Abstract
We sought to determine the frequency by which decreases in left ventricular (LV) end-diastolic volume (LVEDV) with and without increases in end-systolic volume (LVESV) influenced early cancer treatment-associated declines in left ventricular ejection fraction (LVEF) or LV mass. One hundred and twelve consecutively recruited individuals (aged 52 ± 14 years) with cancer underwent blinded cardiovascular magnetic resonance (CMR) measures of LV volumes, mass, and LVEF before and 3 months after initiating potentially cardiotoxic chemotherapy (72 % of participants received anthracyclines). Twenty-six participants developed important declines in LVEF of >10% or to values <50% at 3 months, in whom 19% versus 60%, respectively, experienced their decline in LVEF due to isolated declines in LVEDV versus an increase in LVESV; participants who dropped their LVEF due to decreases in LVEDV lost more LV mass than those who dropped their LVEF due to an increase in LVESV (p=0.03). Nearly a fifth of individuals experience marked LVEF declines due to an isolated decline in LVEDV after initiating potentially cardiotoxic chemotherapy. Since reductions in intravascular volume (which could be treated by volume repletion) may account for LVEDV related declines in LVEF, these data indicate that LV volumes should be reviewed along with LVEF when acquiring imaging studies for cardiotoxicity during treatment for cancer.
Keywords: Cardiovascular magnetic resonance imaging, chemotherapy, cardiotoxicity, cardiac function, cardiac volumes
Introduction
Cancer therapeutics-related cardiac dysfunction (CTRCD) is usually identified through the assessment of left ventricular ejection fraction (LVEF).1-4 It is often assumed that patients experiencing declines in LVEF during cancer treatment is related to cardiomyocyte injury promoting decreases in LV contractility that cause an increase in LV end-systolic volume (LVESV, Figure 1).5-7 It is important to recognize that LVEF may also decline during cancer treatment7,8 due to a decrease in LV end-diastolic volume (LVEDV) or pre-load related to hypovolemia (due to poor PO oral intake and or emesis, Figure 1).9 Since cancer-related survival can depend on the amount of chemotherapy received and this therapy can be withheld if LVEF declines during cancer treatment, we sought to investigate the frequency by which important (>10% or to values <50%) declines in LVEF occurring after receipt of potentially cardiotoxic chemotherapy were due to a decrease in LV pre-load (decrease in LVEDV) as opposed to a decrease in contractility (increase in LVESV). We utilized cardiovascular magnetic resonance (CMR) to determine the pre- and 3-month post-cancer treatment measurements of LVEDV, LVESV and EF in individuals receiving potentially cardiotoxic chemotherapy. Additionally, because LV mass loss has been reported in cancer patients treated with anthracyclines and image derived measures of LV mass can be influenced by intravascular volume status, the relationship of LV mass and LV volumes was also evaluated.10,11
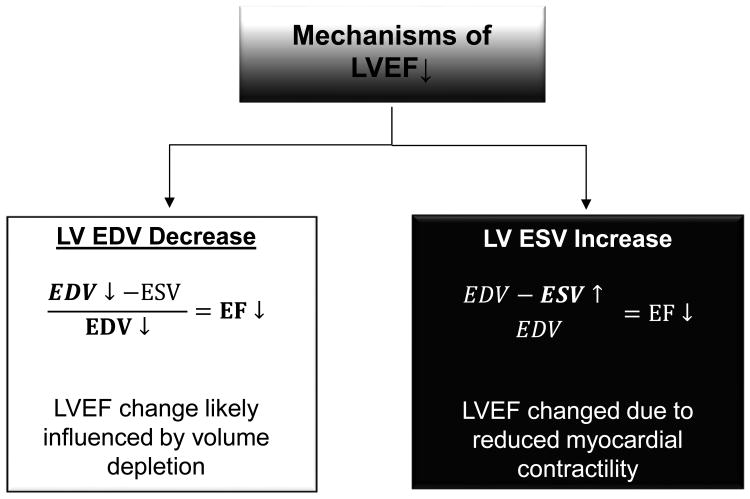
Figure 1. Determinants of declines in left ventricular ejection fraction during receipt of potentially cardiotoxic chemotherapy.
