In rheumatoid arthritis (RA), current clinical practice as well as the vast majority of published studies focus on the ability of certain treatments to improve existing RA disease activity, or to prevent worsening disease in patients with established and classifiable disease. In addition, though, several trials have tried to address the prevention of the development of a classifiable RA in individuals with undifferentiated arthritis (UA), or joint symptoms defined as arthralgia and autoantibody positivity without inflammatory arthritis, with overall mixed results (1–4). In particular, one important contribution to RA prevention is designated PROMPT (Probable RA: Methotrexate Versus Placebo Treatment), an innovative and forward-looking clinical trial designed to stop disease progression from UA to classifiable RA.
The findings from this study have been published in several stages. In the original study that was published in 2007, 110 individuals with DMARD-naïve synovitis at baseline yet not meeting 1987 criteria for RA were randomized to receive methotrexate (starting dose 15 mg/week orally) or placebo, without other DMARDs or steroids (5). After follow-up of up to 30 months, there was no overall difference between groups in those who progressed to classifiable RA (6). However, there was a delay in progression to RA in the MTX-treated group, and at 18 months, patients treated with MTX had less radiographic progression. Furthermore, in subgroup analyses of just individuals who were seropositive for antibodies to citrullinated protein/peptide antigens (ACPA), those who received MTX progressed to classifiable RA less frequently (67% vs. 93%; p<0.001).
The authors concluded in their original manuscript that intervention in this ‘early’ period of RA, especially in individuals who are ACPA positive, may result in longer-term benefits. However, in a follow-up manuscript (2012) that included 5 years of follow-up (7), there was no lasting benefit from the initial course of MTX in terms of development of RA, radiographic damage or drug-free remission, and the conclusion that followed was that perhaps early treatment did not result in lasting improvement in longer-term outcomes, which was somewhat contrary to many findings that demonstrate that earlier treatment in RA leads to improved long term outcomes (8).
In this issue of Arthritis and Rheumatology, the PROMPT investigators have further analyzed the data from this study, this time to address the issue that perhaps earlier analyses were falsely negative because patients at low-risk of developing classifiable RA were included; as such, if the highest risk individuals for future RA were able to be identified, a benefit of treatment may be apparent. With this new approach, they stratified individuals at baseline as ‘high-risk’ for future RA by applying the ‘Leiden prediction rule’, a 9-item instrument that includes clinical and biomarker factors and was developed and validated to determine which patients with UA may progress to classifiable RA (9, 10). In this re-analysis, the primary outcome was the fulfillment of the 1987 criteria, and the secondary outcome was the proportion of patients who achieved drug-free remission.
After applying the Leiden rule, 22 out of the original 110 subjects were identified as ‘high-risk’, and fortuitously this small subgroup was originally randomized equally to receive MTX or placebo (11 in each group). Within these subjects, during the overall follow-up of 5 years, 6/11 (55%) MTX-treated subjects developed classifiable RA compared to 11/11 (100%) in the placebo arm (p<0.001). In addition, classifiable RA was significantly delayed in the MTX-treated subjects (~23 months vs. 3 months; p<0.001), and drug-free remission was more common (36% vs. 0%, p=0.027). Furthermore, when the data were analyzed using only high-risk patients who were additionally ACPA positive high-risk (N=18), classifiable RA was again noted to be significantly delayed, and there was a trend towards greater preventive effect of MTX use. Finally, in the non-high risk subjects, there was no benefit of MTX identified. Based on these findings, the authors conclude that in order to see a benefit of intervention, it will be necessary in future studies to more accurately classify subjects as high risk, and only enter those individuals into RA prevention trials.
