Abstract
PURPOSE
To evaluate the dynamic characteristics of T2*-weighted signal change in exercising skeletal muscle of healthy subjects and peripheral artery disease (PAD) patients under a low-intensity exercise paradigm.
MATERIAL AND METHODS
Nine PAD patients and nine age- and sex-matched healthy volunteers underwent a low-intensity exercise paradigm while MRI (3.0 T) was obtained. T2*-weighted signal time-courses in lateral gastrocnemius, medial gastrocnemius, soleus and tibialis anterior were acquired and analyzed. Correlations were performed between dynamic T2*-weighted signal and changes in heart rate, mean arterial pressure, leg pain, perceived exertion.
RESULTS
A significant signal decrease was observed during exercise in soleus and tibialis anterior of healthy participants (p = 0.0007 to 0.04 and 0.001 to 0.009, respectively). In PAD, negative signals were observed (p = 0.008 to 0.02 and 0.003 to 0.01, respectively) in soleus and lateral gastrocnemius during the early exercise stage. Then the signal gradually increased above the baseline in the lateral gastrocnemius during and after exercise in 6 of the 8 patients who completed the study. This signal increase in patients’ lateral gastrocnemius was significantly greater than in healthy subjects’ during the later exercise stage (two-sample t-tests, p = 0.001 to 0.03). Heart rate and mean arterial pressure responses to exercise were significantly higher in PAD than healthy subjects (p = 0.036 and 0.008, respectively) and the patients experienced greater leg pain and exertion (p = 0.006 and p = 0.0014, respectively).
CONCLUSION
During low-intensity exercise, there were different dynamic T2*-weighted signal behavior in the healthy and PAD exercising muscles.
Keywords: T2*-weighted signal, skeletal muscle, low-intensity exercise, peripheral artery disease, lower extremities
INTRODUCTION
First applied in the brain (1), blood-oxygen-level-dependent (BOLD) magnetic resonance imaging (MRI) contrast using T2*-weighted imaging has become a powerful tool for the study of physiology and function in normal and disease conditions in vivo. Of note, similar to the BOLD contrast in the brain, the dynamic changes of T2*-weighted contrast is also present in exercising skeletal muscles; however, the application of T2*-weighted contrast for the study of the function of skeletal muscle has been limited (2). One limiting factor of the use of this method is the dynamic coupling between the muscle T2*-weighted signal and systemic physiologic changes during exercise. The major physiologic contributors to the T2*-weighted signal in the tissue are blood flow, blood volume and oxygenation. The latter depends on the ratio of the oxygenated and deoxygenated hemoglobin in blood in the tissue being observed. This, in turn, depends on systemic cardiovascular modulations in response to local metabolic activity in the muscle (3, 4).
Unlike the brain, where metabolic processes are normally high and relatively stable, the regulation of blood flow and oxygenation in skeletal muscle is very complex. Indeed, skeletal muscle has a very low metabolic rate at rest and this can increase >10 fold in response to muscle contraction. During exercise, heart rate (HR) and blood pressure (BP), as systemic variables, yet are influenced by the metabolic milieu within the skeletal muscle (5). Thus, the T2*-weighted signal in muscle is highly dependent on the workload, the muscle length and local metabolism in the muscle (6). For these reasons, the T2*-weighted signal should be an informative tool for the investigation of systemic blood flow regulation in health and disease.
One potential clinical application of T2*-weighted contrast MRI is in the assessment of patients with peripheral artery disease (PAD). Affecting more than 200 million people worldwide, PAD causes high morbidity and mortality (7, 8), it is reported that PAD patients are more risky of myocardial infarction and stroke than healthy people (9). In PAD, a classic clinical presentation is the development of intermittent pain (claudication) in the lower limbs. Claudication is exercise induced and is relievable after a brief period of rest (10). The contributors to functional limitation (intermittent claudication or other atypical symptoms) include ischemia due to the narrowed vascular lumen and the subsequent metabolic changes within the muscle. In such cases, patients usually start to complain of muscle pain and discomfort at low-intensity daily activity such as walking. Exercise impairment can worsen and patients can develop rest pain and eventually tissue necrosis necessitating limb amputation. However, the outcome of conventional treatments such as exercise training and peripheral revascularization (angioplasty, stent, or bypass surgery) on oxygenation of leg skeletal muscle are mixed (11–13). As evaluated with MRI, percutaneous intervention is shown to improve the blood supply (measured with rest ABI) as well as ATP metabolism (measured with 31P magnetic resonance spectroscopy) in PAD patients with symptomatic claudication, while the change of tissue perfusion (measured with first-pass Gd-enhanced MRI) was not significant (14). On the other hand, a recent study has shown that blood-oxygen-level-dependent MRI can be a promising tool for detection of perfusion deficits in the PAD (15).
In order to address the technical and physiological issues of using T2-weighted contrast in skeletal muscle in PAD, our primary purpose was to evaluate the dynamic characteristics of T2*-weighted signal change in exercising skeletal muscle of healthy participants and PAD patients under a low-intensity exercise paradigm (2 kg for 14 min), simulating the rhythmic pattern of exercise as would be seen with walking. Our experiment is driven by following hypotheses: 1) The low-intensity paradigm will evoke a hemodynamic steady state in the exercising muscles that can be observed in T2*-weighted imaging and 2) such a steady state in the exercising muscles could be disrupted in PAD.
MATERIAL AND METHODS
Human Participants
All study protocols were approved by the Institutional Review Board (IRB). Written informed consent was obtained from all volunteers prior to participation in accordance with IRB guidelines. Nine PAD patients and nine age- and sex-matched healthy participants were recruited into this study (Table 1). According to our inclusion criteria, the participants in the healthy group were normotensive and non-smokers. As previously reported, activity level could impact T2*-weighted signal (16), our healthy subjects were recreationally active who exercise regularly (mostly walk or bike) but not competitive athletes. Their height, weight, and body mass index were within normal limits, and their ankle branchial index (ABI) was between 1.0 and 1.4. The ABI is the ratio of the blood pressure at the ankle to the blood pressure in the upper arm (brachium), the PAD participants were diagnosed clinically with the ABI of affected limb < 0.9 (17). Two PAD participants had stents in peripheral or coronary arteries. All participants were told to avoid nicotine or caffeine consumption for 12 hours prior to the study. For patients with bilateral disease, both legs were imaged but only data from the leg with worse symptoms and/or a greater exercise impairment were used in the analyses.
