Abstract
BACKGROUND & AIMS
There is controversy over the role of the type 2 immune response in pathogenesis of ulcerative colitis (UC)—few data are available from treatment-naïve patients. We investigated whether genes associated with a type 2 immune response in the intestinal mucosa are upregulated in treatment-naïve pediatric patients with UC, compared to patients with Crohn’s disease (CD)-associated colitis or without inflammatory bowel disease (IBD), and whether expression levels associate with clinical outcomes.
METHODS
We used a real-time reverse transcription quantitative PCR array to analyze mRNA expression patterns in rectal mucosal samples from 138 treatment-naïve pediatric patients with IBD and macroscopic rectal disease, as well as those from 49 children without IBD (controls), enrolled in a multicenter prospective observational study from 2008 and 2012. Results were validated in real-time reverse transcription quantitative PCR analyses of rectal RNA from and independent cohort of 34 pediatric patients with IBD and macroscopic rectal disease and 17 controls from Cincinnati Children’s Hospital Medical Center.
RESULTS
We measured significant increases in mRNAs associated with a type 2 immune response (interleukin 5 gene [IL5], IL13, and IL13RA2) and a type 17 immune response (IL17A and IL23) in mucosal samples from patients with UC compared to patients with colon-only CD. In a regression model, increased expression of IL5 and IL17A mRNAs distinguished patients with UC from patients with colon-only CD (P=.001; area under the receiver operating characteristic curve, 0.72). We identified a gene expression pattern in rectal tissues of patients with UC, characterized by detection of IL13 mRNA, that predicted clinical response to therapy after 6 months (OR, 6.469; 95% CI, 1.553–26.94), clinical response after 12 months (OR, 6.125; 95% CI, 1.330–28.22), and remission after 12 months (OR, 5.333; 95% CI, 1.132–25.12).
CONCLUSION
In an analysis of rectal tissues from treatment-naïve pediatric patients with IBD, we observed activation of a type 2 immune response during the early course of UC. We were able to distinguish patients with UC from those with colon-only CD based on increased mucosal expression of genes that mediate type 2 and type 17 immune responses. Increased expression at diagnosis of genes that mediate a type 2 immune response is associated with response to therapy and remission in pediatric patients with UC.
Keywords: immune regulation, gene expression profile, prognostic factor, AUROC
Type 2 inflammation has been implicated in the pathogenesis of ulcerative colitis (UC).1,2 Classic type 2 immune responses are defined by production of the cytokines IL-4, IL-5, and IL-13 by T helper cells and innate lymphoid cells and are involved in the expulsion of helminthes and the pathogenesis of allergic diseases.3 UC, however, has been associated with an atypical type 2 immune response, with increased IL-5 and IL-13, but not IL-4.2 Further studies have indicated pathogenic effects of IL-13 including activation of inflammatory colon mucosal natural killer T cells and impairment of epithelial barrier function.4 The initial observation of this atypical type 2 immune response in UC originated from ex-vivo experiments with lamina propria immune cells isolated from surgical specimens from adults with IBD.2 Whether type 2 inflammation is involved in UC patients at diagnosis, prior to treatments that affect the inflammatory response, or in pediatric UC remains unknown. Furthermore, it is unknown if patients with heightened type 2 inflammatory responses attain different clinical outcomes.
Distinguishing UC from Crohn’s colitis can be a diagnostic challenge in pediatric patients. Regardless of diagnosis with UC or Crohn’s disease (CD), a colitis phenotype is a common feature of pediatric IBD. UC and IBD-unclassified (IBD-U) account for 30-40 percent of pediatric IBD in the United States and Europe.5-7 Furthermore, approximately 80 percent of children with CD have colonic involvement with 25 percent exhibiting a colon-only phenotype with no small intestinal involvement.8,9 Colon-only CD phenotype is even more common with younger age, occurring in about 40 percent of children under 10 years of age.8,9 Taken together, approximately 50% of pediatric IBD patients exhibit an isolated colitis phenotype. Many of these children exhibit overlapping or atypical features, which hinder rendering a specific diagnosis of CD or UC.10 It is not known whether mucosal expression of genes associated with type 2 inflammation can distinguish UC and CD pediatric patients with isolated colitis phenotypes.
We hypothesized that treatment naïve pediatric patients with UC would exhibit increased mucosal type 2 immune responses compared to patients without IBD and Crohn’s colitis, and that high expression of type 2 associated genes would be associated with poor response to therapy in pediatric UC. Here we applied a microfluidic real-time RT-qPCR array platform to determine rectal mucosal expression of genes associated with type 1, type 2, and type 17 inflammation in patients with UC, Crohn’s colitis, and non-IBD controls from a large multi-center North American pediatric IBD inception cohort. We report that expression of genes associated with type 2 and type 17 immune responses distinguished two colon-only phenotypes of pediatric IBD: UC and colon-only CD Furthermore, we observed that a gene expression profile marked by detectable IL13 expression is associated with improved clinical outcomes in pediatric UC.
Materials and Methods
RISK Cohort Rectal RNA Samples
Rectal mucosal RNA samples from treatment-naïve IBD patients and non-IBD controls and associated clinical data were obtained from the RISK Study, a prospective observational IBD inception cohort sponsored by the Crohn’s & Colitis Foundation of America. 1812 children and adolescents younger than 17 years, newly diagnosed with IBD and non-IBD controls, were enrolled at 28 North American pediatric gastroenterology centers between 2008 and 2012. All participants underwent baseline colonoscopy with confirmation of characteristic chronic active colitis and/or ileitis by histology prior to diagnosis and treatment. Institutional Review Board approval was obtained locally at each participating site. All endoscopic tissues obtained in the RISK study were stored in RNALater (ThermoFisher Scientific, Waltham, MA), thus RNA and DNA, but not protein, is available for study. This analysis included a representative subgroup of RISK participants with UC (n = 56), colon-only CD (CDc, n = 36), ileocolonic CD (CDic, n = 46), and non-IBD controls (n = 49). This constitutes all participants within RISK with rectal RNA that met study criteria for UC and CDc, and a random sample of those that met study criteria for CDic. Only patients with a confirmed diagnosis of CD, UC, or non-IBD based on standard clinical and pathologic criteria after a median 3.3 years follow-up were included in this analysis. To meet the study definition of CD in RISK, patients must eventually have been found to have at least two of the following: signs or symptoms consistent with CD (diarrhea, abdominal pain, rectal bleeding, malaise, weight loss, or linear growth failure), endoscopic findings of discontinuous ulceration or cobblestoning, and/or histopathologic findings of patchy inflammatory cell infiltrates or epithelial granuloma. Detailed granular data regarding anatomic disease involvement was obtained for all participants in the RISK cohort. For the purposes of this analysis, all participants with UC and CD must have exhibited macroscopic inflammation in the rectum at the time of biopsy collection. Participants with UC must have carried a latest diagnosis of UC, exhibited macroscopic inflammation in the rectum (since RNA analyzed in this study was isolated from rectal biopsies), absence of macroscopic inflammation in the ileum at their enrollment endoscopy, and absence of evidence of jejunal inflammation. Participants with CDc, must have carried a latest diagnosis of CD, and exhibited macroscopic inflammation in rectum with absence of macroscopic inflammation in the ileum or jejunum. Participants with CDic, must have carried a latest diagnosis of CD, and exhibited macroscopic inflammation in both the rectum and ileum. Non-IBD control participants must have carried a latest diagnosis of non-IBD and exhibited macroscopically and microscopically normal ileum and colon.
