Abstract
RTOG 3505 is a randomized phase 3 study of concurrent chemoradiation followed by immune checkpoint inhibitor therapy or placebo in patients with locally advanced non-small cell lung cancer (NSCLC). Patients with surgically unresectable stage 3 NSCLC will receive thoracic radiation therapy (RT) to 60 Gy with concurrent cisplatin 50 mg/m2 IV on Days 1, 8, 29, and 36, and etoposide 50 mg/m2 IV on Days 1–5 and 29–33. Between 4 and 12 weeks after completion of concurrent chemoradiation, eligible patients will be randomized to the anti-programmed death 1 (PD1) monoclonal antibody nivolumab 240 mg IV or placebo every 2 weeks for up to 1 year. Co-primary endpoints are overall survival (OS) and progression-free survival (PFS), as determined by central independent radiology review. Secondary objectives include toxicity assessment, patient-reported outcomes and quality of life, and OS and PFS in PD-L1-expressors (≥1%) and PD-L1-non-expressors (<1%). Assuming a rate of 16.7% due to ineligibility and drop-out before randomization, a total of 660 patients will be enrolled to ensure 550 patients will be randomized after completion of chemoradiation. This sample size will provide ≥90% power to detect (1) a hazard ratio (HR) of 0.7 for OS with two-sided type I error of 0.04, and (2) HR of 0.667 for PFS two-sided type I error of 0.01. NCT02768558
Keywords: Abscopal effect, checkpoint inhibitor, co-primary endpoints, immunotherapy, programmed death 1 (PD1)
Study Rationale
We have reached a plateau in outcomes for locally advanced non-small cell lung cancer (NSCLC). Despite aggressive therapy with concurrent chemoradiation, fewer than 20–25% of patients with stage 3 NSCLC achieve 5-year survival and are presumably cured. To date, modifications of chemotherapy have not improved these outcomes. The addition of 3 cycles of consolidation docetaxel after concurrent cisplatin-etoposide with thoracic radiation has no impact on overall survival compared to chemoradiation alone.1 There is no survival advantage for consolidative docetaxel/cisplatin after definitive chemoradiation compared to chemoradiation alone.2 In unselected patients with stage 3 NSCLC, the addition of the epidermal growth factor receptor inhibitor gefitinib as maintenance therapy after completion of cisplatin-based concurrent chemoradiation demonstrates a decrease in survival.3 Adding the anti-EGFR monoclonal antibody cetuximab to chemoradiation does not extend survival.4 Furthermore, there is no evidence that achievable radiation therapy (RT) doses above 60 Gy are better than 60 Gy. In an interim analysis of the Radiation Therapy Oncology Group (RTOG) 0617 clinical trial, patients receiving an RT dose of 74 Gy had a median progression-free survival of 9.8 months, compared to 11.8 months for patients receiving 60 Gy.4
There is a strong rationale to combine immunotherapy and RT. While NSCLC is typically considered relatively non-immunogenic, RT may augment tumor immunogenicity.5 The abscopal effect refers to the observation that RT to a local area results in an antitumor effect distant to the radiation site. One proposed mechanism for this phenomenon is induction of release of circulating tumor antigen or inflammatory factors that could then mediate an augmented immune response against distant malignant lesions expressing similar tumor antigens. Supporting this hypothesis, local RT has been shown to increase the activity of natural killer cells, and T cells are required to mediate distant tumor effects of radiotherapy.6,7 Ablative RT dramatically increases T-cell priming in draining lymphoid tissues, leading to reduction of the primary tumor or distant metastases in a CD8+ T-cell-dependent fashion. These RT-initiated immune responses were greatly amplified by local immunotherapy.8 Finally, RT has been shown to increase tumor expression of programmed death ligand 1 (PD-L1), with combined RT plus PD-1-pathway targeting resulting in synergistic suppression of tumor-infiltrating myeloid-derived suppressor cells (MDSCs), thereby promoting anti-tumor immunity.9
With the availability of immune checkpoint inhibitors such as the anti-cytotoxic T-lymphocyte antigen 4 (CTLA-4) monoclonal antibody, ipilimumab, and agents targeting the PD-1 pathway, the potential benefit of combining these agents with RT has become evident clinically. Case reports suggest the induction of abscopal effect with administration of these agents.10 Among cancer types, lung cancer may be a particularly attractive setting to incorporate immunotherapy into treatment paradigms. A number of studies have suggested that tumor mutational burden is associated with benefit from immunotherapy.11,12 Presumably, increased mutational burden results in increased tumor antigenicity, thereby priming the tumor for immune attack. Lung cancer carries one of the highest mutational burdens of any malignancy, second only to melanoma.13 With two anti-PD1 agents already established and approved in advanced NSCLC, there is clear rationale to explore in earlier stages and in novel combinations.