Table 1.
Demographics and clinical status of participants
| Healthy | PAD | |
|---|---|---|
| Number of participants (male/female) | 9 (7/2) | 9 (7/2) |
| Age (years) | 64 ± 2.3 | 67 ± 2 |
| Ankle-brachial index | 1.1 ± 0.0 | 0.6 ± 0.0* |
| Body mass index (kg/m2) | 25 ± 2 | 28 ± 2.3 |
| Current smoker | 0 | 2 |
| Diagnosed hypertension | 0 | 5 |
| Diagnosed diabetes | 0 | 3 |
| Resting Heart Rate (beats/min) | 59 ± 2 | 65 ± 3 |
| Resting Systolic Blood Pressure (mmHg) | 121 ± 4 | 135± 3 |
| Resting Diastolic Blood Pressure (mmHg) | 71 ± 3 | 69 ± 2 |
| Resting Mean Arterial Pressure (mmHg) | 91 ± 3 | 95 ± 3 |
Data are shown as Mean ± SEM.
indicates p < 0.05 between groups
Plantar Flexion Exercise Paradigm
The MRI-compatible exercise device was placed next to the examination bed behind the magnet for MRI scanning. Baseline HR was obtained by a pulse oximeter on the finger and BP was measured in the supine position using an MRI compatible device (Precess MRI Compatibility, Invivo Corporation). These measurements were performed after a 15-min rest period. The foot of the exercising leg was secured to a wooden pedal connected to a rotary lift device allowing isolated plantar flexion exercise (18).
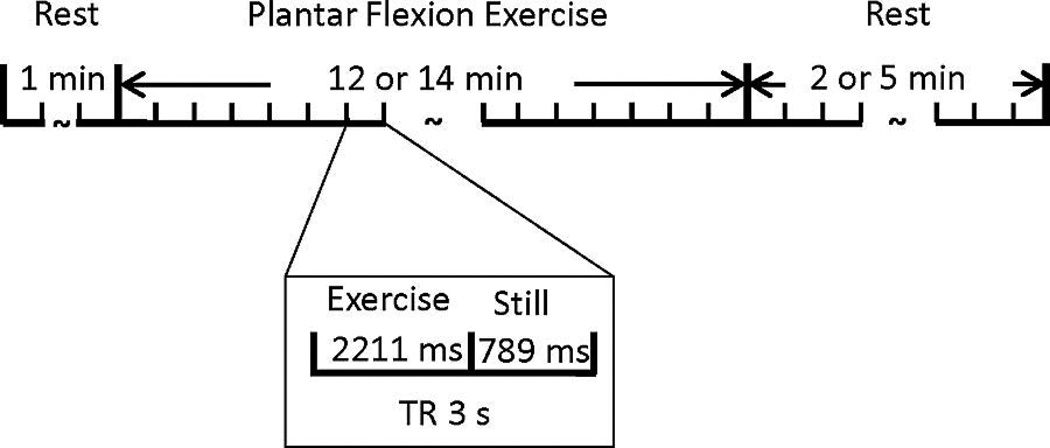
The exercise workload (2 kg) for use here was determined in a separate study as it produced relatively low intensity exercise for 14 min without causing any discernible fatigue for the healthy participants. As shown in Figure 1, the paradigm lasted 20 min and consisted of a 1-min rest period (baseline) followed by a 14-min dynamic plantar flexion exercise and a 5-min recovery period. During exercise, 280 contractions were performed at a rate of 1/3 Hz. For two patients with stents previously placed in peripheral or coronary arteries, the exercise period was shortened to 12-min followed by a 2-min recovery. This modification was made due to data quality and safety considerations.
Figure 1.
Exercise paradigm. The paradigm consisted of a 1-min baseline followed by a 14-min exercise period then a 5-min recovery. Due to safety considerations for 2 PAD patients with stents placed in coronary or peripheral arteries, the exercise and recovery periods were shortened to 12 min and 2 min, respectively.
In order to reduce motion-related image artifacts, the exercise was paced by the cue of the scanner noise during image acquisition. The participants performed one plantar-flexion only during the 2211-ms “quiet” time of each time of repetition (TR), during contractions no MRI images were acquired. A familiarization period was conducted prior to image acquisition to ensure the participants could maintain the cadence. Two investigators stayed in the scanning room during data collection to ensure the participants were exercising in between the scanning and their legs were completely still while being scanned. A potentiometer attached to the shaft of the rotary lift device sensed the angle of rotation and the signal was collected into PowerLab (ADInstruments Inc.) along with the sound of the MRI scanner. To assess the reliability of our experimental procedures, paradigm and exercise device, a pilot test-retest study was first performed on five healthy normal subjects with three subjects using the same leg and two using opposite legs for each test. Both tests produced consistently negative responses of T2*-weighted “BOLD” signal change in muscles during the exercise paradigm. Based on linear mixed-effect model with random intercept, the intraclass correlation coefficient between the two sets of time-series of T2*-weighted signal in the lateral gastrocnemius was 0.60 for all five subjects and 0.67 for the three subjects with the tests on the same legs, which is considered as fair reliability in general.
Data acquisition
All MRI data were acquired on a 3T scanner (Siemens Magnetom Trio, Siemens Medical Solutions, Erlangen, Germany). The participants were supine with their calf muscle fitted into an 8-channel knee coil. Padding was carefully arranged around the calf muscle in order to minimize motion without constricting blood flow. Gradient-echo T2*-weighted echo planar imaging (EPI) was used to acquire time-course images of calf muscle with a voxel size = 2.5 × 2.5 mm2, 10 axial slices, slice thickness = 5.0 mm, field of view = 160 × 160 mm2, flip angle = 70°, TR = 3 s, and echo time = 25 ms). In each TR, the participants performed one plantar flexion movement during the 2211-ms quiet interval with no MRI noise, and stayed still during the next 789-ms while one set of MRI images were acquired.
Measurements of HR and BP of upper arm were logged into PowerLab every 2 min. At the end of each exercise session, the subject provided subjective rating of leg pain on a scale of 0 to 10 (0—no pain, 10—worst possible pain) and rating of perceived exertion (Borg scale from 6–20, 6—very, very light, to 20—fatiguing) (19). Furthermore, for subjects who rated leg pain higher than 0, they were asked to describe the exact place in the leg where pain was noted. For participants who rated effort higher than 12 on the Borg scale, subjects were asked to describe the exact part of muscle groups that worked hardest during the paradigm. One patient’s data were excluded in statistical analysis due to a significant artifact from a knee replacement.