As shown, the left ventricular ejection fraction (LVEF) is calculated by subtracting the end-systolic volume (LVESV) from the end-diastolic volume (LVEDV) and dividing by the original LVEDV. During cancer treatment, there are two mechanisms by which LVEF could decline: 1) the LVEDV could decline (diminished left ventricular preload) and result in a decreased in LVEF (white box). In cancer patients, this may occur due to decreased PO intake, vomiting, or diarrhea; 2) alternatively, the LVEF can also decline if the LVESV increases. This situation occurs when there is diminished left ventricular contractility (black boxes). In patients receiving cancer treatment, this could be due to the adverse consequences of chemotherapy, sepsis, or underlying ischemic heart disease.
Methods
This cohort study was approved by the Institutional Review Board of the Wake Forest School of Medicine; all participants provided witnessed, written informed consent. Cardiovascular magnetic resonance (CMR) images were acquired before and 3 months after initiating cancer treatment. The study population consisted of 112 participants with cancer who were scheduled to receive potentially cardiotoxic chemotherapy. Participants were ineligible if they had a contraindication to CMR (e.g., implanted metal or electronic devices) or if they had an LVEF <50% at baseline. They were consecutively recruited over 5 years from the hematology/oncology outpatient clinics and inpatient hospital facilities within the Comprehensive Cancer Center at the Wake Forest School of Medicine.
To determine LV volumes, EF and mass, all participants underwent scanning on a 1.5-T Magnetom Avanto scanner (Siemens Medical Solutions USA, Malvern, PA) using cine white blood steady-state free precession techniques with a 256 x 128 matrix, a 40-cm field of view, a 10-ms repetition time (TR), a 4-ms echo time (TE), a 20-degree flip angle, a slice 8-mm thick, and a 40-ms temporal resolution. Volumes and mass were also indexed to body surface area (BSA). According to previously published techniques, LV endo- and epicardial contours were drawn at end-diastole and end-systole on offline workstations and summed using Simpson's rule.2,12,13
Descriptive statistics were calculated for all patients. The baseline to 3-month changes in outcomes (body weight, BSA, heart rate, systolic and diastolic blood pressures, pulse pressure, LVEF, EDV, ESV and mass [both unadjusted and indexed by BSA] and arterial elastance) were compared using paired Student's t-tests. Prospectively, CTRCD at 3 months was defined as an LVEF decline of >10% or a drop in LVEF to an absolute value of <50%.3 We assessed the frequency of LVEDV declines (associated with diminished LV preload) or LVESV increases (associated with reductions in myocardial contractility). Large changes in LV volume were defined as an LVEDV drop below the 25th percentile (>19 ml) of all changes in LVEDV, and an LVESV increase above the 75th percentile (>10 ml) of all changes in LVESV. The baseline to 3-month change in LV mass was determined among participants with CTRCD. As previously described, arterial Elastance (EaI) was calculated as end systolic pressure (0.9 × brachial systolic blood pressure)/LV stroke volume indexed to body surface area.14,15 The cumulative anthracycline dose administered to participants from baseline to 3 months was compared among those exhibiting a LVEDV drop or a LVESV increase in individuals with and without CTRCD. Pearson correlations were examined to determine whether changes in LVEDV were correlated with changes in blood pressure and heart rate. We also fit a series of logistic regression models to examine characteristics associated with overall LVEF drop in all participants and in those who experienced an LVEF drop due to an LVEDV decrease without an increase in LVESV or those who experienced an increase in LVESV without a decline in LVEDV. In these models, we examined whether the following were associated with changes in LV volumes: demographic or cardiovascular risk factors (age, gender, race, smoking status, body mass index, coronary artery disease, diabetes and hypertension), the use of cardioprotective medications (beta-blockers, angiotensin converting enzyme inhibitors, or statins), or the receipt of various potentially cardiotoxic chemotherapeutic agents or the use of a diuretic. All values are reported as mean ± standard deviation unless stated otherwise; p-values of ≤0.05 were considered significant. All analyses were performed in SAS version 9.3 (SAS Institute, Cary, NC).