There are several issues to consider when applying the specific findings from the PROMPT study to future prevention studies in RA, and in particular studies that target the stage of disease development between the appearance of the first clinically-apparent synovitis, and classifiable RA. First, this was a very small study using retrospective analyses that may introduce bias, and therefore much larger studies will need to be done to determine the best approaches for treating individuals who present with UA. Second, in this latest analysis, 6/11(~55%) of these ‘high-risk’ subjects did not develop classifiable RA, and 4/11 (~36%) had drug-free remission. Small numbers notwithstanding, these are exciting findings; however, ~45% of MTX-treated subjects still progressed to classifiable RA. This may have been due to too low of a dose of MTX (starting dose of 15 mg/week), too short of a course (1 year), or that MTX targets the wrong pathway at this pathophysiologic stage of RA development. Further studies are needed to identify the best therapeutic agent(s) to optimize responses in this early stage of RA development. Third, in this re-analysis 18/22 (~82%) of the subjects that the PROMPT investigators determined as ‘high-risk’ based on the Leiden rule also met the 2010 RA Classification Criteria (11) at the time of study entry. The authors also found that the high-risk designation from the Leiden score performed better than the 2010 RA criteria in identifying those in whom MTX was most effective at preventing RA by 1987 Criteria, although the small sample size in the sub-analysis limits the conclusions that can be made. However, because the 2010 Criteria are used widely in clinical practice to guide treatment, it remains to be studied in larger trials how the Leiden prediction rule compares to the 2010 Criteria in guiding clinical care. Furthermore, given the growing understanding of the pathophysiology of RA and the expansion of blood-based biomarkers and imaging that are available to assess and monitor disease (12), it will be important to determine how models that include additional autoantibodies, autoantibody levels, breadth of autoantibody reactivity, and other imaging or blood-based measures of inflammation can be used to identify individuals with UA who are at high risk of progressing to classified RA. And, all of these factors may ultimately need to be incorporated into the ‘standard’ classification criteria for RA in order to ensure that classification criteria truly match the biology of disease. Finally, while the Leiden rule incorporates duration of symptoms as one of the 9-items assessed, the authors did not formally assess duration of symptoms in this re-analysis, although in the original publication it was reported that the duration of symptoms was longer in the MTX-treated group (312 days versus 263 days, no p-value provided). This could impact findings because in other studies, duration of disease prior to the initiation of therapy has been found to be an important factor in response to therapy (8).
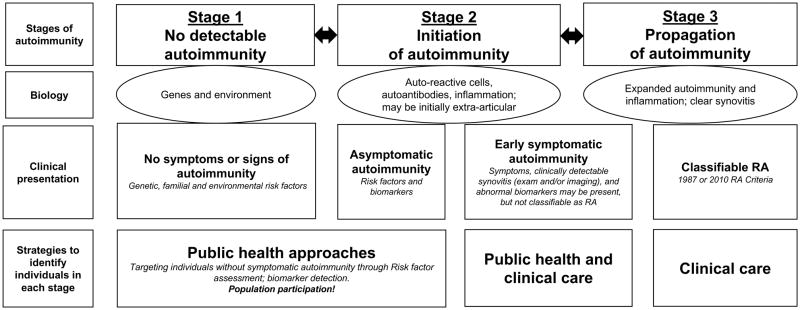
Of note, to this point in this editorial, the use of the ‘classic’ definitions of the stages of prevention, which include primary, secondary and tertiary prevention, have been avoided in consideration of the PROMPT study as a prevention trial. That is because the application of these terms depends to a large extent on when disease is considered to occur. Importantly, the determination of the presence or absence of a disease depends on an understanding of the disease’s natural history and pathophysiology, as well as classification criteria that may be determined in an arbitrary way (13, 14). In particular, defining when disease is present in RA has been affected by the use of two classification schemes (1987 and 2010 Criteria) that have some variability in their performance and therefore may not identify exactly the same subjects, or the same stage of disease development (6, 11, 15). The definition of “disease” in RA has been further challenged by the identification of a preclinical phase in which RA-related biomarkers that include RF and ACPA are elevated years prior to the development of clinically-detectable synovitis, or classifiable RA (Figure 1) (16). Specific definitions that apply to the preclinical phase of RA are in development (17), although as of yet there is no widely accepted approach to uniformly define preclinical RA. Because of these issues, until consensus guidelines are developed, it may be more appropriate in RA to define prevention more broadly as a means to avoid progression from one stage of disease to another, more severe, stage.