Data processing, analysis, and statistics
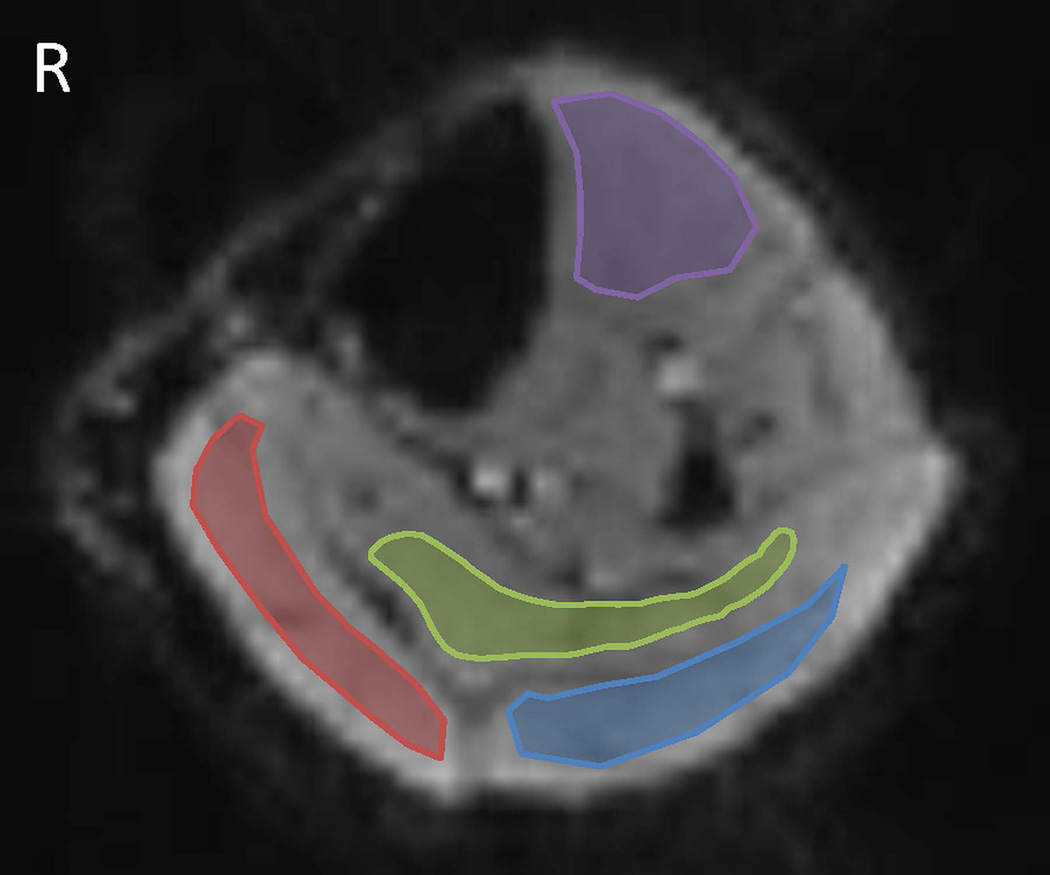
The time-course EPI data were first motion-corrected using a self-written program based on wavelet analysis (20). The timing of each slice was adjusted using SPM8 (The Wellcome Trust Centre for Neuroimaging, University College London, UK). Regions of interest (ROIs) within the lateral head of the gastrocnemius, the medial head of gastrocnemius, the soleus, and the tibialis anterior muscle from each subject were manually segmented on EPI images with MRIcron (Neuropsychology Lab, Columbia, SC, USA) as shown in Figure 2. Care was taken to avoid large blood vessels, nerves, epimysium, and subcutaneous fat tissue. For each individual subject, the mean signal intensity for each ROI (400 data points for healthy controls and 6 PAD patients, and 300 data points for 2 PAD patients with stents) were obtained. Average T2*-weighted signal for the one minute baseline, every minute of exercise, and recovery were calculated for data analyses. Percent change of T2*-weighted signal intensity (SI) as compared to the baseline during every minute of exercise and recovery (ΔSI) were calculated as below:
| (1) |
where n = 1, 2, 3…, denoting the n-th minute of the exercise paradigm. Group comparisons of the T2*-weighted signal changes in each ROI between PAD and healthy were analyzed with two-sample t-test. Ratings for pain and perceived exertion were compared with the Mann-Whitney nonparametric test. Exploratory bivariate correlations were conducted between physiology and perceptual variables. Data are shown as mean ± SEM and p-values < 0.05 were considered statistically significant.
Figure 2.
Regions of interest (ROIs) of calf muscle groups. ROIs were manually drawn for four muscle groups: lateral gastrocnemius (blue) and medial gastrocnemius (red), soleus (green), and tibialis anterior (purple).
For statistics, ANOVA (Bonferroni corrected) was used for comparing the signal change of each time point during exercise to baseline level, paired t-tests (Bonferroni corrected) were used for comparing the signal, HR and MAP change over exercise stage to baseline level, Mann-Whitney nonparametric tests (two-tailed) was used for comparing leg pain rating and Borg value to baseline (0 as the baseline of leg pain rating and 12 as the baseline of Borg value), two-sample t-tests were used for comparing T2*-weighted signal temporal patterns during exercise between healthy participants and PADs.