Results
Demographic data from the 112 participants are displayed in Table 1. The potentially cardiotoxic chemotherapeutic agents received by participants included an admixture of anthracyclines (72%), antimicrotubule agents (60%), alkylating agents (74%), and tyrosine-kinase inhibitors (51%). Among those that received tyrosine-kinase inhibitors, 28% received trastuzumab, 20% rituximab, 4% sunitinib and 3% bevacizumab.
Table 1. Characteristics and cardiac magnetic resonance findings of 112 participants before and 3 months after initiating cancer treatment (mean ± standard deviation).
| Characteristic | Prior to initiating chemotherapy n=112 | 3 months after initiating chemotherapy n=112 | p-value |
|---|---|---|---|
| Age (years) | 52 ± 14 | ||
| Women | 70% | ||
| White/black | 82% / 18% | ||
| Coronary Artery Disease | 1 % | ||
| Diabetes Mellitus | 17% | ||
| Hypertension | 41% | ||
| Hyperlipidemia | 29% | ||
| Smoking | 28% | ||
| Cancer Type | |||
| Breast | 49% | ||
| Leukemia | 11% | ||
| Lymphoma | 34% | ||
| Renal cell | 3% | ||
| Sarcoma | 4% | ||
| Cardiac Magnetic Resonance Values | |||
| Body weight (Kg) | 83 ± 23 | 81 ± 21 | <0.0001 |
| Body surface area (m2) | 1.9 ± 0.3 | 1.9 ± 0.3 | 0.7 |
| Heart rate (bpm) | 77 ± 15 | 80 ± 14 | 0.02 |
| Systolic blood pressure (mmHg) | 120 ± 20 | 113 ± 18 | 0.001 |
| Diastolic blood pressure (mmHg) | 69 ± 13 | 66 ± 13 | 0.03 |
| Pulse pressure (mmHg) | 51 ± 15 | 46 ± 13 | 0.01 |
| Left ventricular ejection fraction (%) | 60 ± 6 | 58 ± 7 | <0.0001 |
| Left ventricular end diastolic volume (ml) | 117 ± 38 | 114 ± 35 | 0.23 |
| Left ventricular end diastolic volume index (ml/m2) | 59 ± 15 | 58 ± 15 | 0.34 |
| Left ventricular end systolic volume (ml) | 46 ± 18 | 48 ± 18 | 0.10 |
| Left ventricular end systolic volume index (ml/m2) | 23 ± 8 | 25 ± 8 | 0.03 |
| Stroke volume (ml) | 70 ± 21 | 66 ± 20 | 0.006 |
| Stroke volume index (ml/m2) | 36 ± 9 | 34 ± 9 | 0.009 |
| Left ventricular mass (grams) | 123 ± 39 | 120 ± 38 | 0.26 |
| Left ventricular mass index (gram/m2) | 62 ± 15 | 61 ± 16 | 0.39 |
| Arterial elastance (mmHg/ml) | 3.2 ± 0.9 | 3.2 ± 1.2 | 0.85 |
Overall body weight, BSA, heart rate, systolic and diastolic blood pressures, LVEF, EDV, ESV and mass [both unadjusted and indexed by BSA] and arterial elastance for the baseline and 3-month exams are shown in Table 1. Individuals with large declines in LVEF experiencing our pre-defined decline in LVEDV or increase in LVESV are shown in Figure 2. In Figure 3, those experiencing marked changes in LV volumes without a substantive change in LVEF are shown. Overall, baseline to 3-month changes in LVEDV were correlated with systolic blood pressure changes (r=0.2, p=0.05) and heart rate changes (r=-0.3, p=0.003). We found no association between changes in brachial diastolic blood pressure or pulse pressure and LVEDV. Similarly, we found no changes in EaI (p=0.85) in the population as a whole or in the subgroup of participants that dropped their LVEF due to LVESV increases (p=0.46).