Figure 1. Model for rheumatoid arthritis (RA) development.
In this model, RA develops in three general Stages: 1) No detectable autoimmunity, 2) Initiation and then 3) Propagation of autoimmunity. At each Stage, the biology of disease can be defined through a variety of factors. Each Stage is also characterized by clinical presentation that may be asymptomatic, unclassifiable signs and symptoms (where the PROMPT study falls), and ultimately classifiable RA using established criteria (e.g. 1987 or 2010 Criteria). Identifying individuals at each Stage requires different approaches such as autoantibody screening to identify asymptomatic or early symptomatic autoimmunity that may be difficult to recognize as related to RA, or clinical evaluation if signs and symptoms of synovitis are present. At each Stage, major challenges are to 1) accurately identify individuals who are in each stage, 2) precisely define the risk for progression to the next ‘worse’ stage, and 3) define the biology so that effective interventions can be applied to maintain, improve or even facilitate an individual’s transition back to a prior Stage.
*Of note, genes and environment likely act throughout all Stages. In addition, the specific duration of each Stage may vary across individuals.
With these issues in mind, it is not yet clear how the findings from the PROMPT study will directly apply to other types of prevention trials in RA that may target different, and earlier, stages of disease. In particular, several studies are currently underway, or have recently been completed, evaluating whether interventions that are given prior to the first clinical evidence of synovitis may prevent future classifiable disease (18–20). Since these studies target an earlier phase of RA development than the PROMPT study, the same methods (e.g. Leiden score) to identify someone with clinically-apparent synovitis that will progress to classifiable RA may not be appropriate to identify someone with preclinical RA who is at risk for developing their first clinically-apparent joint with synovitis. Rather, identifying individuals who will progress from preclinical RA to classifiable disease or even to UA may rely more heavily on biomarkers than clinical symptoms. Indeed, in the prevention studies mentioned above, the inclusion criteria depend heavily on RA-related autoantibody positivity, and in particular ACPA positivity. Furthermore, it will be imperative for future prevention studies in RA to identify the right targets for prevention for each stage of disease development. Specifically, as discussed above, MTX halted progression to classifiable RA in some of the high-risk PROMPT patients, and it is well-known to be useful in treating patients with classified RA; however, MTX may not be appropriate to use in the earlier stages of RA because this drug and others have been optimized for the treatment of clinically-apparent synovitis, a disease process that may be distinctly different from preclinical autoimmunity (12). Additionally, with the increasing understanding that preclinical RA is associated with and perhaps driven by mucosal inflammation (21), in some cases it may be more appropriate to approach prevention through modulation of that process. Furthermore, studies are needed to assess how changes in biomarkers or other measures over time can be used to assess efficacy of an intervention. The field is currently accustomed to ‘treating to target’ in patients with classifiable RA, and in other diseases such as cardiovascular disease, specific lipid biomarkers are repeatedly assessed over time to gauge response to therapy. Similar approaches may be applied in RA prevention, although the targets may need to change in order to reflect underlying immunopathology rather than counts of tender and swollen joints that are highly weighted in most tools that are currently used to assess disease activity in individuals who have clinically-apparent synovitis.