In contrast to CTLA-4 (which exerts its regulatory effects in the priming phase of the immune response in regional lymph nodes), the negative regulatory effects of the PD-1 pathway occur in the effector phase of the immune response in peripheral tissues. Following T-cell stimulation, PD-1 recruits the tyrosine phosphatases SHP-1 and SHP-2, resulting in dephosphorylation of multiple effector molecules involved in the CD3 T-cell signaling cascade.14 Nivolumab (BMS-936558, previously MDX-1106 and ONO-4538), an anti-PD-1 monoclonal antibody, has emerged as one of the most promising immunotherapies for lung cancer. Nivolumab is a fully human, IgG4 (kappa) monoclonal antibody that binds PD-1 on activated immune cells to disrupt engagement of receptor with ligands PD-L1 (B7-H1/CD274) and PD-L2 (B7-DC/CD273). This action results in counteracting inhibitory signals and augmenting host antitumor responses. In early clinical trials, nivolumab monotherapy demonstrated clinical activity in multiple tumor types, including melanoma, renal cell carcinoma, and NSCLC.15 In a multicenter phase I dose-escalation trial enrolling NSCLC, melanoma, renal cell carcinoma, castration-resistant prostate cancer, and colorectal cancer, radiographic response rate in advanced, previously treated NSCLC was 17% (22 of 129 patients).16 Among the 22 patients with objective responses, the Kaplan-Meier estimated median duration of response was 17.0 months.
Two phase 3 clinical trials (Checkmate 057 and Checkmate 017) comparing nivolumab to single-agent docetaxel chemotherapy in previously treated advanced NSCLC have demonstrated improved overall survival and improved tolerability with nivolumab compared to docetaxel, leading to U.S. FDA approval and category 1 recommendations by the National Comprehensive Cancer Network. Both trials have demonstrated improved overall survival with nivolumab compared to docetaxel. The Checkmate 057 trial enrolled patients with non-squamous NSCLC. In the unselected population, for patients receiving nivolumab, median overall survival (OS) was 12.2 months compared with 9.4 months for docetaxel (HR 0.73, HR 0.59–0.89, P=0.002). Fewer grade 3 to 5 adverse events were reported for nivolumab (10%) when compared with docetaxel (54%). Clinical benefit was particularly evident for patients whose tumors had PD L1 staining of 1% or more (HR 0.59).17 There did not appear to be a survival benefit for nivolumab over docetaxel in the PDL1-negative population (HR 0.9), although duration of response was substantially longer and tolerability was better in the nivolumab arm. Nivolumab-related select adverse events observed included infusion-related reactions (3%; no grade 3–4), rash (9%; <1% grade 3–4), pneumonitis (3%; 1% grade 3–4), ALT/AST increased (3%; <1% grade 3–4), diarrhea (8%; 1% grade 3–4), and hypothyroidism (7%; no grade 3–4). Checkmate 017 enrolled patients with squamous NSCLC. Compared to docetaxel, nivolumab resulted in a 41% reduction in the risk of death (HR 0.59; 95% CI, 0.44–0.79; P<0.001).18 Median OS was 9.2 months in the nivolumab arm (95% CI, 7.3–13.3 months) and 6 months in the docetaxel arm (95% CI, 5.1–7.3 months). Toxicity rates were similar to those in Checkmate 057. Although Checkmate 026 did not demonstrate superior PFS or OS of nivolumab over platinum doublet chemotherapy in previously untreated advanced NSCLC with ≥5% cells PDL1 positive, there may still be benefit of nivolumab monotherapy when administered after chemoradiation,19 a clinical scenario in which observation is the current standard of care.