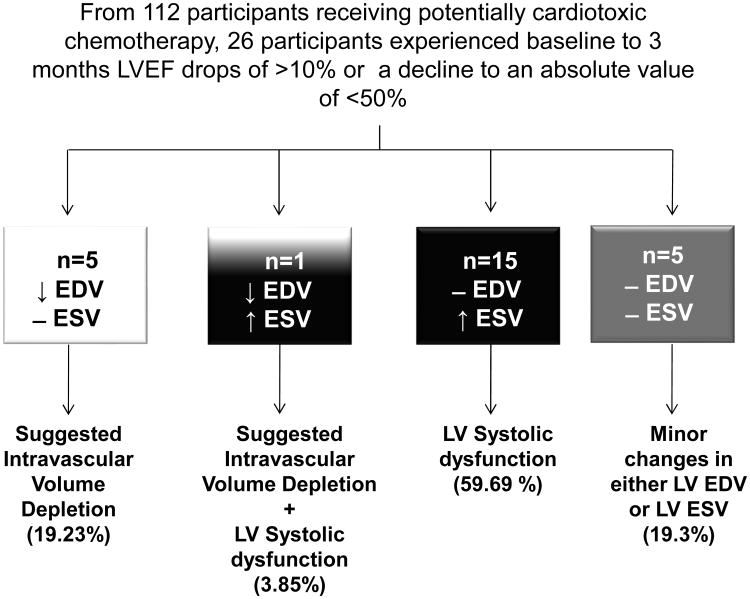
Figure 2. Individuals that experience cancer therapeutics-related cardiac dysfunction (CTRCD) defined as LVEF drops of >10% or a decline to an absolute value of <50%, 3 months after initiating potentially cardiotoxic chemotherapy.
Participants who experienced CTRCD due to isolated LVEDV declines are shown in the white box; those who experience increases in LVESV with minimal changes to LVEDV (impaired LV contractility) are shown in the black box; participants who had a combination of LVEDV drop and LVESV increase are shown in white and black box and those with minor LVEDV and LVESV changes are shown in the gray box.
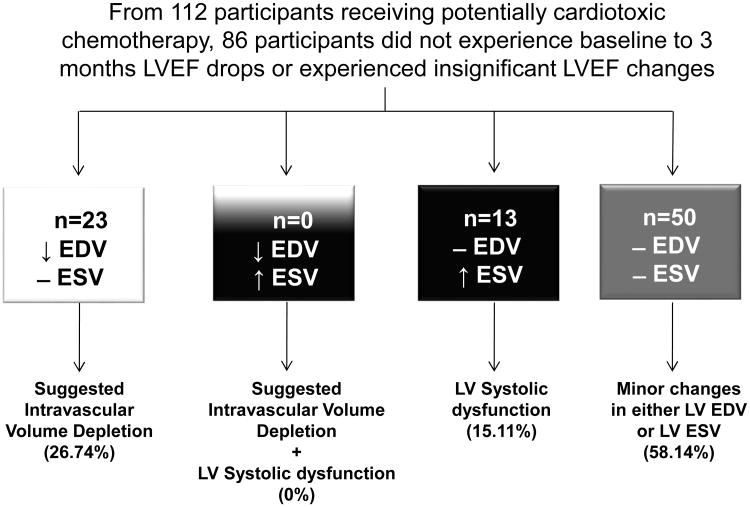
Figure 3. Individuals that did not experience LVEF changes or that LVEF drops did not meet cancer therapeutics-related cardiac dysfunction (CTRCD) criteria (LVEF drops of >10% or a decline to an absolute value of <50%) 3 months after initiating potentially cardiotoxic chemotherapy.