Despite these caveats, this re-evaluation of the PROMPT data addresses a critically important concept of identifying the right individuals to include in any type of prevention study in RA. Certainly, this concept is not new as investigators have long tried to enroll the ‘right’ patients in studies in order to optimize the identification of meaningful interventions. However, in prevention trials where the success of the trial may rest heavily on the number of strictly defined outcomes as dichotomous variables (e.g. RA present or absent), it is likely even more important to accurately identify adequate risks for events in order to have sufficient outcomes for analyses during defined study periods. Moreover, given the temporal limits of clinical trials, inclusion criteria will need to incorporate both the likelihood of an important outcome, as well as the timing of that outcome. For example, in studies of preclinical RA, this means that inclusion criteria will need to provide estimates of the number of individuals who will develop classifiable RA, as well as the estimate how many of those events will occur during the study period. Furthermore, when inclusion criteria adequately reflect the underlying biology of disease, they can help identify the subjects with the right biology that will respond to a specific intervention. These critical issues for prevention trials can be summed up as: right individuals, right time, and right drug/intervention – all of which can be informed by robust inclusion criteria.
As such, great efforts should be put into identifying the right set of inclusion criteria for clinical prevention trials, with these criteria being based on sound understanding of the natural history of disease and its underlying biology. The several trials seeking to prevent the development of classifiable RA in autoantibody positive individuals who do not have clinically-apparent synovitis at baseline, are already building on significant data that the presence of ACPA with or without RF positivity is highly predictive of the future onset of classifiable RA (16). However, as the knowledge about preclinical RA grows from these and other studies on the natural history of RA, we should be prepared to perform additional stratification that can be informed by a variety of clinical, biomarker and imaging factors that will allow for delivery of ‘precision medicine’ in prevention (22).
Related to the above issues, it is important to consider the planned duration of preventive trials, especially if a planned intervention may take a long time to demonstrate its effect. For example, while one may expect a large and ‘quick’ preventive effect on future classifiable RA from a DMARD, it may take much longer for a lifestyle intervention to result in demonstrable outcomes (23). Specifically, retrospective studies suggest that it may take 10–20 years to see an effect of smoking cessation on the future development of RA (24). As such, an RA prevention trial where the intervention was smoking cessation would require very prolonged follow-up in order to adequately assess outcomes. Although this appears to be a daunting issue, improvements in cardiovascular disease outcomes using interventions such as statins have been measured not only in years but rather decades (25), so such approaches are possible. In addition, to date, the majority of RA prevention studies, including the PROMPT study, have enrolled subjects who have come to attention because they have sought medical care for symptoms. However, to identify individuals even earlier in the natural history of RA, different strategies will have to be employed, that may require more public health-type approaches (Figure 1) and investment by clinicians, investigators and funding agencies, as well as individuals who are at-risk for rheumatic diseases whose participation in preventive trials will be critical to move the field forward. In particular, some prevention studies that are underway, including StopRA (Strategy for the Prevention of Onset of Clinically-Apparent RA) (18), are seeking to identify some study subjects even prior to presentation to clinical care through serum ACPA screening of higher risk populations such as first-degree relatives of patients with RA, an approach which has both advantages and disadvantages but is an approach more akin to cardiovascular disease and cancer prevention.
Overall, however, it is exciting that rheumatology is to a point where prevention in RA can more comprehensively be addressed. Hopefully, as the understanding of the pathophysiology and natural history of RA as well as other rheumatic diseases (e.g. lupus (26)) improves, the development of robust inclusion criteria for prevention studies as well as addressing other issues will find the right balance between 1) accurately classifying individuals in each stage of RA development, 2) precisely predicting risk for progression to the next ‘worse’ stage of disease, and 3) understanding the biology of disease in each stage and on an individual level so that optimal interventions can be applied. Hopefully, addressing these issues will soon culminate in investigators being able to demonstrate conclusively that preventive interventions work for rheumatic disease.
Acknowledgments
Funding
Drs. Deane Striebich, and Holers’ work on this publication was supported by the University of Colorado Autoimmunity Center of Excellence under the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health Award Number UM1 AI110503.In addition, Drs Deane and Holers are supported by NIAID U01 AI101981. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Contributor Information
Kevin D. Deane, Division of Rheumatology, University of Colorado Denver School of Medicine.
Christopher C. Striebich, Division of Rheumatology, University of Colorado Denver School of Medicine.