Cincinnati Cohort
Under a protocol approved by the Cincinnati Children’s Hospital Medical Center Institutional Review Board, patients presenting for routine colonoscopy for clinical indications were enrolled in a separate independent local Cincinnati cohort for the purposes of validating the findings from the RISK cohort. Patients in the Cincinnati cohort were not in the RISK cohort. Clinical information was collected at the time of enrollment. Rectal biopsies were placed in RNALater and stored at −80°C. Only patients with macroscopic inflammation in the rectum at endoscopy were included in the analysis. H&E stained sections from rectal biopsies were scored by a pediatric pathologist blinded to diagnosis using the validated Robarts Histopathology Index (RHI).11
Real-time RT-qPCR
For all RISK cohort RNA samples studied, rectal RNA integrity was determined using an Agilent 2100 Bioanalyzer instrument (Agilent Technologies, Santa Clara, CA). Only samples with an RNA integrity number ≥ 7 were included (95.5% of samples tested). Expression of 24 genes related to type 2, type 1, type 17, and regulatory immune responses (Supplementary Table 1) was determined by quantitative real-time reverse transcription PCR (RT-qPCR) in duplicate from 100 ng starting RNA using custom TaqMan array 384-well microfluidic cards on a 7900HT Real-Time PCR System (Thermo Fisher Scientific, Waltham, MA). The endogenous reference control was selected empirically by analyzing a subset of 16 samples (4 from each diagnosis group) with the TaqMan Human Endogenous Control Array (Thermo Fisher Scientific), which assesses expression of 16 genes known to exhibit minimal differential expression across tissues. Real-time RT-qPCR on rectal RNA from the Cincinnati Cohort was performed using a subset of individual gene expression assays from the custom microfluidic array. Relative expression was determined using a modification of the 2−ΔΔCq method as previously described.12 Briefly, ΔCq values were obtained by subtracting the target quantification cycle (Cq) from that of the reference gene. ΔCq values were then shifted such that expression in samples with undetectable expression (Cq > 40) was considered half that of the sample with the least detectable expression.
Outcomes
Outcomes assessed in the UC group included steroid-free, surgery free clinical response and clinical remission at 6 and 12 months. Clinical remission was defined as a physician’s global assessment (PGA) of quiescent. The four PGA categories were quiescent, mild, moderate, and severe. Clinical response was defined a PGA of quiescent or mild with at least a 1 category drop in PGA between baseline and the indicated time point (i.e. if the patient was mild at baseline, they must have improved to quiescent). For both endpoints, patients must have been off systemic corticosteroids, with no prior surgery at the indicated time point.
Statistical Analysis
Statistical analyses were performed using SAS v9.3 (SAS Institute, Cary, NC). For gene expression array data from the Risk Cohort, we assessed global differences in expression for each gene amongst the 4 diagnosis groups using the nonparametric Kruskal-Wallis H test with false discovery rate (FDR) to control the type 1 error rate at 0.05. We then performed pairwise comparisons between diagnosis groups only for genes passing the omnibus test using the Mann-Whitney U test with FDR correction. Correlation of expression between genes was assessed by Spearman's rank correlation coefficient. Principle component analysis incorporating all array gene expression data was performed using Genespring GS (Agilent Technologies, Santa Clara, CA). We applied unsupervised hierarchical clustering (Genespring GS) to identify patient clusters with unique gene expression patterns. To validate gene expression differences discovered in the RISK Cohort in the independent Cincinnati Cohort, we compared expression of selected genes and RHI using the Kruskal-Wallis H test followed by pairwise comparisons using the Mann-Whitney U test only if the omnibus test was significant. In a sensitivity analysis to control for histologic disease activity within the local Cincinnati Cohort, patients with CD and UC were matched on RHI (within 1 point) and gene expression was compared using the Wilcoxon signed rank test. We assessed the performance of gene expression to distinguish UC from CDc and to predict clinical outcomes in UC patients using logistic regression. The regression model for distinguishing UC from CDc was internally validated across 1000 random samples of equal size using a random sampling with replacement bootstrap technique. To assess the contribution of degree of inflammation to the observed differences in gene expression between UC and CDc, bivariate logistic regression was performed for each target gene with significant differences in gene expression between UC and CDc and S100A8. A change in effect estimate between univariate and bivariate analysis with S100A8 of <10%, 10-20%, or >20% were interpreted as no, mild, or moderate confounding by S100A8, respectively.13 S100A8 was forced into the final multivariate model for predicting UC from CDc to determine contribution of degree of inflammation to final model. Baseline characteristics amongst gene expression patient clusters were compared using the Kruskal-Wallis H test for continuous variables or chi-square test for nominal variables. The association between gene expression cluster and clinical outcomes in the UC group was assessed by Fisher's exact test.
Sample Size and Power
We determined that a sample size of 40 patients per-diagnosis group would provide 90% power to detect a 1.5-fold difference in expression while controlling the type I error rate at 1% for multiplicity.
Results
RISK Cohort rectal mucosal gene expression
Demographics and baseline characteristics of the subset of RISK Cohort participants studied for this analysis are detailed in Table 1. There were no meaningful differences between the subset of RISK cohort patients included in this study and the overall RISK cohort other than for characteristics within the specific inclusion or exclusion criteria for this study (Supplementary Table 2). All participants with CDic, CDc and UC in the studied subset exhibited macroscopic rectal inflammation at endoscopy, compared to 67.5, 74.8, and 88.5 percent, respectively, in the overall RISK cohort (relative rectal sparing can be an atypical feature of pediatric UC). We only included participants exhibiting macroscopic rectal inflammation in order to limit the effect of differences in rectal inflammation on the analysis. Additionally, no patients in the studied subset of CDc participants exhibited jejunal inflammation compared to 9.5 percent of CDc participants in the overall RISK cohort. While jejunal involvement (Paris L4b) does preclude the designation of colon-only CD (Paris L2) based on the Paris classification,14 we excluded participants with jejunal disease from our CDc group because these patients can be easily distinguished from UC patients based on anatomic disease distribution alone.
Table 1.
Baseline characteristics of RISK cohort patients studied
| Non-IBD (n = 49) | CDic (n = 46) | CDc (n = 36) | UC (n = 56) | |
|---|---|---|---|---|
| Age, y | 12.8 (10.8,15) | 12.4 (10.9,13.6) | 12.7 (10.8,14.5) | 13.5 (10.8,15.5) |
| A1a: 0–<10 y | 11 (23.9) | 6 (17.1) | 13 (23.2) | |
| A1b: 10–<17 y | 35 (76.1) | 30 (85.7) | 43 (76.8) | |
| Male sex | 23 (46.9) | 25 (54.3) | 17 (47.2) | 30 (53.6) |
| CD location | ||||
| L1: terminal ileal ± limited cecal disease | 0 (0) | 0 (0) | ||
| L2: colonic | 0 (0) | 36 (100) | ||
| L3: ileocolonic | 46 (100) | 0 (0) | ||
| L4a: upper disease proximal to ligament of Treitz | 32 (69.6) | 17 (47.2) | ||
| L4b: upper disease distal to ligament of Treitz | 12 (26.1) | 0 (0) | ||
| UC extent | ||||
| E1: ulcerative proctitis | 3 (5.3) | |||
| E2: left-sided colitis | 7 (12.5) | |||
| E3: extensive colitis | 9 (16.1) | |||
| E4: pancolitis | 35 (62.5) | |||
| Data not available | 2 (3.6) | |||
| Macroscopic rectal involvement | 46 (100) | 36 (100) | 56 (100) | |
| PGA | ||||
| Quiescent | 0 (0) | 1 (2.8) | 1 (1.8) | |
| Mild | 11 (23.9) | 7 (19.4) | 20 (35.7) | |
| Moderate | 22 (47.8) | 19 (52.8) | 23 (41,1) | |
| Severe | 13 (28.3) | 9 (25.0) | 12 (21.4) | |
| PUCAI | 45 (35,60) | |||
| Rectal deep ulcers | 5 (10.9) | 12 (33.3) | 10 (17.9) |
Quantitative variables expressed as median (quartile 1, quartile 3) and dichotomous variables as n (%).
