should not affect the fold of the target protein and interfere with its function.
(iv) The interaction between the tagged protein and the affinity resin should be sufficiently strong even in the presence of detergents, glycerol, salts and other additives used to solubilize and stabilize membrane receptors. This is especially important for low-expressing proteins like GPCR in which case the affinity of interaction should be ideally in picomolar- or low nanomolar range.
(v) The tag should dissociate from the affinity resin under mild conditions that ensure preservation of the functional structure of the target protein.
These requirements limit the choice of purification systems for membrane protein targets. For instance, several excellent systems that are based on a small peptide/ antibody interaction are not practical for purification of large quantities of target proteins due to prohibitively high cost of antibody-based resins, with the possible exception of Rho-tag/ 1D4 (13, 19). However, the affinity of interaction for this tag/ antibody pair (Kd ~ 20 nM even in the presence of detergents and glycerol) may be too high for purification of the target proteins from very dilute solutions.(19)
In addition to a single tag (17), the use of a combination of two or more affinity tags flanking the target protein gains increased popularity (9, 13, 20). We previously described the tandem affinity chromatography for purification of CB2 from detergent-containing solutions, using a combination of the IMAC and the chromatography on a Strep-Tactin resin. (3, 8, 16, 19). This allows isolation of the full-length CB2 receptor with high yield and purity. One notable restriction for chromatography on the Strep-Tactin resin is the apparently low affinity of the Strep-tag for the resin. Therefore, the concentration of the target protein in the detergent extract should be quite high, in the high-nanomolar or low-micromolar range.(21) Thus, in the case of very dilute protein solutions it is difficult to attain satisfactory yield and purity of the target protein (6). Therefore, there is a need for a better performing, high affinity resin for purification of proteins expressed at low titers. In this study we demonstrate that the Twin-Strep-tag/ Strep-Tactin XT system is an appropriate choice for purification of membrane proteins, especially for those expressed at low levels.
The Strep-tag system has been thoroughly described in various articles and reviews (8, 16-18, 22-26). Currently, two different streptavidin binding tags are being used most commonly: the Strep-tag II (WSHPQFEK)(22), and its tandem variant– the Twin-Strep-tag (WSHPQFEKGGGSGGGSGGSAWSHPQFEK) (22). The latter is characterized by higher affinity but still reversible binding, and increasingly gains popularity(22). Through its enhanced binding properties, the Twin-Strep-tag enables a wide spectrum of applications, and is the preferred choice when larger tag size on a target protein is acceptable(22).
Among the streptavidin-based affinity reagents currently available are native streptavidin, the affinity engineered Strep-Tactin (22), as well as its modified version with higher affinity for the Strep-tag.(27) These resins cover affinities ranging from micromolar to picomolar thereby enabling the most common purification tasks under native conditions. Competitive elution is performed by biotin(27) and biotin analogs, i. e. diaminobiotin for streptavidin (28-30) and desthiobiotin for Strep-Tactin (31-34), resulting in a displacement of Twin-Strep-tag fusion proteins from the column and, simultaneously, allowing for easy regeneration of the streptavidin affinity resin for the next purification cycle.(5, 8)
The bivalent Twin-Strep-tag comprised of two 8 amino acid-long epitopes separated by a small linker enhances binding to the Strep-Tactin through the avidity effect. Due to the comparatively fast off-rate of each individual Strep-tag II module, the Twin-Strep-tagged protein can be efficiently displaced from the complex with Strep-Tactin upon addition of desthiobiotin, resulting in sharp elution (18). To exploit this concept for even more demanding applications, Strep-Tactin was further engineered resulting in the development of Strep-Tactin XT(27). The new resin has reportedly double digit-picomolar affinity to the Twin-tag, while preserving reversibility of binding as determined for interaction with soluble proteins containing the Twin-tag(27).
In addition to affinity purification, some assays for membrane receptors may be quite challenging, particularly when extensive washing of the surface is required. Examples are ELISA or BiaCoreTM experiments where the recombinant Twin-Strep-tag fusion protein is immobilized on a microtiter plate or a sensor chip, coated with Strep-Tactin XT. Low off-rate helps to reduce the protein loss and improve the performance of the assay.
In this presentation we describe the application of the Strep-Tactin XT resin for purification of the integral membrane cannabinoid receptor CB2 either as one-step or a two-step affinity purification procedure. We also present the application of the strong Twin-Strep-tag/ Strep-Tactin XT interaction for Biacore binding experiments. The effect of the length and the position of the Twin-Strep-tag on the expression and binding properties of the fusion protein will also be discussed.
Materials and Methods
1. Chemicals and reagents
Oligonucleotides were purchased from Operon Biosciences. Restriction enzymes and DNA-modifying enzymes were obtained from New England Biolabs. The Complete His resin was from Roche. The purified Strep-Tactin XT, Strep-Tactin XT Superflow and MagStrep “type3” XT beads were kindly provided by IBA GmbH. Research grade sensor chip CM4, immobilization reagents NHS, EDC, and ethanolamine and HBS-N buffers for SPR experiments were from GE Healthcare.
Cholesteryl hemisuccinate Tris salt (CHS) and detergents 3 [(cholamidopropyl) dimethylammonio]-1-propanesulfonate (CHAPS) and n-dodecyl-b-D-maltosyde (DDM) were obtained from Anatrace. Lipids 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) and 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoserine sodium salt (POPS) were purchased from Avanti Polar Lipids Inc. Synthetic cannabinoid ligand CP-55,940 was from Tocris. All other chemicals of reagent grade were purchased from Sigma.
2. Expression constructs
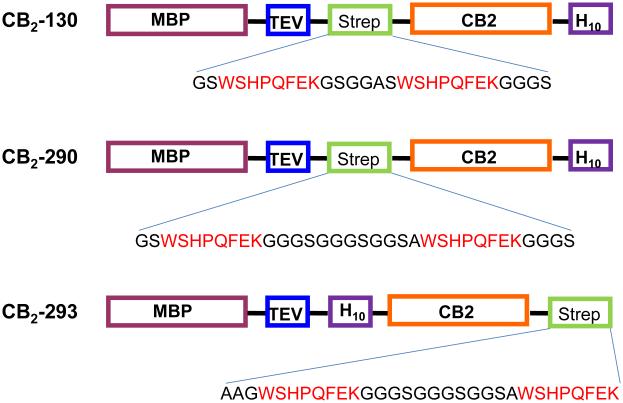
Fusion constructs for expression of CB2 in E. coli and subsequent purification (Fig. 1) were constructed as follows. The construction of CB2-130 was previously described (8).
Fig. 1.