Participants who experienced isolated LVEDV declines are shown in the white box; those who experience increases in LVESV with minimal changes to LVEDV (impaired LV contractility) are shown in the black box; participants who had a combination of LVEDV drop and LVESV increase are shown in the white and black box and those with minor LVEDV and LVESV changes are shown in the gray box.
The development of CTRCD due to isolated increases in LVESV were associated with smoking (p=0.02) and borderline associated with the receipt of anthracyclines (p=0.06). In multivariable analyses, no factors (p=NS) were associated with LVEDV mediated CTRCD, including the receipt of tyrosine-kinase inhibitor and diuretics.
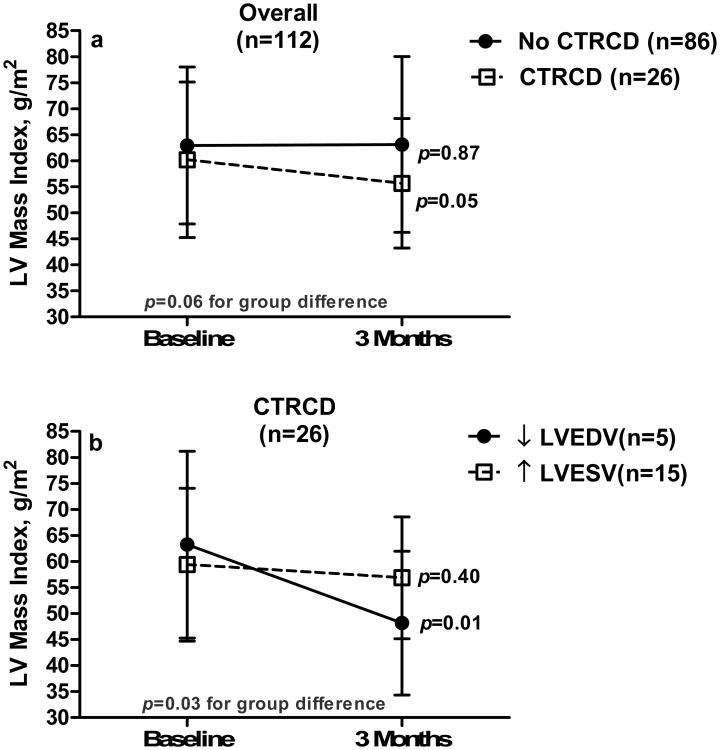
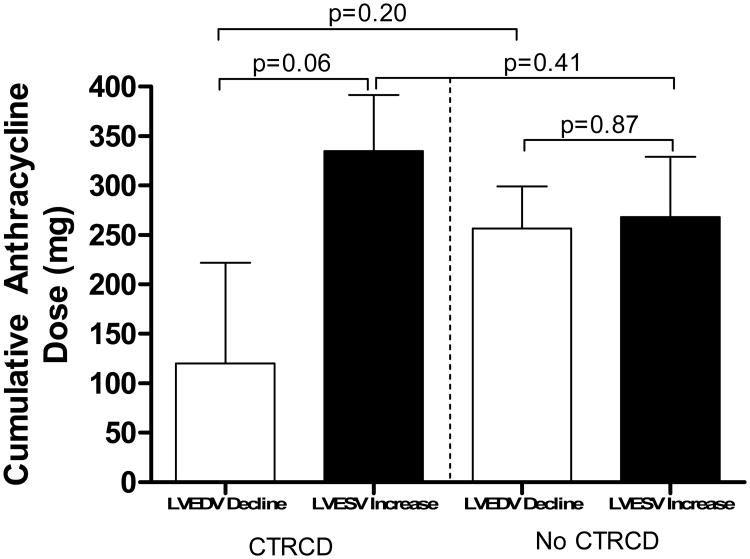
Figure 4a illustrates the LV mass index changes from baseline to 3-months in individuals with and without CTRCD, and Figure 4b depicts the sub-group analysis of LV mass index changes from baseline to 3-months post-chemotherapy among CTRCD individuals. The cumulative anthracycline dose administered to participants from baseline to 3-months and the change in LVEF related to LV volume fluctuations is depicted in Figure 5.