CD, Crohn’s disease; CDc, colon-only Crohn’s disease; CDic, ileocolonic Crohn’s disease; IBD, inflammatory bowel disease; PGA, physicians global assessment; PUCAI, Pediatric Ulcerative Colitis Activity Index; UC, ulcerative colitis
Amongst 16 candidate reference gene controls assessed, GAPDH exhibited the least variable expression across diagnosis groups (standard deviation of Cq = 0.55) and was included in the gene expression array as the endogenous control against which we normalized results.
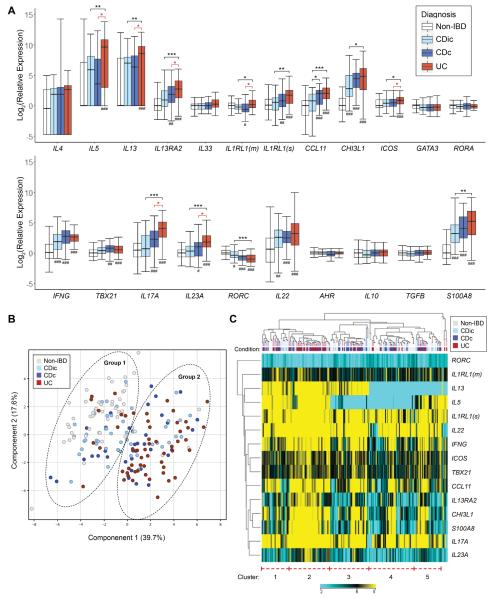
Rectal relative expression of the 22 genes assayed normalized to median of non-IBD patients is detailed in Figure 1A (The assay for CLDN2 did not amplify and is excluded from the results). Compared to non-IBD patients, UC patients were the only group that exhibited significantly increased rectal expression of the genes for the type 2 cytokines IL5 and IL13 (no group exhibited significantly increased expression of IL4.) IL17A and IL23A were significantly increased in UC and CDc, but not CDic, compared to non-IBD. IFNG and IL22 expression were similarly increased in all 3 groups compared to non-IBD.
Figure 1.
Results of microfluidic RT-qPCR gene expression array on rectal mucosal RNA from RISK cohort patients. (A) Box and whisker chart depicting gene expression for each target gene on the array for each IBD diagnostic sub-phenotype normalized to median expression of the non-IBD patient group (boxes represent median and interquartile range, whiskers represent the 95% confidence interval). (B) PCA plot demonstrating separation of two groups of patients with UC and CDc clustering in group 1 and non-IBD in group 2. (C) Dendrogram and heatmap depicting the results of unsupervised hierarchical clustering using genes with differential expression between at least two diagnosis groups. UC patients aggregate within cluster 2, which exhibits high expression of IL13, IL5, and IL17A. IL1RL1(m) and ILRL1(s), transcripts for the membrane-bound and soluble versions of the IL-33 receptor, respectively. *P < .05, **P < .01, ***P < 0.001; #P < .05, ##P < .01, and ###P < 0.001 vs. non-IBD (all P values are FDR-corrected)
Compared to CDc patients with macroscopic rectal involvement, UC patients exhibited significantly increased rectal expression of genes associated with type 2 (IL5, IL13, IL13RA2, ICOS, and the transcript for membrane-bound IL-33 receptor, IL1RL1(m) and type 17 (IL17A, IL23A) immune responses. Of note, expression of the inflammatory marker S100A8, which encodes a subunit of calprotectin, was not significantly different between UC and CDc patients.
Consistent with the finding of elevated IL13 and IL17A expression in UC patients, we observed moderate correlation (r2 = .18, P < .0001) between IL13 and IL17A expression amongst all IBD patients. However, amongst UC patients, we observed no significant correlation between IL13 and IL17A expression (r2 = .057, P = .076).
Principal component analysis (Figure 1B), incorporating all genes analyzed, identified two groups of patients. Group 1 included most of the UC and CDc patients, whereas group 2 included most of the non-IBD patients. CDic patients were intermixed between the two principle component groups.
Unsupervised hierarchical clustering segregated patients into 5 clusters based on gene expression (Figure 1C). The largest aggregate of a single diagnosis was UC patients within cluster 2, which was defined by high expression of IL13 and IL5, and also high expression of CCL11, IL13RA2, CHI3L1, S100A8, IL23A and IL17A. Cluster 2 was composed of 59.1% UC patients (46.4% of all the UC patients) and 6.8% non-IBD patients. The remaining clusters were defined by differences in IL5 and IL13 expression, with more variable, and lower expression of the remaining genes compared to cluster 2. Cluster 4, which exhibited undetectable expression of IL5 and IL13, was comprised of the largest group of non-IBD patients (43.5% non-IBD, 40.8% of all the non-IBD patients). This unequal distribution of diagnoses amongst the clusters was statistically significant, while other baseline characteristics were similar (Table 2).
Table 2.
Comparison of baseline characteristics between gene expression patient clusters
| Gene expression cluster |
||||||
|---|---|---|---|---|---|---|
| 1 (n = 25) | 2 (n = 44) | 3 (n = 39) | 4 (n = 46) | 5 (n = 27) | P value | |
| Age, y | 12.1 (9.3, 14.8) | 12.8 (9.4,15.4) | 13.8 (10.7,15.2) | 12.3 (10.8,14.8) | 12.9 (11.3,15.2) | .757 |
| Male sex | 11 (44.0) | 23 (52.3) | 25 (64.1) | 28 (60.9) | 12 (44.4) | .342 |
| Diagnosis | ||||||
| Non-IBD | 6 (24.0) | 3 (6.8) | 9 (23.1) | 20 (43.5) | 7 (25.9) | < .001 |
| CDic | 8 (32.0) | 9 (20.5) | 11 (28.2) | 10 (21.7) | 8 (29.6) | |
| CDc | 4 (16.0) | 6 (13.6) | 12 (30.8) | 8 (17.4) | 6 (22.2) | |
| UC | 7 (28.0) | 26 (59.1) | 7 (17.9) | 8 (17.4) | 6 (22.2) | |
| PGA | ||||||
| Quiescent | 0 (0) | 1 (2.4) | 0 (0) | 1 (3.8) | 0 (0) | .119* |
| Mild | 3 (15.8) | 17 (41.5) | 7 (23.3) | 6 (23.1) | 5 (25.0) | |
| Moderate | 14 (73.7) | 13 (31.7) | 15 (50.0) | 10 (38.5) | 10 (50.0) | |
| Severe | 2 (10.5) | 10 (24.4) | 8 (26.7) | 9 (34.6) | 5 (25.0) | |
| Rectal deep ulcers | 4 (21.1) | 7 (17.1) | 6 (20.0) | 6 (23.1) | 3 (15.0) | .957 |
Quantitative variables expressed as median (quartile 1, quartile 3) and dichotomous variables as n (%).