Expression constructs for CB2 protein
Plasmid CB2-130 contains a short linker GSGGAS separating the double repeat of the Strep-tag WSHPQFE. For construction of CB2-290, plasmid CB2-130 was subjected to three consecutive rounds of site-directed mutagenesis using pairs of primers 290a-For: 5’-CCGCAGTTTGAAAAAGGAGGCGGATCGGGCGCGAGCTGG-3’ and 290a-Rev: 5’-CCAGCTCGCGCCCGATCCGCCTCCTTTTTCAAACTGCGG-3’; 290b-For: 5’-GGAGGCGGATCGGGCGGAGGATCGTGGTCGCATCCGCAGTTT-3’ and 290b-Rev: 5’-AAACTGCGGATGCGACCACGATCCTCCGCCCGATCCGCCTCC -3’; and 290c-For: 5’-TCGGGCGGCGGATCGGGAGGCTCGGCTTGGTCGCATCCGCAG-3’ and 290c-Rev: 5’-CTGCGGATGCGACCAAGCCGAGCCTCCCGATCCGCCGCCCGA-3’.The resulting plasmid encodes the following fusion construct: MBP-Asn10-TEV-GS-WSHPQFEK-GGGSGGGSGGSA-WSHPQFEK-GGGS-CB2-His10, where MBP corresponds to the full sequence of the E. coli maltose binding protein, Asn10- the sequence of a linker containing ten asparagine residues, CB2-sequence of human cannabinoid receptor CB2, and His10- a decahistidine tag. The sequence of the plasmid was verified.
For construction of plasmid CB2-293 that encodes the fusion protein MBP-CB2 with the His10 tag at the N-terminus, and the Twin-Strep-tag - at the C-terminus of CB2, the previously published plasmid pAY-109(35) was modified as follows. The plasmid was digested with BamHI, dephosphorylated by phosphatase, and ligated with the duplex DNA fragment encoding the His10 tag, and also introducing the unique NheI site using oligonucleotides 5’-P- GATCACACCATCACCATCACCATCACCATCACCATGCTAGCA-3’ and 5’-P- GATCTGCTAGCATGGAGATGGAGATGGAGATGGAGATGGAGT-3’ forming the plasmid pAY260a. This plasmid was digested with NotI, and the singly cut plasmid dephosphorylated with phosphatase to reduce the background. The duplex DNA formed by the oligonucleotides 5’-P- GGCCGGGATGGTCGCATCCGCAGTTTGAAAAAGGAGGCGGATCGGGAGGCGGATCGGGCGGATCCGCTTGGTCGCATCCGCAGTTTGAAAAGGG-3’ and 5’-P- GGCCCCTTTTTCAAACTGCGGATGCGACCAAGCGGATCCGCCCGATCCGCCTCCCGATCCGCCTCCTTTTTCAAACTGCGGATGCGACCATCC-3’was inserted by ligation. The inserted fragment encodes the Twin-Strep-tag in which two repeats WSHPQFEK are separated by a linker GGGSGGGSGGSA. The oligonucleotides were designed so that both NotI sites flanking the insert in the newly formed plasmid were inactivated upon ligation. This allowed for selection for the insert-containing plasmid by digesting the ligation mixture by NotI enzyme prior to transformation into the E. coli DH5α cells.
The sequences of all plasmids were verified.
3. Expression of CB2 in E. coli
CB2 was expressed as a fusion with the N-terminal MBP and affinity tags in E. coli BL21 (DE3) cell cultivated in 2xYT medium supplemented with glucose and ampicillin as described previously (4). Cells were collected by centrifugation, washed with phosphate-buffered saline, and stored at −80°C. Membranes containing CB2 protein were prepared as described previously(3, 8), the expression levels of the protein determined by semi-quantitative Western blot, and the functional activity of CB2 measured by the G protein activation assay as described previously.(5)
Preparation of crude extract and solubilisation in detergents
Cells were disrupted with Avestin homogenizer, and CB2 fusion protein was solubilized at 4°C in 50 mM Tris-HCl pH 7.5, 150 mM NaCl, 30% glycerol, 1% dodecyl maltosyde (DDM), 0.5% CHAPS, 0.1% cholesteryl hemisuccinate (CHS) with 10 μM cannabinoid agonist CP-55,940 added for CB2 stabilization. The crude extract was centrifuged at 170,000x-g for 1h, and the protein purified from the cleared cell extract as described below.
4. Binding to Strep-Tactin resin
Determination of the binding capacity of Strep-Tactin XT resin
The CB2-130 fusion protein was partially purified on a Ni-NTA resin (Qiagen) and eluted with 250 mM imidazole in buffer A (50 mM Tris pH 7.5, 150 mM NaCl, 30% glycerol, 0.1% DDM, 0.5% CHAPS, 0.1% CHS) as described earlier (4). Fractions containing fusion protein were combined (19 mL) and dialyzed against buffer C (50 mM Tris pH 7.5, 100 mM NaCl, 10% glycerol, 0.1% DDM, 0.5% CHAPS, 0.1% CHS) to remove imidazole and reduce the concentration of glycerol and NaCl.
To study the binding of the fusion CB2 to the Strep-Tactin XT resin, 1 mL of the dialyzed protein solution was applied directly onto the Strep-Tactin XT resin. To study the binding of the CB2 devoid of the MBP fusion partner, the remaining 8 mL of protein solution were first incubated with 2 mg of TEV protease for 4 h at 4°C and then reacted with the resin. One mL of the protein extract (either treated with TEV protease or untreated as specified in the text) was slowly passed by gravity through the disposable mini-column packed with 500 μL of the Strep-Tactin XT resin. The columns were then washed successively with 8 mL of buffer A, 8 mL of buffer A supplemented with 500 mM NaCl, and 8 mL of buffer A supplemented with 1M NaCl. All wash fractions were collected and analysed separately. The protein was eluted by gravity flow by applying 1 column volume of buffer A supplemented with 1M NaCl and 50 mM biotin. Upon 15 min incubation the eluate was collected and the elution procedure repeated 4 more times. All samples were processed in duplicates. Further studies showed that the bound protein can be equally effectively eluted by passing the buffer A containing 50 mM biotin through the column without stopping the flow.
For evaluation of the binding capacity of Strep-Tactin XT magnetic beads, 300 μL of a 5% suspension of the beads were mixed with 1 mL of the protein extract on ice and incubated for 30 min. Because of the small volume of the beads they were not packed into the column but separated from the solution by brief centrifugation, and the supernatant carefully removed. The beads were then washed four times with 1.7 mL of buffer A, four times with buffer A + 500 mM NaCl, and four times with 1.7 mL of buffer A + 1M NaCl. All wash fractions were collected separately and analysed. Protein was eluted by resuspending the beads in 150 μL of buffer A + 1M NaCl + 50 mM biotin, incubation for 15 min followed by brief centrifugation. The supernatant was collected, and the elution repeated 3 more times. Samples were processed in duplicates.
Protein concentration was determined in the elution fractions by absorbance measurements at 280 nm, and by the Bio Rad Dc detergent-compatible protein assay kit.