Figure 4.
a-Left ventricular (LV) mass changes among individuals with (dotted line) and without (solid line) cancer therapeutics-related cardiac dysfunction (CTRCD) and b- LV mass changes among participants with CTRCD due to decreases in LV end diastolic volume (EDV, solid line) and increases in LV end systolic volume (ESV, dotted line).
Figure 5.
Mean cumulative doses of anthracyclines administered to participants with and without CTRCD due to LVEDV decline (white bars) and LVESV increase (black bars).
Discussion
In this consecutively recruited cohort of individuals receiving potentially cardiotoxic chemotherapy for the treatment of cancer, 23% experienced CTRCD defined as a pre-treatment to 3 months after initiation of treatment LVEF decline of >10% or a drop in LVEF to an absolute value of <50%. Among participants with CTRCD, 19% of the 3-month declines in LVEF occurred due to LVEDV declines of >19 mL which was within the top 25th percentile of all LVEDV declines in the cohort (Figure 2). Furthermore, LV mass loss among CTRCD participants was associated with LVEDV declines of >19 ml (Figure 4). These data indicate that clinically relevant drops in LVEF and loss of LV mass in patients receiving cardiotoxic chemotherapy may be related to hypovolemia rather than increases in LVESV, which often reflect reduced LV myocardial contractility due to LV myocellular injury from receipt of cardiotoxic chemotherapy. We found no univariable associations between any of the risk factors for the development of CTRCD due to a decrease in LVEDV including the receipt of oral diuretics. We found no changes in EaI, suggesting that an increase in arterial elastance was not necessarily responsible for the declines in LVEF we observed in our study population that were mediated by increases in LVESV. Participants averaged 52 years of age and ∼70% were white women. These demographic metrics are consistent with the fact that 49% of the participants in the study were women treated for breast cancer, and 45% were treated for leukemia or lymphoma (Table 1). The mildly reduced overall LVEF for the cohort in this study (60 ± 7% which is somewhat lower than the LVEF for women [71 ± 7%] or men [67 ± 7%] in other population-based studies without cancer, such as from the Multi-Ethnic Study of Atherosclerosis [MESA]),13 may be related to the fact that this cancer population exhibited multiple CV co-morbidities before initiating their cancer treatment (Table 1).13 Participants also exhibited weight loss and slightly lower blood pressure 3 months after initiating their chemotherapeutic regimen.
There are important clinical implications regarding the results of this study. Although LVEF is commonly utilized to identify “cardiotoxicity” after receiving treatment for cancer,3 simultaneous assessments of LV volumes have been less emphasized. The results of this study indicate that observed declines in LVEF should be evaluated in relation to changes in LV preload (as reflected by LVEDV measures) and myocardial contractility (as assessed by changes in LVESV). If one simply reports the LVEF, then those responsible for managing the patient receiving chemotherapy could inadvertently alter patient management for potentially incorrect reasons. For example, stopping potentially life-saving but cardiotoxic chemotherapy or initiation of cardio-protective medications that can lower blood pressure4,16 may not be advised if the LVEF declined as a result of a decrease in LVEDV (or preload). In this situation, oral or intravenous hydration to restore intravascular volume, LV preload, and LVEF may be more appropriate.
The cumulative dose of anthracycline trended toward an association of LVEF drop due to increases in LVESV (Figure 5); however, due to the small number of participants receiving anthracycline-based chemotherapy in this study, clarity regarding the relationship between the cumulative dose of anthracyclines and the change in LVEDV or LVESV mediated declines in LVEF requires further study. In the current study, overall LV mass declines observed 3 months after initiation of chemotherapy were more frequently associated with declines in LVEF associated with drops in LVEDV (p=0.01) as compared to increases in LVESV (p=0.40). These findings suggest that during treatment with potentially cardiotoxic chemotherapy, imaging measures of LV mass may fall in relation to reductions in intravascular volume that diminish sarcomere size or reduce the volume of the interstitial space between the sarcomeres. Additional studies incorporating MRI mapping methods17 could measure the myocardial extracellular volume simultaneously with LV mass during receipt of potentially cardiotoxic chemotherapy and provide additional insight into these questions. We did not observe increases in LVEDV (Table 1) in this study suggesting that at 3 months post-initiation of potentially cardiotoxic chemotherapy myocardial remodeling/dilation were not present in this cohort.