Quiescent and Mild combined for chi-square test
CDc, colon-only Crohn’s disease; CDic, ileocolonic Crohn’s disease; IBD, inflammatory bowel disease; PGA, physicians global assessment; UC, ulcerative colitis
Validation of RISK Cohort differential rectal gene expression in Cincinnati Cohort
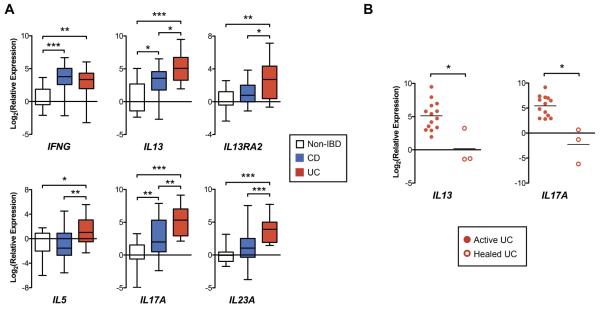
Demographics and baseline characteristics of patients in the Cincinnati cohort are detailed in Supplementary Table 3. Rectal biopsies were analyzed from 17 non-IBD, 20 CD, and 14 UC patients. All CD and UC patients exhibited macroscopic inflammation in the rectum. As in the RISK Cohort, UC patients in our Cincinnati cohort exhibited increased rectal expression of IL5, IL13, IL13RA2, IL17A, and IL23A compared to CD patients with colitis and non-IBD patients (Figure 2A). Also consistent with the RISK Cohort findings, UC and CD patients exhibited similarly increased IFNG expression compared to non-IBD patients.
Figure 2.
Real-time RT-qPCR of rectal mucosal RNA from patients in the Cincinnati validation cohort. (A) Box and whisker chart depicting gene expression normalized to median expression of the non-IBD patient group (boxes represent median and interquartile range, whiskers represent the 95% confidence interval). (B) Dot plot depicting gene expression (normalized to median expression of the non-IBD patient group) in patients with active UC compared to UC patients with endoscopic healing (each dot represents a single patient, and lines represent median). *P < .05
*P < .05, *P < .01, ***P < 0.001
We sought to compare rectal histopathologic disease activity between the macroscopically involved rectums of CD and UC patients in the Cincinnati Cohort, acknowledging the challenge that there exists no histopathologic index validated for pediatric UC or CD, or for comparing between CD and UC patients. Therefore, histopathologic activity was compared between patient groups using the RHI, a validated UC index that assesses the following features common to both UC and CD: chronic inflammatory infiltrate, lamina propria neutrophils, neutrophils in the epithelium, and erosion or ulceration.11 Median RHI was significantly higher in the macroscopically inflamed rectums of UC compared to CD patients (Supplementary Figure 2A). Therefore, to determine whether differences in gene expression are explained by differences in histopathologic severity, we performed a sensitivity analysis comparing gene expression between a subset of 10 UC and CD patients matched on RHI (Supplementary Figure 2B and 2C). In this smaller subset of RHI-matched patients, there was significantly increased rectal relative expression of IL13 and IL13RA2 in UC compared to CD patients. Furthermore, other gene expression relationships between UC and CD were maintained with numerically (but not statistically significantly) increased relative expression of IL17A, IL23A and IL5, and equivalent IFNG expression in UC compared to CD.
To determine if healing on treatment is associated with changes in IL13 and IL17A expression, we compared mucosal gene expression between UC patients in the Cincinnati cohort, all with active endoscopic disease, to three additional UC patients with complete mucosal healing on treatment (Mayo endoscopic score = 0; all female; ages 7, 9, and 20 years; healing achieved on 6-mercaptopurine + infliximab, 5-ASA alone, and 5-ASA and oral corticosteroids, respectively). Both IL13A and IL17A expression were significantly decreased in the patients with mucosal healing compared to those with active endoscopic disease (Figure 2B).
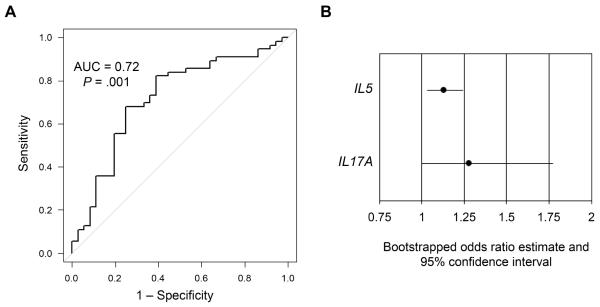
IL5 and IL17 expression distinguish UC from Colitis-only CD
To determine the ability of gene expression to discriminate UC from CDc, we applied univariate logistic regression using only the genes with significant differential expression between UC and CDc (Supplementary Table 4). Using multivariate logistic regression, we determined that a model including IL5 and IL17A gene expression best balanced parsimony with discriminatory ability (P = .001, area under the curve [AUC] = 0.72) (Table 3, Figure 3A). We internally validated the model using bootstrap random sampling with replacement and demonstrate that the bootstrapped estimates for the ORs for both IL5 and IL17A were significant with 95% confidence intervals not crossing 1 (Figure 3B).
Table 3.
Multivariate logistic regression for discriminating UC from CDc
| Gene | OR* | 95% CI | P-value |
|---|---|---|---|
| IL5 | 1.130 | 1.032–1.238 | .009 |
| IL17A | 1.196 | 0.976–1.467 | .085 |
Odds of a diagnosis of UC over CDc per unit increase in Cq value for the listed gene
CI, confidence interval; Cq, quantification cycle; OR, odds ratio
Figure 3.
Logistic regression model including IL5 and IL17A gene expression distinguishes UC from CDc. (A) Receiver operator curve of logistic regression model. (B) Chart depicting estimates of the odds ratios (odds of UC per unit increase in Cq) and 95% confidence intervals for IL5 and IL17A after bootstrap random sample with replacement internal validation.
Although there was not a significant difference in S100A8 expression between UC and CDc, we sought to determine the degree to which difference in other genes were confounded by general inflammatory activity. For genes that exhibited significantly different expression between UC and CDc, we compared the effect estimate obtained by univariate analysis, to that obtained by bivariate analysis with S100A8 (Supplementary Table 5). We detected no evidence of confounding by S100A8 (<10% change in effect estimate) for IL5, IL1RL1(m), IL17A and IL23A, evidence of only mild confounding (10-20% change) for IL13, and evidence of moderate confounding (20-30%) for IL13RA2 and ICOS. Furthermore, when S100A8 is forced into our final model with IL5 and IL17A, S100A8 does not contribute any predictive value for UC over CDc to the model, and the overall model characteristics and performance are unchanged with an unchanged AUC of .72 (Supplementary Table 6).
Gene expression predicts clinical outcome in UC patients
Of the 56 UC patients in the RISK cohort we studied, outcome data was available for 44 and 37 patients at 6 and 12 months, respectively. We applied univariate logistic regression to determine whether any of the 6 genes with differential expression between UC and CDc predicted steroid-free clinical remission or response at 6 or 12 months (Supplementary Table 7). We found that higher IL13 expression was significantly associated with increased likelihood of clinical response at 6 (odds ratio [OR] 1.182, 95% confidence interval [CI] 1.028–1.359) or 12 months (OR 1.172, 95% CI 1.012– 1.359), and a trend toward association with clinical remission at 12 months (OR 1.126, 95% CI .978–1.297). We then assessed whether the unsupervised clustering based on gene expression predicted clinical outcomes in UC patients. We observed that patients in clusters 1, 2 and 3 were significantly more likely to demonstrate clinical response at 6 months (trend for remission), and clinical response and remission at 12 months, compared to those in clusters 4 and 5 (Table 4). The major distinguishing gene expression difference between these groups is increased IL13 gene expression in the clusters 1-3 with essentially undetectable IL13 expression in clusters 4-5. Baseline characteristics and medication exposures by 6 and 12 months were similar between UC patients in clusters 1-3 compared to clusters 3-4, with the exception of exposure to anti-TNF biologics, which occurred numerically, but not statistically significantly, more often in cluster 4-5 (Supplementary Table 8). By 6 months 16.1 percent of patients in clusters 1-3 and 30.8 percent of patients in clusters 4-5 were exposed to an anti-TNF biologic drug (P = .414), and 25.0 percent of patients in clusters 1-3 and 54.5 percent of patients in clusters 4-5 by 12 months (P = .131). It is likely that the numerically increased and earlier infliximab exposure in clusters 4-5 is an additional reflection of the poorer clinical response of this group to first line therapies (i.e. corticosteroids, 5-aminosalicylates, and thiopurines) compared to that of patients in clusters 1-3. We did not find any association between IL13 expression or gene expression cluster and clinical outcomes in CD.