RESULTS
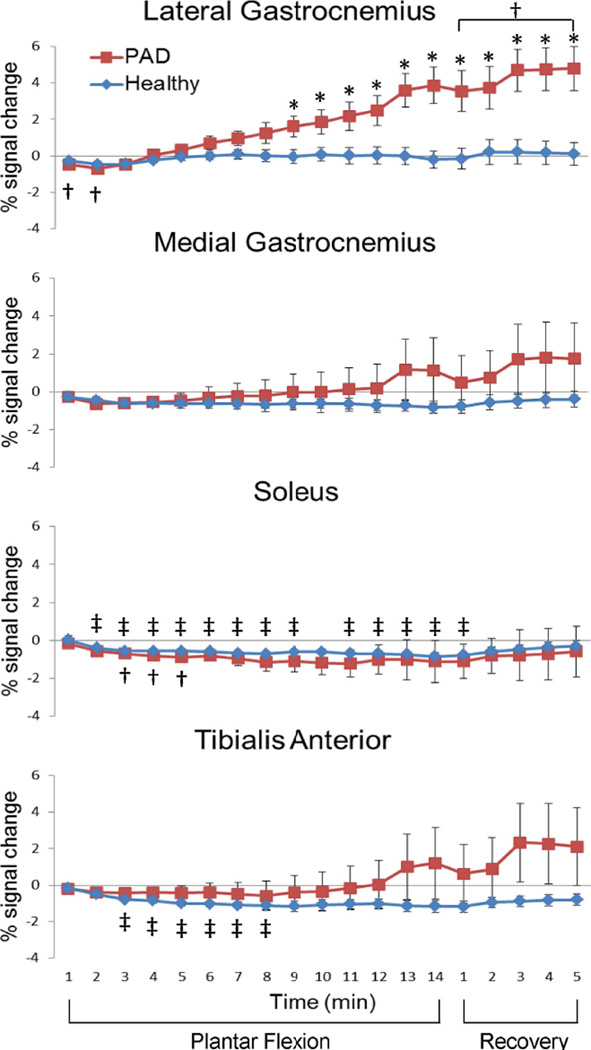
Figure 3 shows the T2*-weighted signal time-course of the four muscle groups in healthy and PAD participants during our low-intensity exercise paradigm. During the exercise paradigm most ROIs showed significant T2*-weighted signal changes as compared to baseline (p = 0.0007 to 0.04). In general, there was a T2*-weighted signal decrease after exercise started. Significant T2*-weighted signal decreases during exercise were observed in the soleus and tibialis anterior of healthy participants (p = 0.0007 to 0.04 and 0.001 to 0.009, respectively), and in the soleus and lateral gastrocnemius of PAD patients during the early phase of exercise (p = 0.008 to 0.02 and 0.003 to 0.01, respectively).
Figure 3.
T2*-weighted signal time courses of four calf muscles. % signal change comparing to the one-minute baseline (mean ± SEM) in a temporal resolution of one minute from healthy participants (n = 9, blue diamonds) and PAD patients (n = 8, red squares). †, significant signal change comparing to the baseline of the PAD participants, paired t-tests with Bonferroni correction, p < 0.05; ‡, significant signal change comparing to the baseline of the HC participants, paired t-tests with Bonferroni correction, p < 0.05; *, T2*-weighted signal in PAD patients significantly higher than that in HC participants, two-sample t-tests, p < 0.05.
After the initial signal drop during the first 2–3 mins of exercise, the T2*-weighted signal in lateral gastrocnemius started to increase gradually above the baseline level (positive T2*-weighted signal change) during exercise and recovery periods in 6 of the 8 PAD patients that successfully completed the study. This T2*-weighted signal increase in PADs was statistically greater than baseline during the 5-min recovery period (p = 0.04). The average BOLD signal in lateral gastrocnemius of the PAD group was significantly greater than that in the healthy group during the last 6 min of exercise and during the 5 min of recovery (p = 0.001 to 0.03).
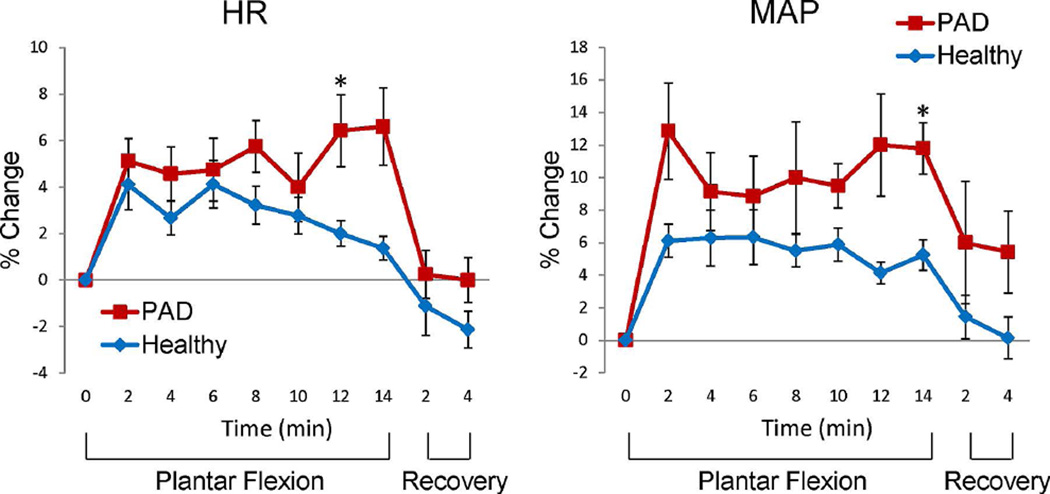
Figure 4 shows the HR and mean arterial pressure (MAP) changes from baseline during and after exercise. The HR in both healthy and PAD participants significantly increased during exercise (p < 0.048 for healthy participants and p < 0.012 for PAD). Accordingly, The MAP also increased significantly (p < 0.015 for healthy participants and p < 0.038 for PAD subjects). The HR and MAP increase in PADs were significantly higher than those in healthy participants (two-sample t-tests, p = 0.036 and 0.008, respectively).
Figure 4.
Heart rate (HR) and mean arterial pressure (MAP) change from the baseline during exercise paradigm (mean ± SEM). The * symbol, indicates the change of HR and MAP in the PAD patients were significantly higher than those in the healthy participants (two-sample t-tests, p < 0.05).