Strengths of the study include the use of CMR to accurately measure LV volumes and EF, which have been assessed in large population studies.10,13,17,18 Limitations to the study include the fact that we did not assess LV diastolic function in our analyses.4 As a result, it is uncertain how LV diastolic dysfunction impacts our results. Additionally, long-term follow-up of the participants is not yet available, and therefore the clinical relevance of these pre- to 3-month post-initiation of chemotherapy changes in LV volumes, EF, and mass are uncertain.
Acknowledgments
Financial Support: This research was supported in part by National Institutes of Health grants R01CA167821, R33CA12196, and R01HL118740. Winston-Salem, NC, USA.
Footnotes
Disclosures: Conflicts of interest: none. All authors have approved the final article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vera T, D'Agostino RB, Jr, Jordan JH, Whitlock MC, Meléndez GC, Lamar ZS, Porosnicu M, Bonkovsky HL, Poole LB, Hundley WG. Relation of Pre-anthracycline Serum Bilirubin Levels to Left Ventricular Ejection Fraction After Chemotherapy. Am J Cardiol. 2015;116:1752–1755. doi: 10.1016/j.amjcard.2015.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drafts BC, Twomley KM, D'Agostino R, Jr, Lawrence J, Avis N, Ellis LR, Thohan V, Jordan J, Melin SA, Torti FM, Little WC, Hamilton CA, Hundley WG. Low to moderate dose anthracycline-based chemotherapy is associated with early noninvasive imaging evidence of subclinical cardiovascular disease. JACC Cardiovasc Imaging. 2013;6:877–885. doi: 10.1016/j.jcmg.2012.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Plana JC, Galderisi M, Barac A, Ewer MS, Ky B, Scherrer-Crosbie M, Ganame J, Sebag IA, Agler DA, Badano LP, Banchs J, Cardinale D, Carver J, Cerqueira M, DeCara JM, Edvardsen T, Flamm SD, Force T, Griffin BP, Jerusalem G, Liu JE, Magalhães A, Marwick T, Sanchez LY, Sicari R, Villarraga HR, Lancellotti P. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2014;27:911–939. doi: 10.1016/j.echo.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz RG, Venci N. Can serial changes of diastolic dysfunction signal incremental risk of chemotherapy-induced heart failure missed by the timing of declining LV ejection fraction? J Nucl Cardiol. 2016;23:833–836. doi: 10.1007/s12350-015-0194-4. [DOI] [PubMed] [Google Scholar]
- 5.Lightfoot JC, D'Agostino RB, Jr, Hamilton CA, Jordan J, Torti FM, Kock ND, Jordan J, Workman S, Hundley WG. Novel approach to early detection of doxorubicin cardiotoxicity by gadolinium-enhanced cardiovascular magnetic resonance imaging in an experimental model. Circ Cardiovasc Imaging. 2010;3:550–558. doi: 10.1161/CIRCIMAGING.109.918540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vasu S, Hundley WG. Understanding cardiovascular injury after treatment for cancer: an overview of current uses and future directions of cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2013;15:1–18. doi: 10.1186/1532-429X-15-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nguyen KL, Hu P, Ennis DB, Shao J, Pham KA, Chen JJ. Cardiac MRI: A Translational Imaging Tool for Characterizing Anthracycline-Induced Myocardial Remodeling. Curr Oncol Rep. 2016;18:1–14. doi: 10.1007/s11912-016-0533-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwartz RG, Jain D, Storozynsky E. Traditional and novel methods to assess and prevent chemotherapy-related cardiac dysfunction noninvasively. J Nucl Cardiol. 2013;20:443–464. doi: 10.1007/s12350-013-9707-1. [DOI] [PubMed] [Google Scholar]