Table 4.
Gene expression cluster and clinical response
| Remission |
Response |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cluster | 6 mo | OR* (95% CI) |
P value |
12 mo | OR (95% CI) |
P value |
6 mo | OR (95% CI) |
P value |
12 mo | OR (95% CI) |
P value |
| All patients | 20/44 (45.5) |
21/38 (55.3) |
27/44 (61.4) |
25/38 (65.8) |
||||||||
| 1, 2 or 3 | 17/31 (54.8) |
4.048 (.929–17.63) |
.096 | 18/27 (66.7) |
5.333 (1.132–25.12) |
.037 | 23/31 (74.2) |
6.469 (1.553–26.94) |
.016 | 21/27 (77.8) |
6.125 (1.330–28.22) |
.024 |
| 4 or 5 | 3/13 (23.1) |
3/11 (27.3) |
4/13 (30.8) |
4/11 (36.4) |
||||||||
Frequencies are reported as n meeting endpoint / n in group (%)
Odds of attaining endpoint for patients in cluster 1, 2, or 3 compared to those in cluster 4 or 5.
CI, confidence interval; Cq, quantification cycle; OR, odds ratio
Discussion
In a well-characterized treatment-naïve pediatric IBD inception cohort, we demonstrate that the rectal mucosa of pediatric UC patients is distinguished from that of patients with colon-only Crohn’s disease by increased expression of genes associated with type 2 and type 17 immune responses. This finding is not explained by differences in overall inflammation as measured by S100A8 expression. Furthermore, in an analysis of prospective data from this cohort, we show that heightened rectal mucosal IL13 expression at baseline is associated with improved clinical outcomes in pediatric UC.
The involvement of type 2 inflammation in the pathogenesis of UC has been debated in the literature. Fuss and colleagues first described disparate cytokine secretion from lamina propria mononuclear cells (LPMCs) isolated from surgical specimens of adults with UC and CD, with those from UC patients producing increased IL-5 and IL-13 and those from CD patients producing IFN-γ.1,2 The same group went on to demonstrate that the IL-13 is produced by natural killer T (NKT), and that both IL-13 and NKT cells disrupt epithelial barrier function.2,4 Accordingly, we have previously demonstrated increased epithelial activation of STAT6, a transcription factor downstream of IL-13 signaling, in pediatric UC. 15 However, other groups have not detected increased IL-13 production either from LPMCs or colon tissue of adult or pediatric patients, respectively, with UC, perhaps due to differences in the ex vivo experimental techniques for studies.16,17 Here we provide strong evidence from a large well-characterized cohort that mucosal type 2 immune responses are involved in the early course of pediatric UC.
Our finding of increased IL17A expression in UC is in line with recent observations by others. The ratio of mucosal IL17A to IL17F expression has been shown to significantly correlate with endoscopic disease activity in adult UC.18 In addition, increased dual expression of IL17A by CD4+CD25− regulatory T cells expressing surface TGF-β in its latent form (LAP+) reduces the suppressor activity of these cells in UC.19
A number of groups have examined the ability of measures of immune response type to discriminate CD from UC in adults, but none have made distinctions between CD anatomic sub-phenotypes or studied exclusively newly diagnosed treatment-naïve patients.12,20,21 A cross-sectional study of adult patients from Japan similarly showed increased mucosal IL13 gene expression in UC compared to Crohn’s disease, and that a panel of genes associated with adaptive immune responses could distinguish the two.12 Similarly, another group demonstrated that the higher mucosal type 2 and lower type 1 T cells as measured by flow cytometry distinguishes UC from CD in adult patients.20 Using a discovery cohort of established patients, they showed that a model including the percent of CD4+ T cells positive for IFN-γ, T-bet, IL-13, and Gata3 was predictive of CD over UC, and validated the model in a small cohort of newly diagnosed patients. However, in the former study, tissues samples were taken from both the colon and the ileum, and in both studies, patients had a mean disease duration of at least 8 years, were on variety of immune suppressive treatments, and the CD groups included patients with ileitis, ileocolitis, and colitis. Our study substantially builds on these prior findings with the demonstration that in a large cohort of newly diagnosed treatment naïve pediatric patients, rectal type 2 and type 17 gene expression not only distinguishes UC from CD, but distinguishes UC from colon-only CD. By studying tissues from newly diagnosed pediatric patients, our findings provide insight regarding the mucosal immune response from arguably the earliest practical opportunity in the disease course to study IBD. Since patients are treatment naïve, we are assured that the findings are not influenced by medications with profound effects on immune function. Furthermore, since colitis-only CD is more common in children than adults and can be difficult to distinguish from UC, our study addresses an important clinical problem.
Some have proposed that Crohn’s colitis should be considered a distinct disease entity from ileal CD. A recent genome wide association study meta-analysis demonstrated that the based on the relative genetic risk for CD versus UC, CDc and CDic are best characterized as intermediate phenotypes between UC and CD ileitis (with CDc being intermediate between UC and CDic).22 In our immune gene expression panel, IL17A was intermediately expressed in CDc and significantly different from expression in UC and CDic. CCL11 also exhibited significantly increased expression in CDc compared to CDic, similar to in UC. Our PCA analysis, which incorporates the collective differences from all the genes analyzed, aggregates CDc patients closer to UC patients, supporting the notion of CDc representing an intermediate phenotype based on gene expression as well as genetic make-up.
Contrary to our initial hypothesis, we observed that pediatric UC patients with a gene expression pattern marked by increased IL13 expression achieve higher rates of steroid-free clinical response and remission, and that IL13 gene expression alone was directly associated with clinical response at 6 and 12 months. The newly diagnosed patients in this study received standard of care treatment at the discretion of the treating physician. Others groups have examined markers of adaptive immune responses primarily with regard to response to anti-TNF therapy in adult UC. In a cohort of adults patients with UC, higher mucosal IL17A and IFNG gene expression was associated with remission after infliximab induction therapy.23 IL13 expression was not assessed in this study. With regard to markers of type 2 immune responses, one group observed fewer Gata3+ lamina propria T cells in UC patients responding to anti-TNF agents compared to those without response.20 Additionally, IL13RA2 was amongst several genes identified in a mucosal genome-wide expression study as associated with non-response to infliximab in UC.24 We did not observe any significant associations between IL17A, IFNG or IL13RA2 and clinical outcomes in this pediatric UC cohort. The discrepancies between our results and those of other groups is likely due to our patients being assessed prior to any IBD-directed therapy, with many achieving remission on either 5-ASA or immunomodulator drugs. Only one third of the UC patients in our group were ever exposed to an anti-TNF drug, whereas the patients in these studies were refractory to first-line therapies with response specifically to anti-TNF drugs being assessed.