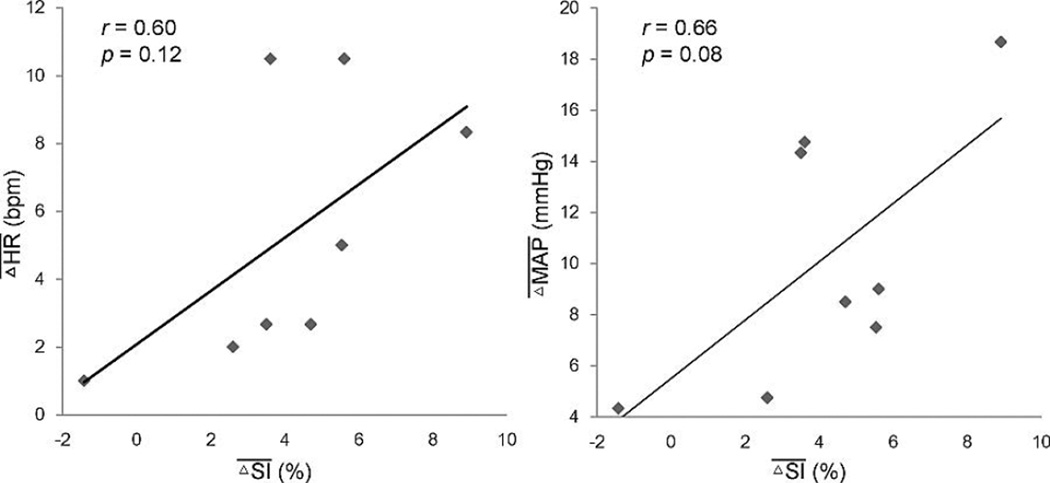
In Figure 5 we present the correlation data between the mean positive T2*-weighted signal change in the exercising calf muscles and mean and in the PAD patients. These mean values of T2*-weighted signal, MAP and HR for each PAD subjects were calculated over the time period where the T2*-weighted signal change was positive. The positive T2*-weighted signal changes during this stage of exercise in PAD participants were positively correlated with the concurrent increase of HR and MAP but did not reach statistical significance (r = 0.596, p = 0.119 for HR change, and r = 0.657, p = 0.077 for MAP change). However, the maximum T2*-weighted signal change in the calf muscles during exercise in the PAD participants was significantly correlated with the MAP change (r = 0.843, p = 0.009). In contrast, no significant correlations were found in the healthy participants during exercise (r = 0.023, p = 0.859 for HR change, and r = 0.112, p = 0.412 for MAP change).
Figure 5.
Relationship between T2*-weighted signal change and heart rate and blood pressure change during the phase of positive T2*-weighted signal change in PAD. The value of and from each PAD patient were obtained by averaging the corresponding measurements over the time period with positive T2*-weighted signal.
The rating of leg pain for the PAD participants ranged from no pain to some pain, whereas the perceived level of exertion ranged from very light to fatiguing. In contrast, the ratings from the healthy participants were significantly lower than those from the patients: no pain to little pain (p = 0.006); and very light to fairly light (p = 0.014, Borg scale).
The T2*-weighted signal-time-course varied greatly across subjects in both PAD and healthy group, with the inter-subject variability significantly larger in the PAD group (maximum −7.3% to 12.4%) as compared to the healthy participants (maximum −3.3% to 3.9%). We were able to detect significantly different T2*-weighted signal temporal patterns during our low-intensity exercise paradigm between healthy and PAD participants in the later phase of exercise (p = 0.016 for exercise time point 12 min, n = 17, Cohen’s d = 1.30; p = 0.001 for exercise time point 14 min, n = 15, Cohen’s d = 2.03).
DISCUSSION
In this work we invested hypotheses that a low-intensity exercise paradigm that could be sustained for long time without involvement significant sympathetic activities will evoke a hemodynamic steady-state in the exercising muscles, and that such a steady-state could be disrupted in PAD. With the low-intensity paradigm, our data (Figure 3) revealed that the exercising muscles evoked in a state during which a negative T2*-weighted signal change was observed. The time-course of T2*-weighted image seemed paradoxical at first glance, as one would expect to see more decreases in T2*-weighted signal with PAD subjects than healthy subjects during exercise because of known impaired hyperemic responses in PAD patients (21–23). Evidently our data revealed a different aspect of PAD impairment from that was obtained previously with hyperemia studies. Our paradigm evoked a steady-state that likely simulates an exercise conditions that we do in our daily life such as slow-paced walking during which the exercising muscles can sustain for long period time without the feeling of fatigue in the normal conditions. Under such a state, the T2*-weighted signal was negative in normal subjects, while, in PAD subjects, a higher sympathetic tone and stronger hyperemia activity were evoked to maintain the task demand. In general, at the beginning of low-intensity exercise, the T2*-weighted signal in exercising muscles decreased in both groups. The observation of negative T2*-weighted signal change in the muscles of the healthy participants might seem counterintuitive as it is known that the arterial blood flow is a contributing factor to the T2*-weighted signal and flow increases during the transition from rest to exercise (24–29). In the brain, an increase in flow causes an increase in the T2*-weighted signal (30, 31). However, in skeletal muscle, both flow and oxygen extraction increase during exercise in order to meet the increased metabolic demand (32–34) and deoxygenation in the exercising muscles produces a negative T2*-weighted signal (35, 36). Poole et al. has demonstrated that the partial oxygen pressure in the active muscle decreases rapidly (37), depending on the intensity of the workload. During exercise these two competing factors contribute to the T2*-weighted signal change. Under the current low-intensity exercise paradigm, the observed negative T2*-weighted signal is likely due to increased muscle oxygen extraction that lead to a net negative T2*-weighted signal change to a degree without involving significant increases in sympathetic activates.
Later in exercise, the T2*-weighted signal in lateral gastrocnemius of the PAD participants steadily increased above baseline. For those muscle groups exhibiting positive T2*-weighted signal change in PAD, it is important to note that the positive T2*-weighted did not start at the beginning of exercise, but during later stages of the exercise period. Clinically claudication is not usually seen right after walking started. As stated above, at the later stage of exercise both MAP and HR increased to a greater extent in PAD than in controls. These findings suggest greater sympathetic nervous system activation and possibly greater muscle recruitment in the PAD patients as compared to the controls. The positive T2*-weighted signals during this stage of exercise in PAD participants were positively correlated with HR and MAP increase, with the maximum T2*-weighted signal change in the calf muscles during exercise significantly correlated with the MAP change. In contrast, no significant correlations were observed in healthy participants during exercise. Of note, our PAD patient data resemble the data for a higher intensity calf workload in normal subjects published by Schmid et al. (38). In that study, the exercise consisted of plantar flexion with 30% of individual maximum voluntary contraction force for 5 min, which is a greater workload but shorter duration compared to our paradigm. In that report a brief negative T2*-weighted signal was first observed at exercise onset (SI dropped to minimum within 1 min) which then increased to a level of 20% above the baseline at exercise end. Another important finding in Schmid’s study is the strong correlation between T2*-weighted signal and pH kinetics during exercise. Based on the similarity in the T2*-weighted signal change, it is likely that our PAD participants under the low-intensity exercise condition also experienced acidosis during the later part of exercise. Under such conditions, the sensations of fatigue and limb pain are not surprising.