Effect of the position of the twin-Strep-tag on binding of CB2 to Strep-Tactin XT
Detergent-solubilized CB2-130 and CB2-290 proteins were purified by immobilized metal-affinity chromatography (IMAC) on a Complete His resin (Roche) as described above, and 1, 4, or 14 mL extract were mixed with 0.2 mL Strep-Tactin XT resin. In the case of the construct CB2-293 that lacks the C-terminal His-tag, a biotin blocking solution (BioLockTM, IBA GmbH) was added to the extract according to the manufacturer’s protocol, to sequester endogenous biotin present in E. coli cells. Then either 4, 40, or 200 mL of solubilized extract were mixed with 0.2 mL resin. Incubation continued for 1 hour at 4°C. We later demonstrated that shorter incubation for 10-15 min were equally effective in capturing the protein (results not shown). The resin was collected by centrifugation, transferred into a disposable column, washed with 10 mL of buffer A + 0.5 M NaCl + CP-55,940, and protein was eluted with 4x column volumes of buffer A + 1M NaCl + CP-55,940 + 50 mM biotin.
Regeneration of the Strep-Tactin XT resin
Regeneration of the Strep-Tactin XT resin was performed according to the manufacturer’s instructions.
Purification of CB2 using conventional Strep-Tactin resin
The purification of CB2-130 using conventional Strep-Tactin resin and a Twin-Strep-tag was performed as described earlier(8).
Large-scale purification of CB2
E. coli BL21(DE3) cells expressing CB2-290 were grown in minimal salt medium in a 7.5-L fermentation vessel and protein production induced as described earlier(6). The biomass was collected, resuspended in 50 mM Tris-HCl pH 7.5 and 150 mM NaCl supplemented with protease inhibitors and DNAse I, and disrupted by passing twice through an Avestin cell homogenizer. Detergents 1% DDM, 0.5% CHAPS, protein stabilizers 0.1% CHS, 30% (v/v) glycerol were added, protein extracted on ice for 1 h, and the solution cleared by centrifugation at 155,000xg for 1 h. The supernatant was filtered through a 0.22 μm pore size filter and applied to a column packed with CompleteHis resin connected to an Akta Purifier 100 chromatography system. The column was washed with buffer A supplemented with 7.5 mM imidazole, and the protein eluted with buffer A + 250 mM imidazole. The fractions containing fusion CB2 protein were combined, dialyzed against buffer C, and the fusion protein cleaved upon addition of 2 mg of TEV protease.(8) The resulting extract (50 mL) was mixed with 4 mL of Strep-Tactin XT resin and incubated overnight at 4°C on a shaker. The resin was transferred into a disposable column and washed with buffer A by gravity flow. Buffer A supplemented with 1M NaCl and 50 mM biotin was added, the flow stopped for 15 min, and the eluate collected. The elution cycle was repeated 4 more times, the elution fractions collected and the protein concentration determined by the BioRad DC assay.
5. Surface plasmon resonance (SPR) experiments
CM4 sensor chips were used for SPR experiments performed in a Biacore X-100 biosensor (GE Healthcare) at a temperature 10 °C as indicated in the text.
Immobilization of Strep-Tactin XT onto the sensor chip
The purified Strep-Tactin XT protein was immobilized on the CM4 chip surface at 25 °C by amine coupling using 10 mM HEPES, pH 7.4, and 150 mM NaCl (HBS-N) as a running buffer at a flow rate of 5 μL/ min following the procedure recommended by the manufacturer. Upon activation of surfaces with 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC) and N-hydroxysuccinimide (NHS) for 8 min, two consecutive injections, 10 min each, of the Strep-Tactin XT protein diluted in 10 mM sodium acetate to 2 μg/mL at a flow rate of 2 μL/min were performed. Excess activated groups were blocked with two consecutive injections (35 and 5 μL) of 1 M ethanolamine-HCl, pH 8.5. A density of Strep-Tactin XT corresponding to 170 response units (RU) was achieved. The surface was stabilized by several consecutive 50-μL injections of 2 M NaCl and running buffer HBS-N at a flow rate 50 μL/min. A reference surface was generated by treatment with the amine coupling reagent without the protein.
Preparation of CB2 for SPR binding experiments
CB2-130 and CB2-290 were expressed in E. coli cells and purified by tandem affinity chromatography on Complete His. The MBP tag was removed by TEV protease treatment, and the released CB2 purified on Strep-Tactin resin as described previously(8). To remove biotin from the protein solutions eluted from Strep-Tactin, the proteins were then immobilized onto Complete His resin and washed extensively with buffer A supplemented with 10 μM CP-55,940. The proteins were eluted with 250 mM imidazole in buffer A and concentrated on centrifugal micro spin concentrators to 1-2 mg/mL. Prior to the SPR experiments, the protein samples were dialyzed against four changes of the buffer C (50 mM Tris pH 7.5, 150 mM NaCl, 10% (v/v) glycerol, 0.1% DDM, 0.5% CHAPS, 0.1% CHS) supplemented with 10 μM CP-55,940.
CB2-290 fusion protein was purified on Complete His resin and then on Strep-Tactin XT resin. Treatment with TEV protease was not performed. The protein was then captured again onto Complete His resin and processed the same way as described above for CB2-130 and CB2-290.
CB2-293 was purified as follows. The MBP-CB2 fusion was captured onto Strep-Tactin XT resin, treated with TEV protease, and then captured onto Complete His resin. The resin was washed extensively to remove impurities, and the eluted protein concentrated and dialyzed against SPR running buffer as described above.
Affinity of CB2 to Strep-Tactin XT
Binding experiments were performed at 10 °C at a flow rate of 5 μL/ min using a surface coated with Strep-Tactin XT and a reference generated by mock coupling without the protein. Running buffer C supplemented with 10 μM cannabinoid ligand CP-55,940 was used in all experiments. The running buffer was kept on ice during the entire experiment, and samples were kept on ice until injection. Solutions of the purified CB2 (0.05, 0.3, 5, 30, 100 nM and 1 μM) were injected over both the active and reference surfaces; the contact time for each injection was 20 min, the maximal time allowed by the instrument to allow the sensogram to approach a steady reading. Then the running buffer was passed through the cell for 1 h or longer. The CB2 was eluted by injecting 50 mM biotin in running buffer for 10 minutes or longer. The injections were repeated one or several times until steady readings were achieved. The surfaces were regenerated as described below.
Regeneration of the sensor chip surface
The removal of captured CB2 was performed by displacement of the protein with biotin at 10 °C. A solution of 50 mM biotin in buffer C was injected at a flow rate of 1 μL/-min. Biotin was then removed at a flow rate of 50 μL/-min injection of the running buffer, 10 mM NaOH twice followed by two more injections of running buffer.
Results
Fusion constructs for expression of CB2
To study the effects of the length of the linker between the two binding epitopes WSHPQFEK in Twin-Strep tag on the affinity of the fusion protein to the resin, we employed two expression constructs CB2-130 and CB2-290 (Fig. 1). In the construct CB2-130, two Strep-tag epitopes are separated by six amino acid-long linker, while in CB2-290 the length of the linker is 12 residues. Furthermore, to explore the effect of the position of Twin-Strep-tag relative to CB2, we placed the tag either in the middle of the fusion protein (CB2-290) or downstream of CB2 at the C-terminus of the fusion (CB2-293). In addition, all expression constructs contain the E. coli maltose-binding protein (MBP) as an N-terminal fusion partner that directs the newly synthesized protein to the cytoplasmic membranes of E. coli and stabilizes the fusion.(35) The TEV protease recognition sequence was placed downstream of the MBP in all constructs to allow the removal of the MBP part of the fusion during CB2 purification.