There are two potential explanations as to why increased mucosal IL13 expression at baseline predicts improved clinical outcomes. Investigations by our group and others have supported a pathogenic role for IL-13 and type 2 immune responses in human UC and several murine models of colitis including oxazolone-induced colitis and spontaneous colitis in Wiskott-Aldrich syndrome protein-deficient and T cell receptor-α-deficient mice.2,4,15,25-31 If IL-13 is indeed part of a pathogenic type 2 immune response, then our results suggest this pathway is sufficiently suppressed by standard initial therapy, and that a subset of patients with increased IL13 expression may be more responsive to treatment. Indeed, we observed that mucosal expression of IL13 was significantly decreased in patients with therapy-induced mucosal healing compared to those with active disease.
However, the failure of two phase IIa clinical trials of anti-IL-13 monoclonal antibodies for the treatment of UC to meet their primary endpoints, draws into question the notion of a pathogenic role of IL13 in UC (although one study did meet significance for important secondary endpoints including clinical remission).32,33 It is also possible improved outcomes in high IL13-expressers is due to a protective effect exerted by IL-13 induced in the context of inflammation. Amongst UC patients in this study, we did not observe a correlation between IL13 and IL17A expression, suggesting the production of IL13 may be independent of a Th17 immune response. Indeed, some groups have observed beneficial roles for IL-13 with regard to epithelial wound healing and goblet cell function (the latter particularly with regard to helminth expulsion).34-36 IL-10-deficient mice also deficient for IL13RA2, the gene for a neutralizing receptor for IL-13, exhibit decreased inflammation when challenged a parasite or a non-steroidal anti-inflammatory drug, suggesting IL-13 activity is protective in these models.37 Lastly, IL-33-dependent group 2 innate lymphoid cells (ILCs) that produce IL-5, IL-13, and the epidermal growth factor ligand amphiregulin limit inflammation induced from epithelial damage in dextran sodium sulfate-induced colitis in mice.38 In line with this last concept, we observed increased expression of the transcript for membrane-bound IL-33 receptor, one marker of group 2 ILCs, in UC compared to both CD and non-IBD.
There are several notable strengths to our study. First, our findings are from newly diagnosed treatment-naïve pediatric patients, and, thus, are not influenced by treatment. Second, the cohort was meticulously characterized allowing us to examine distinct CD anatomic sub-phenotypes. Third, the differences we observed were derived from examination of exclusively rectal samples all from patients with documented rectal involvement. Fourth, we validated gene expression differences between colitis diagnoses in an independent local cohort. A weakness of this study is that only gene expression and not protein abundance was assessed. Tissue samples from the RISK cohort were not collected in a manner conducive to protein analysis. While cytokines and other proteins may be regulated at the translational or post-translational level, cytokine gene expression measured by real-time RT-qPCR generally correlates quite well with measures of protein abundance.39-41 Additionally, while we did not observe differences in the expression of the type 1 cytokine INFG or transcription factor TBX21 between UC and CD, we did not measure expression of other type 1 cytokines such as IL12 or TNFB, thus limiting the conclusions we can draw regarding the relative contribution of a type 1 immune response to UC and CD.
In conclusion, our data support a role for mucosal type 2 inflammatory responses in the early course of pediatric UC. In treatment-naïve pediatric patients, UC is distinguished from Crohn’s colitis, and specifically colon-only CD by increased expression of genes associated with type 2 and type 17 immune responses. Furthermore, an immune gene expression profile marked by elevated expression of the type 2 cytokine IL13 is associated with improved clinical outcomes in pediatric UC. Future studies are warranted from large UC cohorts to determine whether a type 2 gene expression predicts response to specific UC therapies with the ultimate goal of directing therapies based on patient immunophenotype.
Supplementary Material
Table S1. Targets on gene expression array
Table S2. Comparison between the studied RISK cohort subset and overall RISK cohort
Table S3. Characteristics of the Cincinnati Cohort patients
Table S4. Univariate logistic regression for discriminating UC from CDc
Table S5. Change in effect estimate after bivariate analyses with S100A8
Table S6. Inclusion of S100A8 in multivariate logistic regression model for discriminating UC from CDc
Table S7. Univariate logistic regression of gene expression for predicting UC clinical outcomes
Table S8. Comparison of baseline characteristics and medication exposures between UC patient gene expression clusters
Figure S1. Identification of GAPDH as reference gene with least expression variation across diagnosis groups. (A) Dot plot depicting mean Cq and standard errors of 16 candidate reference genes from an endogenous control real-time RT-qPCR microfluidic array (n = 4 each of non-IBD, CDic, CDc, UC). (B) Bar chart depicting standard deviations of the Cq for each candidate reference gene. GAPDH exhibited the lowest variability in expression across samples.
Figure S2. Analysis of Cincinnati cohort rectal mucosa real-time RT-qPCR controlling for histologic disease activity. (A) Dot plot of Robarts Histologic Index scores for CD and UC patients in the Cincinnati cohort. (B) Dot plot of a subset of CD and UC patients in the Cincinnati cohort matched on Robarts Histologic Index scores. (C) Box and whisker chart depicting gene expression normalized to median expression of the non-IBD patient group in CD and UC patients matched on Robarts Histologic Index scores (boxes represent median and interquartile range, whiskers represent the 95% confidence interval).
Acknowledgements
We thank the following RISK Study investigators for their efforts enrolling, phenotyping, and following participants in this cohort: Anthony R. Otley, Scott B. Snapper, Stephen L. Guthery, David J. Keljo, Barbara S. Kirschner, Marian D. Pfefferkorn, Maria Oliva-Hemker, Ashish S. Patel, Shervin Rabizadeh, Stanley A. Cohen, David A. Ziring, and Jonathan Evans. We also thank the patients and their families who participated in this study.
Grant Support: Research reported in this publication was supported primarily by the National Institute Of Diabetes And Digestive And Kidney Diseases (NIDDK) of the National Institutes of Health (NIH) under award number K23DK094832 (MJR). Biospecimens were obtained from the RISK Stratification Study, funded by the Crohn’s & Colitis Foundation of America. This project was also supported in part by the NIH Clinical and Translational Science Award (CTSA) 1UL1TR001425-01 (REDCap Database), NIDDK P30DK078392 (Gene and Protein Expression Core) of the Digestive Disease Research Core Center in Cincinnati, NIH P30ES006096 of the University of Cincinnati College of Medicine Center for Environmental Genetics (Genomics, Epigenomics and Sequencing Core), NIH R01DK090119 and Crohn’s & Colitis Foundation of America Senior Research Award (SPH), NIH K23DK105229 (PM), and the Gutsy Kids Fund including philanthropic donation from the Karen and Brock Wagner Family (RK).
Abbreviations
- AUC
area under the curve
- CD
Crohn’s disease
- CDc
colon-only Crohn’s disease
- CDic
ileocolonic Crohn’s disease
- CI
confidence interval
- Cq
quantification cycle
- FDR
false discovery rate
- IBD
inflammatory bowel disease
- IBD-U
inflammatory bowel disease unclassified
- LPMC
lamina propria mononuclear cells
- OR
odds ratio
- PGA
physician’s global assessment
- PUCAI
Pediatric Ulcerative Colitis Activity Index
- RHI
Robarts Histopathology Index
- RT-qPCR
quantitative reverse transcription PCR
- UC
ulcerative colitis
Footnotes
Conflicts of Interest: Michael J. Rosen has served on an advisory board for Abbvie and receives research support from Prometheus Laboratories. Jeffrey S. Hyams has served on advisory boards for Janssen and Abbvie, and as a consultant for Lilly, Celgene, Takeda, Astra Zeneca and Receptos. David R. Mack has served on advisory boards for Abbvie, Janssen and Mead Johnson, as a consultant for UCB, and is a shareholder of Biotagenics. Melvin B. Heyman has received research support from Janssen, Genentech, Sucampo, and Abbvie, and has served as an advisor for Gilead. Michael D. Kappelman has received research support from and served as a consultant for Janssen and Abbvie. Joel R. Rosh has received research support from and served as an advisor for Janssen and Abbvie. Thomas D. Walters has received speaker fees and research support from and served on advisory boards for Janssen Canada and Abbvie Canada. Lee A. Denson has received research support from Janssen and royalties from Glycosyn, LLC. The remaining authors have no conflicts of interest to disclose.