Due to potential overlapping pneumonitis with radiation therapy, nivolumab pulmonary toxicity is a particular concern in the setting of locally advanced NSCLC. To date, across the clinical trial experience in 2,166 patients with solid tumors, fatal immune-mediated pneumonitis has occurred in 0.2% (5/2166) of patients receiving nivolumab. All five fatal cases occurred early in the nivolumab development program. Perhaps reflecting cumulative experience with and knowledge of this potential toxicity, there were no fatal cases of pneumonitis in the phase 3 Checkmate 017 and Checkmate 057 trials.17,18 In Checkmate 017, pneumonitis occurred in 5% of patients receiving nivolumab, of which there was only one Grade 3 case and no Grade 4 cases. The median time to onset of treatment-related pulmonary events was 15.1 weeks (range, 2.6 to 85.1 weeks). All cases resolved upon discontinuation of nivolumab and administration of corticosteroids, with median time to resolution of 5.0 weeks (range 0.6 to 12.1 weeks). In Checkmate 057, any grade pneumonitis was 3% and grade 3 and above pneumonitis was 1%. The low rate of pneumonitis and the good response to supportive therapy seen in the most recent nivolumab trials suggests that, with appropriate monitoring and precautions, this agent could be studied in combination with thoracic radiation therapy.
Although nivolumab has not yet been studied in combination with RT, data are available for nivolumab-treated patients who previously received RT. While the precise timing and nature of treatments were not captured, of the 129 patients with NSCLC treated on the phase 1, dose-escalation, cohort expansion trial (CA209-003), 75 (58%) had previously received RT. In the overall patient population of 129 NSCLC cases, there were nine cases of pneumonitis (7.0%) and three cases of grade 3–4 pneumonitis (2.3%), suggesting that the overwhelming majority of NSCLC patients treated with nivolumab after prior RT do not develop pneumonitis. There also is limited experience for patients receiving RT during or after nivolumab therapy. Among approximately 3,000 patients across tumor types (NSCLC, renal cell carcinoma, melanoma) treated with nivolumab to date, approximately 50 were treated with palliative RT during or immediately following nivolumab therapy, with irradiated sites including brain, bone, and lung. No serious adverse events were associated with these cases of palliative RT administration.
In the present trial, enrollment is not restricted to PD-L1 expressors for numerous reasons. Despite early reports suggesting that PD-L1 expressors (≥1%) appear to derive particular benefit from PD-1- and PD-L1-targeted therapies, the definition of PD-L1 expression remains unclear. Furthermore, in preclinical models, RT has been shown to increase tumor expression of PD-L1,9 which would limit the correlation of outcomes with pre-RT tissue biomarkers. The observation that tumor PD-L1 expression may be prognostic in general lung cancer populations—but not among cases treated with radiation or chemotherapy20—further suggests that baseline assessment of this biomarker may not adequately define the target population most likely to benefit.