Expression levels of CB2 and activity
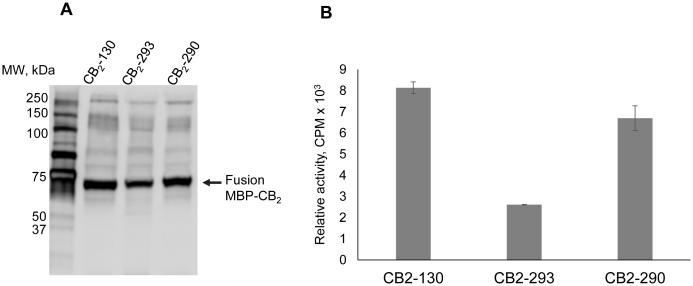
The expression of CB2 in E. coli, determination of the levels of protein accumulation in E. coli membranes, and determination of functional activity of CB2 were performed as described in Materials and Methods. The accumulation of CB2-290 in membranes was almost identical to that of CB2-130, while CB2-293 expressed at somewhat lower levels (Fig. 2 A). These results are confirmed by the activity tests measuring the rates of G protein activation by CB2, with the activity levels of CB2-293 being about 2.5-times lower than those of CB2-130. Lower expression levels of the fusion CB2-293 protein may be attributed to lower rates of translation and/-or folding of the nascent polypeptide due to an unfavourable effect of the decahistidine tag located in the middle of the fusion protein.
Fig. 2.
Expression and activity of CB2.
A, Western blot performed on E. coli membranes expressing CB2. Proteins were probed with rabbit polyclonal antibody against His5 tag.
B, activation rates of G protein on E. coli membrane expressing CB2. The receptor was activated by addition of 10 μM agonist CP-55,940 and the rates of activation of G protein analysed by measuring the accumulation of 35S-γ-GTP-Gαi1 complex as described previously.(5)
Determination of the binding capacity of resin variants
To compare the binding of the Strep-Tactin Superflow resin and the magnetic beads the following experiment was performed: The CB2-130 construct was pre-purified by chromatography on Ni-NTA as described in Materials and Methods. The Strep-Tactin Superflow resin as well as the Strep-Tactin XT magnetic beads were tested. The protein solution in detergent-containing buffer (Buffer A as described in Materials and Methods) was applied to the resin in a batch mode.
To study the effect of the position of the Twin-Strep tag in the fusion protein (Fig.1) on binding of CB2 to the resin, we compared the binding of the fusion MBP-CB2-130 protein to the resin with that of the CB2 protein pre-treated with TEV protease. TEV protease cleaves the fusion protein downstream of MBP, leaving only three amino acid residues (SGS) upstream of the Twin-Strep-tag in case of CB2-130. In the uncleaved MBP-CB2 fusion protein, the Twin-Strep tag is located approximately in the middle of the fusion. The results of these experiments are summarized in Table 1.
Table 1.
Determination of the binding capacity of Streptactin-XT resin.
| Variant of the Strep-Tactin XT resin |
Binder | Resin binding capacity, nmol protein/ mL resin* |
|---|---|---|
| Strep-Tactin XT | CB2-130 | 37.5+/− 1.1 |
| Strep-Tactin XT | CB2-130-MBP fusion |
11.4 +/−1.8 |
| Strep-Tactin XT magnetic beads |
CB2-130 | 72.7 +/−4.3 |
The results represent an average of two samples.
For the Strep-Tactin XT Superflow, the binding capacity for the N-terminally located Twin-Strep-tag in CB2-130 was in the range of 37-38 nmol per mL of resin, corresponding to 1.65-1.75 mg of the CB2 protein (44,065 Da) per 1 mL of resin. For the fusion protein MBP-CB2 (86,336 Da) the binding capacity decreased by about 3-fold, most likely because of steric hindrance to interaction of the Strep-tag with binding sites on the resin from the MBP fusion partner. The binding capacity of the Strep-Tactin XT magnetic beads for the CB2 protein was almost two-fold higher than for regular Strep-Tactin XT chromatography resin which can be attributed to a higher density of the Strep-tag binding sites on the functionalized beads(27).
The resin samples containing bound protein were extensively washed with a total of 48 column volumes of wash buffer (see Materials and Methods). In all cases we detected only traces of CB2 in wash fractions, indicating tight binding of the protein to the resin. The elution was achieved by five sequential cycles of treatment of the resin with 1 column volume of the elution buffer containing 50 mM biotin. Over 95% of the protein was eluted in the first 4 fractions.
Position of the Strep-tag and the length of the linker
The position of the Strep-tag in the fusion protein and the length of the linker between the binding epitopes may affect the exposure of the tag for interaction with binding sites on the Strep-Tactin XT resin which, in turn, may influence the binding capacity of the resin and the affinity of interaction. We compared the binding of several CB2-expressing fusion constructs to the Strep-Tactin XT resin.
E. coli cells expressing CB2-130, CB2-290 or CB2-293 were grown in 2xYT medium, and the recombinant CB2 protein production induced as described in Materials and Methods. The expression levels of these proteins were determined by Western blot and the activity tests (Fig. 2). Cells were collected, and the fusion proteins solubilized in buffers containing detergents DDM, CHAPS and CB2 stabilizers CHS and high affinity cannabinoid ligand CP-55,940.
The experiments were designed such that for each sample the extract applied onto the resin contained a large (3-4 fold) excess of CB2 protein relative to the amount of resin. In order to accomplish this, CB2-130 and CB2-290 fusion proteins were purified from the respective crude extracts by IMAC using the C-terminal decahistidine tag and eluted in buffer A supplemented with 250 mM imidazole as described in the Materials and Methods. Imidazole was removed from the eluates by dialysis, and part of the sample was incubated with TEV protease to remove the MBP fusion partner while the other portion of the extract was left intact. The resulting concentration of CB2 in these eluates was approximately ten-fold higher (0.5-1 μM) than in the crude extracts prior to the Ni-NTA affinity purification.
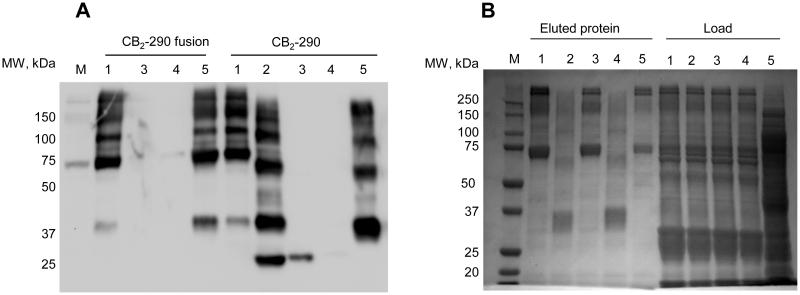
For CB2-293 that lacks the C-terminal polyhistidine tag, the pre-purification by IMAC could not be performed. The crude extract cleared by ultracentrifugation and filtration was treated with BioLock (biotin blocking solution) to remove the biotin that may be present in cell extracts, and mixed with the Strep-Tactin XT resin as described in the Materials and Methods. After the protein binding step was completed, the resin was extensively washed with buffers with progressively increasing concentrations of NaCl, and the CB2 protein eluted with biotin. The results are summarized in Table 3 and in Fig. 3.