We suspect that at this low workload PAD patients did not effectively utilize oxygen throughout the entire lateral gastrocnemius and this lead to the late rise in the T2*-weighted signal. In other words, an underutilization or a mismatch of oxygen utilization is likely the key factor for the positive T2*-weighted signal change observed in PAD. Whether the data in this study is reflective of mitochondria dysfunction, oxidative stress or changes in muscle fiber type cannot be determined from these data (39–41).
Previous studies have shown that there was a delay in patients’ oxygen saturation washout during reactive hyperemia (42, 43). This may help explain why T2*-weighted signal increase in the exercising muscles remained positive even after the exercise stopped. Another contributing factor to the positive T2*-weighted signal could be the delayed phosphocreatine recovery seen in PAD patients during post-exercise (38, 44).
For PAD patients, intermittent claudication is frequently seen during low intensity daily activity such as slow-paced walking. For healthy people, such activities are unlikely to activate the sympathetic nervous system, but in PAD we believe the sympathetic nervous system is activated with low-intensity exercise. As compared to previous studies that used higher workloads (20–30% maximum voluntary contraction force), our paradigm produced distinctive dynamic T2*-weighted signal patterns in PAD subjects in the lateral gastrocnemius.
In most prior studies, a cuff-induced ischemia-reperfusion model (postischemic reactive hyperemia) has been used to assess the skeletal muscle microcirculation function with MRI (45, 46). Quantitative models of exercising calf muscle T2*-weighted signal under ischemic conditions have also been performed, which showed an increase of signal intensity after cuff release (47). A recent normative study at 7.0 T (48) showed that in gastrocnemius, T2*-weighted signal decreased during exercise, while after exercise both T2*-weighted signal and perfusion increased. The above studies on muscle T2*-weighted signal dynamics did not study low-intensity exercise which would more closely simulate the walking induced intermittent claudication in PAD. Under our paradigm, a stable negative T2*-weighted signal was found in the active calf muscles during exercise for the healthy group while in PAD patients, a more dynamic T2*-weighted signal-time-course was found in the lateral gastrocnemius.
The inter-subject variability of T2*-weighted signal-time-course in the PAD group was significantly larger than the healthy group. The inter-subject variability in PAD group could be due to several factors, including disease severity, differences in muscle mass and strength, and the overall physical condition of the subjects. Prior studies have also shown that patients with different severities of PAD exhibited different muscle recruitment strategies to accomplish a given exercise task (49). Future studies should be carried out with a larger cohort and more comprehensive concurrent physiological and clinical measurements to delineate these confounding variables. Nevertheless, we were able to detect significantly different T2*-weighted signal temporal patterns in the healthy participants and the PAD subjects in the later phases of a low intensity exercise paradigm. With all these considerations, we speculate that the positive T2*-weighted signal change during exercise in PAD is due to impaired oxygen utilization. The higher variance in the dynamic T2*-weighted signal in the PAD patients during exercise could reflect differences in disease severity.
There are several limitations for the current study. A single workload (2-kg) was used for every subject. Although the 2-kg load was experimentally determined as a “low” intensity via a preliminary tests using healthy participants, it may not reflect the workload for the PAD subjects. Future studies should verify that the PAD patients and healthy subjects are performing the same relative workload during exercise, for which the duration of the exercise must be also considered as indicated in this study. Because the workload is generally low for the most of the participants, in order to characterize the dynamic physiological behavior of PAD over time, the duration of the paradigm was relatively long, which is inefficient. Thus, more studies should be carried out to find an optimal combination of workload and exercise duration in the future. Lastly, the clinical sample size of the current study is relatively small. For further application of this low-intensity exercise paradigm to PAD studies, a systematic evaluation of the methodology on larger and better characterized human subject samples of both normal healthy and PAD subjects will be needed.
In conclusion, we developed a low intensity plantar flexion exercise paradigm that allows for measurements of the dynamic T2*-weighted signal change during the paradigm. In healthy participants, negative T2*-weighted signal changes were found in exercising muscles with minimal sympathetic activities during this exercise paradigm. In PAD patients, the lateral gastrocnemius showed a positive T2*-weighted signal during the later stages of exercise, accompanied with higher HR, MAP, perceived pain and fatigue. The positive T2*-weighted signal characteristics in these exercising muscles in PAD is likely a presentation of the poor oxygen utilization in the tissue under low-intensity exercise. We demonstrated that, with innovative exercising paradigm designs, T2*-weighted signal dynamics can be an informative avenue for investigating skeletal muscle physiology in this common and important disease.
Acknowledgments
Grant support:
This work was supported, in part, by State Scholarship Fund No.201306940013 (to Z. Li) from China Scholarship Council, NIH Grants UL1 TR000127 and P01 HL096570 (to L. I. Sinoway) and KL2 TR000126 from the National Center for Advancing Translational Sciences (NCATS) and also under a grant with the Pennsylvania Department of Health using Tobacco CURE funds (to M.D. Muller). The Pennsylvania Department of Health and the NIH specifically disclaim responsibility for any analyses, interpretations, or conclusions.
The authors appreciate the technical support provided by Jeff Vesek, Mick Herr, and Amanda Ross, the nursing support provided by Cheryl Blaha and Aimee Cauffman, the statistical support provided by Dr. Ming Wang, and also the support and guidance provided by Dr. Tielian Yu (Tianjin Medical University General Hospital, Department of Radiology).
Footnotes
Informed Consent:
All study protocols were approved by the Pennsylvania State University College of Medicine Institutional Review Board (IRB). Written informed consent was obtained from all volunteers prior to participation in accordance with IRB guidelines.