The selection of a cisplatin-etoposide chemotherapy backbone reflects multiple considerations. This regimen has been successfully combined with “consolidation” chemotherapy, such as docetaxel 75 mg/m2 every 21 days with acceptable toxicity profiles.1 In a randomized phase 2 trial, the regimen had improved outcomes compared to a carboplatin-paclitaxel-based regimen (3-year OS 33% vs 13%),21 although survival differences between the regimens have not been noted in population-based observational studies.22,23 Additionally, cisplatin-etoposide is ideally suited to combination with consolidation nivolumab because, in contrast to carboplatin-paclitaxel chemoradiation regimens, (a) the entire chemotherapy course is administered during the 6 to 7 weeks of thoracic radiation and (b) the steroid requirement (which could hypothetically reduce the efficacy of nivolumab) is lower. Finally, rates of radiation pneumonitis—a toxicity that could hypothetically be exacerbated by immunotherapy—may be lower with concurrent cisplatin-etoposide than with concurrent carboplatin-paclitaxel (approximately 5% versus 15%).1,24
There is preclinical evidence supporting the administration of immunotherapy before, during, and after RT. However, administration of immunotherapy before chemoradiation could delay potentially curative treatment for this aggressive disease. Furthermore, clinical considerations support the evaluation of administering nivolumab as consolidation therapy after concurrent chemoradiation. As no chemotherapy will be given with nivolumab, no steroid premedication will be administered, thereby theoretically optimizing immune stimulation. Patients with severe toxicities from concurrent chemoradiation will be identified prior to administration of nivolumab and will have additional time to recuperate from their toxicities. Finally, clinical observations of anti-CTLA-4 antibodies suggest that immunotherapy administration following RT may provide synergistic effects.10 The initiation of nivolumab 4–12 weeks after completion of chemoradiation reflects previous experience with combined modality regimens for locally advanced disease, prior experience with nivolumab and palliative radiation therapy, and optimization of potential synergistic effects. In concurrent chemoradiation regimens that continue chemotherapy after completion of thoracic radiation, the first post-radiation chemotherapy cycle is commonly given 3–4 weeks after thoracic radiation has ended.1,24 In the START phase 3 trial of tecemotide (MUC1 vaccine) after chemoradiotherapy, vaccine was initiated 4–12 weeks after chemoradiotherapy completion and was well tolerated.25 Administration of palliative RT with at least a 14-day window between RT and nivolumab dosing in multiple disease settings has not been associated with increased toxicity. Finally, relatively early initiation of immunotherapy may also capitalize on residual and ongoing radiation-induced tumor antigenic stimulation.5,6 The administration of nivolumab for up to one year covers the time period when patients are at greatest risk of recurrence or progression (approximately 75% of cases that eventually progress do so within 12 months25). Post-treatment fluorodeoxyglucose (FDG) uptake not representing disease recurrence or progression has been reported up to 15 months after completion of chemoradiation.26 If such radiographic findings correspond to physiologic effects related to tumor antigenic stimulation, then this period of time might represent the optimal period to capitalize on the abscopal effect. Prolonged anti-PD-1 therapy also appears tolerable. In nivolumab phase 1 trials, the number of patients who continued nivolumab beyond 6 months (and a smaller number beyond 12 months) due to prolonged radiographic response or stable disease were able to do so without apparent cumulative toxicity.27
Objectives
The co-primary endpoints of this trial are OS (defined as the time from randomization to death due to any cause) and PFS (defined as the time from randomization to documented progressive disease or death due to any cause, whichever occurs first). The PFS co-primary endpoint is based on an independent radiology review committee according to RECIST 1.1.28 PFS was included as a co-primary endpoint because of the possibility of cross-over after progression in the control arm and because some international regulatory bodies incorporate it into consideration for drug label indication. An independent radiology review committee is employed for this endpoint because assessment of disease status post-chemoradiation is notoriously complex (up to 65% of patients have treatment-related radiographic changes29) and the addition of sequential immunotherapy may further complicate the assessment because of potential “pseudo-progression.”30
Secondary objectives of this study include the following:
Compare toxicities in the control and experimental arms
Compare patient reported outcomes and quality of life (using the Functional Assessment of Cancer Therapy-Trial Outcome Index [FACT-TOI] for lung cancer, the Patient-Reported Outcomes Measurement Information System [PROMIS] fatigue short form, and the EuroQol five dimensions questionnaire [EQ-5D] index and visual analog scale [VAS]) in the control and experimental arms
Evaluate OS and PFS in (1) PD-L1 expressors (≥1%) and (2) PD-L1 non-expressors (<1%)
Exploratory objectives include the following:
Evaluate predictive and pharmacodynamic biomarkers
Determine the proportion of patients alive at 12 and 24 months
Determine the proportion of patients progression free at 12 and 24 months according to RECIST 1.1
Compare PFS based on investigator assessment and blinded independent central review
Study Design
This is a multi-center phase 3 randomized, double-blind, placebo-controlled clinical trial sponsored by the RTOG Foundation and Bristol-Myers Squibb. Tumor PD-L1 expression will be determined for all patients but is not required for enrollment. Analysis of PD-L1 expression will be performed by LabCorp by use of the 28–8 antibody to PD-L1 (BMS) using the Dako IHC platform.31 PD-L1 membrane staining will be assessed by light microscopy. Either complete circumferential or partial linear plasma membrane staining will constitute positive PD-L1 staining.