Fig. 3.
Constructs of CB2 purified on Strep-Tactin XT resin.
A, Western blot detection of CB2: Lane 1, extract applied onto Complete His resin; 2,eluate from CompleteHis treated with TEV protease; 3, flowthrough from Strep-Tactin XT; 4-wash fractions from Strep-Tactin XT; 5-eluate from Strep-Tactin XT.
B, Eluates from Strep-Tactin XT analysed by SDS-PAGE, gel stained with Instant Blue: Lane 1, CB2-130-MBP fusion protein; lane 2, CB2-130; lane 3, CB2-290-MBP fusion protein; lane 4, CB2-290; lane 5, CB2-293 fusion protein.
The results suggest that steric hindrance by MBP negatively affects the binding of the fusion proteins. The location of the Strep-tag in the middle of the fusion is not optimal for binding since the apparent capacity of the resin is significantly lower for the fusion protein. In the case of CB2-130, the removal of MBP increased the binding of CB2 by about 4-fold. A slightly lower increase by about a factor of 2 was observed for the CB2-290 construct. Interestingly, the increase in length of the linker from 6 to 12 amino acids slightly lowers binding of CB2 protein but appears to be beneficial for binding of the larger fusion protein.
The C-terminal location of the tag (CB2-293) appears to be less favourable for binding as opposed to the N-terminal location (CB2-130 and CB2-290). However, in the case of CB2-293 binding occurred from dilute crude extracts, and other proteins may have obstructed interaction of the tag with the resin.
Importantly, the purity of CB2 in all cases is high (Fig. 3), and leaching of CB2 from the resin during washes is minimal.
Regeneration of the Strep-Tactin XT resin
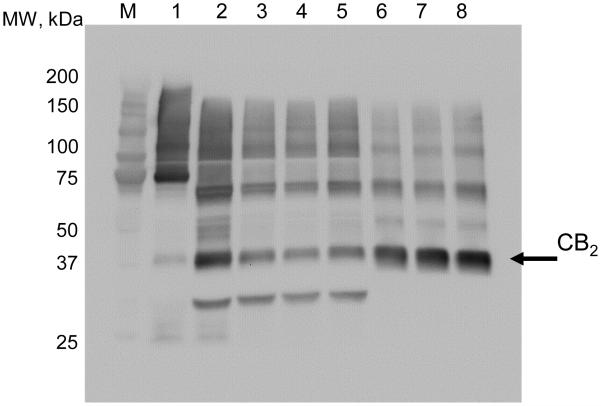
Regeneration of the Strep-Tactin XT resin was performed by repeated treatments with NaOH according to the manufacturer’s instructions. To estimate the performance of the regenerated resin, it was reacted with IMAC-pre-purified CB2-130, washed, and the eluted protein quantified. The resin packed in a column was subjected to three consecutive cycles of regeneration/-binding; the binding capacity slightly decreased with each cycle. For CB2-130 the binding capacity decreased from about 60 nmol/mL to ~45 nmol/mL after 3 cycles of regeneration (Fig. 4)
Fig. 4.
Regeneration of Strep-Tactin XT resin. Western blot probed with anti-His-tag antibody. Lane 1, eluate from IMAC; 2-eluate from IMAC treated with TEV protease; 3-5, flow-through from the Strep-Tactin resin regenerated 0, 1 and 3 times; 6-8 eluate from Strep-Tactin XT resin regenerated 0, 1 and 3 times.
Large-scale purification of CB2 from dilute solutions
For NMR studies, the CB2 protein must be labelled with stable isotopes. This was achieved by expression in E. coli cultivated in minimal salt medium supplemented with labelled nutrients.(6) The titer of the recombinant protein expressed in minimal medium typically decreases several-fold compared to rich medium. To compensate for this decrease and to achieve high protein yield, cells are cultivated to a high density in a fermenter under controlled conditions of aeration, pH, temperature and nutrient supply. (6) The large amount of biomass generated in the fermentation process provides additional challenges for downstream purification. Our typical protocols call for processing of as much as 300-400-g of wet cell paste for preparation of 3-4 mg of pure CB2. The procedure for the purification is described in Materials and Methods.
The biomass obtained from fermentation in minimal medium typically contains lower titers of CB2 compared to the rich medium, and solubilization of the recombinant receptor requires large volume of detergent-containing buffers. Typically, proteins from 300-400 g of wet biomass are solubilized in 2-2.5 L of buffer, which corresponds to an effective concentration of CB2 of 50 nM or lower, depending on expression levels of the target protein.
The construct CB2-293 with the C-terminal Twin-Strep tag was successfully purified directly from the cell extracts on a Strep-Tactin XT resin (Fig. 3). Because the level of expression of CB2-293 is lower than for CB2-130 or CB2-290 (see Fig. 2), the latter two constructs are preferred for large-scale production of CB2. Because in these two fusion proteins the Twin-Strep tag is located in the middle of the construct, binding capacity of the resin for those constructs is low. Furthermore, it would be challenging to pass a large volume of extract through the column because of the very long interaction time required to achieve efficient binding.
Therefore, we purified the CB2 protein by tandem affinity chromatography, using successively the C-terminal His10 tag and the N-terminal twin-Strep-tag. The first stage of purification (IMAC) is performed on a large excess of relatively inexpensive Ni-resin. The eluates containing CB2 protein captured from the crude extracts are then dialyzed, treated with TEV protease, and applied onto the Strep-Tactin XT column. While the purity of the CB2 protein in the IMAC eluate is low, this step increases the concentration of CB2 by about 20 fold which makes the final affinity purification on the Strep-Tactin XT resin much more efficient (Fig. 5). We estimated that this strategy recovers over 70% of CB2 present in the original crude extract allowing purification of large quantities of CB2. About 4 mg of CB2 protein was obtained from 400 g of wet biomass using this protocol. The purified CB2 was reconstituted in proteoliposomes (7) and was shown to be fully functional by G protein-activation tests (results not shown).
Fig. 5.
Purification of CB2 from dilute solutions. A, SDS-PAGE stained with Instant Blue. B, Western blot probed with anti-His antibody.
M, molecular weight marker; 1, crude extract; 2, flowthrough from IMAC; 3-combined wash fractions from IMAC; 4, 5, elution fractions from IMAC; 6, flowthrough from 5 mL Strep-Tactin XT resin; 7-wash fractions from Strep-Tactin XT resin; 8-eluate from Strep-Tactin XT.