Author Contributions: MJR – obtained funding, study supervision, study concept and design, analysis and interpretation of data, and drafting of the manuscript; RK, MHC, YH, TDW - analysis and interpretation of data and critical revision of the manuscript for important intellectual content; JEV, AW – technical support, acquisition of data, and drafting of the manuscript; RB, PM, RNB, JSH, SSB, RK, JDN, AMG, JRR, WVC, MBH, DRM, MDK, JM – enrolled research participants and secured biospecimens, and critical revision of the manuscript for important intellectual content. KTW - study concept and design and critical revision of the manuscript for important intellectual content. SK, DEM – RISK Study concept and design and supervision, enrolled research participants and secured biospecimens, and critical revision of the manuscript for important intellectual content. SPH, LAD - study concept and design, analysis and interpretation of data, and critical revision of the manuscript for important intellectual content.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fuss IJ, Neurath M, Boirivant M, et al. Disparate CD4+ lamina propria (LP) lymphokine secretion profiles in inflammatory bowel disease. Crohn's disease LP cells manifest increased secretion of IFN-gamma, whereas ulcerative colitis LP cells manifest increased secretion of IL-5. J Immunol. 1996;157:1261–1270. [PubMed] [Google Scholar]
- 2.Fuss IJ, Heller F, Boirivant M, et al. Nonclassical CD1d-restricted NK T cells that produce IL-13 characterize an atypical Th2 response in ulcerative colitis. J Clin Invest. 2004;113:1490–1497. doi: 10.1172/JCI19836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wynn TA. Type 2 cytokines: mechanisms and therapeutic strategies. Nat Rev Immunol. 2015;15:271–282. doi: 10.1038/nri3831. [DOI] [PubMed] [Google Scholar]
- 4.Heller F, Florian P, Bojarski C, et al. Interleukin-13 Is the Key Effector Th2 Cytokine in Ulcerative Colitis That Affects Epithelial Tight Junctions, Apoptosis, and Cell Restitution. Gastroenterology. 2005;129:550–564. doi: 10.1016/j.gastro.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 5.Kappelman MD, Moore KR, Allen JK, et al. Recent trends in the prevalence of Crohn's disease and ulcerative colitis in a commercially insured US population. Dig Dis Sci. 2012;58:519–525. doi: 10.1007/s10620-012-2371-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adamiak T, Walkiewicz-Jedrzejczak D, Fish D, et al. Incidence, Clinical Characteristics, and Natural History of Pediatric IBD in Wisconsin. Inflamm Bowel Dis. 2013;19:1218–1223. doi: 10.1097/MIB.0b013e318280b13e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Winter DA, Karolewska-Bochenek K, Lazowska-Przeorek I, et al. Pediatric IBD-unclassified Is Less Common than Previously Reported; Results of an 8-Year Audit of the EUROKIDS Registry. Inflamm Bowel Dis. 2015;21:2145–2153. doi: 10.1097/MIB.0000000000000483. [DOI] [PubMed] [Google Scholar]
- 8.Gupta N, Bostrom AG, Kirschner BS, et al. Presentation and disease course in early- compared to later-onset pediatric Crohn's disease. Am J Gastroenterol. 2008;103:2092–2098. doi: 10.1111/j.1572-0241.2008.02000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Bie CI, Pærregaard A, Kolacek S, et al. Disease Phenotype at Diagnosis in Pediatric Crohn’s Disease. Inflamm Bowel Dis. 2013;19:378–385. doi: 10.1002/ibd.23008. [DOI] [PubMed] [Google Scholar]
- 10.Levine A, Koletzko S, Turner D, et al. The ESPGHAN Revised Porto Criteria for the Diagnosis of Inflammatory Bowel Disease in Children and Adolescents. J Pediatr Gastroenterol Nutr. 2014;58:795–806. doi: 10.1097/MPG.0000000000000239. [DOI] [PubMed] [Google Scholar]
- 11.Mosli MH, Feagan BG, Zou G, et al. Development and validation of a histological index for UC. Gut. 2015;66:50–58. doi: 10.1136/gutjnl-2015-310393. [DOI] [PubMed] [Google Scholar]
- 12.Iboshi Y, Nakamura K, Ihara E, et al. Multigene analysis unveils distinctive expression profiles of helper T-cell-related genes in the intestinal mucosa that discriminate between ulcerative colitis and Crohn's disease. Inflamm Bowel Dis. 2014;20:967–977. doi: 10.1097/MIB.0000000000000028. [DOI] [PubMed] [Google Scholar]
- 13.Bliss R, Weinberg J. Determining the Probability Distribution and Evaluating Sensitivity and False Positive Rate of a Confounder Detection Method Applied To Logistic Regression. J Biomet Biostat. 2012;03 doi: 10.4172/2155-6180.1000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levine A, Griffiths A, Markowitz J, et al. Pediatric modification of the Montreal classification for inflammatory bowel disease: the Paris classification. Inflamm Bowel Dis. 2011;17:1314–1321. doi: 10.1002/ibd.21493. [DOI] [PubMed] [Google Scholar]
- 15.Rosen MJ, Frey MR, Washington MK, et al. STAT6 activation in ulcerative colitis: A new target for prevention of IL-13-induced colon epithelial cell dysfunction. Inflamm Bowel Dis. 2011;17:2224–2234. doi: 10.1002/ibd.21628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Biancheri P, Di Sabatino A, Ammoscato F, et al. Absence of a role for interleukin-13 in inflammatory bowel disease. Eur J Immunol. 2014;44:370–385. doi: 10.1002/eji.201343524. [DOI] [PubMed] [Google Scholar]
- 17.Kadivar K, Ruchelli ED, Markowitz JE, et al. Intestinal interleukin-13 in pediatric inflammatory bowel disease patients. Inflamm Bowel Dis. 2004;10:593–598. doi: 10.1097/00054725-200409000-00014. [DOI] [PubMed] [Google Scholar]
- 18.Iboshi Y, Nakamura K, Fukaura K, et al. Increased IL-17A/IL-17F expression ratio represents the key mucosal T helper/regulatory cell-related gene signature paralleling disease activity in ulcerative colitis. J Gastroenterol. 2016 May 13; doi: 10.1007/s00535-016-1221-1. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 19.D’Ambrosio A, Cossu A, Amendola A, et al. Lamina Propria CD4+LAP+ Regulatory T Cells Are Increased in Active Ulcerative Colitis but Show Increased IL-17 Expression and Reduced Suppressor Activity. J Crohns Colitis. 2016;10:346–353. doi: 10.1093/ecco-jcc/jjv216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li J, Ueno A, Fort Gasia M, et al. Profiles of Lamina Propria T Helper Cell Subsets Discriminate Between Ulcerative Colitis and Crohn's Disease. Inflamm Bowel Dis. 2016;22:1779–1792. doi: 10.1097/MIB.0000000000000811. [DOI] [PubMed] [Google Scholar]
- 21.Verdier J, Begue B, Cerf-Bensussan N, et al. Compartmentalized Expression of Th1 and Th17 Cytokines in Pediatric Inflammatory Bowel Diseases. Inflamm Bowel Dis. 2012;18:1260–1266. doi: 10.1002/ibd.21905. [DOI] [PubMed] [Google Scholar]