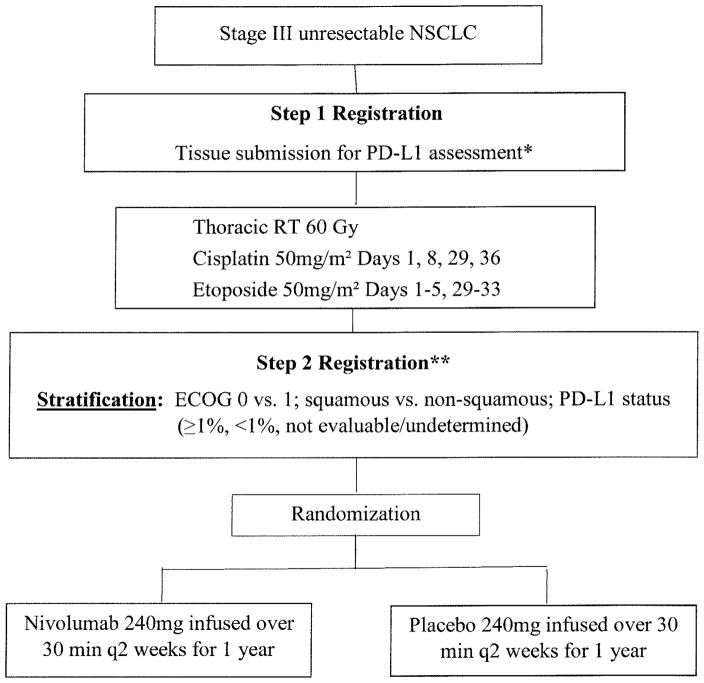
Figure 1 shows the study schema. All patients will receive concurrent chemoradiation as follows: thoracic radiation therapy (RT) to 60 Gy with concurrent cisplatin 50 mg/m2 IV on Days 1, 8, 29, and 36, and etoposide 50 mg/m2 IV on Days 1–5 and 29–33. Between 4 and 12 weeks after completion of concurrent therapy, eligible patients will be randomized 1:1 to nivolumab 240 mg IV over 30 minutes or placebo every 2 weeks for up to 1 year.
Figure 1.
Study schema
* Verification that archived tissue block is available for submission as the completed submission will be required at least 5 weeks prior to Step 2 Registration
** Assessment for progression after chemoRT; if evidence of distant metastases or local disease progression, will not be randomized
Randomization will be stratified by performance status (ECOG 0 vs 1), histology (squamous vs non-squamous), and tumor PD-L1 expression (≥1%, <1%, not evaluable/undetermined). There are no nivolumab/placebo dose modifications. Depending on toxicities, nivolumab/placebo is given at full dose, withheld, or discontinued. The study requires the use of photons delivered either using 3D-conformal radiation therapy (CRT) or intensity-modulated radiation therapy (IMRT) techniques. Tomotherapy is allowed if appropriately credentialed for its use. Use of stereotactic radiation therapy is not permitted for this trial. The use of image-guided radiation therapy (IGRT) is highly encouraged but not required. Patients are permitted to continue treatment beyond initial RECIST 1.1-defined (PD) as long as the following criteria are met: (a) investigator-assessed clinical benefit and do not have rapid disease progression; (2) tolerance of study drug; (3) stable performance status; (4) treatment beyond progression will not delay an imminent intervention to prevent serious complications of disease progression.