Surface Plasmon Resonance studies of CB2 binding to Strep-Tactin
Binding affinity of the interaction of CB2 with Strep-Tactin was further studied by surface plasmon resonance (SPR). The CM-4 sensor chip was functionalized with Strep-Tactin XT, and the CB2 protein prepared as described in the Materials and Methods.
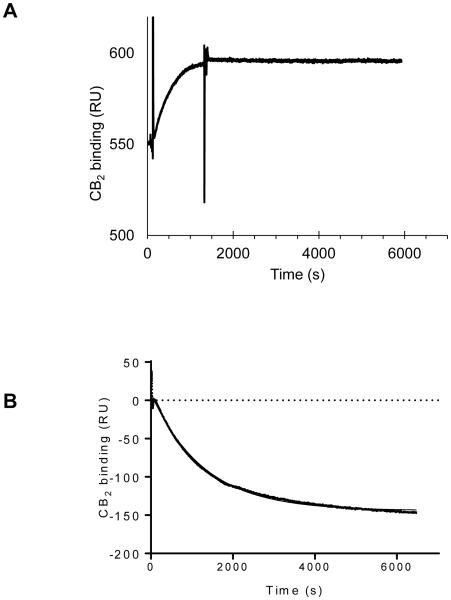
A typical binding curve of the construct CB2-130 to the functionalized chip is shown in Fig. 6 A. The protein sample was applied at a flow rate of 5 μL/ min for 20 min followed by the injection of the running buffer. The concentrations of the protein in the injected samples ranged from 0.05 nM to 1 μM. Depending on the concentration of CB2, an increase in the signal from 1 response units (RU) up to ~350 RU was observed (Supplemental Fig. 1). This surface coverage corresponds to 80-90% of the binding capacity of the functionalized sensor chip assuming 1:1 interaction between binding site and CB2 and a kon= 3.1× 105 M−1s−1.(19) During the dissociation phase initiated by the application of running buffer over the sensor chip surface that lasted for 1 h or longer, only a slight decrease in the signal was observed with an estimated koff =2.3 × 10−4 s−1. The dissociation of the receptor from the chip surface was slow, as expected based on the previous studies with soluble proteins. (31). The regeneration of the surface was performed for more than 10 times according to the protocol described in Materials and Methods, with only a small decline of binding capacity.
Fig. 6.
Binding of CB2 to Strep-Tactin XT studied by surface plasmon resonance.
A, CB2-130 at a concentration 100 nM was injected over 20 min over both the functionalized and the reference surfaces of the sensor chip. The dissociation stage was performed in running buffer C.
B, elution of CB2 from the chip by injection of 50 mM biotin in running buffer. All experiments performed at 10 °C.
Because of the very slow dissociation of the receptor from the surface, an accurate determination of the binding affinity of the protein to the functionalized chip at the experimental conditions was not possible. We estimated that the Kd of interaction between the CB2 and Strep-Tactin XT in the presence of 10% glycerol and various detergents is at least in the low nanomolar to high picomolar range, consistent with experiments performed with the Strep-Tactin XT resin. Our attempts to more accurately quantify the binding of various constructs of CB2 to the surface by equilibrium titration at 10 °C (Supplemental figures 1-4) were not successful because a steady signal at each concentration of analyte was not reached on a reasonable time scale.
Among the CB2 constructs tested, we observed highest binding affinity for CB2-130 and only slightly lower binding – for CB2-290 (Supplemental figures S1A-S2A). Binding of the MBP-CB2-290 construct (figure S3A) corresponded to about 50% of binding density of CB2-290, probably reflecting steric hindrance effects due to the presence of maltose binding protein in the fusion. Binding capacity of CB2-293 (Supplemental figure S4A) was about 3 times lower than that of CB2-290 and CB2-130 suggesting weaker interactions with the surface due to steric hindrance of the tag located at the C terminus of CB2.
The injection of biotin resulted in elution of all four constructs tested (S1,B-S4,B). However, the decrease in the signal corresponded to release of no more than 50% of chip-bound receptor. Subsequent injections of biotin-containing buffer over longer periods of time resulted in further release of CB2. For complete regeneration of the sensor surface repeated cycles of injection NaOH and running buffer (without biotin) as described in Materials and Methods are effective.
Discussion
GPCR are very flexible proteins that respond to binding of specific ligands by changing conformation that ensures transmittal of the signal from the extracellular milieu to intracellular responding elements. To study conformation and dynamics of GPCR, an array of spectroscopic methods is used including nuclear magnetic resonance spectroscopy. Application of this method, however, benefits enormously from the ability to introduce stable isotopes (13C, 15N, 2H) either uniformly or in selected positions of the protein. This is achieved by expressing the protein in E. coli cells cultivated in a medium of defined composition (minimal salt medium, MSM) supplemented with stable isotope-labelled nutrients. While this approach enables the incorporation of stable isotope(s) into the target protein, cultivation in MSM typically results in lower levels of target protein expression per unit of biomass. Even at carefully optimized conditions, the titers of CB2 in MSM reach no more than 40% of that of rich medium. Since purification of the protein requires solubilization of the biomass in detergent-containing buffers, the concentration of CB2 extracted from biomass obtained from minimal medium is 20 nM or less. This requires a very efficient purification protocol with an affinity to the resin used for purification in the lower nanomolar- or better picomolar range. The resin has to possess a sufficiently high binding capacity, allow elution of the captured protein at mild conditions, and should be priced to permit purification of milligrams of target protein at reasonable cost. We are not aware of other commercially available resins that can fully satisfy these stringent requirements.
We have previously demonstrated a superior performance of the Twin-Strep-tag (formerly known as Strep-Tag III) over the Strep-tag II for purification of CB2 on a Strep-Tactin resin, likely due to the avidity effect.(8) While the binding affinity of the Twin-Strep-tag to the resin was not quantified, we routinely observed 40-50% higher recovery of the constructs of CB2 containing the Twin-Strep-tag relative to the single tag. However, even for the constructs with Twin-Strep-tag, the previously available version of the Strep-Tactin resin was not optimal due to the requirement of relatively high concentrations (about 0.3-1 μM) of CB2 protein applied to the resin, and a larger number of column volumes to elute the protein. Thus, a further increase in affinity of interaction between the tag and the resin was highly desirable.
Here we demonstrate that the Strep-Tactin XT resin that recently became commercially available satisfies stringent requirements for affinity and binding capacity, enabling mild purification of CB2 receptor at high purity and yield. For the N-terminally located, well exposed tag, we estimated a recovery of 1.5 mg receptor from 1 mL of resin. The elution of the protein is efficient in the presence of 50 mM biotin, and the bulk of the protein (over 80-90%) can be recovered in the first 3 column volumes of eluate. The resin can be efficiently regenerated with only a slight decline in binding capacity over 3 regeneration cycles.
The binding affinity of the Strep-Tactin XT resin is very high. The resin with bound CB2 was washed extensively, as much as 48 column volumes of detergent-, glycerol- and salt-containing buffers, yet we did not detect any significant CB2 in the wash fractions.