- 22.Cleynen I, Boucher G, Jostins L, et al. Inherited determinants of Crohn's disease and ulcerative colitis phenotypes: a genetic association study. Lancet. 2016;387:156–167. doi: 10.1016/S0140-6736(15)00465-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rismo R, Olsen T, Cui G, et al. Mucosal cytokine gene expression profiles as biomarkers of response to infliximab in ulcerative colitis. Scand J Gastroenterol. 2012;47:538–547. doi: 10.3109/00365521.2012.667146. [DOI] [PubMed] [Google Scholar]
- 24.Arijs I, Li K, Toedter G, et al. Mucosal gene signatures to predict response to infliximab in patients with ulcerative colitis. Gut. 2009;58:1612–1619. doi: 10.1136/gut.2009.178665. [DOI] [PubMed] [Google Scholar]
- 25.Heller F, Fuss IJ, Nieuwenhuis EE, et al. Oxazolone colitis, a Th2 colitis model resembling ulcerative colitis, is mediated by IL-13-producing NK-T cells. Immunity. 2002;17:629–638. doi: 10.1016/s1074-7613(02)00453-3. [DOI] [PubMed] [Google Scholar]
- 26.Mannon PJ, Hornung RL, Yang Z, et al. Suppression of inflammation in ulcerative colitis by interferon-β-1a is accompanied by inhibition of IL-13 production. Gut. 2011;60:449–455. doi: 10.1136/gut.2010.226860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fuss IJ, Joshi B, Yang Z, et al. IL-13R 2-bearing, type II NKT cells reactive to sulfatide self-antigen populate the mucosa of ulcerative colitis. Gut. 2014;63:1728–1736. doi: 10.1136/gutjnl-2013-305671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosen MJ, Chaturvedi R, Washington MK, et al. STAT6 deficiency ameliorates severity of oxazolone colitis by decreasing expression of claudin-2 and Th2-inducing cytokines. The Journal of Immunology. 2013;190:1849–1858. doi: 10.4049/jimmunol.1201373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mizoguchi A, Mizoguchi E, Bhan AK. The critical role of interleukin 4 but not interferon gamma in the pathogenesis of colitis in T-cell receptor alpha mutant mice. Gastroenterology. 1999;116:320–326. doi: 10.1016/s0016-5085(99)70128-9. [DOI] [PubMed] [Google Scholar]
- 30.Nguyen DD, Maillard MH, Cotta-de-Almeida V, et al. Lymphocyte-dependent and Th2 cytokine-associated colitis in mice deficient in Wiskott-Aldrich syndrome protein. Gastroenterology. 2007;133:1188–1197. doi: 10.1053/j.gastro.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kawashima R, Kawamura YI, Oshio T, et al. Interleukin-13 Damages Intestinal Mucosa via TWEAK and Fn14 in Mice-A Pathway Associated With Ulcerative Colitis. Gastroenterology. 2011;141:2119–2129. doi: 10.1053/j.gastro.2011.08.040. [DOI] [PubMed] [Google Scholar]
- 32.Danese S, Rudziński J, Brandt W, et al. Tralokinumab for moderate-to-severe UC: a randomised, double-blind, placebo-controlled, phase IIa study. Gut. 2015;64:243–249. doi: 10.1136/gutjnl-2014-308004. [DOI] [PubMed] [Google Scholar]
- 33.Reinisch W, Panés J, Khurana S, et al. Anrukinzumab, an anti-interleukin 13 monoclonal antibody, in active UC: efficacy and safety from a phase IIa randomised multicentre study. Gut. 2015;64:894–900. doi: 10.1136/gutjnl-2014-308337. [DOI] [PubMed] [Google Scholar]
- 34.Seno H, Miyoshi H, Brown SL, et al. Efficient colonic mucosal wound repair requires Trem2 signaling. Proc Natl Acad Sci USA. 2009;106:256–261. doi: 10.1073/pnas.0803343106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Webb RA, Hoque T, Dimas S. Expulsion of the gastrointestinal cestode, Hymenolepis diminuta by tolerant rats: evidence for mediation by a Th2 type immune enhanced goblet cell hyperplasia, increased mucin production and secretion. Parasite Immunol. 2007;29:11–21. doi: 10.1111/j.1365-3024.2006.00908.x. [DOI] [PubMed] [Google Scholar]
- 36.McKay DM, Khan WI. STAT-6 is an absolute requirement for murine rejection of Hymenolepis diminuta. J. Parasitol. 2003;89:188–189. doi: 10.1645/0022-3395(2003)089[0188:SIAARF]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 37.Wilson MS, Ramalingam TR, Rivollier A, et al. Colitis and intestinal inflammation in IL10−/− mice results from IL-13Rα2-mediated attenuation of IL-13 activity. Gastroenterology. 2011;140:254–264. doi: 10.1053/j.gastro.2010.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Monticelli LA, Osborne LC, Noti M, et al. IL-33 promotes an innate immune pathway of intestinal tissue protection dependent on amphiregulin–EGFR interactions. Proc Natl Acad Sci USA. 2015;112:10762–10767. doi: 10.1073/pnas.1509070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Young S-H, Antonini JM, Roberts JR, et al. Performance evaluation of cytometric bead assays for the measurement of lung cytokines in two rodent models. Journal of Immunological Methods. 2008;331:59–68. doi: 10.1016/j.jim.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 40.Sullivan KE, Cutilli J, Piliero LM, et al. Measurement of cytokine secretion, intracellular protein expression, and mRNA in resting and stimulated peripheral blood mononuclear cells. Clin. Diagn. Lab. Immunol. 2000;7:920–924. doi: 10.1128/cdli.7.6.920-924.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Flores MG, Zhang S, Ha A, et al. In vitro evaluation of the effects of candidate immunosuppressive drugs: flow cytometry and quantitative real-time PCR as two independent and correlated read-outs. Journal of Immunological Methods. 2004;289:123–135. doi: 10.1016/j.jim.2004.04.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Targets on gene expression array
Table S2. Comparison between the studied RISK cohort subset and overall RISK cohort
Table S3. Characteristics of the Cincinnati Cohort patients
Table S4. Univariate logistic regression for discriminating UC from CDc
Table S5. Change in effect estimate after bivariate analyses with S100A8
Table S6. Inclusion of S100A8 in multivariate logistic regression model for discriminating UC from CDc
Table S7. Univariate logistic regression of gene expression for predicting UC clinical outcomes
Table S8. Comparison of baseline characteristics and medication exposures between UC patient gene expression clusters
Figure S1. Identification of GAPDH as reference gene with least expression variation across diagnosis groups. (A) Dot plot depicting mean Cq and standard errors of 16 candidate reference genes from an endogenous control real-time RT-qPCR microfluidic array (n = 4 each of non-IBD, CDic, CDc, UC). (B) Bar chart depicting standard deviations of the Cq for each candidate reference gene. GAPDH exhibited the lowest variability in expression across samples.
Figure S2. Analysis of Cincinnati cohort rectal mucosa real-time RT-qPCR controlling for histologic disease activity. (A) Dot plot of Robarts Histologic Index scores for CD and UC patients in the Cincinnati cohort. (B) Dot plot of a subset of CD and UC patients in the Cincinnati cohort matched on Robarts Histologic Index scores. (C) Box and whisker chart depicting gene expression normalized to median expression of the non-IBD patient group in CD and UC patients matched on Robarts Histologic Index scores (boxes represent median and interquartile range, whiskers represent the 95% confidence interval).