Imaging exams are collected at the following time points: baseline (prior to study treatment); post-chemoradiation; every 3 months for 2 years, every 6 months for years 3–5, then yearly; at disease progression; at request when treatment occurs beyond progression and subsequent tumor evaluations. At the time of investigator-assessed progression, sites request a real-time independent review of progression, which is conducted by a blinded, third party, independent radiologist. If there is discordance between the local and central reviews (ie, central review does not confirm progression), a central adjudicator will review both local and central results to render a final decision on progression. In those cases when the site investigators disagree with final adjudicated central review interpretation, subsequent therapy may be initiated per investigator’s clinical decision, and protocol therapy may be discontinued. Local site investigators also have the option of continuing therapy beyond their local interpretation of progression.
Study Population
Detailed study eligibility criteria are listed in Table 1. Eligible patients have a pathologically (histologically or cytologically) proven diagnosis of stage IIIA or stage IIIB disease (AJCC 7th edition32) NSCLC with that is unresectable, medically inoperable, or for which resection is refused. Unresectable stage IIIA disease is defined by multiple and/or bulky N2 mediastinal lymph nodes on computed tomography (CT) scan. N2 and/or N3 disease must be documented by biopsy, by fluorodeoxyglucose positron emission tomography (FDG-PET), or by CT (if nodes are more than 2 cm). Core or excisional biopsy is required for this study because fine needle aspirates (FNA) and cytology specimens are not adequate for PD-L1 analysis. Notably, patients are ineligible if they have an active, known, or suspected autoimmune disease.33 Exceptions include vitiligo, type I diabetes mellitus, residual hypothyroidism due to autoimmune condition only requiring hormone replacement, and psoriasis not requiring systemic treatment.
Table 1.
Trial Eligibility Criteria
| Step 1—Registration Prior to Chemoradiation |
| Inclusion Criteria |
|
| Exclusion Criteria |
|
| Step 2—registration after completion of chemoradiation, before randomization |
| Inclusion Criteria |
|
| Exclusion Criteria |
|
Statistical Analysis Plan
The study accounts for two primary endpoints: OS and PFS. Overall (2-sided) alpha is 0.05, which is split with 0.01 for evaluating PFS and with 0.04 for evaluating OS. Assuming a rate of 16.7% due to ineligibility and drop-out between initial registration and randomization, a total of 660 patients will be enrolled to ensure 550 patients are randomized after completion of chemoradiation. This sample size will provide ≥90% power to detect a hazard ratio (HR) of 0.667 for PFS with two-sided α=0.01, and 90% power to detect an HR of 0.7 for OS with two-sided α=0.01. The trial will be declared positive if either the OS or PFS comparison of the treatments is statistically significant favoring the experimental arm.
To determine whether the treatment effect is consistent across various subgroups, the estimate of the between-group treatment effect for the primary endpoints will be estimated within each category of the following classification variables: age (≤65, >65 years), sex, race (white, non-white), ECOG status (0, 1), histology (squamous, non-squamous), smoking status (never, former, current).
If the trial is declared positive based on either OS or PFS in the overall intent-to-treat (ITT) population, a step-down procedure will be applied to OS and PFS in the ITT subgroups in the following order: (1) PD-L1 expressors; (2) PD-L1 non-expressors, (3) PD-L1 undetermined, with an overall 2-sided α of 0.05 for each subgroup analysis. In the event that study is not declared positive based on either OS or PFS in the ITT population, the presence of qualitative interactions between PD-L1 status and nivolumab will be assessed by analyzing OS and PFS within each PD-L1 subgroup separately for exploratory purposes. The statistical analysis plan will be same except that no formal hypothesis testing will be performed.
For the QOL secondary endpoint, it is hypothesized that the experimental arm will have a clinically meaningful better QOL due to reduction in disease progression and associated symptoms.