- 9.Jacob M, Chappell D. Effects of perioperative fasting on haemodynamics and intravascular volumes. Best Pract Res Clin Anaesthesiol. 2012;26:421–430. doi: 10.1016/j.bpa.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 10.Jordan JH, Vasu S, Morgan TM, D'Agostino RB, Jr, Meléndez GC, Hamilton CA, Arai AE, Liu S, Liu CY, Lima JA, Bluemke DA, Burke GL, Hundley WG. Anthracycline-Associated T1 Mapping Characteristics Are Elevated Independent of the Presence of Cardiovascular Comorbidities in Cancer Survivors. Circ Cardiovasc Imaging. 2016;9:e004325. doi: 10.1161/CIRCIMAGING.115.004325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neilan TG, Coelho-Filho OR, Pena-Herrera D, Shah RV, Jerosch-Herold M, Francis SA, Moslehi J, Kwong RY. Left ventricular mass in patients with a cardiomyopathy after treatment with anthracyclines. Am J Cardiol. 2012;110:1679–1686. doi: 10.1016/j.amjcard.2012.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jordan JH, D'Agostino RB, Jr, Hamilton CA, Vasu S, Hall ME, Kitzman DW, Thohan V, Lawrence JA, Ellis LR, Lash TL, Hundley WG. Longitudinal assessment of concurrent changes in left ventricular ejection fraction and left ventricular myocardial tissue characteristics after administration of cardiotoxic chemotherapies using T1-weighted and T2-weighted cardiovascular magnetic resonance. Circ Cardiovasc Imaging. 2014;7:872–879. doi: 10.1161/CIRCIMAGING.114.002217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heckbert SR, Post W, Pearson GD, Arnett DK, Gomes AS, Jerosch-Herold M, Hundley WG, Lima JA, Bluemke DA. Traditional cardiovascular risk factors in relation to left ventricular mass, volume, and systolic function by cardiac magnetic resonance imaging: the Multiethnic Study of Atherosclerosis. J Am Coll Cardiol. 2006;48:2285–2292. doi: 10.1016/j.jacc.2006.03.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bombardini T, Costantino MF, Sicari R, Ciampi Q, Pratali L, Picano E. End-systolic elastance and ventricular-arterial coupling reserve predict cardiac events in patients with negative stress echocardiography. Biomed Res Int. 2013;2013:1–14. doi: 10.1155/2013/235194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen CH, Fetics B, Nevo E, Rochitte CE, Chiou KR, Ding PA, Kawaguchi M, Kass DA. Noninvasive single-beat determination of left ventricular end-systolic elastance in humans. J Am Coll Cardiol. 2001;38:2028–2034. doi: 10.1016/s0735-1097(01)01651-5. [DOI] [PubMed] [Google Scholar]
- 16.Prisant LM, Kleinman DJ, Carr AA, Bottini PB, Gross CM. Assessment of echocardiographic left ventricular mass before and after acute volume depletion. Am J Hypertens. 1994;7:425–428. doi: 10.1093/ajh/7.5.425. [DOI] [PubMed] [Google Scholar]
- 17.Meléndez GC, Jordan JH, D'Agostino RB, Jr, Vasu S, Hamilton CA, Hundley WG. Progressive 3-Month Increase in LV Myocardial ECV After Anthracycline-Based Chemotherapy. JACC Cardiovasc Imaging. 2016 doi: 10.1016/j.jcmg.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Armstrong GT, Plana JC, Zhang N, et al. Screening adult survivors of childhood cancer for cardiomyopathy: comparison of echocardiography and cardiac magnetic resonance imaging. J Clin Oncol. 2012;30:2876–2884. doi: 10.1200/JCO.2011.40.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]