The significantly higher binding of CB2 onto magnetic beads relative to the Strep-Tactin XT Superflow resin can be explained by a much denser coating of the beads with Strep-Tactin XT in comparison to the Superflow resin, and also by the smaller size of the beads (25-30 μm vs. 60-150 μm). However, the application of the magnetic beads for the purification of large quantities of CB2 is not practical due to the relatively high price of the beads.
While we demonstrated the feasibility of a one-step purification of CB2 with Twin-Strep-tag at the N-terminus from crude cell extracts using Strep-Tactin XT resin, this approach is not practical due to relatively low binding capacity of the resin for CB2 with C-terminal Twin-Strep-tag. A purification in column mode can be performed instead, but it harbours yet another problem: a large volume of extract has to be processed in order to capture the target protein which will lengthen the purification time unreasonably. A similar problem was observed with purification of CB2 harbouring the C-terminal Rho-tag(19). The purity of the CB2 fusion protein captured on the 1D4 resin is high. However, the affinity of Rho-tag binding to 1D4 antibody in the presence of detergents was not very high (Kd approximately 20 nM(19)) and it can be used C-terminally only. Therefore, this tag is not as practical for recovery of CB2 from dilute solutions as compared to the Twin-Strep-tag that can be used at the N-terminus as well.
Therefore, our current purification protocol relies on two successive chromatography steps that, in turn, require two affinity tags on the protein. The first step is purification via IMAC that allows recovery of most of the protein from the solution albeit at low purity only, using a large excess (relative to protein) of the inexpensive chromatography resin. The enriched material is further purified by chromatography using Strep-Tactin XT. Due to the slow off-rate, the protein bound to the Strep-Tactin XT resin is not lost during extensive washing, resulting in much higher purity and overall yield of the target protein. This is a significant improvement over the previous version of the Strep-Tactin resin that was characterized by much lower affinity for the tag and hence much greater losses of the protein during the wash step.
A similar two-step purification protocol has been employed in this laboratory with the previously available Strep-Tactin resins. The recovery of CB2 from cells expressed in rich 2xYT medium was on average 0.25-0.3 mg/L cell culture. However, the superior binding properties of the recently released Strep-Tactin XT allows for much more stringent washes of the protein bound to the resin, yielding higher purity. Furthermore, the recovery of CB2 is about 30-40% higher, typically in 0.45-0.5 mg/L range.
Our SPR results confirm the high affinity of interaction between the Twin-Strep tag attached to CB2 and the Strep-Tactin XT. The interaction is efficient in the presence of high concentrations of detergents, glycerol and salts. It appears that the N-terminal-, – rather than the C-terminal – position of the Twin-Strep tag is preferred for higher density of binding. As expected, the location of the Twin-Strep tag in the middle of the fusion protein results in lower density of surface coverage, most likely due to unfavourable steric effects of the large MBP tag. All surface-captured constructs of CB2 were stable, and very little of the surface-captured protein was lost during prolonged washing with the running buffer. The protein was eluted under mild conditions by addition of biotin.
To summarize, we demonstrated significant advantages of the Strep-Tactin XT for purification of the difficult-to express cannabinoid CB2 membrane receptor. The use of the twin-Strep-tag is preferred over the single Strep-tag, since the higher affinity of interaction with the resin allows efficient capture and recovery of the target protein. The location of the tag at the N-terminus of CB2 leads to higher density of binding compared to the C-terminal location. However, this effect is likely to be specific to CB2 protein due to the steric hindrance of the tag. The length of the spacer between the two epitopes of the twin-Strep-tag does not significantly affect the affinity of binding.
The twin-Strep-tag/ Strep-Tactin XT system can be especially useful for purification of recombinant proteins expressed at low titers, and for purification of membrane proteins in the presence of high concentrations of detergents, salts and glycerol.
Supplementary Material
Table 2.
Binding of various constructs of CB2 to Strep-Tactin XT resin
| CB2 expression construct |
Binding capacity of the resin* | |
|---|---|---|
| mg/mL | nmol/mL | |
| CB2-130-MBP fusion | 1.3 mg/mL | 15 |
| CB2-130 | 2.8 mg/mL | 63.5 |
| CB2-290-MBP fusion | 2.2 mg/mL | 25.3 |
| CB2-290 | 1.9 mg/mL | 42.9 |
| CB2-293-MBP fusion | 1.7 mg/mL | 19.6 |
The results are the average of two samples
Highlights.
A novel high affinity resin Strep-Tactin® XT was tested for purification of integral membrane receptor CB2
We developed an efficient protocol for capture and purification of recombinant cannabinoid receptor CB2 from dilute solutions
The N-terminal position of the Twin-Strep-tag is preferred for efficient binding to the resin
Surface plasmon resonance studies with the purified protein confirm the high affinity of interaction between the Twin-Strep-tag fused to the CB2 and Strep-Tactin XT.
Acknowledgements
A.Y. and L.Z. are supported by DICBR, NIAAA, NIH. Authors appreciate the contribution by the IBA GmbH that provided samples of the Strep-Tactin XT resin, purified Strep-Tactin XT and shared the unpublished protocols. We thank Klaus Gawrisch (NIAAA, NIH) for critical reading of the manuscript and discussion.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ashton JC, Glass M. The cannabinoid CB2 receptor as a target for inflammation-dependent neurodegeneration. Current neuropharmacology. 2007;5:73–80. doi: 10.2174/157015907780866884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guzior N, Wieckowska A, Panek D, Malawska B. Recent development of multifunctional agents as potential drug candidates for the treatment of Alzheimer's disease. Current medicinal chemistry. 2015;22:373–404. doi: 10.2174/0929867321666141106122628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yeliseev AA. Methods for recombinant expression and functional characterization of human cannabinoid receptor CB2. Computational and structural biotechnology journal. 2013;6:e201303011. doi: 10.5936/csbj.201303011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yeliseev AA, Wong KK, Soubias O, Gawrisch K. Expression of human peripheral cannabinoid receptor for structural studies. Protein Sci. 2005;14:2638–2653. doi: 10.1110/ps.051550305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vukoti K, Kimura T, Macke L, Gawrisch K, Yeliseev A. Stabilization of Functional Recombinant Cannabinoid Receptor CB2 in Detergent Micelles and Lipid Bilayers. Plos One. 2012;7 doi: 10.1371/journal.pone.0046290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berger C, Ho JT, Kimura T, Hess S, Gawrisch K, Yeliseev A. Preparation of stable isotope-labeled peripheral cannabinoid receptor CB2 by bacterial fermentation. Protein Expr Purif. 