References
- 1.Ogawa S, Lee TM, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc NatI Acad Sci USA. 1990;87(24):9868–9872. doi: 10.1073/pnas.87.24.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jacobi B, Bongartz G, Partovi S, et al. Skeletal muscle BOLD MRI: from underlying physiological concepts to its usefulness in clinical conditions. J Magn Reson Imaging. 2012;35(6):1253–1265. doi: 10.1002/jmri.23536. [DOI] [PubMed] [Google Scholar]
- 3.Delp MD, Laughlin MH. Regulation of skeletal muscle perfusion during exercise. Acta Physiol Scand. 1998;162(3):411–419. doi: 10.1046/j.1365-201X.1998.0324e.x. [DOI] [PubMed] [Google Scholar]
- 4.Wagner PD. Muscle intracellular oxygenation during exercise: optimization for oxygen transport, metabolism, and adaptive change. Eur J Appl Physiol. 2011;112:1–8. doi: 10.1007/s00421-011-1955-7. [DOI] [PubMed] [Google Scholar]
- 5.Mortensen SP, Svendsen JH, Ersbøll M, et al. Skeletal muscle signaling and the heart rate and blood pressure response to exercise: insight from heart rate pacing during exercise with a trained and a deconditioned muscle group. Hypertension. 2013;61(5):1126–1133. doi: 10.1161/HYPERTENSIONAHA.111.00328. [DOI] [PubMed] [Google Scholar]
- 6.Duling BR, Klitzman B. Local control of microvascular function: role in tissue oxygen supply. Annu Rev Physiol. 1980;42:373–382. doi: 10.1146/annurev.ph.42.030180.002105. [DOI] [PubMed] [Google Scholar]
- 7.Fowkes FG, Rudan D, Rudan I, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013;382:1329–1340. doi: 10.1016/S0140-6736(13)61249-0. [DOI] [PubMed] [Google Scholar]
- 8.Hirsch AU, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; Vascular Disease Foundation. Circulation. 2006;113:e463–654. doi: 10.1161/CIRCULATIONAHA.106.174526. [DOI] [PubMed] [Google Scholar]
- 9.Criqui MH, Langer RD, Fronek A, et al. Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med. 1992;326(6):381–386. doi: 10.1056/NEJM199202063260605. [DOI] [PubMed] [Google Scholar]
- 10.Smith GD, Shipley MJ, Rose G. Intermittent claudication, heart disease risk factors, and mortality. The Whitehall Study. Circulation. 1990;82:1925–1931. doi: 10.1161/01.cir.82.6.1925. [DOI] [PubMed] [Google Scholar]
- 11.Bondke Persson A, Buschmann EE, Lindhorst R, et al. Therapeutic arteriogenesis in peripheral arterial disease: combining intervention and passive training. Vasa. 2011;40:177–187. doi: 10.1024/0301-1526/a000092. [DOI] [PubMed] [Google Scholar]
- 12.Parmenter BJ, Dieberg G, Smart NA. Exercise training for management of peripheral arterial disease: a systematic review and meta-analysis. Sports Med. 2015;45(2):231–244. doi: 10.1007/s40279-014-0261-z. [DOI] [PubMed] [Google Scholar]
- 13.Aherne T, McHugh S, Kheirelseid EA, et al. Comparing Supervised Exercise Therapy to Invasive Measures in the Management of Symptomatic Peripheral Arterial Disease. Surg Res Pract. 2015;2015:960402. doi: 10.1155/2015/960402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.West AM, Anderson JD, Epstein FH, et al. Percutaneous intervention in peripheral artery disease improves calf muscle phosphocreatine recovery kinetics: a pilot study. Vasc Med. 2012;17(1):3–9. doi: 10.1177/1358863X11431837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bajwa A, Wesolowski R, Patel A, et al. Blood Oxygenation Level-Dependent CMR-Derived Measures in Critical Limb Ischemia and Changes With Revascularization. J Am Coll Cardiol. 2016;67(4):420–431. doi: 10.1016/j.jacc.2015.10.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stacy MR, Caracciolo CM, Qiu M, et al. Comparison of regional skeletal muscle tissue oxygenation in college athletes and sedentary control subjects using quantitative BOLD MR imaging. Physiol Rep. 2016;4(16):pii: e12903. doi: 10.14814/phy2.12903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Al-Qaisi M, Nott DM, King DH, Kaddoura S. Ankle brachial pressure index (ABPI): An update for practitioners. Vasc Health Risk Manag. 2009;5:833–841. doi: 10.2147/vhrm.s6759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muller MD, Li Z, Sica CT, et al. Muscle oxygenation during dynamic plantar flexion exercise: combining BOLD MRI with traditional physiological measurements. Physiol Rep. 2016;4(18):pii: e13004. doi: 10.14814/phy2.13004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14(5):377–381. [PubMed] [Google Scholar]
- 20.Satterthwaite TD, Elliott MA, Gerraty RT, et al. An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. Neuroimage. 2013;64:240–256. doi: 10.1016/j.neuroimage.2012.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Komiyama T, Shigematsu H, Yasuhara H, Muto T. An objective assessment of intermittent claudication by nearinfrared spectroscopy. Eur J Vasc Surg. 1994;8(3):294–296. doi: 10.1016/s0950-821x(05)80144-6. [DOI] [PubMed] [Google Scholar]
- 22.Komiyama T, Onozuka A, Miyata T, Shigematsu H. Oxygen saturation measurement of calf muscle during exercise in intermittent claudication. Eur J Vasc Endovasc Surg. 2002;23(5):388–392. doi: 10.1053/ejvs.2002.1645. [DOI] [PubMed] [Google Scholar]
- 23.Osada T, Katsumura T, Murase N, et al. Post-exercise hyperemia after ischemic and non-ischemic isometric handgrip exercise. J Physiol Anthropol Appl Human Sci. 2003;22(6):299–309. doi: 10.2114/jpa.22.299. [DOI] [PubMed] [Google Scholar]
- 24.Villar R, Hughson RL. Repeatability of popliteal blood flow and lower limb vascular conductance at rest and exercise during body tilt using Doppler ultrasound. Physiol Meas. 2013;34(3):291–306. doi: 10.1088/0967-3334/34/3/291. [DOI] [PubMed] [Google Scholar]
- 25.Hiatt WR, Marsh RC, Brammell HL, Fee C, Horwitz LD. Effect of aerobic conditioning on the peripheral circulation during chronic beta-adrenergic blockade. J Am Coll Cardiol. 1984;4:958–963. doi: 10.1016/s0735-1097(84)80057-1. [DOI] [PubMed] [Google Scholar]