Effect of This Study
Recent attempts to improve clinical outcomes in patients with stage 3 NSCLC—including the addition of consolidation chemotherapy, maintenance EGFR inhibitor therapy, sequential vaccine therapy, incorporating trimodality therapy, and increasing radiation dose—have failed. Preclinical models and early clinical experience suggest that locally advanced NSCLC treated with concurrent chemoradiation may represent the optimal setting for anti-PD1 immune checkpoint inhibitor therapy, which is already approved for previously treated advanced NSCLC. This trial offers unprecedented insight into this therapeutic approach, including not only OS and PFS endpoints, but also patient-reported outcomes, quality of life, and relevant biomarker studies.
Acknowledgments
Funding: Funded in part by the RTOG Foundation, Bristol-Myers Squibb, and a National Cancer Institute (NCI) Midcareer Award in Patient-Oriented Research (K24CA201543-01; to DEG). The authors thank Ms. Dru Gray for assistance with manuscript preparation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could a3ect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hanna N, Neubauer M, Yiannoutsos C, et al. Phase III study of cisplatin, etoposide, and concurrent chest radiation with or without consolidation docetaxel in patients with inoperable stage III non-small-cell lung cancer: the Hoosier Oncology Group and U.S. Oncology J Clin Oncol. 2008;26:5755–60. doi: 10.1200/JCO.2008.17.7840. [DOI] [PubMed] [Google Scholar]
- 2.Ahn JS, Ahn YC, Kim JH, et al. Multinational Randomized Phase III Trial With or Without Consolidation Chemotherapy Using Docetaxel and Cisplatin After Concurrent Chemoradiation in Inoperable Stage III Non-Small-Cell Lung Cancer: KCSG-LU05-04. J Clin Oncol. 2015;33:2660–6. doi: 10.1200/JCO.2014.60.0130. [DOI] [PubMed] [Google Scholar]
- 3.Kelly K, Chansky K, Gaspar LE, et al. Phase III trial of maintenance gefitinib or placebo after concurrent chemoradiotherapy and docetaxel consolidation in inoperable stage III non-small-cell lung cancer: SWOG S0023. J Clin Oncol. 2008;26:2450–6. doi: 10.1200/JCO.2007.14.4824. [DOI] [PubMed] [Google Scholar]
- 4.Bradley JD, Paulus R, Komaki R, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol. 2015;16:187–99. doi: 10.1016/S1470-2045(14)71207-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iyengar P, Gerber DE. Locally advanced lung cancer: an optimal setting for vaccines and other immunotherapies. Cancer J. 2013;19:247–62. doi: 10.1097/PPO.0b013e318292e51a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Uchida A, Mizutani Y, Nagamuta M, Ikenaga M. Effects of X-ray irradiation on natural killer (NK) cell system. I. Elevation of sensitivity of tumor cells and lytic function of NK cells. Immunopharmacol Immunotoxicol. 1989;11:507–19. doi: 10.3109/08923978909005381. [DOI] [PubMed] [Google Scholar]
- 7.Demaria S, Ng B, Devitt ML, et al. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys. 2004;58:862–70. doi: 10.1016/j.ijrobp.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 8.Lee Y, Auh SL, Wang Y, et al. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood. 2009;114:589–95. doi: 10.1182/blood-2009-02-206870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deng L, Liang H, Burnette B, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest. 2014;124:687–95. doi: 10.1172/JCI67313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Postow MA, Callahan MK, Barker CA, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med. 2012;366:925–31. doi: 10.1056/NEJMoa1112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124–8. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Le DT, Uram JN, Wang H, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015 doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alexandrov LB, Nik-Zainal S, Wedge DC, et al. Signatures of mutational processes in human cancer. Nature. 2013;500:415–21. doi: 10.1038/nature12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Talmadge JE, Donkor M, Scholar E. Inflammatory cell infiltration of tumors: Jekyll or Hyde. Cancer Metastasis Rev. 2007;26:373–400. doi: 10.1007/s10555-007-9072-0. [DOI] [PubMed] [Google Scholar]