2010;70:236–247. doi: 10.1016/j.pep.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kimura T, Yeliseev AA, Vukoti K, Rhodes SD, Cheng K, Rice KC, Gawrisch K. Recombinant Cannabinoid Type 2 Receptor in Liposome Model Activates G Protein in Response to Anionic Lipid Constituents. J Biol Chem. 2012;287:4076–4087. doi: 10.1074/jbc.M111.268425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yeliseev A, Zoubak L, Gawrisch K. Use of dual affinity tags for expression and purification of functional peripheral cannabinoid receptor. Protein Expression and Purification. 2007;53:153–163. doi: 10.1016/j.pep.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kimple ME, Brill AL, Pasker RL. Current protocols in protein science / editorial board. Vol. 73. John E. Coligan; 2013. Overview of affinity tags for protein purification. [et al.] Unit 9 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Terpe K. Overview of tag protein fusions: from molecular and biochemical fundamentals to commercial systems. Appl Microbiol Biot. 2003;60:523–533. doi: 10.1007/s00253-002-1158-6. [DOI] [PubMed] [Google Scholar]
- 11.Arnau J, Lauritzen C, Petersen GE, Pedersen J. Current strategies for the use of affinity tags and tag removal for the purification of recombinant proteins. Protein Expr Purif. 2006;48:1–13. doi: 10.1016/j.pep.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 12.Waugh DS. Making the most of affinity tags. Trends Biotechnol. 2005;23:316–320. doi: 10.1016/j.tibtech.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 13.Molday LL, Molday RS. 1D4: a versatile epitope tag for the purification and characterization of expressed membrane and soluble proteins. Methods Mol Biol. 2014;1177:1–15. doi: 10.1007/978-1-4939-1034-2_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Asher WB, Bren KL. Affinity purification of heme-tagged proteins. Methods Mol Biol. 2014;1177:17–33. doi: 10.1007/978-1-4939-1034-2_2. [DOI] [PubMed] [Google Scholar]
- 15.Goodfellow I, Bailey D. Detection of protein-protein interactions using tandem affinity purification. Methods Mol Biol. 2014;1177:121–133. doi: 10.1007/978-1-4939-1034-2_10. [DOI] [PubMed] [Google Scholar]
- 16.Locatelli-Hoops SC, Yeliseev AA. Use of Tandem Affinity Chromatography for Purification of Cannabinoid Receptor CB2. Methods Mol Biol. 2014;1177:107–120. doi: 10.1007/978-1-4939-1034-2_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Young CL, Britton ZT, Robinson AS. Recombinant protein expression and purification: A comprehensive review of affinity tags and microbial applications. Biotechnol J. 2012;7:620–634. doi: 10.1002/biot.201100155. [DOI] [PubMed] [Google Scholar]
- 18.Schmidt TGM, Skerra A. The Strep-tag system for one-step purification and high-affinity detection or capturing of proteins. Nat Protoc. 2007;2:1528–1535. doi: 10.1038/nprot.2007.209. [DOI] [PubMed] [Google Scholar]
- 19.Locatelli-Hoops SC, Gorshkova I, Gawrisch K, Yeliseev AA. Expression, surface immobilization, and characterization of functional recombinant cannabinoid receptor CB2. Bba-Proteins Proteom. 2013;1834:2045–2056. doi: 10.1016/j.bbapap.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yeliseev A, Vukoti K. Expression of G-protein-coupled receptors. In: Robinson AS, editor. Production of membrane proteins. Wiley-VCH; Weinheim: 2011. pp. 219–248. [Google Scholar]
- 21.LifeSciences, Inc. http://www.iba-lifesciences.com/details/product/2-1201-002.html, Manual StrepTactin.
- 22.Schmidt TGM, Batz L, Bonet L, Carl U, Holzapfel G, Kiem K, Matulewicz K, Niermeier D, Schuchardt I, Stanar K. Development of the Twin-Strep-tag (R) and its application for purification of recombinant proteins from cell culture supernatants. Protein Expression and Purification. 2013;92:54–61. doi: 10.1016/j.pep.2013.08.021. [DOI] [PubMed] [Google Scholar]
- 23.Bell MR, Engleka MJ, Malik A, Strickler JE. To fuse or not to fuse: What is your purpose? Protein Sci. 2013;22:1466–1477. doi: 10.1002/pro.2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miladi B, El Marjou A, Boeuf G, Bouallagui H, Dufour F, Di Martino P, Elm'selmi A. Oriented immobilization of the tobacco etch virus protease for the cleavage of fusion proteins. J Biotechnol. 2012;158:97–103. doi: 10.1016/j.jbiotec.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 25.Walls D, Loughran ST. Tagging Recombinant Proteins to Enhance Solubility and Aid Purification. Protein Chromatography: Methods and Protocols. 2011;681:151–175. doi: 10.1007/978-1-60761-913-0_9. [DOI] [PubMed] [Google Scholar]
- 26.Schmidt TGM, Skerra A. The Random Peptide Library-Assisted Engineering of a C-Terminal Affinity Peptide, Useful for the Detection and Purification of a Functional Ig Fv Fragment. Protein Eng. 1993;6:109–122. doi: 10.1093/protein/6.1.109. [DOI] [PubMed] [Google Scholar]
- 27.IBA GmbH Manual "New 3rd Generation StrepTag system" [Google Scholar]
- 28.Torreggiani A, Fini G. The binding of biotin analogues by streptavidin: A Raman spectroscopic study. Biospectroscopy. 1998;4:197–208. doi: 10.1002/(SICI)1520-6343(1998)4:3%3C197::AID-BSPY5%3E3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 29.Rodriguez-Melendez R, Lewis B, McMahon RJ, Zempleni J. Diaminobiotin and desthiobiotin have biotin-like activities in Jurkat cells. J Nutr. 2003;133:1259–1264. doi: 10.1093/jn/133.5.1259. [DOI] [PubMed] [Google Scholar]
- 30.Reznik GO, Vajda S, Sano T, Cantor CR. A streptavidin mutant with altered ligand-binding specificity. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:13525–13530. doi: 10.1073/pnas.95.23.13525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmidt T, Skerra A. The Strep-tag System for One-Step Affinity Purification of Proteins from Mammalian Cell Culture. Affinity Chromatography: Methods and Protocols. (3rd) 2015;1286:83–95. doi: 10.1007/978-1-4939-2447-9_8. [DOI] [PubMed] [Google Scholar]
- 32.Hirsch JD, Eslamizar L, Filanoski BJ, Beechem JN, Haugland RP, Haugland RP. Desthiobiotin: An easily reversible ligand for biotin-binding proteins. Faseb J. 2002;16:A1182–A1182. doi: 10.1016/s0003-2697(02)00201-4. [DOI] [PubMed] [Google Scholar]
- 33.Yoon HC, Hong MY, Kim HS. Reversible association/dissociation reaction of avidin on the dendrimer monolayer functionalized with a biotin analogue for a regenerable affinity-sensing surface. Langmuir. 2001;17:1234–1239. [Google Scholar]
- 34.Voss S, Skerra A. Mutagenesis of a flexible loop in streptavidin leads to higher affinity for the Strep-tag II peptide and improved performance in recombinant protein purification. Protein Eng. 1997;10:975–982. doi: 10.1093/protein/10.8.975. [DOI] [PubMed] [Google Scholar]
- 35.Krepkiy D, Wong K, Gawrisch K, Yeliseev A. Bacterial expression of functional, biotinylated peripheral cannabinoid receptor CB2. Protein Expression and Purification. 2006;49:60–70. doi: 10.1016/j.pep.2006.03.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.