- 26.Granger HJ, Goodman AH, Cook BH. Metabolic models of microcirculatory regulation. Fed Proc. 1975;34(11):2025–2030. [PubMed] [Google Scholar]
- 27.Haddy FJ, Scott JB. Metabolic factors in peripheral circulatory regulation. Fed Proc. 1975;34(11):2006–2011. [PubMed] [Google Scholar]
- 28.Honig CR. Contributions of nerves and metabolites to exercise vasodilation: a unifying hypothesis. Am J Physiol. 1979;236(5):H705–H719. doi: 10.1152/ajpheart.1979.236.5.H705. [DOI] [PubMed] [Google Scholar]
- 29.Johnson PC, Henrich HA. Metabolic and myogenic factors in local regulation of the microcirculation. Fed Proc. 1975;34(11):2020–2024. [PubMed] [Google Scholar]
- 30.Menon RS, Ogawa S, Hu X, Strupp JP, Anderson P, Uğurbil K. BOLD based functional MRI at 4 Tesla includes a capillary bed contribution: echo-planar imaging correlates with previous optical imaging using intrinsic signals. Magn Reson Med. 1995;33(3):453–459. doi: 10.1002/mrm.1910330323. [DOI] [PubMed] [Google Scholar]
- 31.Buxton RB, Uludağ K, Dubowitz DJ, Liu TT. Modeling the hemodynamic response to brain activation. Neuroimage. 2004;23(Suppl 1):S220–S233. doi: 10.1016/j.neuroimage.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 32.Cain SM, Chapler CK. O2 extraction by canine hindlimb during alpha-adrenergic blockade and hypoxic hypoxia. J Appl Physiol Respir Environ Exerc Physiol. 1980;48(4):630–635. doi: 10.1152/jappl.1980.48.4.630. [DOI] [PubMed] [Google Scholar]
- 33.Schaffartzik W, Barton ED, Poole DC, et al. Effect of reduced hemoglobin concentration on leg oxygen uptake during maximal exercise in humans. J Appl Physiol (1985) 1993;75(2):491–498. doi: 10.1152/jappl.1993.75.2.491. [DOI] [PubMed] [Google Scholar]
- 34.King-Vanvlack CE, Curtis SE, Mewburn JD, Cain SM, Chapler CK. Role of endothelial factors in active hyperemic responses in contracting canine muscle. J Appl Physiol (1985) 1995;79(1):107–112. doi: 10.1152/jappl.1995.79.1.107. [DOI] [PubMed] [Google Scholar]
- 35.Frahm J, Merboldt KD, Hänicke W, Kleinschmidt A, Boecker H. Brain or vein--oxygenation or flow? On signal physiology in functional MRI of human brain activation. NMR Biomed. 1994;7(1–2):45–53. doi: 10.1002/nbm.1940070108. [DOI] [PubMed] [Google Scholar]
- 36.Zheng Y, Martindale J, Johnston D, Jones M, Berwick J, Mayhew J. A model of the hemodynamic response and oxygen delivery to brain. Neuroimage. 2002;16(3 Pt 1):617–637. doi: 10.1006/nimg.2002.1078. [DOI] [PubMed] [Google Scholar]
- 37.Poole DC, Richardson RS. Determinants of oxygen uptake. Implications for exercise testing. Sports Med. 1997;24(5):308–320. doi: 10.2165/00007256-199724050-00003. [DOI] [PubMed] [Google Scholar]
- 38.Schmid AI, Schewzow K, Fiedler GB, et al. Exercising calf muscle T2* changes correlate with pH, PCr recovery and maximum oxidative phosphorylation. NMR Biomed. 2014;27(5):553–560. doi: 10.1002/nbm.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pipinos II, Judge AR, Selsby JT, et al. The myopathy of peripheral arterial occlusive disease: part 1. Functional and histomorphological changes and evidence for mitochondrial dysfunction. Vasc Endovascular Surg. 2007–2008;41(6):481–489. doi: 10.1177/1538574407311106. [DOI] [PubMed] [Google Scholar]
- 40.Pipinos II, Judge AR, Selsby JT, et al. The myopathy of peripheral arterial occlusive disease: Part 2. Oxidative stress, neuropathy, and shift in muscle fiber type. Vasc Endovascular Surg. 2008;42(2):101–112. doi: 10.1177/1538574408315995. [DOI] [PubMed] [Google Scholar]
- 41.Brass EP, Wang H, Hiatt WR. Multiple skeletal muscle mitochondrial DNA deletions in patients with unilateral peripheral arterial disease. Vasc Med. 2000;5(4):225–230. [PubMed] [Google Scholar]
- 42.Englund EK, Langham MC, Li C, et al. Combined measurement of perfusion, venous oxygen saturation, and skeletal muscle T2* during reactive hyperemia in the leg. J Cardiovasc Magn Reson. 2013;15:70. doi: 10.1186/1532-429X-15-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Englund EK, Langham MC, Ratcliffe SJ, et al. Multiparametric assessment of vascular function in peripheral artery disease: dynamic measurement of skeletal muscle perfusion, blood-oxygen-level dependent signal, and venous oxygen saturation. Circ Cardiovasc Imaging. 2015;8(4):pii: e002673. doi: 10.1161/CIRCIMAGING.114.002673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Isbell DC, Berr SS, Toledano AY, et al. Delayed calf muscle phosphocreatine recovery after exercise identifies peripheral arterial disease. J Am Coll Cardiol. 2006;47(11):2289–2295. doi: 10.1016/j.jacc.2005.12.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thompson RB, Aviles RJ, Faranesh AZ, et al. Measurement of skeletal muscle perfusion during postischemic reactive hyperemia using contrast-enhanced MRI with a step-input function. Magn Reson Med. 2005;54(2):289–298. doi: 10.1002/mrm.20535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ledermann HP, Schulte AC, Heidecker HG, et al. Blood oxygenation level-dependent magnetic resonance imaging of the skeletal muscle in patients with peripheral arterial occlusive disease. Circulation. 2006;113(25):2929–2935. doi: 10.1161/CIRCULATIONAHA.105.605717. [DOI] [PubMed] [Google Scholar]
- 47.Schewzow K, Andreas M, Moser E, Wolzt M, Schmid AI. Automatic model-based analysis of skeletal muscle BOLD-MRI in reactive hyperemia. J Magn Reson Imaging. 2013;38(4):963–969. doi: 10.1002/jmri.23919. [DOI] [PubMed] [Google Scholar]
- 48.Schewzow K, Fiedler GB, Meyerspeer M, et al. Dynamic ASL and T2-weighted MRI in exercising calf muscle at 7 T: a feasibility study. Magn Reson Med. 2015;73(3):1190–1195. doi: 10.1002/mrm.25242. [DOI] [PubMed] [Google Scholar]
- 49.Celis R, Pipinos II, Scott-Pandorf MM, et al. Peripheral arterial disease affects kinematics during walking. J Vasc Surg. 2009;49(1):127–132. doi: 10.1016/j.jvs.2008.08.013. [DOI] [PubMed] [Google Scholar]