- 15.Brahmer JR, Drake CG, Wollner I, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28:3167–75. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gettinger SN, Horn L, Gandhi L, et al. Overall Survival and Long-Term Safety of Nivolumab (Anti-Programmed Death 1 Antibody, BMS-936558, ONO-4538) in Patients With Previously Treated Advanced Non-Small-Cell Lung Cancer. J Clin Oncol. 2015;33:2004–12. doi: 10.1200/JCO.2014.58.3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373:1627–39. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373:123–35. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Socinski MA, Creelan B, Horn L, et al. CheckMate 026: A Phase 3 Trial of Nivolumab vs Investigator's Choice of Platinum-Based Doublet Chemotherapy as First-line Therapy for Stage IV/Recurrent Programmed Death Ligand 1-Positive NSCLC European Society of Medical Oncology Congress. 2016 [Google Scholar]
- 20.Sun J-M, Zhou W, Choi Y-L, et al. PD-L1 expression and survival in patients with non-small cell lung cancer (NSCLC) in Korea. J Clin Oncol. 2014:32. [Google Scholar]
- 21.Wang L, Wu S, Ou G, et al. Randomized phase II study of concurrent cisplatin/etoposide or paclitaxel/carboplatin and thoracic radiotherapy in patients with stage III non-small cell lung cancer. Lung Cancer. 2012;77:89–96. doi: 10.1016/j.lungcan.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 22.Santana-Davila R, Devisetty K, Szabo A, et al. Cisplatin and etoposide versus carboplatin and paclitaxel with concurrent radiotherapy for stage III non-small-cell lung cancer: an analysis of Veterans Health Administration data. J Clin Oncol. 2015;33:567–74. doi: 10.1200/JCO.2014.56.2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ezer N, Smith CB, Galsky MD, et al. Cisplatin vs. carboplatin-based chemoradiotherapy in patients >65 years of age with stage III non-small cell lung cancer. Radiother Oncol. 2014;112:272–8. doi: 10.1016/j.radonc.2014.07.014. [DOI] [PubMed] [Google Scholar]
- 24.Belani CP, Choy H, Bonomi P, et al. Combined chemoradiotherapy regimens of paclitaxel and carboplatin for locally advanced non-small-cell lung cancer: a randomized phase II locally advanced multi-modality protocol. J Clin Oncol. 2005;23:5883–91. doi: 10.1200/JCO.2005.55.405. [DOI] [PubMed] [Google Scholar]
- 25.Butts C, Socinski MA, Mitchell PL, et al. Tecemotide (L-BLP25) versus placebo after chemoradiotherapy for stage III non-small-cell lung cancer (START): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2014;15:59–68. doi: 10.1016/S1470-2045(13)70510-2. [DOI] [PubMed] [Google Scholar]
- 26.Larici AR, del Ciello A, Maggi F, et al. Lung abnormalities at multimodality imaging after radiation therapy for non-small cell lung cancer. Radiographics. 2011;31:771–89. doi: 10.1148/rg.313105096. [DOI] [PubMed] [Google Scholar]
- 27.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1. 1) Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 29.McDonald S, Rubin P, Phillips TL, Marks LB. Injury to the lung from cancer therapy: clinical syndromes, measurable endpoints, and potential scoring systems. Int J Radiat Oncol Biol Phys. 1995;31:1187–203. doi: 10.1016/0360-3016(94)00429-O. [DOI] [PubMed] [Google Scholar]
- 30.Wolchok JD, Hoos A, O'Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15:7412–20. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 31.Taube JM, Anders RA, Young GD, et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Science translational medicine. 2012;4:127ra37. doi: 10.1126/scitranslmed.3003689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rami-Porta R, Crowley JJ, Goldstraw P. The revised TNM staging system for lung cancer. Ann Thorac Cardiovasc Surg. 2009;15:4–9. [PubMed] [Google Scholar]
- 33.Khan SA, Pruitt SL, Xuan L, Gerber DE. Prevalence of Autoimmune Disease Among Patients With Lung Cancer: Implications for Immunotherapy Treatment Options. JAMA oncology. 2016 doi: 10.1001/jamaoncol.2016.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]