V. Michael Holers, Division of Rheumatology, University of Colorado Denver School of Medicine.
References
- 1.Emery P, Durez P, Dougados M, Legerton CW, Becker JC, Vratsanos G, et al. Impact of T-cell costimulation modulation in patients with undifferentiated inflammatory arthritis or very early rheumatoid arthritis: a clinical and imaging study of abatacept (the ADJUST trial) Ann Rheum Dis. 2010;69(3):510–6. doi: 10.1136/ard.2009.119016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Machold KP, Landewe R, Smolen JS, Stamm TA, van der Heijde DM, Verpoort KN, et al. The Stop Arthritis Very Early (SAVE) trial, an international multicentre, randomised, double-blind, placebo-controlled trial on glucocorticoids in very early arthritis. Ann Rheum Dis. 2010;69(3):495–502. doi: 10.1136/ard.2009.122473. [DOI] [PubMed] [Google Scholar]
- 3.Verstappen SM, McCoy MJ, Roberts C, Dale NE, Hassell AB, Symmons DP, et al. Beneficial effects of a 3-week course of intramuscular glucocorticoid injections in patients with very early inflammatory polyarthritis: results of the STIVEA trial. Ann Rheum Dis. 2010;69(3):503–9. doi: 10.1136/ard.2009.119149. [DOI] [PubMed] [Google Scholar]
- 4.Bos WH, Dijkmans BA, Boers M, van de Stadt RJ, van Schaardenburg D. Effect of dexamethasone on autoantibody levels and arthritis development in patients with arthralgia: a randomised trial. Ann Rheum Dis. 2010;69(3):571–4. doi: 10.1136/ard.2008.105767. [DOI] [PubMed] [Google Scholar]
- 5.van Dongen H, van Aken J, Lard LR, Visser K, Ronday HK, Hulsmans HM, et al. Efficacy of methotrexate treatment in patients with probable rheumatoid arthritis: a double-blind, randomized, placebo-controlled trial. Arthritis Rheum. 2007;56(5):1424–32. doi: 10.1002/art.22525. [DOI] [PubMed] [Google Scholar]
- 6.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31(3):315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 7.van Aken J, Heimans L, Gillet-van Dongen H, Visser K, Ronday HK, Speyer I, et al. Five-year outcomes of probable rheumatoid arthritis treated with methotrexate or placebo during the first year (the PROMPT study) Ann Rheum Dis. 2014;73(2):396–400. doi: 10.1136/annrheumdis-2012-202967. [DOI] [PubMed] [Google Scholar]
- 8.van Nies JA, Krabben A, Schoones JW, Huizinga TW, Kloppenburg M, van der Helm-van Mil AH. What is the evidence for the presence of a therapeutic window of opportunity in rheumatoid arthritis? A systematic literature review. Ann Rheum Dis. 2014;73(5):861–70. doi: 10.1136/annrheumdis-2012-203130. [DOI] [PubMed] [Google Scholar]
- 9.van der Helm-van Mil AH, le Cessie S, van Dongen H, Breedveld FC, Toes RE, Huizinga TW. A prediction rule for disease outcome in patients with recent-onset undifferentiated arthritis: how to guide individual treatment decisions. Arthritis Rheum. 2007;56(2):433–40. doi: 10.1002/art.22380. [DOI] [PubMed] [Google Scholar]
- 10.McNally E, Keogh C, Galvin R, Fahey T. Diagnostic accuracy of a clinical prediction rule (CPR) for identifying patients with recent-onset undifferentiated arthritis who are at a high risk of developing rheumatoid arthritis: a systematic review and meta-analysis. Semin Arthritis Rheum. 2014;43(4):498–507. doi: 10.1016/j.semarthrit.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 11.Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO, 3rd, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62(9):2569–81. doi: 10.1002/art.27584. [DOI] [PubMed] [Google Scholar]
- 12.Arend WP, Firestein GS. Pre-rheumatoid arthritis: predisposition and transition to clinical synovitis. Nature reviews Rheumatology. 2012;8(10):573–86. doi: 10.1038/nrrheum.2012.134. [DOI] [PubMed] [Google Scholar]
- 13.Starfield B, Hyde J, Gervas J, Heath I. The concept of prevention: a good idea gone astray? J Epidemiol Community Health. 2008;62(7):580–3. doi: 10.1136/jech.2007.071027. [DOI] [PubMed] [Google Scholar]
- 14.Tinetti ME, Fried T. The end of the disease era. Am J Med. 2004;116(3):179–85. doi: 10.1016/j.amjmed.2003.09.031. [DOI] [PubMed] [Google Scholar]
- 15.Biliavska I, Stamm TA, Martinez-Avila J, Huizinga TW, Landewe RB, Steiner G, et al. Application of the 2010 ACR/EULAR classification criteria in patients with very early inflammatory arthritis: analysis of sensitivity, specificity and predictive values in the SAVE study cohort. Ann Rheum Dis. 2013;72(8):1335–41. doi: 10.1136/annrheumdis-2012-201909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deane KD, El-Gabalawy H. Pathogenesis and prevention of rheumatic disease: focus on preclinical RA and SLE. Nature reviews Rheumatology. 2014;10(4):212–28. doi: 10.1038/nrrheum.2014.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gerlag DM, Raza K, van Baarsen LG, Brouwer E, Buckley CD, Burmester GR, et al. EULAR recommendations for terminology and research in individuals at risk of rheumatoid arthritis: report from the Study Group for Risk Factors for Rheumatoid Arthritis. Ann Rheum Dis. 2012;71(5):638–41. doi: 10.1136/annrheumdis-2011-200990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Strategy for the prevention of onset of clinically-apparent rheumatoid arthritis (StopRA) https://clinicaltrials.gov/ct2/show/NCT02603146.
- 19.Arthritis Prevention in the Pre-Clinical Phase of RA with Abatacept (APIPPRA) ISRCTN No. 46017566. http://www.isrctn.com/ISRCTN46017566.
- 20.European League Against Rheumatism (EULAR) Congress 2016: Abstract OP0182. Presented June 9, 2016. Prevention of clinically manifest rheumatoid arthritis by B cell directed therapy in the earliest phase of the disease (PRAIRI); http://www.trialregister.nl/trialreg/admin/rctview.asp?TC=2442. [Google Scholar]
- 21.Demoruelle MK, Deane KD, Holers VM. When and where does inflammation begin in rheumatoid arthritis? Curr Opin Rheumatol. 2014;26(1):64–71. doi: 10.1097/BOR.0000000000000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mirnezami R, Nicholson J, Darzi A. Preparing for precision medicine. N Engl J Med. 2012;366(6):489–91. doi: 10.1056/NEJMp1114866. [DOI] [PubMed] [Google Scholar]
- 23.Gerlag DM, Norris JM, Tak PP. Towards prevention of autoantibody-positive rheumatoid arthritis: from lifestyle modification to preventive treatment. Rheumatology (Oxford) 2016;55(4):607–14. doi: 10.1093/rheumatology/kev347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Costenbader KH, Feskanich D, Mandl LA, Karlson EW. Smoking intensity, duration, and cessation, and the risk of rheumatoid arthritis in women. Am J Med. 2006;119(6):503e1–9. doi: 10.1016/j.amjmed.2005.09.053. [DOI] [PubMed] [Google Scholar]
- 25.Chou R, Dana T, Blazina I, Daeges M, Jeanne TL. Statins for Prevention of Cardiovascular Disease in Adults: Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA. 2016;316(19):2008–24. doi: 10.1001/jama.2015.15629. [DOI] [PubMed] [Google Scholar]
- 26.Olsen NJ, Karp DR. Autoantibodies and SLE: the threshold for disease. Nature reviews Rheumatology. 2014;10(3):181–6. doi: 10.1038/nrrheum.2013.184. [DOI] [PubMed] [Google Scholar]